Abstract
The architecture of the subterranean nests of the Florida harvester ant, Pogonomyrmex badius, was studied through excavation and casting. Nests are composed of two basic units: descending shafts and horizontal chambers. Shafts form helices with diameters of 4 to 6 cm, and descend at an angle of about 15–20° near the surface, increasing to about 70° below about 50 cm in depth. Superficial chambers (< 15 cm deep) appear to be modified shafts with low angles of descent, and are distinct from deeper chambers. In larger nests, they have a looping, connected morphology. Chambers begin on the outside of the helix as horizontal-floored, circular indentations, becoming multi-lobed as they are enlarged. Chamber height is about 1 cm, and does not change with area. Chamber area is greatest in the upper reaches of the nest, and decreases with depth. Vertical spacing between chambers is least in the upper reaches and increases to a maximum at about 70 to 80% of the maximum depth of the nest. The distribution of chamber area is top-heavy, with about half the total area occurring in the top quarter of the nest. Each 10% depth increment of the nest contains 25 to 40% less area than the decile above it, no matter what the size of the nest.
Nests grow by simultaneous deepening, addition of new chambers and/or shafts and enlargement of existing chambers. As a result, the vertical spacing between chambers is similar at all nest sizes, and the relative distribution of chamber area with relative nest depth did not change during colony growth (that is, the size-free nest shape was the same at all colony sizes). Total chamber area increased somewhat more slowly than the population of workers excavating the nest. The branching of shafts was consistently shallow (< 40 cm), somewhat more so in large nests than small. Large colonies rarely had more than 4 shaft/chamber series. Each new series contributed less to the total chamber area because its chambers were smaller. Incipient colonies were usually 40 to 50 cm deep while mature colonies were commonly 2.5 to 3.0 m deep.
Workers captured near the top of a mature nest (and therefore older) and penned in escape proof enclosures, excavated larger nests than did young workers captured from the bottom of the nest. Most of this difference was due to a larger fraction of older workers engaging in digging, rather than an increase in their rate of work. All ages of workers produced similar top-heavy nests. When different ages of workers from different levels of a mature colony were allowed to re-assort themselves in a vertical test apparatus buried in the soil, older workers moved upward to assume positions in the upper parts of the nest, much as in the colonies from which they were taken. The vertical organization of workers based on age is therefore the product of active movement and choice. A possible template imparting information on depth is a carbon dioxide gradient. Carbon dioxide concentrations increased 5-fold between the surface and the depths of the nest. A preference of young workers for high carbon dioxide concentrations, and a tendency for workers to dig more under low carbon dioxide concentrations could explain both the vertical age-distribution of workers, and the top-heaviness of the nest's architecture.
Keywords: Formicidae, nest excavation, colony size, colony depth, chambers, shafts, nest structure, division of labor
Introduction
The construction of the conspicuous paper, carton or wax nests by worker wasps, termites and bees has long invited investigation, and has generated many fertile ideas, including templates, self-organization, self-assembly and stigmergy (indirect communication through the environment). What wasps, termites and bees build and how they build it has been reviewed several times (Downing and Jeanne 1988; Theraulaz et. al. 1999; Seeley 1995; Karsai and Penzes 1993; Winston 1987; Hansell 1984; Wilson 1971; Korb, 2003). The nests of these social insects are examples of how complex results can emerge from the actions of many independent workers responding using simple rules to local information. In recent years, there has been intense interest in such emergent, self-organizing phenomena, in part because of the perception that they are central to social insect colonies.
In contrast, the architecture of subterranean ant nests is largely terra incognita, and the literature on it is sparse, unsystematic, unquantitative and non-experimental. These subterranean nests are hidden from view, and are fundamentally different because they are made by the removal of soil, not by construction. As a result, these nests are more difficult to study, and have received little attention. By and large, information on them is collected incidentally, rather than as a primary goal of research. Moreover, interior spaces are difficult to render from excavations, and most reports are simply verbal descriptions of nest structure or drawings of variable detail, with little quantitative or comparative information (McCook 1879; Wheeler 1910; Talbot and Kennedy 1940; 1964; Scherba 1961; Ettershank 1968; Dlussky 1968; MacKay 1981; Kugler and Hincapie 1983; Conway 1983; Lavigne, 1969; Nielsen and Jensen, 1975; McCahon and Lockwood, 1990; Bristow et al., 1992: Peeters 1994; Antonialli-Junior and Gianotti 1997; Crosland 1995; Nielsen and Jensen 1975; Ettershank 1971). At one extreme, excavation of the nests of Atta species, in spite of being a heroic undertaking, has been reported several times (Autuori 1942; Jonkman 1980; Moser, 1963).
A few studies provided some quantitative description of the architecture of ant nests. Tschinkel (1987; 1999b) and Mikheyev and Tschinkel (2004) determined chamber area and shape in relation to depth and colony size in Prenolepis imparis, Pogonomyrmex badius and Formica pallidefulva, respectively. In P. badius and F. pallidefulva, average chamber size decreased downward, whereas spacing between chambers increased, so that over half the total chamber area was in the top quarter of the nest. In both, nest size was strongly correlated to the number of workers in the colony. The use of dental plaster to make casts of ant nests, first reported by Williams and Lofgren (1988), introduced a superior method for rendering the interior space of nests in three dimensions, and is one of the methods used in the work described here.
In spite of the shortcomings of most nest architectural studies, it is clear that ant nests are highly structured and species-typical in both size and morphology. It is much less apparent that the distribution of colony members and activities within natural nests are also spatially structured. Such structure takes on more significance when the ant colony is viewed as a superorganism. As observed by Bonner (1974) and Sendova-Franks and Franks (1995), the parts of a “regular” organism are attached to one another to create a spatial structure, but the individuals in a social insect colony move freely. Spatial structure of a social insect colony, unlike the nest it occupies, is thus not immediately apparent. However, the generally centrifugal movement of aging workers away from the brood (Hölldobler and Wilson 1990) creates an association of worker age, location and task, that is, a spatial social structure, even in laboratory colonies (Hölldobler and Wilson 1990; Sendova Franks and Franks 1995).
Most field studies, even those based on careful excavations, provide no quantitative information on the distribution of ants among nest chambers (Dlussky 1981; Darlington 1997; Conway 1983). Exceptions include Kondoh's (1968a, b) study of the seasonal distribution of fat-repletes within the nest of Formica japonica. Pogonomyrmex harvester ants show consistent patterns of distribution of seeds within their subterranean nests (Lavigne 1969; Tschinkel, 1999a, b). In several species of harvester ants, and in Prenolepis imparis, workers are stratified by age and season: young workers are found deep in the nest and migrate upward as much as 3 to 4 meters as they age (Chew 1960; Golley and Gentry 1964; MacKay 1981ab; Tschinkel 1987; Tschinkel 1998; 1999a, b; Porter and Jorgesen 1981). In Pogonomyrmex badius nests, seeds are stored exclusively in chambers between 40 and 100 cm below ground, and brood and newly emerged workers are located primarily in the bottom third. Movement of aging workers away from the brood therefore causes them to migrate upward in the nest, leading to an increase in older workers in the upper regions of the nest (Tschinkel 1999b). Worker density increases dramatically with depth, with young workers densely packed in the lower chambers and older workers sparsely occupying the upper chambers. By inserting barriers to vertical movement from the side of a pit, Tschinkel showed that these distributions are not artifacts of driving ants downward during the excavation procedure. The great vertical spread of the nest is thus associated with an equally great vertical organization of the colony within.
A few descriptions of worker nest-excavation behavior exist, most notably those of Sudd (1969; 1982), who tried to derive the nest's architecture from the orientations of individual ants digging in moist sand in the laboratory. However, these ants never produced chambers. Mikheyev and Tschinkel (2004) found that chambers in F. pallidefulva nests tend to be oriented like the shafts leading to them. Franks & Deneubourg (1997) examined the construction (not excavation) of the simple nest walls of an ant that nests between flat rocks, and showed that simple behaviors accounted for most of the nest attributes. Several researchers estimated the work involved in nest excavation (Sabiti 1980; Nielsen and Jensen 1975; Lockaby and Adams 1985), but the emphasis in these was ecological, not architectural. Sudd (1969) estimated that a worker might expend 10% of its daily energy intake on nest excavation. Nests of F. pallidefulva that moved twice a year would expend about 20% of their energy intake and 6% of their time on nest excavation (Mikheyev and Tschinkel, 2004). Sudd and Franks (1987) summarized construction of above ground mounds.
Materials and Methods
Study Site
All study colonies were located at a site about 16 km southwest of Tallahassee, Florida, USA, within the Apalachicola National Forest. The site consists of excessively drained sandy soil occupying a slope to a wetland and stream, causing its water table to be depressed, thereby making it suitable for P. badius, as well as several xeric species of plants such as Opuntia and Nolina. The remaining vegetation included young longleaf pines, broomsedge (Andropogon sp.) and several other successional species.
Methods of study
North Florida is an ideal location for studying ant nest architecture because of its deep, homogeneously sandy soils. There are no stones, barriers or layers of varying resistance. The nests in this soil are strictly the result of the ants' intrinsic behavioral programs, unconstrained by variations in the medium.
The architecture of the nest void was rendered by two methods. In the first, a pit was dug next to the nest and the chambers were exposed horizontally, one at a time, starting from the top. The depth and orientation of each chamber were recorded, its contents collected for later counting and measuring, and its outlines traced on transparent acetate sheets. Using this method, detailed excavations were completed and complete census of 33 Florida harvester ant nests (Pogonomyrmex badius), ranging from 2 to 3.02 m deep and consisting of 5 to 150 chambers. This method produced a reasonable rendering of the two-dimensional outlines and spatial distribution of the horizontal chambers, and a complete record of the distribution of ants and seeds within the nest. It did not record the vertical shafts connecting chambers. Except for the details of nest architecture, the results of these excavations have been published (Tschinkel 1998; 1999a; b)
In the second method, the nest void was filled with a thin slurry of orthodontal plaster poured into the nest entrance (Williams and Lofgren 1988). This produced a (usually) perfect three-dimensional rendering of the nest's voids. The hardened cast was excavated and then reassembled to produce the finished cast. For reassembly, chambers were supported with steel rods driven into holes in a backboard. Because the shafts of P. badius are large in diameter, the thin slurry often completely filled a 3 m-deep nest in a single pour. The sandy soils of the Florida coastal plains allow the plaster slurry to displace the air within the chambers, filling them completely. Heavy clay soils often produce incomplete casts and voids within the plaster because of trapped air. Although a few images of the nest casts of several ant species have been published (Tschinkel 2003), the details of the nest architecture of P. badius are presented here for the first time.
Because reconstruction of a plaster cast often required considerable time, a method was developed for melting zinc or aluminum in a portable kiln fired with charcoal and provided with an air blast from a battery-powered automobile heater fan. As with plaster, the molten metal is poured into the nest entrance and flows until it freezes. Each metal has its advantages and limitations. Zinc is brittle, but because of its lower melting point, flows deeper into a nest, whereas aluminum makes extremely strong casts, but does not penetrate as deeply. For very deep nests, both metals require excavation of the cast followed by a second episode of pouring. Pouring red-hot aluminum in the bottom of a 2-meter pit runs the risk of having ones socks catch on fire from the radiant heat.
Pen experiments
The worker population of P. badius colonies is vertically organized by age, with the youngest workers in the bottom chambers of the nest and the oldest in the upper chambers (Tschinkel 1999a). In order to test the digging characteristics of workers of different mean ages, a pit was dug adjacent to a colony, and workers were extracted from upper, middle and lower chambers from the side of the pit. The workers from each level were penned with food and water in an escape-proof, 30 × 30 cm sheet-metal enclosure and allowed to dig for 4 to 5 days. At the end of this period, all workers that came to the surface in a half-hour period were recaptured, the nest filled with dental plaster and excavated. This procedure was replicated with four colonies, with 125 to 250 workers per pen.
Analysis of the nest architecture
Using either plaster casts or outline drawings of chambers, several morphological nest features were measured: (1) chambers: number, chamber floor area; (2) chamber outline shape: ratio of perimeter to area as an estimate of outline complexity; (3) shafts: number of branches, length; slope and diameter of the shaft helix; (4) location of each chamber in relation to the surface; vertical spacing between chambers. This set of features captures most of what seems significant about nest morphology.
Data analysis
Most of the data were analyzed with standard statistical methods, including analysis of variance, regression and t-tests. Data were transformed as needed to stabilize variance. For several analyses, colonies were grouped into size classes based roughly on the logarithms of the number of workers, as in Tschinkel (1999a, b). The classes were as follows: class 0, 0–100 workers; class 1, 101–800; class 2, 801–2000; class 3, 2001–4000; class 4, > 4000 workers.
Results
Nest overview
The nest created by a mature colony of harvester ants is undeniably spectacular (Fig. 1). It is made more so by understanding that the nest in Fig. 1 was created by about 5000 workers (15–20 g), who excavated about 20 kg of sand in 4 to 5 days (see below), and that, because a colony moves once or twice a year, it creates one or two of these nests a year. This highlights two more significant facts: (1) the nest, with its characteristic structure and top-heavy arrangement of chambers is the “intentional” outcome of a short period of digging, not of long-term growth of the original founding nest. The relatively large size of the upper parts is not the result of having been in existence longer and therefore enlarged more. No colony occupies an enlargement of its founding nest. (2) No matter whether a colony moved a short or long time before excavation, the vertical arrangement of workers of different ages, seeds and brood is always the same (Tschinkel, 1999a). It follows that although the vertical arrangement becomes completely unglued during the move to a new nest, it is recreated in the new nest. This vertical arrangement is therefore not an accident of maintaining brood in the bottom of the nest, but is “deliberate”, and thus part of the structure of the superorganism.
Figure 1.

A plaster cast of a large Pogonomyrmex badius nest. This nest consisted of 135 chambers and 12 meters of vertical shafts. The top-heavy distribution of chamber area and spacing is typical for the species, as are the helical shafts and the decrease of chamber size with depth (photo by Charles Badland).
Basic Nest Structural Units: shafts
Nests are composed of two types of units, shafts, and chambers. Shafts are defined as elongated voids with a circular, oval or flattened-oval cross-section, with a long axis usually inclined from the vertical by 20° to 70° (rarely 90°). Shaft diameters average a little less than 1 cm. In the upper parts of the nest, shafts are usually larger and may have a flattened-oval cross-section up to about 2 cm wide.
Descending shafts
Nests usually had a single entrance, though two or three entrances were sometimes present. From the entrance, a shaft descended at an angle of about 20–30° from horizontal, gradually increasing to 45 to 60° by about 50 cm and below. The shaft spiraled, more often to the left than to the right, forming a helix about 4–6 cm in diameter. Because the shaft was steeper at greater depth, the shaft length per turn gradually increased from about 8–10 cm per turn near the surface to about 20 cm per turn deeper in the nest.
Branches
Occasionally, and usually in the top half of the nest, shafts branched, usually at the base of a chamber, creating an additional descending shaft-and-chamber series. A branch may be equal to the original, or shorter. It may spiral to the right or left. There are rarely more than 2 branches in a shaft. The number of vertical chamber series ranged from one in small nests to a typical maximum of 4 in the largest nests.
Basic Structural Units: Chambers
Superficial Chambers
Chambers within 10 to 15 cm of the surface arise differently than deeper ones. Near-surface chambers begin as horizontal or nearly horizontal shafts, and are gradually widened and branched until they appear like complex, lobed, interconnecting, looping chambers. Unlike deeper chambers, lobes, branches and extensions were often located on both sides of these superficial shafts (or chambers). Fig. 2 shows a series of such superficial chambers from nests of increasing size, and demonstrates that the highly connected chambers of larger nests arise through the enlargement and fusion of superficial shafts. It is likely that these superficial chambers result from a modification of the behavioral program that produces shafts. They often lie within the soil excavated from deeper and dumped around the nest entrance.
Figure 2.
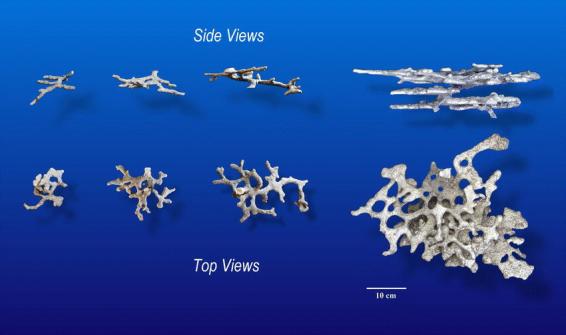
Superficial chambers from Pogonomyrmex badius nests of increasing size, shown in side and top views. These “chambers” are probably produced by a modification of the behavioral program producing shafts throughout the deeper portions of the nest. Note the increasingly interconnected and loop-like morphology as the shafts become extended and enlarged.
Deeper chambers
In contrast to shafts, chambers had more or less horizontal floors and a horizontal outline ranging from near circular when small, to multi-lobed when large. In vertical cross-section, they were flattened or slightly domed, with the horizontal dimension much greater than the vertical. All chambers were about 1 cm high, floor to ceiling, no matter what the floor area. Shafts usually intersected chambers at an edge, and connected sequential chambers. Below about 15 to 20 cm, chambers always began as lateral, horizontal-floored extensions from the outside of a spirally-descending shaft (Fig. 3A) that therefore intersected the inner edge of the chamber at an angle ranging from 25°to about 70° (Fig. 3B–D).
Figure 3.
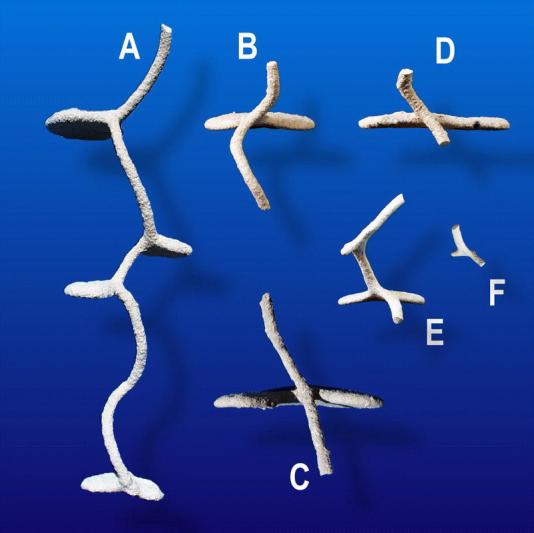
A. The descending shafts of Pogonomyrmex badius nests form a helix. B–D. Chambers are constructed outward from the outside of this helix, so that the intersection between chambers and the helical shaft forms an angle between 25 and 70°. The angle may be either right or left of vertical (compare A and B with C and D). E–F. Chambers are initiated at the outside of the helix as small, flat-bottomed niches.
Chamber morphology was rarely elongated and shaft-like, but began as a niche in the outer wall of a shaft (Fig. 3E–F). Initially, these incipient chambers had a circular outline, but as workers enlarged them, they became increasingly lobed (Fig. 4), deviating ever more from the initial circular outline. This deviation from circularity was measured as the ratio of the actual perimeter of a chamber to the perimeter of a circle of the same area (i.e. the minimum possible perimeter). Fig. 5 shows that, especially in the upper third of the nest where most of the large chambers were located, the actual perimeter is frequently 2 to 7 times as long as a circle of the same area.
Figure 4.

Chambers of Pogonomyrmex badius nests become more lobed in outline as workers enlarge them. Chambers begin as small indentations in the wall of the shaft (left). Large chambers often appear to wrap around the shaft (right).
Figure 5.
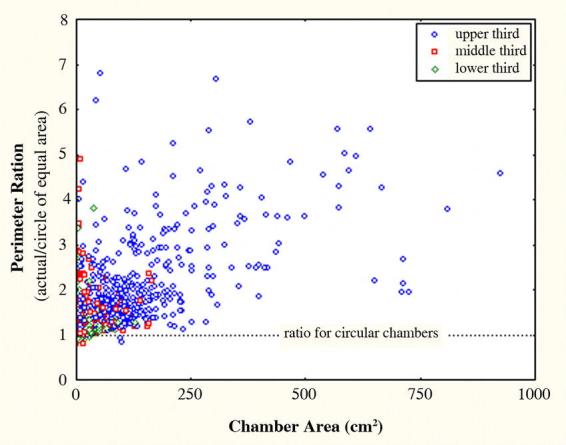
The ratio of the actual perimeter of Pogonomyrmex badius nest chambers to the perimeter of a circle of equal area estimates the complexity of the chamber outlines. Most of this increase results from an increase in the number of lobes. Because the largest chambers are located in the upper third of the nest, the greatest deviations from circularity occur in this part of the nest.
Chambers were spaced ever farther apart as depth increased, from 2–4 cm vertical separation near the surface, to 20–30 cm deeper in the nest. Deeper chambers were smaller, so that average chamber size was about 5–6 times as great near the surface as near the bottom.
Patterns of nest growth
Overall size measures
Some of the size-related architectural features have been reported in Tschinkel (1999a, b) and will only be summarized here. Larger colonies excavate larger nests as a result of nest deepening, chamber enlargement and the addition of new vertical series of chambers, all of which occur more or less simultaneously. These and many other architectural changes associated with colony growth can be seen in the casts of a small, medium and large nest shown in Fig. 6. Especially conspicuous is the increase of total chamber area, which ought to be most strongly related to the number of older, dark workers, because these carry out most of the excavation. For every 10-fold increase in the number of dark workers, total chamber area increased about 7.5 fold (log Total Area= 0.55 + 0.87 log DW; p< 0.00001; R2 = 93%) (Fig. 7). Thus, floor area per ant decreased with nest size, although workers are not distributed evenly (Tschinkel, 1999a). The relationship is almost identical for nest volume, as calculated from a mean chamber height of 1 cm and shaft diameter of 1 cm. The number of dark workers is also strongly related to the maximum depth of the nest. Every 10-fold increase in the dark worker population is associated with a 2.4-fold increase in maximum nest depth (log MaxDep= 0.95 + 0.37 log DW; p<0.00001, R2 = 72%). The deepest nest was 3.06 meters. Incipient nests were 29 to 37 cm deep.
Figure 6a.

Casts of Pogonomyrmex badius nests of increasing size: (A) a very small nest with a single vertical chamber series and shaft; (B) a medium-small nest with the beginning of a second vertical series and shaft; (C) a large-mature nest with 4 vertical series and shafts. The right nest (C) cast is of dental plaster, the middle (B) of aluminum, and the left (A) of zinc. The middle cast is incomplete, the lowest chambers having failed to fill with metal. Its maximum depth was probably intermediate between the large and small casts (photos by Charles Badland). (continued on page 8)
Figure 6b.

Figure 6c.
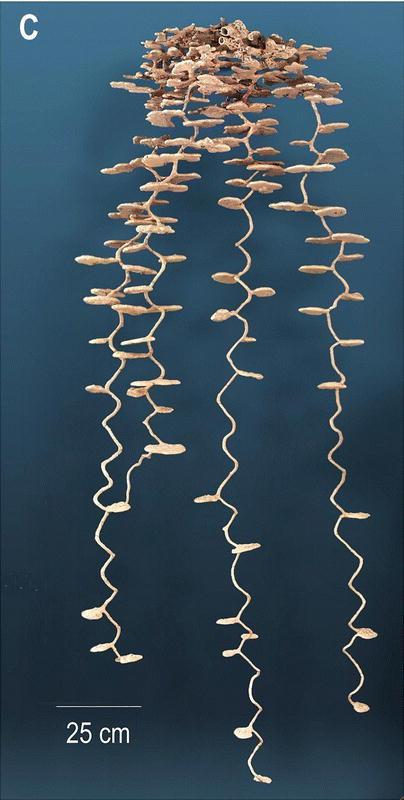
Figure 7.
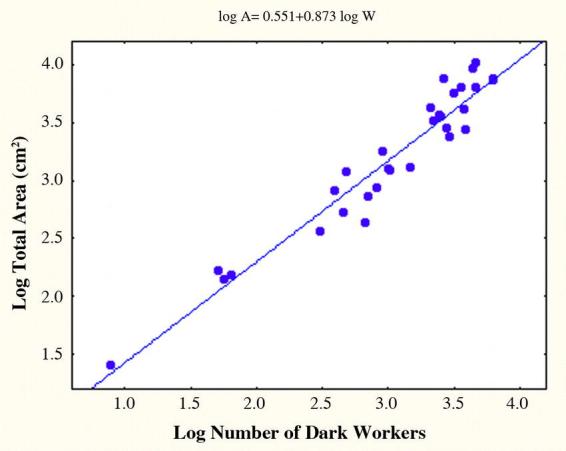
The total chamber area of Pogonomyrmex badius nests grows more slowly than does the population of workers that excavate the nest. For every 10-fold increase in the number of mature workers, the area increases 7.5-fold. The space per workers therefore decreases as nests grow. However, workers are very unevenly distributed within the nest.
Chamber size in relation to colony size
Superficial chambers
Chambers less than 10 to 15 cm deep were distinct in their morphology (Fig. 2), and increased greatly in relation to colony size. The mean size of these superficial chambers was 26 cm2 in the smallest colony size class, and approximately doubled in area with each increase of size class up to the largest, in which the mean area was about 330 cm2, or about 13 times as large as in size class 0. However, it is not always easy to distinguish the limits of superficial chambers because they fused as they were enlarged.
Shafts
Other than being elongated, shafts change little with nest growth, retaining a similar diameter at all nest sizes. However, shafts in the upper parts of the nest were exceptional, taking on a racetrack cross section in larger nests. Casts of these shafts have the appearance of a twisted ribbon (compare Figs. 8 top, bottom).
Figure 8.
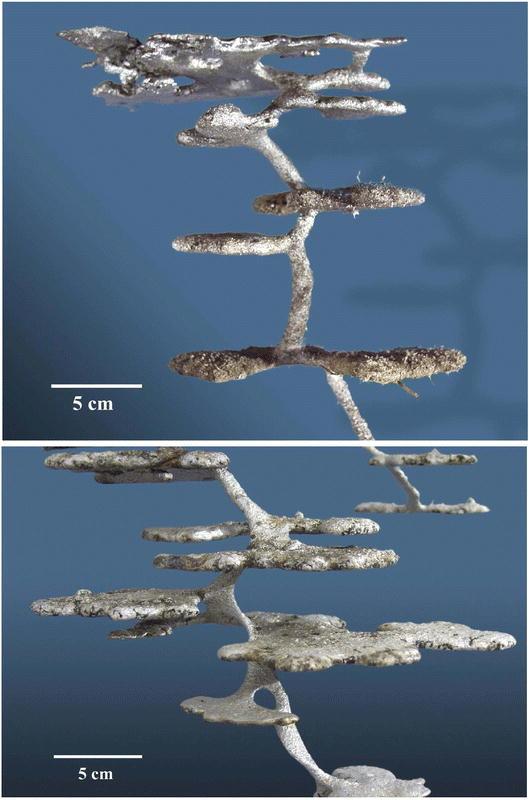
The uppermost portions of a medium-small Pogonomyrmex badius nest (top) and a small one (bottom). Note the ribbon-like descending shaft in the larger nest, but not the smaller (photos by Charles Badland).
Deciles. Nests grow in total chamber area through the addition of more chambers, deepening of the nest and an increase in the mean area of chambers (Fig. 9A–D). All occur simultaneously, but chamber enlargement contributed much more to the increase in total nest area than did the addition of new chambers (Tschinkel 1999a). Nests are top-heavy at all sizes because chambers are larger and closer together in the upper nest regions. Interestingly, this chamber spacing does not change much as the nest grows, because deepening occurs simultaneously with the addition of chambers. A plot of the vertical separation of chambers in relation to decile (Fig. 10) shows that all size classes (with 2 outlying points) have overlapping distributions. In other words, nest deepening is accompanied by the addition of sufficient new chambers in each decile to preserve the same approximate spacing. No matter what the size, the maximum inter-chamber spacing occurs in the 7th or 8th deciles rather than the 10th.
Figure 9.
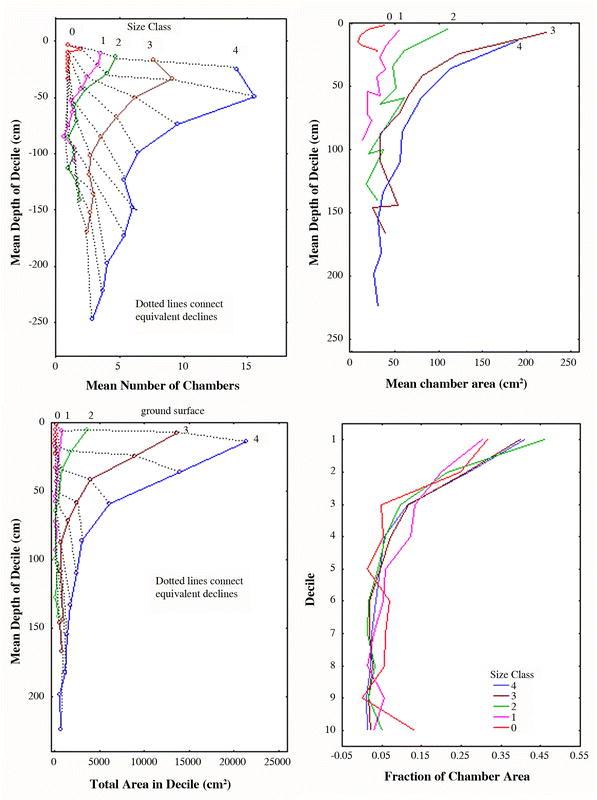
Pogonomyrmex badius nests grow through the addition of chambers, chamber enlargement and nest deepening. Patterns are shown by decile (increments of 10% of the maximum nest depth) and colony size class. A. More chambers are added in the upper regions of the nest than the lower. B. The mean size of chambers at each depth increases with nest size. C. The combination of increased numbers and mean size of chambers greatly increases the total area in each decile, much more in the upper regions than the lower. D. The increase of total area and maximum depth are proportional, so that the distribution of the percent of the total area in relation to the percent of the maximum depth does not change much as colonies grow. In other words, the size-free shape of the depth-area distribution does not change with colony size.
Figure 10.
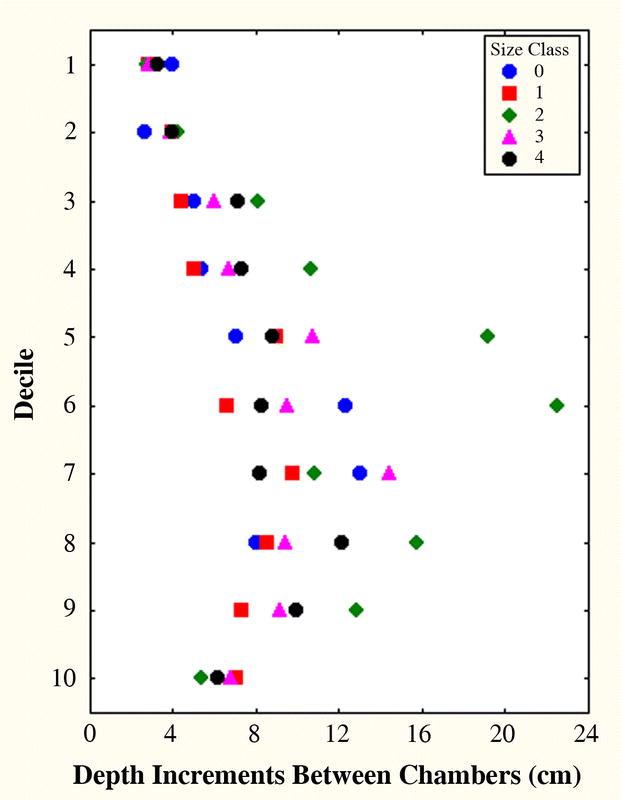
The vertical spacing between Pogonomyrmex badius nest chambers increases to a maximum in the 7th or 8th deciles then decreases again. This pattern does not change much with nest size because nest deepening and chamber addition occur simultaneously, preserving spacing. One nest in size class 2 contained outliers in the 5th and 6th deciles. None of the other size classes were different in how spacing varied with depth.
Together, these processes assure that the total area of chambers is always greatest in the first decile and decreases with depth. The nature of this decrease can be seen in a log-log plot (for each size class) of total chamber area in each decile against the mean depth of the decile (Fig. 11). These differ only in the intercept; the slopes do not differ from each other. The common slope is −0.59 (R2= 62%; p<0.000001), which means that, for all sizes of colonies, a 10-fold increase in mean decile depth is accompanied by a 75% decrease in decile area. Overall, each decile averaged half the area of the one above it, but at a smaller scale, this proportional decrease increased from an average of 10% from the 1st to the 2nd decile to 90% from the 9th to the 10th. Although this relationship was significant, it only explained 16% of the variation in decile-to-decile area decrease (Prop. Decrease = 0.10 (decile) − 0.12; F1,34 = 7.72; R2 = 0.16; p < 0.01). Variation was high, and lower deciles sometimes contained more area than those above. The area in the top decile increased with size class as follows (Fig. 11): class 0, 38 cm2; class 1, 190 cm2; class 2, 515 cm2; class 3, 1700 cm2; class 4, 2670 cm2. Because maximum nest depth increased with size class, the mean depth of these top deciles (and all others) also increased, as did the depths bracketed by the deciles.
Figure 11.
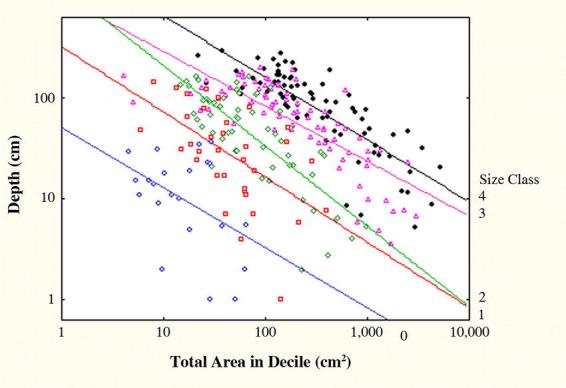
The proportional decrease in chamber area with depth of Pogonomyrmex badius nests is similar no matter what the colony size. Every 10-fold increase in depth is associated with a 75% decrease in summed chamber area in the decile (note log-log scale). Depths are the mean depths for each decile and size class. None of the slopes are significantly different from each other: slope of the log-log regression was − 0.59. Intercepts were: size class 0 = 1.69; size class 1 = 2.40; size class 2 = 2.72; size class 3 = 3.09; size class 4 = 3.35. These estimate the area at 1 cm depth, and have only mathematical reality. Actual mean areas in the uppermost deciles were 38, 190, 516, 1700 and 2670 cm2 for size classes 0 to 4, respectively.
Size-free shape during colony growth
Dividing the area in each decile by the total nest area, and the depth of each decile by the maximum nest depth creates size-free shape variables (proportions). When these are plotted against one another (Fig. 9D), it can be seen that the overall “shape” of the nest changed little during nest growth. These patterns are discussed in more detail in Tschinkel (1999a).
Addition of vertical series
Nests grew, in part, through branching of the descending shafts and the addition of another vertical chamber series. The number of vertical chamber series ranged from 1 to 4 (a single nest out of 32 excavated had 5). Nests with a single vertical series averaged 1700 workers, those with two, 3200, with three, 4600 and with four, 5000 (the standard deviation on all mean values were 1200 to 1600). Very small nests always consisted of a single descending shaft, but size classes 1 and 2 often had two descending series. In size class 3, two or three series was typical, and in size class 4, there four or even five could be present.
Figure 12B shows that the depth at which descending shafts branch is independent of the size of the nest, with the single exception that all shaft branches begin less than 40 cm below the ground surface, no matter what the nest size. When the outlier at 94 cm was excluded, deeper nests branched closer to the surface than shallower ones (regression of branch depth vs. maximum depth: p< 0.05, R2= 10%; Fig. 12A).
Figure 12.
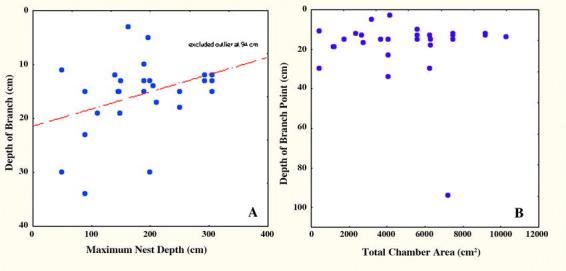
The depth of shaft branch points is deeper in shallower nests of Pogonomyrmex badius nests than in deep ones (A), but is independent of total nest area (B).
Below the branch, each additional chamber series contributed less to the total nest area because it consisted of fewer and smaller chambers than higher series. Thus, in the first series, each chamber increased the total area in the series by a mean of 98 cm2, in the second by 73 cm2, in the third by 57 and in the fourth by 43 (Fig. 13). These slopes were significantly lower for series 3 and 4 than for 1 and 2. Because the series are numbered in the order of the depths of their branch points, this means that series that start deeper had smaller chambers, on average. In all but the largest size class of colonies, the first branch appeared at a depth of 12 to 17 cm, and the second at 26 to 33 cm. When there was a third, it began at 35 cm. In the largest size class, the second series began at variable depths, and all the rest at 10 to 15 cm. The deeper the branch point, the fewer the chambers in the series (slope of regression of number on branch depth= −0.66, p=0.05, R2= 9.9%), and the less the total chamber area contributed by the series.
Figure 13.

Each additional vertical series of chambers of Pogonomyrmex badius nests contributes less to the total chamber area because the mean size of its chambers is smaller than higher series.
Why Are Nests Top-heavy?
Pogonomyrmex colonies are vertically stratified by age, with the youngest workers (callows) occur mostly in the bottom third of the nest (Tschinkel 1999b). Workers move up in the nest as they age, so that only the oldest workers occur near the surface, spending the last weeks of their lives as foragers (Porter and Jorgensen 1981). If workers developed an increasing tendency to dig as they aged, this would combine with their upward movement to produce the typical top-heavy nest morphology. Young, mixed-aged and old workers were therefore extracted from the bottom, middle and top chambers, respectively. Each group of 125 to 250 (constant for each replicate set) was penned in a sheet-metal enclosure in the field, where they invariably dug a nest. After 4 to 7 days, I made a plaster cast of the nest. This experiment was replicated 4 times with 4 different source colonies.
The old workers from the upper levels always dug the largest, most complex nests, and the youngest workers the smallest and least complex (Fig. 14). Because the number of workers and elapsed times varied among replicates, the total weight of the plaster casts was converted to grams of plaster per worker-day, or cm shaft per worker-day. Workers taken from the top level constructed nests about twice as fast as the middle group, and 3 times as fast as the bottom group (Fig. 15A). Rates of gallery construction followed similar patterns (Fig. 15B). These rates were significantly different (one-way ANOVA, F2,8 = 4.86; p < 0.05), with the bottom and top differing significantly (Tukey's HSD test). Relative to the nest excavated by the top group within each replicate, those of the middle and bottom groups were 50% and 26% as large, respectively. To confirm that this was the result of worker age rather than the nest level from which workers were taken, workers were separated from only the middle level into young (pale colored) and old (dark colored) workers. As predicted, the old, dark workers dug larger, more complex nests than the younger ones (one replicate).
Figure 14.
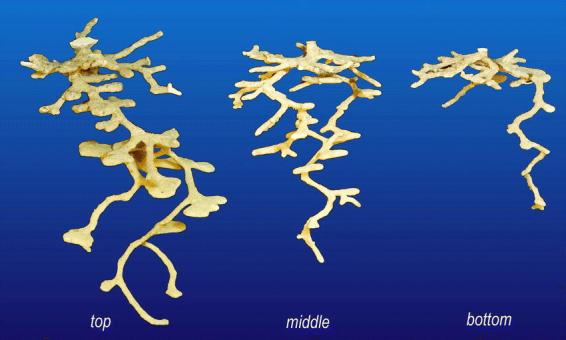
Plaster casts of Pogonomyrmex badius nests excavated by 250 workers taken from the uppermost chambers, the middle-depth and the bottom of a mature nest. Each group was penned in an escape-proof enclosure for 4 days, after which workers coming to the surface were recaptured, and a plaster cast of their nest was made. The figure shows one of 4 similar replicate sets.
Figure 15.
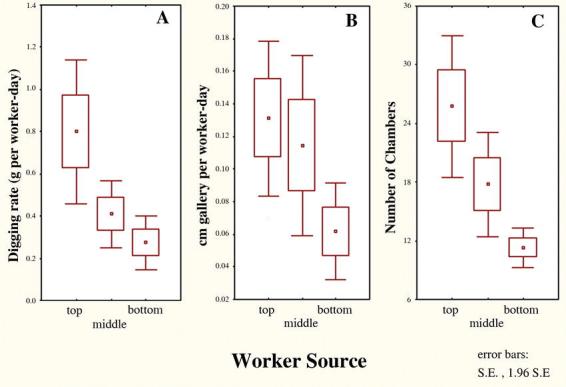
All measures of nest excavation were highest for the older workers taken from the top of a mature Pogonomyrmex badius nest, intermediate for the middle group and lowest for the youngest group from the nest bottom. A: rate of excavation, as grams of plaster per worker per day; B: cm of shaft per worker per day; C: number of chambers in nest.
At the termination of the penning experiment, all workers that came to the surface as they dumped sand were collected. About 82% of old workers were recaptured in this manner, 25% of the middle group, but only 19% of young group, indicating that younger workers engaged less frequently in digging. The digging rate (g plaster per day per worker) was positively related to the fraction recaptured (Fig. 16) (R= 0.78 F + 0.18; F1,10= 17.9; p<0.002; R2= 0.61). When the digging rate was calculated using only the number of workers recaptured, the significant effect of fraction recaptured disappeared, as did the differences between the three groups (one-way ANOVA, F2,8 = 2.17, n.s.). A worker either digs consistently or does not dig at all.
Figure 16.
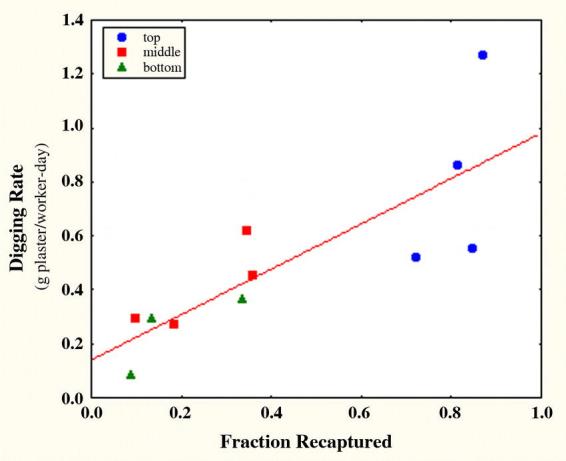
Most of the differences in the size of the excavated Pogonomyrmex badius nests were associated with the number of workers actively digging. Only actively digging workers could be recaptured at the surface as they brought up soil to discard. Younger workers from deeper in the nest were rarely recaptured. Because replicates began with varying numbers of workers (125 to 250), digging rate is plotted against fraction recaptured.
These experiments also established the rate of excavation. Older workers, as a group, excavate about 0.45 cm2 of chamber and 0.13 cm of shaft per worker-day, while for young worker groups, these rates are 0.15 cm2 and 0.06 cm (Fig 15). The average worker removes about 300 to 400 times its own weight in sand every day during construction. These rates are such that the workers of any colony, no matter what its size, can excavate a complete nest in only 3 to 6 days. The total weight of sand removed ranges from about 40 g in incipient nests, to about 40 kg in the largest.
Templates for nest architecture and vertical assortment
Architecture
Nests created by all age groups in all replicates were top-heavy, suggesting that an age-related increase of “digginess” is not a sufficient explanation of the top-heaviness of nests of all sizes. No matter what their age, all workers seem to have generally similar excavation algorithms, and therefore create nests of generally similar shape. Nevertheless, there are minor differences in nest morphology— the ratio of shaft length to chamber ratio increased with nest size (chamber/shaft = 0.0035 Wt. + 0.311; F1,10 = 6.15; p< 0.05; R2 = 0.32), and was higher for the top than the middle and bottom groups. However, test group and nest size are confounded, and it is not possible to say whether these shape differences were the result of nest size (i.e. allometric growth) or worker age.
Hangartner (1969) showed that fire ants dig in response to carbon dioxide, and that they dig more under lower concentrations. This suggested that the top-heavy shape might be the result of the diggers' response to a soil CO2 gradient, and that this gradient provides a template for digging. If this is so, then only the total volume of the nest, rather than its morphology, is the result of age-related differences in “digginess”. To test for such CO2 gradients in field harvester ant nests, I inserted a thin steel tube into chambers at the desired depth (resistance suddenly slackens when a chamber is entered), and sucked 25 ml of chamber air into an evacuated, septum-stoppered bottle. In the lab, these gas samples were analyzed for carbon dioxide with a Horiba Infrared Gas Analyzer (IRGA) calibrated with standard air-CO2 mixtures.
Carbon dioxide concentrations were ambient near the surface and increased 5-fold toward the bottom of the nest (Fig. 17a), suggesting that the CO2 concentration gradient could serve as a nest construction template. The shape of the gradient in Fig. 17A is almost the mirror image of the distribution of chamber area in Fig. 17B. Especially within a meter or less of the surface, the carbon dioxide concentration could readily serve as a depth cue.
Figure 17.
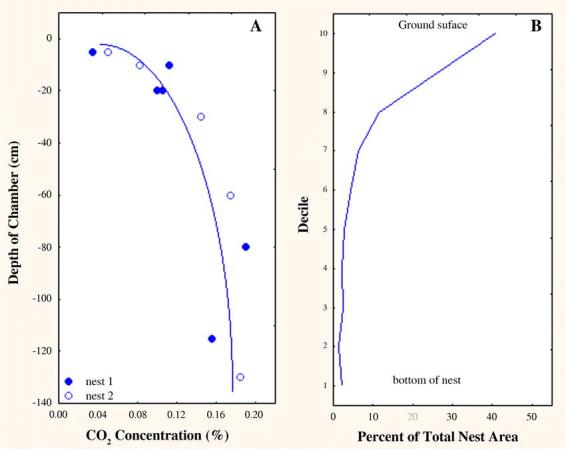
A. The carbon dioxide concentration in air taken from Pogonomyrmex badius nest chambers of increasing depth. The concentration in the deepest chambers is about 5 times that at the surface (2 replicates). B. The distribution of nest area by decile is roughly the mirror image of the carbon dioxide concentration, suggesting that the carbon dioxide concentration gradient might serve as a template for nest excavation and worker assortment. The steepest gradient is in the top half-meter or so, and this is also the zone with the most rapid decrease in chamber area (see Fig. 9).
Worker vertical assortment by age
P. badius colonies move one or more times a year (personal observations). During the move, the vertical organization of workers, brood and seeds is completely scrambled, but is recreated in the new nest. It follows that workers have cues about their own vertical location and have an age-based preference for certain locations. Such a preference was tested with the apparatus shown in Fig 18A. Screened chambers were connected to the vertical tube by a lateral hole. The vertical tube consists of a tube-within-a-tube, such that when the inner tube was turned, the openings into the chambers could be closed, like a stopcock. With the stopcock closed, 25 each of marked old and young workers and 20 brood were added to each chamber along with food and water. The initial ratio of young to old workers was therefore 1:1.
Figure 18.
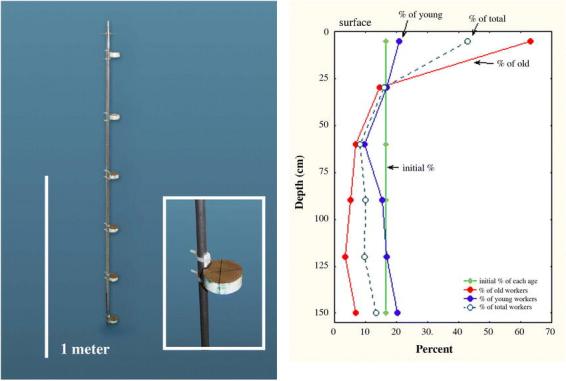
A. The “ant hotel” used to test the depth preference of young and old workers (see text for description). B. Initially, each “hotel room” contained 25 young workers taken from the bottom of a mature Pogonomyrmex badius nest, and 25 old workers from the top (each also contained 10 larvae and pupae). Each chamber thus contained 17% of each age group. After allowing 4 days for reassortment, old workers moved upward so that a large proportion of them were recaptured in the upper chambers. The downward movement of young workers was less dramatic.
The entire apparatus was buried vertically. After a day of acclimation, the stopcock was opened so that the ants could reassort themselves. Four days later, the stopcock was turned to “off” and the apparatus excavated. The workers in the chambers had re-assorted themselves such that 78% were recaptured in the top two chambers, and only 22% in the lower four (Fig. 18B). In other words, the ants distributed themselves within this experimental nest as they do in natural nests. Old workers had a greater tendency to move up than young worker had to move down, so that most of the change in distribution among chambers resulted from upward movement of old workers. At the end of the experiment, 95% of the brood had been aggregated into four chambers, but there was no clear relationship with depth. Perhaps the association of young workers with brood care assured that young workers moved less than old ones.
Discussion
The subterranean nests of the Florida harvester ant, P. badius, show clear, consistent, species-typical structural patterns. Among these are a top-heaviness of chamber-area distribution, a decrease of chamber size and complexity with depth, an increase of vertical inter-chamber spacing with depth, helical shafts with chambers emanating from the outside of the helix, superficial chambers modified from shafts, deeper chambers initiated with a circular outline, and an increase in shaft inclination with depth. Many architectural features are nest-size related, and change as the colony grows. These include the mean size and variation of chambers at any specified depth, the maximum depth of the nest, the number of vertical shafts and the complexity of chamber outlines. A few features do not vary with nest size: the percent of total chamber area as a function of percent of maximum depth (nest “shape”), shaft diameter (except in the top decile), chamber height and vertical separation of chambers in relation to depth. Altogether, these features give casts of P. badius nests an unmistakable, easily recognized appearance (Figs. 1 and 6).
Several of these features are shared with the nests of other species. Like those of P. badius the nests of F. pallidefulva, Pheidole morrissi and Solenopsis invicta are top-heavy in the distribution of chamber area, and grow by adding shafts with chambers (Mikheyev and Tschinkel 2004; Tschinkel 2003). Superficial chambers that differ greatly in morphology from deeper chambers are also present in Prenolepis imparis (Tschinkel 2003). Vertical spacing between chambers typically increases with depth as well. However, although these features are probably common to many species, they are by no means universal as some species show no top-heaviness, multiple shafts or differences in chamber spacing.
The nest's architecture represents a considerable investment of energy and time, and provides shelter, microclimate choice, and defendability (Sudd and Franks 1987). In addition to these obvious services, nest architecture is likely also to be a mechanism for integrating the colony members into an efficient functioning whole, the superorganism. The large amounts of time and effort invested in nest construction surely must pay dividends in fitness, for this energy could have been invested in other fitness-enhancing functions instead. An as yet unmet challenge is to identify how particular features of the architecture serve particular colony functions, and how this service contributes to colony fitness. The towers built by several tropical termite species function as ventilation and heat-exchange structures, and are thus adaptive (Korb, 2003), but similar information on subterranean ant nests is not available. For ants, a reasonable first hypothesis is that the natural nest architecture organizes colony functions to make them (ultimately) more efficient in converting labor and resources into sexuals.
That the colony is organized within the nest is clear. The vertical migration of aging workers within the nest creates vertical social structure by worker age and life stage, and therefore creates a division of labor in space as well as by worker age (Tschinkel 1999a, b; Porter and Jorgesen 1988). The “ant hotel” experiment showed that this stratification is not an incidental outcome of upward drift, but is a product of active movement and choice by the workers. Sendova-Franks and Franks (1995) found analogous patterns in single-chamber laboratory colonies of Leptothorax unifasciatus. In this species, brood was kept in a central pile, each worker had a limited movement zone, and these zones formed partially overlapping sequences from the center of the nest to the periphery. The tasks performed by a worker depended upon the location of her movement zone. Most significantly, when the colony was transferred to a new nest, workers resumed the same positions they occupied in the previous nest (Sendova-Franks and Franks 1994), recreating the original social structure, much as harvester ant workers do when moving to a new nest. These observations imply that the social structure is functional, and not a meaningless epiphenomenon.
Nest architecture regulates a second attribute of social structure, group size, because chamber size sets an upper limit on the number of ants that can coexist in one group, a limit that is often approached in the lowest chambers of most P. badius nests, where practically all floor space is often covered with ants. Average group size in P. badius increases with depth. Averaged over the entire year, the mean number of brood per brood-containing chamber ranges from 20 to 40 in the top half of the nest to 60 to 110 in the bottom half. In parallel, the mean numbers of workers in these same chambers in the bottom half ranges from 90 to 130 for all workers, and 25 to 50 for callow workers. Although variation is large, it is clear that there are brackets around group size and worker/brood ratios.
Whether the social structure generated by the nest architecture is functional or not has rarely been tested. Exceptions include the report by Brian (1956) that brood are reared more efficiently when they are dispersed among several smaller chambers, rather than grouped in a single large chamber of equal total area. Porter and Tschinkel (1985) showed that small, single-chamber laboratory nests of fire ant workers reared brood more efficiently than did large ones. These two studies imply that nest architecture, through subdivision of the worker force into efficient-sized rearing groups, may be linked to colony function in specific ways. In field colonies of fire ants, the average nest chamber contains about 200 workers and 100 brood (Cassill and Tschinkel 2002). Laboratory nests of this size are highly efficient. Whereas single-chamber laboratory nests become less and less efficient with increased size, field colonies do not (Tschinkel 1993). This difference begs for explanation, and nest architecture is a prime suspect.
The functional significance of the top-heaviness is mysterious. This large space seems under-used, typically harboring very low worker density. Perhaps this area is important for regulating brood development rate during the cooler months, for these chambers are exposed to the greatest diurnal variation in temperature. Alternately, a unit volume of upper chamber is cheaper to excavate than deeper ones because the soil is brought up over smaller vertical distances, but if this space is not heavily used, why create it? If top-heaviness is not functional, but merely a non-adaptive epiphenomenon of some other behavior, it is an expensive one. Whatever the functional reason, all ages of workers excavate nests that are top-heavy, although perhaps not to the same degree. Interestingly, Tschinkel (1987) noted that the upper chambers of P. imparis nests were also sparsely occupied.
Whether the vertical age structure is functional has not been explicitly tested, although it seems intuitive that separating different tasks into different parts of the nest ought to increase colony efficiency, much as it increases the efficiency of a factory. The possible link between colony function and nest architecture needs to be tested in experimental nests of different architectures, and between single-chamber laboratory nests and natural nests.
Whatever the functional meaning of vertical social structure, workers clearly have cues as to their own vertical location within the nest; colonies resume a similar vertical arrangement of workers, brood and seeds after moving to a new nest, and workers in the “ant hotel” experiment returned to the positions they had originally occupied in their home nest. A number of soil gradients might embody information on depth. Such factors as temperature and soil moisture probably vary too much on a daily, seasonal and weather basis to serve as a template for the highly regular patterns of worker and chamber distribution. In contrast, carbon dioxide gradients are probably the result of microbial and physical processes within the soil. Governed by the balance between microbial production and diffusion to the atmosphere, these gradients are probably rather stable. In harvester ant colonies, carbon dioxide concentration increases 5-fold with depth, providing ants with a strong depth cue that would allow them to arrange themselves as observed, and to expend excavation labor to produce the top-heavy nest as observed. The observed patterns would result if young workers preferred high concentrations of carbon dioxide and this preference declined with age, and if all ages of workers dug more under low carbon dioxide concentrations.
Ants have been shown to respond to carbon dioxide concentration. Hangartner (1969) showed that fire ants (Solenopsis geminata) settle preferentially at higher concentrations of carbon dioxide, and dig in response to lower concentrations. Raffy (1929) measured carbon dioxide concentrations as high as 1–2% in ant nests. Attine ant nests, because of their huge worker populations, deep nests and metabolizing fungus gardens accumulate concentrations of carbon dioxide up to 5% (Kleineidam and Roces 2000). Atta vollenweideri can detect absolute carbon dioxide concentrations (Kleineidam and Tautz 1996), and provides its nest with turrets that create wind-induced nest ventilation (Kleineidam et al. 2001).
As a subject of behavioral study, nest architecture offers an appealing feature that practically no other behaviors offer; namely, the nest is a perfect record of the collective digging effort of a colony, and once cast, is ready for study. By studying a series of casts of increasing size, it is possible to describe the nest's growth and ontogeny, infer its species-typical characteristics, and bracket the range of variation. By doing this under different environments and soil types, possibly with transplanted colonies, it is possible to tease out the variation that the environment imposes on the architecture. The current study is only a small, initial step toward creating a field of nest architectural studies, whose ultimate goal is an understanding of how the nest emerges from self-organizing behavior, what functions it serves, how it varies within and between species, and how it evolves. In addition, these nest casts reveal something previously unseen. The study of nest architecture is thus a true exploration of a hidden world that holds unsuspected beauty, pattern and complexity.
References
- Antonialli-Junior WF, Giannotti E. Nest architecture and population dynamics of the Ponerine ant, Ectatomma opaciventre Roger (Hymenoptera : Formicidae) Journal of Advanced Zoology. 1997;18:64–71. [Google Scholar]
- Autuori M. Contribuição para o conhecimento da saúva (Atta spp. Hymenoptera - Formicidae). III. Excavação de um saúveiro (Atta sexdens rubropilosa Forel, 1908. Arquivo Instituto Biologia. 1942;13:137–148. [Google Scholar]
- Bonner JT. 1974 On Development: the Biology of Form. Harvard Univ. Press, Cambridge. [Google Scholar]
- Brian MV. Group form and causes of working inefficiency in the ant Myrmica rubra. Physiological Zoology. 1956;29:173–194. [Google Scholar]
- Bristow CM, Cappaert D, Campbell NJ, Heise A. Nest structure and colony cycle of the Allegheny mound ant, Formica exsectoides Forel (Hymenoptera: Formicidae) Insectes Sociaux. 1992;39:385–402. [Google Scholar]
- Cassill DL, Tschinkel WR, Vinson SB. Nest complexity, group size and brood rearing in the fire ant, Solenopsis invicta. Insectes Sociaux. 2002;49:158–163. [Google Scholar]
- Chew RM. Note on colony size and activity in Pogonomyrmex occidentalis (Cresson) New York Entomological Society. 1960;68:81–82. [Google Scholar]
- Conway JR. Nest architecture and population of the honey ant, Myrmecocystus mexicanus Wesmael (Formicidae), in Colorado. Southwestern Naturalist. 1983;28:21–31. [Google Scholar]
- Crosland MWJ. Nest and colony structure in the primitive ant, Harpegnathos venator (Smith) (Hymenoptera: Formicidae) Pan Pacific Entomologist. 1995;71:18–23. [Google Scholar]
- Darlington JPEC. Comparison of nest structure and caste parameters of sympatric species of Odontotermes (Termitidae, Macrotermitidae) in Kenya. Insectes Sociaux. 1997;44:393–408. [Google Scholar]
- Dlussky GM. Evolution of ant nest construction (Hymenoptera Formicidae) Proceedings of the 13th International Congress of Entomology, Moscow. 1968;3:359–360. [Google Scholar]
- Dlussky GM. 1981 Ants of Deserts. Moscow, Nauka. [Google Scholar]
- Downing HA, Jeanne RL. Nest construction by the paper wasp, Polistes: a test of stigmergy theory. Animal Behaviour. 1988;36:1729–1739. [Google Scholar]
- Ettershank G. The three-dimensional gallery structure of the nest of the meat ant Iridomyrmex purpureus (Sm.) (Hymenoptera: Formicidae) Australian Journal of Zoology. 1968;16:715–723. [Google Scholar]
- Ettershank G. Some aspects of the ecology and nest microclimatology of the meat ant, Iridomyrmex purpureus (Sm) Royal Society of Victoria Proceedings. 1971;84:137–151. [Google Scholar]
- Franks NR, Deneubourg JL. Self-organizing nest construction in ants: individual worker behaviour and the nest's dynamics. Animal Behaviour. 1997;54:779–796. doi: 10.1006/anbe.1996.0496. [DOI] [PubMed] [Google Scholar]
- Golley FB, Gentry JB. Bioenergetics of the southern harvester ant, Pogonomyrmex badius. Ecology. 1964;45:217–225. [Google Scholar]
- Hangartner W. Carbon dioxide, a releaser for digging behavior in Solenopsis geminata (Hymenoptera: Formicidae) Psyche. 1969;76:58–67. [Google Scholar]
- Hansell MH. 1984 Animal Architecture and Building Behavior. Longman, London. [Google Scholar]
- Hölldobler B, Wilson EO. 1990 The Ants. Cambridge, MA, Belknap/Harvard Press. [Google Scholar]
- Jonkman JCM. The external and internal structure and growth of nests of the leaf-cutting ant Atta vollenweideri Forel, 1893 (Hymenoptera: Formicidae) Zeitschrift fuer Angewandte Entomologie. 1980;89:158–173. [Google Scholar]
- Karsai I, Penzes Z. Comb building in social wasps: self-organization and stigmergic script. Journal of Theoretical Biology. 1993;161:505–525. [Google Scholar]
- Kleineidam C, Tautz J. Perception of carbon dioxide and other “air-condition” parameters in the leaf cutting ant Atta vollenweideri. Insectes Sociaux. 1996;47:241–248. [Google Scholar]
- Kleineidam C, Roces F. Carbon dioxide concentrations and nest ventilation in nests of the leaf-cutting ant Atta vollenweideri. Insectes Sociaux. 2000;47:241–248. [Google Scholar]
- Kleineidam C, Ernst R, Roces F. Wind-induced ventilation of the giant nests of the leaf-cutting ant Atta vollenweideri. Naturwissenschaften. 2001;88:301–305. doi: 10.1007/s001140100235. [DOI] [PubMed] [Google Scholar]
- Kondoh M. Bioeconomic studies on the colony of an ant species, Formica japonica Motschulsky. 1. Nest structure and seasonal change of the colony members. Japanese Journal of Ecology. 1968a;18:124–133. [Google Scholar]
- Kondoh M. Bioeconomic studies on the colony of an ant species, Formica japonica Motschulsky. 2. Allometric study of the body weight and the corpulency relating to the body size of workers. Japanese Journal of Ecology. 1968b;18:171–179. [Google Scholar]
- Korb J. Thermoregulation and ventilation of termite mounds. Naturwissenschaften. 2003;90:212–219. doi: 10.1007/s00114-002-0401-4. [DOI] [PubMed] [Google Scholar]
- Kugler C, Carmen Hincapie Md. Ecology of the ant Pogonomyrmex mayri: distribution, abundance, nest structure, and diet. Biotropica. 1983;15:190–198. [Google Scholar]
- Lavigne RJ. Bionomics and nest structure of Pogonomyrmex occidentalis (Hymenoptera: Formicidae) Annals of the Entomological Society of America. 1969;62:1166–75. [Google Scholar]
- Lockaby BG, Adams JC. Pedoturbation of a forest soil by fire ants. Journal of the Soil Science Society of America. 1985;49:220–223. [Google Scholar]
- MacKay WP. 1981a A comparison of the ecological energetics of three species of Pogonomyrmex harvester ants (Hymenoptera: Formicidae), Ph.D. dissert. University of California at Riverside. 258. p. [DOI] [PubMed] [Google Scholar]
- MacKay WP. A comparison of the nest phenologies of three species of Pogonomyrmex harvester ants (Hymenoptera: Formicidae) Pogonomyrmex montanus, Pogonomyrmex rugosus, Pogonomyrmex subnitidus, Mexico. Psyche. 1981b;88:25–74. [Google Scholar]
- McCahon TJ, Lockwood JA. Nest architecture and pedoturbation of Formica obscuripes Forel (Hymenoptera: Formicidae) Pan-Pacific Entomologist. 1990;66:147–156. [Google Scholar]
- McCook HC. 1879 The natural history of the agricultural ant of Texas: a monograph of the habits, architecture, and structure of Pogonomyrmex barbatus. Academy of Natural Sciences, Philadelphia. [Google Scholar]
- Mikheyev A.S, Tschinkel W.R. Nest architecture of the ant Formica pallidefulva: structure, costs and rules of excavation. Insectes Sociaux. 2004;51:30–36. [Google Scholar]
- Nielsen MG, Jensen TF. Okologiske studier over Lasius alienus (Forst.) (Hymenoptera, Formicidae) Entomoliske Meddeilingen. 1975;43:5–16. [Google Scholar]
- Peeters C, Hölldobler B. Wall-papering and elaborate nest architecture in the ponerine ant Harpegnathos saltator. Insectes Sociaux. 1994;41:211–218. [Google Scholar]
- Porter SD, Jorgensen CD. Foragers of the harvester ant, Pogonomyrmex owyheei: a disposable caste? Behavioral Ecology and Sociobiology. 1981;9:247–256. [Google Scholar]
- Porter SD, Tschinkel WR. Fire ant polymorphism: the ergonomics of brood production. Behavioral Ecology and Sociobiology. 1985;16:323–336. [Google Scholar]
- Raffy A. L'atmosphère interne des fourmilières contient-elle de l'oxyde de carbone. Comptes Rendus Societe Biologique Paris. 1929;102:908–909. [Google Scholar]
- Sabiti JMN. The ecological role of ants in the ecosystem: foraging activity and excavation in Paltothyreus tarsatus. African Journal of Ecology. 1980;18:113–121. [Google Scholar]
- Scherba G. Nest structure and reproduction in the mound-building ant Formica opaciventris Emery in Wyoming. Journal of the New York Entomological Society. 1961;69:71–87. [Google Scholar]
- Seeley TD. 1995 The Wisdom of the Hive. Harvard Univ. Press, Cambridge, MA. [Google Scholar]
- Sendova Franks AB, Franks NR. Spatial relationships within nests of the ant Leptothorax unifasciatus (Latr.) and their implications for the division of labour. Animal Behaviour. 1995;50:121–136. [Google Scholar]
- Sendova-Franks AB, Franks NR. Social resilience in individual worker ants and its role in division of labour. Proceedings of the Royal Society of London B. 1994;256:305–309. [Google Scholar]
- Sendova-Franks AB, Franks NR. Spatial relationships within nests of the ant Leptothorax unifasciatus (Latr.) and their implications for the division of labour. Animal Behaviour. 1995;50:121–136. [Google Scholar]
- Sudd JH. Excavation of nests by ants. International Union for the Study of Social Insects Proceedings. 1969;6:281–286. [Google Scholar]
- Sudd JH. The excavation of soil by ants. Zeitschrift fuer Tierpsychologie. 1969;26:257–276. [Google Scholar]
- Sudd JH. 1982 Ants: foraging, nesting, brood behavior and polyethism. in Social Insects. H. R. Hermann. New York, Academic Press. 108–155. [Google Scholar]
- Sudd JH, Franks NR. 1987 The Behavioural Ecology of Ants. Glasgow, U.K. 206. p., Blackie. [Google Scholar]
- Talbot M. Nest structure and flights of the ant Formica obscuriventris Mayr. Animal Behavior. 1964;12:154–158. [Google Scholar]
- Talbot M, Kennedy CH. The slave-making ant, Formica sanguinea subintegra Emery, its raids, nuptial flights and nest structure. Annals of the Entomological Society of America. 1940;33:560–577. [Google Scholar]
- Theraulaz G, Bonabeau E, and Deneubourg J-L. 1999 The mechanism and rules of coordinated building in social insects. in Information Processing in Social Insects, C. Detrain, Deneubourg, JL Pasteels JM, eds. Birkäuser Verlay, Basel. [Google Scholar]
- Tschinkel WR. Seasonal life history and nest architecture of a winter-active ant, Prenolepis imparis. Insectes Sociaux. 1987;34:143–164. [Google Scholar]
- Tschinkel WR. Sociometry and sociogenesis of colonies of the fire ant Solenopsis invicta during one annual cycle. Ecological Monographs. 1993;64:425–457. [Google Scholar]
- Tschinkel WR. Sociometry and sociogenesis of colonies of the harvester ant, Pogonomyrmex badius: worker characteristics in relation to colony size and season. Insectes Sociaux. 1998;45:385–410. [Google Scholar]
- Tschinkel WR. Sociometry and sociogenesis of colonies of the harvester ant, Pogonomyrmex badius: distribution of workers, brood and seeds within the nest in relation to colony size and season. Ecological Entomology. 1999a;24:222–237. [Google Scholar]
- Tschinkel WR. Sociometry and sociogenesis of colony-level attributes of the Florida harvester ant (Hymenoptera : Formicidae) Annals of the Entomological Society of America. 1999b;92:80–89. [Google Scholar]
- Tschinkel WR. Subterranean ant nests: trace fossils past and future? Paleogeography Paleoclimatology Paleoecology. 2003;192:321–333. [Google Scholar]
- Wheeler WM. 1910 Ants, Their Structure, Development and Behavior. New York, Colombia University Press. [Google Scholar]
- Williams DF, Logfren CS. 1988 Nest casting of some ground-dwelling Florida ant species using dental labstone. Advances in Myrmecology. Leiden, E. J. Brill: pp. 433–443. [Google Scholar]
- Winston ML. 1987 The Biology of the Honeybee. Harvard Univ. Press, Cambridge. [Google Scholar]
- Wilson EO. A chemical releaser of alarm and digging behavior in the ant Pogonomyrmex badius. Psyche. 1958;65:41–51. [Google Scholar]
- Wilson EO. 1971 The Insect Societies. Cambridge, Mass., Belknap/HarvardL. [Google Scholar]


