ABSTRACT
Investigations of environmental microbial communities are crucial for the discovery of populations capable of degrading hazardous compounds and may lead to improved bioremediation strategies. The goal of this study was to identify microorganisms responsible for aerobic benzene degradation in coal tar-contaminated groundwater. Benzene degradation was monitored in laboratory incubations of well waters using gas chromatography mass spectrometry (GC-MS). Stable isotope probing (SIP) experiments using [13C]benzene enabled us to obtain 13C-labled community DNA. From this, 16S rRNA clone libraries identified Gammaproteobacteria and Betaproteobacteria as the active benzene-metabolizing microbial populations. Subsequent cultivation experiments yielded nine bacterial isolates that grew in the presence of benzene; five were confirmed in laboratory cultures to grow on benzene. The isolated benzene-degrading organisms were genotypically similar (>97% 16S rRNA gene nucleotide identities) to the organisms identified in SIP experiments. One isolate, Variovorax MAK3, was further investigated for the expression of a putative aromatic ring-hydroxylating dioxygenase (RHD) hypothesized to be involved in benzene degradation. Microcosm experiments using Variovorax MAK3 revealed a 10-fold increase in RHD (Vapar_5383) expression, establishing a link between this gene and benzene degradation. Furthermore, the addition of Variovorax MAK3 to microcosms prepared from site waters accelerated community benzene degradation and correspondingly increased RHD gene expression. In microcosms using uninoculated groundwater, quantitative (q)PCR assays (with 16S rRNA and RDH genes) showed that Variovorax was present and responsive to added benzene. These data demonstrate how the convergence of cultivation-dependent and -independent techniques can boost understandings of active populations and functional genes in complex benzene-degrading microbial communities.
IMPORTANCE Benzene is a human carcinogen whose presence in contaminated groundwater drives environmental cleanup efforts. Although the aerobic biodegradation of benzene has long been established, knowledge of the identity of the microorganisms in complex naturally occurring microbial communities responsible for benzene biodegradation has evaded scientific inquiry for many decades. Here, we applied a molecular biology technique known as stable isotope probing (SIP) to the microbial communities residing in contaminated groundwater samples to identify the community members active in benzene biodegradation. We complemented this approach by isolating and growing in the laboratory a bacterium representative of the bacteria found using SIP. Further characterization of the isolated bacterium enabled us to track the expression of a key gene that attacks benzene both in pure cultures of the bacterium and in the naturally occurring groundwater microbial community. This work advances information regarding the documentation of microbial processes, especially the populations and genes that contribute to bioremediation.
KEYWORDS: stable isotope probing, biodegradation, subsurface, RT-qPCR, nutrient limitation, dioxygenase, groundwater
INTRODUCTION
Microbial communities play a globally significant role by metabolizing a diverse array of compounds in the Earth's ecosystems (1, 2). The achievement of a more robust understanding of key microbial players and their metabolic processes is constrained by the complexity of microbial communities and by methodological limitations in environmental microbiology, particularly with traditional cultivation-dependent approaches (3, 4). Regarding the carbon cycle, the identification of the microbial population responsible for metabolizing specific compounds has major implications for bioremediation and for predicting ecosystem responses to ongoing anthropogenic release of pollutants, such as hydrocarbons, to terrestrial and aquatic environments (5). Despite the importance of microbial communities in contaminant degradation, much remains to be explored regarding the vast taxonomic and functional genetic diversities of bioremediation agents (4, 6).
Aerobic hydrocarbon degradation is one of the most well-described microbially mediated processes in the environment (7–9). Numerous studies have applied assays with molecular biomarker approaches to contaminated sites, with the objective of exploring the diversities of biodegradation genes and of the naturally occurring populations involved in metabolizing hydrocarbons (10–16). In addition, cultivation efforts have successfully isolated hydrocarbon-degrading organisms from habitats, including from soil, groundwater, marine waters, and deep sea sediments (12, 17–22). Despite the many studies successfully reporting new information on the genetic basis (e.g., dioxygenase) of hydrocarbon metabolism, much remains to be learned regarding both the identities of populations responsible for biodegradation and the diversity of biodegradation genes (4, 10, 14, 16).
Linking the responsible agents to specific metabolic processes in the environment remains difficult, even in the well-described processes of aerobic aromatic hydrocarbon degradation (4, 8, 23, 24). Stable isotope probing (SIP) is a promising tool for targeting and identifying active populations within microbial communities without requiring cultivation (25–28). Studies using SIP dose substrates labeled with heavy isotopes (e.g., 13C and 15N) into mixed microbial populations. Then, assimilative metabolic processes incorporate the heavy atoms into the metabolically active biomass (typically a small subset of the total). The result is an enrichment of heavy atoms in biomarker molecules (e.g., phospholipid fatty acids [PLFAs], DNA, and RNA) that can be extracted and separated from the light (nonlabeled) biomass. Biomarkers are then analyzed to identify the organisms responsible for substrate metabolism (29–31). Previously, SIP investigations of the metabolism of benzene, toluene, ethylbenzene, and xylene (BTEX) compounds largely focused on anaerobic benzene and toluene degradation, using PLFA and rRNA-based fingerprinting techniques to identify the active populations (32–35). DNA- and RNA-based benzene SIP investigations have been conducted in coal tar-contaminated sediment, gasoline-contaminated water, and soil (31, 36, 37). However, the high diversities of habitat types and geochemical conditions where contaminants occur warrant additional SIP investigations.
Here, we present a systematic approach using SIP, cultivation, and molecular techniques to understand the microbial populations responsible for degrading benzene in a naturally occurring groundwater microbial community. The contaminated site is one with a history of coal tar contamination (38–40) that features benzene within a complex mixture of organic compounds. We used SIP-microcosm incubations to identity the bacterial community members responsible for benzene degradation in site waters. After the SIP investigation, we cultivated representatives of the community and verified their ability to degrade benzene. We also used quantitative (q)PCR to determine the expression level of a putative dioxygenase during benzene degradation in a Variovorax sp. isolate that showed high 16S rRNA similarities to the populations identified in the SIP study. Finally, we found enhanced signals for Variovorax and its dioxygenase catabolic gene in site waters dosed with benzene. This study demonstrates the way in which the combination of cultivation-independent and -dependent methodologies can be used to gain insight into the populations and their catabolic genes involved in contaminant biodegradation.
RESULTS
Degradation of benzene by well water communities.
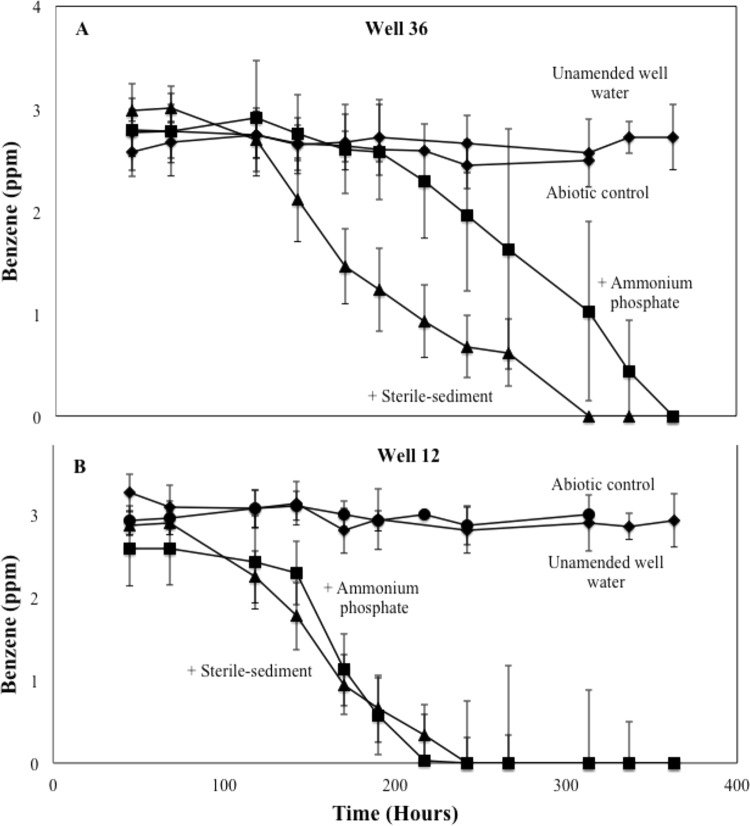
Microcosms were incubated to demonstrate the ability of microbial communities in wells 12 and 36 to degrade benzene. Water samples from both wells were dosed with 3 ppm benzene and treated four ways: (i) with site water only (unamended), (ii) with water amended with 10% (vol/vol) sterile sediment, (iii) with water amended with 10 mM (NH4)3PO4, and (iv) with an abiotic control poisoned with HCl. Benzene persisted in the abiotic treatments for wells 36 and 12 (Fig. 1A and B, respectively) during the >300-h incubation. The community in the sediment- and nutrient-amended treatments from well 36 degraded the benzene completely within 300 and 360 h, respectively (Fig. 1A). Similarly, the community from well 12 degraded benzene within 200 and 250 h for nutrient- and sediment-amended microcosms, respectively (Fig. 1B). Surprisingly, benzene persisted in the well water unamended with nutrients or sterile sediment for >350 h. The lack of benzene biodegradation by microorganisms in the unamended incubations likely resulted from nutrient limitation. Clearly, benzene biodegradation in the nutrient-amended incubation was equivalent to benzene biodegradation in the sediment-amended incubation. We conclude that nitrogen and phosphorus sources associated with the sterile aquifer solids supported benzene metabolism by the native communities. These results demonstrate that the microbial communities in site waters were capable of degrading benzene, but that nitrogen and/or phosphorus limitation can occur if site waters are incubated in the absence of site aquifer solids.
FIG 1.
Benzene biodegradation in serum-bottle incubations containing microorganisms from well 36 (A) and well 12 (B). Gas chromatography mass spectrometry of headspace gases determined the concentration of benzene at each time point. The experiment included 4 treatments: sterile-sediment-amended (10% [vol/vol]), 10 mM (NH4)3PO4-amended, unamended, and an abiotic control (HCl to pH 2). Error bars represent the standard deviations from averages of triplicate microcosms. The final two time points for the abiotic control were not sampled.
Stable isotope probing to identify active benzene-degrading populations.
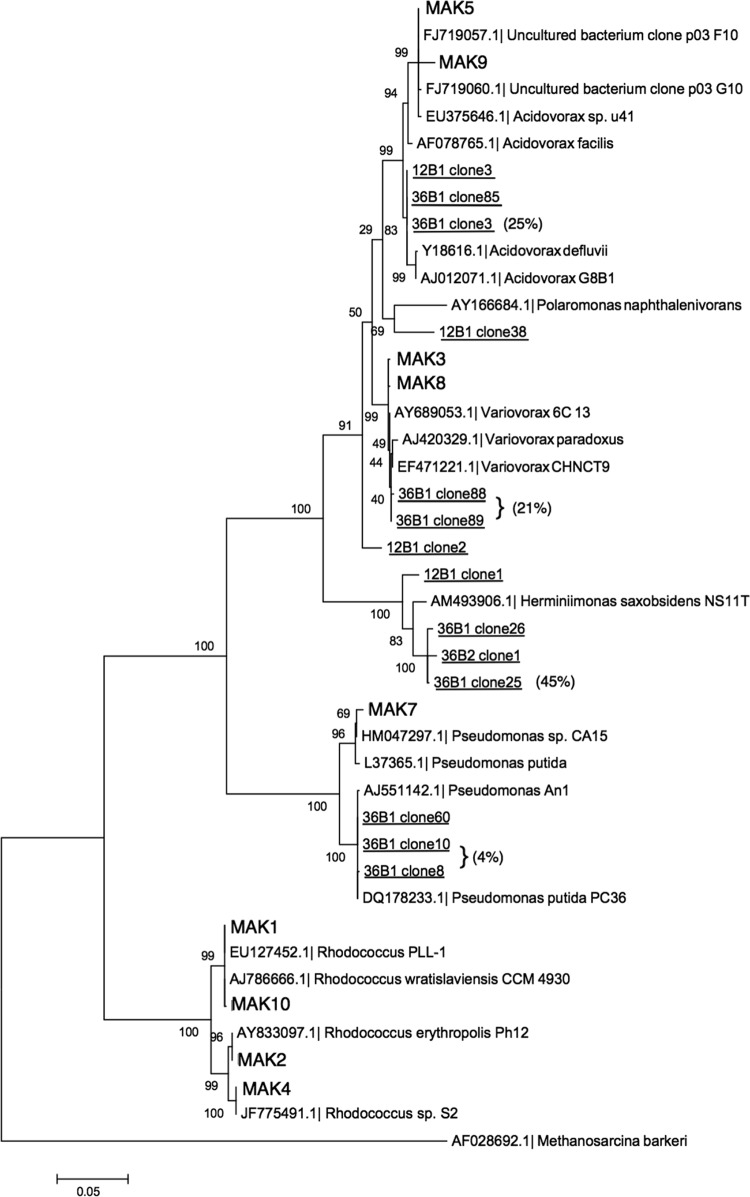
To identify the members of the aquifer microbial community that were active in benzene metabolism, a stable isotope probing (SIP) experiment was performed in parallel with the degradation assays. The treatments were prepared in a manner identical to the above degradation assay, except 13C-labeled liquid benzene was added to a set of microcosms. After 70% of the 13C-labeled benzene was degraded, the microcosms were sacrificed. Parallel (unlabeled) [12C]benzene treatments were also processed. After DNA extraction, CsCl ultracentrifugation enabled the collection of the heavy (13C) fractions from 12C- and 13C-treatments (30). The location of the heavy DNA in the CsCl gradient corresponded to the band where DNA from 13C-grown control cells appeared. Dilutions were prepared. The first dilution that failed to yield 16S rRNA gene amplicons in the 12C treatment, but continued to yield amplicons in the 13C treatments, was operationally defined as enriched in benzene-derived [13C]DNA (30). 16S rRNA genes in the DNA fraction were amplified and cloned using universal bacterial 16S rRNA primers. From a library of 156 clones, 14 displaying unique restriction fragment length polymorphism (RFLP) profiles were chosen for sequencing, including ten from well 36 and four from well 12. A phylogenetic analysis of the 14 SIP-generated (Fig. 2) sequences showed that Gammaproteobacteria and Betaproteobacteria, specifically the genera Herminiimonas, Acidovorax, Variovorax, and Pseudomonas, represented the predominant active benzene-degrading microorganisms. Results from the clone library revealed that groundwater from well 36 was highly enriched with two sequences from Variovorax sp., which together comprised 21% of the clone library, and one sequence from an Acidovorax sp. represented another 25% of the library.
FIG 2.
Phylogenetic affiliations of cultivated benzene-degrading bacteria and 16S rRNA clones derived from the stable isotope probing (SIP) experiment. The figure shows a maximum-likelihood tree of full (>1,400 bp) bacterial 16S rRNA genes from the culture-derived isolates and clones from 10 mM (NH4)3PO4-amended [13C]benzene SIP experiments from monitoring wells 12 and 36 using MEGA 5.05. Bootstrap values estimated node confidence. The comparison consisted of the nine isolate sequences (bold), 10 clones (underlined) from well 36, and four clones from well 12. Nineteen reference sequences are included in the tree; the outgroup was Methanosarcina barkeri. Percentages for representations in a library of 156 clones are shown in brackets.
16S rRNA gene sequencing of benzene-degrading isolates.
Water samples from the well water degradation experiment were incubated to isolate the benzene-degrading organisms. Nine isolates exhibiting distinct morphologies were successfully cultivated and yielded robust colonies in the presence, but not in the absence, of benzene vapor (see Fig. S1 in the supplemental material). The nine isolates were then transferred to basal salt minimal medium (BSM) broth at room temperature with benzene as the sole carbon source. Five of the nine isolates successfully degraded benzene in liquid cultures over the incubation period of 70 h (see Fig. S2). The remaining four isolates, including the control, did not show a significant loss of benzene over the same period. We speculate that the four isolates may not have degraded benzene in liquid culture due to the toxicity of dissolved benzene at 16 ppm or to low growth rates. Alternatively, they may have been able to grow on agar plates (used during isolation) because of carbon scavenging from the agar.
The 16S rRNA genes of the isolates were sequenced and compared with those derived from the 13C-labeled DNA fraction from the SIP experiment (Fig. 2). The results indicated that several of the isolates shared >97% identity with SIP clones. Most notably, isolate MAK5 had 97% identity with a SIP clone (36B1 clone 3), which comprised 25% of the clone library, and MAK3 shared 98% identity with two SIP sequences (36B1 clone 88 and 36B1 clone 89) that comprised 21% of the clone library. The largest group of sequenced isolates (MAK1, MAK2, MAK4, and MAK10) clustered with the genus Rhodococcus, which was not identified in the SIP experiment. The reason for the absence of rhodococci from the SIP library is uncertain; it may have been due to the limited size of the clone library or to resistance of Gram-positive rhodococci to cell lysis.
Dioxygenase gene expression by strain MAK3 during benzene degradation.
The isolate MAK3 was chosen for further investigation because of its high sequence similarity and its representation in the SIP clone library. qPCR primers were designed based on a putative dioxygenase gene annotated in the Variovorax paradoxus S110 genome. The specificity of the primers was verified using conventional PCR on isolate MAK3 and by sequencing the 182-bp amplicon (data not shown). The results confirmed 96% identity to the annotated dioxygenase gene in Variovorax paradoxus S110.
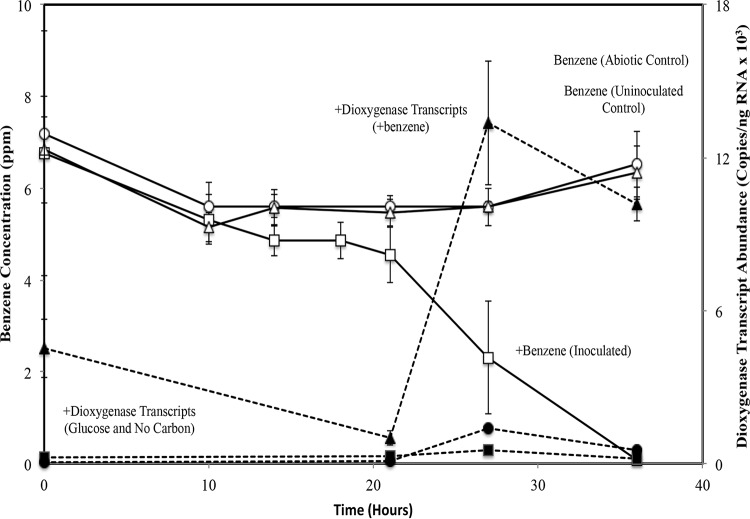
We hypothesized that the ring-hydroxylating dioxygenase (RHD) gene of Variovorax MAK3 would be upregulated (highly expressed) during benzene degradation. To test this, we prepared microcosm incubations in the presence and absence of benzene and two negative-controls, namely one with no added carbon and the other with glucose as the growth substrate. Over the course of the experiment (Fig. 3), the biomass was subsampled at times corresponding with the measurements of benzene concentration. Reverse transcriptase (RT)-qPCR of the putative dioxygenase gene transcripts showed that Variovorax MAK3 cells were most active between 21 and 27 h (14% to 57% benzene depletion); this amounts to a 10-fold increase in RHD transcript abundance (Fig. 3). The elevated transcript levels persisted to the final time point when 98% of the benzene had been consumed. The absolute number of transcript copies of the MAK3 RHD gene increased from approximately 1.0 × 103 copies/ng at 14% benzene depletion to 1.3 × 104 copies/ng at 57% benzene depletion and to 1.0 × 104 copies/ng at 98% depletion. Over the same time periods, the RHD transcript levels in the samples grown with glucose were not increased, and the levels were similar to those in the no-benzene control.
FIG 3.
Benzene degradation by isolate Variovorax MAK3 and corresponding mRNA transcript abundance of its beta subunit ring-hydroxylating dioxygenase (RDH). Isolates (initial cell density ∼106 CFU/ml) were incubated in minimal media (i) with benzene, (ii) with glucose, (iii) with HCl as an abiotic control, or (iv) without benzene. Open symbols represent benzene degradation quantified by GC-MS, and closed symbols represent dioxygenase transcript abundance quantified by RT-PCR. Error bars represent the standard deviations from averages of triplicate microcosms.
Additionally, Variovorax-specific 16S rRNA primers (41) were used in qPCR assays to monitor the abundance of Variovorax cells during the experiment. The results indicated that 16S rRNA copies increased 150% in the benzene treatment, whereas they decreased 62% in the treatment without benzene. The glucose treatment demonstrated the largest increase, where 16S rRNA copies rose 410% (see Fig. S4).
The above results show that an increase in the dioxygenase transcript abundance corresponded to the rapid degradation of benzene and an increase in 16S rRNA copy number; both are associated with cell growth. Comparison of the dioxygenase results between the benzene and glucose treatments strongly suggested that the putative dioxygenase gene was specifically involved in the catabolism of benzene and not in general growth or metabolism. Furthermore, the difference in 16S rRNA gene abundance in the presence versus the absence of benzene confirmed that Variovorax MAK3 derived its carbon and energy solely from benzene.
Dioxygenase gene expression by native populations during benzene degradation.
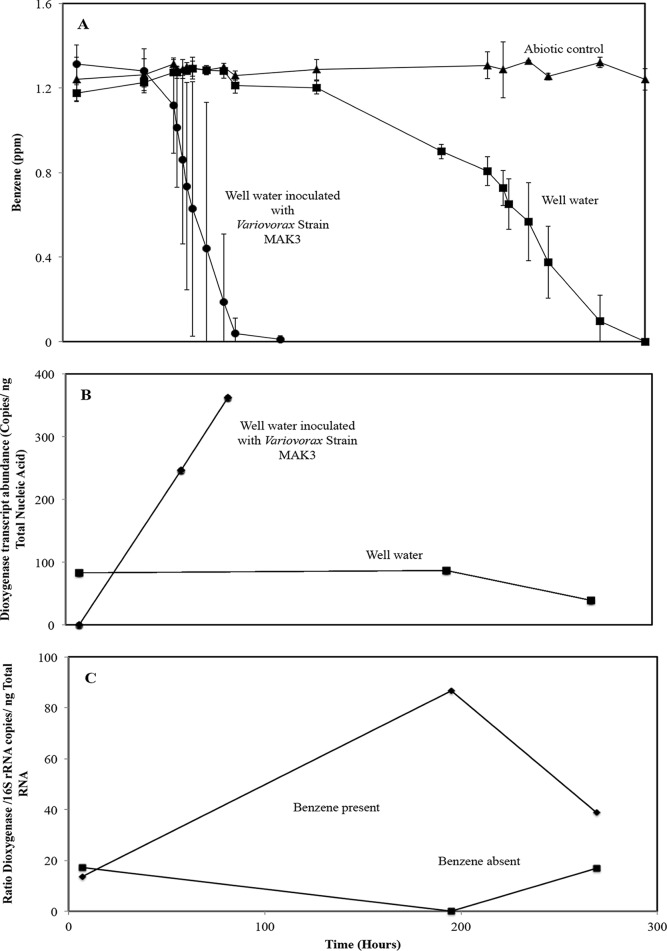
The final series of experiments tested the hypothesis that the Variovorax-related naturally occurring populations are active in degrading benzene in the microbial community native to the site's groundwater. The 16S rRNA and RHD gene qPCR assays described above were applied to nitrogen- and phosphorous-amended well 36 incubations with and without added Variovorax MAK3 cells and with and without added benzene. Samples were sacrificed for biomass extraction at times in the experiment when approximately 0%, 25%, and 90% of the initial benzene concentration were consumed. The addition of Variovorax MAK3 accelerated benzene degradation substantially (3-fold) compared with that of the native community alone (Fig. 4A). The inoculated treatment consumed ∼100% of the benzene in 90 h, while the native community required 300 h to degrade the benzene. In the inoculated treatment, the absolute transcript abundance for the MAK3 RHD gene increased over time, corresponding well with the accelerated loss of benzene during the first 100 h of the experiment (Fig. 4B). RHD transcripts were not detected initially, but they increased to 246 copies/ng when 25% of the benzene had been consumed and to 362 copies/ng when 90% had been consumed. These patterns in RHD gene expression are consistent with the results of the pure culture experiment (Fig. 3) and indicate that benzene loss is mechanistically tied to RHD gene activity. We hypothesized that Variovorax populations related to strain MAK3 were present and active in the uninoculated well water microcosms shown in Fig. 4A and B. qPCR assays of Variovorax-specific 16S rRNA and RHD transcripts showed that at 200 h (when the native community was most active; Fig. 4B), added benzene caused a 6-fold enhancement in the ratio of expressed RHD genes to 16S rRNA copies (Fig. 4C). Clearly, Variovorax MAK3 and related well water populations are adapted to degrading benzene in site waters.
FIG 4.
Experiments assessing the role of Variovorax strain MAK3 and its ring-hydroxylating dioxygenase (RHD) gene in benzene degradation in the naturally occurring well water microbial community. (A) Benzene degradation in freshly gathered contaminated well water sample (well 36) with and without added Variovorax strain MAK3 cells (initial cell density ∼105 CFU/ml). Error bars represent the standard deviations from averages of triplicate microcosms. (B) Dioxygenase transcript abundances (β subunit of ring-hydroxylating dioxygenase) in well 36 water community with and without added Variovorax strain MAK3 cells (initial cell density ∼105 CFU/ml). Data points represent single qPCR measurements. (C) Dioxygenase mRNA (β-subunit of ring-hydroxylating dioxygenase normalized to copies of 16S rRNA transcripts) in the well 36 microbial community induced by benzene addition. Variovorax strain MAK3 cells were not added to these treatments. Data points represent the ratios of single qPCR measurements.
DISCUSSION
Our aim was to use groundwater microcosms as a model system for investigating benzene degradation by microbial communities in coal tar-contaminated groundwater. Although benzene is a minor constituent of coal tar, the continued presence of other BTEX compounds onsite and the ubiquity of benzene as an environmental pollutant make it an important compound to study (42). In these experiments, we attempted to create relevant naturally occurring field conditions in our incubations. However, we fully acknowledge that the behavior and composition of the microbial communities in our laboratory model incubations are not necessarily indicative of the functions of microbial communities in situ (3).
We demonstrated in samples from wells 12 and 36 that separating the groundwater from the sediment matrix altered the biodegradation potentials of the communities (Fig. 1). A nutrient amendment was required to support benzene biodegradation activity. Results from previous investigations demonstrated that nutrient additions do not necessarily lead to drastic alterations of community functions (43, 44). Moreover, the sequences recovered from the SIP experiments represented Gammaproteobacteria and Betaproteobacteria, which is a finding consistent with those of field investigations of benzene-degrading communities (12).
Convergent lines of cultivation-dependent and cultivation-independent inquiries confirmed that the members of Gammaproteobacteria and Betaproteobacteria classes within the groundwater community were responsible for benzene biodegradation in the microcosms. We coupled SIP with cultivation to directly address potential weaknesses in SIP methodological procedures (4, 45). The isolation and pure-culture degradation experiments confirmed that the organisms native to the groundwater, specifically Variovorax MAK3 and Acidovorax MAK5, were capable of degrading benzene (see Fig. S2 and S3 in the supplemental material).
Cultivation approaches that supply BTEX compounds in vapor phase were used in previous investigations (12, 22). Hendrickx et al. (12) reported the cultivation of Acidovorax and Variovorax spp. from soil samples using BTEX mixtures. The results of SIP and cultivation experiments from this study reinforce a growing body of evidence that Acidovorax and Variovorax are active benzene-degrading organisms in many BTEX-contaminated sites (46–48). Variovorax spp. particularly have been associated with soil habitats and characterized as a metabolically diverse group of organisms with promising bioremediation and biotechnological applications (49–51).
To our knowledge, this is the first investigation demonstrating benzene degradation by Variovorax spp. in pure cultures, while simultaneously quantifying the expression of the oxygenase gene involved in benzene attack (Fig. 3). The RT-qPCR data suggest putative dioxygenase (Vapar_5383) plays a role in benzene biodegradation by Variovorax MAK3. However, a correlation does not prove the putative RHD is responsible for benzene degradation, and quantitative PCR has inherent limitations, particularly concerning the primer specificity and SYBR green assays. To verify that the primers were amplifying the target gene, the amplicon was sequenced and the qPCR was optimized to achieve a single peak in the dissociation curve. The transcript abundances from the pure-culture experiment were consistent with the findings of Kong and Nakatsu (52), who investigated RNA-extraction methods for quantifying aromatic oxygenase genes (52). We feel it is reasonable to presume that putative dioxygenase (homologous to Vapar_5383) enables benzene attack by Variovorax MAK3, while acknowledging that only gene-knockout experiments will provide definitive proof.
Interestingly, the widely used toluene/benzene dioxygenase family primer sets, TOD (10) and TODC1 (12), did not produce amplicons in the expected size range when applied to these Variovorax and Acidovorax isolates. The sequencing of the amplicons obtained using TOD and TODC1 primers yielded genes with no relation to aromatic dioxygenase genes (data not shown). Witzig et al. compared the toluene/biphenyl α-subunit-family gene sequences carried by Variovorax paradoxus EC4 with those of the classic dioxygenases in Pseudomonas ML2 (benzene) and F1 (toluene) (11). Results of the sequence analyses revealed little similarity among the dioxygenases (11). This underscores the broad diversity of oxygenase genes in the environment and the potential limitations and biases of PCR-based inquiries (8, 14). Despite the high diversity among genes encoding enzymes involved in the attack of monoaromatic hydrocarbons, there seems to be a high degree of conservation in the biochemical pathways that enable aerobic destabilization of the aromatic ring (8, 14).
Introducing Variovorax MAK3 accelerated the loss of benzene in site-water microcosms, and the accelerated loss coincided with increased putative RHD abundance (Fig. 4A and B). Furthermore, Variovorax populations native to the groundwater microbial community were found, and their RHD transcriptional activity was boosted by benzene addition (Fig. 4C). Surprisingly, the RHD transcript abundances were significantly lower in the assays using groundwater than the corresponding abundances in the pure-culture experiments. This may be due to several contributing factors, including low biomass, lower benzene concentrations in the incubations, increased competition, and/or lower cellular growth rates (53).
Investigations into groundwater microbial communities using SIP face significant challenges given the inherent difficulty for accessing the habitats and controlling substrate dispersal in an open system. In situ approaches into groundwater systems have been performed using Bio-Sep beads carrying 13C-labeled benzene (33–35). Such assays are only successful when the biodegrading populations colonize the Bio-Sep beads and form a biofilm. The studies utilizing Bio-Sep beads have largely focused on anaerobic benzene degradation in anoxic waters and have relied on PLFA analysis for identifying the 13C-labeled community members. SIP-based benzene biodegradation by microorganisms from the coal tar-contaminated field site investigated in this study was previously reported in a study demonstrating that Gammaproteobacteria and Betaproteobacteria were active in both anaerobic laboratory incubations and field SIP (31). However, there are many differences between the physiological conditions and the inocula used by Liou et al. (largely anaerobic sediments) (31) and the inoculum and conditions used in this study. These differences make direct comparisons of the results from the two studies difficult.
The present study demonstrated the ability of cultivation-dependent and cultivation-independent approaches to complement one another to produce new information regarding the identity of microorganisms (and their catabolic genes) carrying out key metabolic processes in complex microbial communities.
MATERIALS AND METHODS
Site description and sample collection.
The study site is a coal tar-contaminated aquifer located in South Glens Falls, New York. The site's microbiology and geochemistry are well documented (13, 54, 55). Well water samples were taken from two monitoring wells (well 12 and well 36) as described by Bakermans and Madsen (55). Previously collected site sediment was stored at 4°C. Sterile sediment was prepared by air drying site samples, dispensing the samples into 50-ml screw-cap glass tubes, and applying gamma irradiation (2.5 Mrad from the 60Co source in the Ward Nuclear Reactor, Cornell University). Sterility was verified by the absence of colonies after plating a 10−1 dilution on LB media and incubating the sample at room temperature for 10 days.
Benzene biodegradation assays.
Laboratory biodegradation assays for groundwater were set up within 24 h of sample collection. Eighty milliliters of groundwater was dispensed into 160-ml serum bottles and sealed with Teflon-faced butyl rubber septa. The following 4 treatments were established for bottles that received both unlabeled (12C) and labeled (13C) benzene: (i) site water only (unamended), (ii) amended with 10% (vol/vol) sterile sediment, (iii) amended with 10 mM (NH4)3PO4, and (iv) an abiotic control that received 10 N HCl to achieve a pH <2. Liquid benzene was added (neat) to septa (or immediately prior to sealing the serum bottles) with a 2-μl syringe to final concentrations ranging from 1.2 to 20 ppm. Bottles were incubated at 10°C (ambient temperature for the study site) in the dark without shaking. At various times over a 360-h period, headspace gases were sampled using a 250-μl gastight syringe (Hamilton) and were analyzed for benzene by gas chromatography mass spectrometry (GC-MS), as described below. Benzene metabolism by isolated bacterial cultures was monitored in serum bottles prepared as described above, but incubated at 21°C and shaken at 120 rpm.
Stable isotope probing.
After ∼70% of the benzene was consumed in the above microcosms, samples from [12C]- and [13C]benzene treatments were obtained for nucleic acid extraction. DNA was extracted using a Fast DNASPIN kit as previously described by DeRito et al. (30). The triplicate samples from each treatment were pooled and processed by CsCl ultracentrifugation to create an isotopic DNA gradient, separating the [12C] and [13C]DNA (30). The location of labeled (heavy) DNA in the gradient was determined by including a tube containing [13C]DNA (from Pseudomonas putida grown on [13C]glucose) in the ultracentrifuge rotor. After pulling these heavy fractions from the [12C]- and [13C]benzene treatments, dilutions were prepared and 16S PCR was performed as per DeRito et al. (30). The pool of 16S rRNA PCR amplicons representing the active benzene-degrading populations was operationally defined as the dilution of DNA from the 13C treatment that successfully amplified, while the corresponding dilution from the 12C treatment failed to amplify (30). This [13C]benzene-derived set of amplicons was cloned and sent for sequencing (on an Applied Biosystems model 3700 analyzer) at the Cornell University biotechnological facility (30).
Cultivation of isolates on solid media.
A site-specific medium consisting of filter-sterilized (0.2 μm; Corning) well water and noble agar (Difco; BD) was prepared. After the sample from well 36 was incubated, two separate dilution series (100-, 10−1-, and 10−2-fold) were spread plated and incubated at 10°C in the presence or absence of benzene vapor in the headspace; details of how benzene vapor was delivered to avoid toxicity are described in the supplemental material. After 15 days of observation, small translucent colonies appeared on the control and benzene-exposed plates. At day 22, sterile colony transfer pads (RepliPlate; FMC) were used to replica plate the cultures onto Stanier's basal salt minimal medium (BSM) agar plates as per Burlage (56). After incubating 18 more days in benzene vapor, the minimal media plates yielded colonies ∼2 mm in diameter. Nine colonies with distinct morphologies that grew only in the presence of benzene were selected and streaked to isolate single colonies. Isolates were restreaked and grown three successive times in the presence of benzene and were picked and grown in R2A medium. A portion of the R2A culture was preserved by adding 50% glycerol and freezing at −80°C. The isolates were revived and streaked on BSM and displayed growth only in the presence of benzene.
Dioxygenase expression in minimal media.
The MAK3 isolate was grown in BSM containing 0.5 g/liter glucose, was washed 3× in BSM, and was added to 80 ml liquid BSM in 1-liter serum bottles for a final concentration of approximately 1 × 106 CFU/ml. The benzene (7 ppm) degradation experiment included the following three treatments: (i) Variovorax strain MAK3 only, (ii) Variovorax strain MAK3 cells killed with 5 M hydrochloric acid, and (iii) without inoculum. Cultures were incubated without shaking at 21°C. A glucose (0.5 g/liter) control treatment for quantifying dioxygenase gene expression was prepared concurrently in BSM. Subsamples of each culture were taken periodically (at 0, 21, 27, and 36 h) and immediately frozen in liquid N2 for RNA extraction.
Dioxygenase expression in nutrient-amended well water.
In the final degradation experiment, 80 ml of water from well 36 amended with filter-sterilized (NH4)3PO4 (final concentration of 10 mM) was added to the serum bottles. Variovorax strain MAK3 cells, grown as described above, were added to attain an initial concentration of 2 × 105 CFU/ml. Liquid benzene was added to each treatment at 1.2 ppm. Cultures were incubated without shaking at 21°C. Subsamples of the Variovorax-amended and unamended treatments were taken at 3 time points corresponding with 15, 75, and 100% benzene loss and were immediately frozen in liquid N2 for RNA extraction.
Phylogenetic analysis of benzene-degrading isolates.
Nine isolates were identified using 16S rRNA gene sequencing. 16S rRNA PCR was performed on individual colonies using 27F/1492R primers (see Table S1 in the supplemental material). The 25-μl 16S rRNA reaction mixtures included 5 μl 5× MyTaq buffer (15 mM MgCl2, 5 mM deoxynucleoside triphosphates [dNTPs]; Bioline), 0.5 μl 27F/1492R primers (20 μM), and 0.1 μl MyTaq DNA polymerase (5 U/μl; Bioline) and were run on a thermocycler (PTC-200; MJ Research) with the following conditions: initial denaturation (95°C, 5 min), 32 cycles of denaturation (94°C, 1 min), annealing (55°C, 1 min 30 s), and extension (72°C, 1 min 30 s), and a final extension (72°C, 10 min). The 16S rRNA PCR amplicons were gel purified using a QIAquick gel extraction kit (Qiagen) and were ligated into a pCR2.1 plasmid vector (TOPO TA cloning; Invitrogen) as per the manufacturer's protocol. Plasmids were transformed into chemically competent Escherichia coli (One shot TOP10; Invitrogen) cells and were grown for blue/white screening. White colonies were picked and verified by PCR with M13F/M13R primers (Invitrogen). The clones containing the insert were grown overnight in Luria broth with kanamycin (50 μg/μl), and the plasmids were extracted for sequencing using a Zyppy plasmid miniprep kit (Zymo). Sequencing was performed by the Cornell University Life Sciences Core Laboratories center with an Applied Biosystems automated 3730 DNA analyzer using BigDye Terminator chemistry and AmpliTaq-FS DNA polymerase. Consensus 16S rRNA sequences were built from 4 independently sequenced reactions employing primer sets M13F/M13R, 27F/1492R, 530F/519R, and 1114F/1100R using Ebiox (version 1.5.1) (see Table S1). The isolate sequences were compared with the GenBank nucleotide database library using BLASTn and with the Ribosomal Database Project for taxonomic identification. Phylogenetic trees were constructed using MEGA 5.05 (57). The isolates, SIP clones, and reference sequences were aligned with ClustalW and were assembled using a neighbor-joining algorithm with 1,000 bootstrap replications.
Variovorax RHD primer design.
Primers were designed to target the aromatic-ring-hydroxylating dioxygenase beta subunit (Vapar_5383) of Variovorax paradoxus S110 (NC_012792.1) using the NCBI Primer-BLAST tool. The primer set VarRHDF/VarRHDR amplifies a 182-bp region of the gene (see Table S1). The 25-μl volume PCR VarRHD reaction conditions included 0.1 μl Taq DNA polymerase (5 U/μl; Bioline), 5 μl 5× MyTaq buffer (15 mM MgCl2, 5 mM dNTPs; Bioline), 0.5 μl 20 μM VarRHDF/VarRHDR, and 1 μl DNA template. Thermocycler conditions were as follows: initial denaturation (95°C, 10 min), then 40 cycles of denaturation (95°C, 1 min), annealing (60°C, 1 min), and extension (72°C, 30 s), and a final extension (72°C, 10 min). To validate the specificity of the primers, the amplicon was gel purified with a QIAquick gel extraction kit (Qiagen) and was cloned, sequenced, and compared to the NCBI reference using BLASTn.
Cell extraction, RNA, RT-PCR, and qPCR.
Cells from the degradation experiments were subsampled from the microcosm experiments, were concentrated by centrifugation (7,000 × g), and immediately frozen in liquid nitrogen for storage at −80°C until processing. RNA extractions were performed with Quick-RNA kits (Zymo). The extraction buffer was applied directly to the frozen pellet, and the procedure was followed as per the manufacturer's protocol. Total nucleic acid extractions were treated with DNase (Invitrogen) and converted to cDNA using SuperScript III first strand reverse transcriptase (Invitrogen); the RT-negative control treatment was prepared in parallel. The cDNA was quantified using qPCR with dioxygenase-specific primers (VarRHDF/VarRHDR) and Variovorax-specific 16S rRNA primers VarF/VarR (41) (see Table S1). Quantitative PCR was performed on an Applied Biosystems 7300 real-time PCR system. Standards were made by serially diluting gel-purified VarF/VarR and VarRHDF/VarRHDR PCR amplicons and comparing them with known quantities of lambda DNA using Quant-iT (PicoGreen double-stranded DNA [dsDNA] reagent, P7581; Invitrogen). The 16S rRNA reaction mixture and RHD PCR mixture included 12.5 μl master mix (SYBR select; Applied Biosystems), 0.3 μl 20 μM RHD/16S (240 nM), 0.3 μl 20 μM VarR (240 nM), 10.9 μl H2O, and 1.0 μl template cDNA. The quantitative PCR thermocycler conditions included 2 min at 50°C, 15 min at 95°C, and 40 cycles of 15 s at 95°C and 30 s at 58°C, and a dissociation curve was included to assess amplification specificity.
Gas chromatography mass spectrometry analysis of benzene.
A Hewlett-Packard HP6890 gas chromatograph (Wilmington, DE) linked with an HP 5973 mass-selective detector was used to quantify benzene. The gas chromatograph was fitted with a Hewlett-Packard HP-5 phenylmethyl-siloxane capillary column (HP 19091J-433, capillary: 30.0 m by 250 μm by 0.25 μm) carrying high-purity helium gas supplied by Airgas (Elmira, NY). The GC parameters included a split injection (20:1), an inlet temperature of 150°C, and an initial GC oven temperature of 50°C that ramped 15°C/min to a final temperature of 100°C. The mass spectrometer detector was operating at 2,000 eV with a vacuum of 2 × 10−5 torr in scan mode from 50 to 500 m/z (benzene m/z = 78). Benzene was measured by taking 100-μl headspace samples with a 250-μl gastight syringe (Hamilton, Reno, NV). Calibration curves were constructed using external standards with known amounts of benzene (EMD, Germany). Benzene concentrations were averaged and the standard deviations from triplicate chambers were compared.
Accession number(s).
The sequences from this study have been deposited in GenBank under accession numbers KX665551 to KX665559 and KX670396 to KX670409 for isolates and clones, respectively.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Fernandes and J. Oh for providing technical assistance in GC-MS measurements and PCR optimization. We also thank A. Hay and B. Hanson for help with obtaining P. putida F1 cultures.
This research was supported by NSF grant DEB-0841999 and by the USDA National Institute of Food and Agriculture, McIntire Stennis project 1001853.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02658-16.
REFERENCES
- 1.Falkowski PG, Fenchel T, Delong EF. 2008. The microbial engines that drive Earth's biogeochemical cycles. Science 320:1034–1039. doi: 10.1126/science.1153213. [DOI] [PubMed] [Google Scholar]
- 2.Madsen EL. 2011. Microorganisms and their roles in fundamental biogeochemical cycles. Curr Opin Biotechnol 22:456–464. doi: 10.1016/j.copbio.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Madsen EL. 1998. Epistemology of environmental microbiology. Environ Sci Technol 32:429–439. doi: 10.1021/es970551y. [DOI] [Google Scholar]
- 4.Madsen EL. 2005. Identifying microorganisms responsible for ecologically significant biogeochemical processes. Nat Rev Microbiol 3:439–446. doi: 10.1038/nrmicro1151. [DOI] [PubMed] [Google Scholar]
- 5.Reid BJ, Jones KC, Semple KT. 2000. Bioavailability of persistent organic pollutants in soils and sediments–a perspective on mechanisms, consequences and assessment. Environ Pollut 108:103–112. doi: 10.1016/S0269-7491(99)00206-7. [DOI] [PubMed] [Google Scholar]
- 6.Rappé MS, Giovannoni SJ. 2003. The uncultured microbial majority. Annu Rev Microbiol 57:369–394. doi: 10.1146/annurev.micro.57.030502.090759. [DOI] [PubMed] [Google Scholar]
- 7.King GM, Kostka JE, Hazen TC, Sobecky PA. 2015. Microbial responses to the Deepwater Horizon oil spill: from coastal wetlands to the deep sea. Ann Rev Mar Sci 7:377–401. doi: 10.1146/annurev-marine-010814-015543. [DOI] [PubMed] [Google Scholar]
- 8.Gibson DT, Parales RE. 2000. Aromatic hydrocarbon dioxygenases in environmental biotechnology. Curr Opin Biotechnol 11:236–243. doi: 10.1016/S0958-1669(00)00090-2. [DOI] [PubMed] [Google Scholar]
- 9.Bamforth SM, Singleton I. 2005. Bioremediation of polycyclic aromatic hydrocarbons: current knowledge and future directions. J Chem Technol Biotechnol 80:723–736. doi: 10.1002/jctb.1276. [DOI] [Google Scholar]
- 10.Baldwin BR, Nakatsu CH, Nies L. 2003. Detection and enumeration of aromatic oxygenase genes by multiplex and real-time PCR. Appl Environ Microbiol 69:3350–3358. doi: 10.1128/AEM.69.6.3350-3358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witzig R, Junca H, Hecht H-J, Pieper DH. 2006. Assessment of toluene/biphenyl dioxygenase gene diversity in benzene-polluted soils: links between benzene biodegradation and genes similar to those encoding isopropylbenzene dioxygenases. Appl Environ Microbiol 72:3504–3514. doi: 10.1128/AEM.72.5.3504-3514.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendrickx B, Junca H, Vosahlova J, Lindner A, Rüegg I, Bucheli-Witschel M, Faber F, Egli T, Mau M, Schlömann M, Brennerova M, Brenner V, Pieper DH, Top EM, Dejonghe W, Bastiaens L, Springael D. 2006. Alternative primer sets for PCR detection of genotypes involved in bacterial aerobic BTEX degradation: distribution of the genes in BTEX degrading isolates and in subsurface soils of a BTEX contaminated industrial site. J Microbiol Methods 64:250–265. doi: 10.1016/j.mimet.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Yagi JM, Madsen EL. 2009. Diversity, abundance, and consistency of microbial oxygenase expression and biodegradation in a shallow contaminated aquifer. Appl Environ Microbiol 75:6478–6487. doi: 10.1128/AEM.01091-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwai S, Johnson TA, Chai B, Hashsham SA, Tiedje JM. 2011. Comparison of the specificities and efficacies of primers for aromatic dioxygenase gene analysis of environmental samples. Appl Environ Microbiol 77:3551–3557. doi: 10.1128/AEM.00331-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar M, Khanna S. 2010. Diversity of 16S rRNA and dioxygenase genes detected in coal tar-contaminated site undergoing active bioremediation. J Appl Microbiol 108:1252–1262. doi: 10.1111/j.1365-2672.2009.04523.x. [DOI] [PubMed] [Google Scholar]
- 16.Meynet P, Head IM, Werner D, Davenport RJ. 2015. Re-evaluation of dioxygenase gene phylogeny for the development and validation of a quantitative assay for environmental aromatic hydrocarbon degraders. FEMS Microbiol Ecol 91:pii=fiv049. doi: 10.1093/femsec/fiv049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arenghi FL, Berlanda D, Galli E, Sello G, Barbieri P. 2001. Organization and regulation of meta cleavage pathway genes for toluene and o-xylene derivative degradation in Pseudomonas stutzeri OX1. Appl Environ Microbiol 67:3304–3308. doi: 10.1128/AEM.67.7.3304-3308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D, Kim Y-S, Kim S-K, Kim SW, Zylstra GJ, Kim YM, Kim E. 2002. Monocyclic aromatic hydrocarbon degradation by Rhodococcus sp. strain DK17. Appl Environ Microbiol 68:3270–3278. doi: 10.1128/AEM.68.7.3270-3278.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taki H, Syutsubo K, Mattison RG, Harayama S. 2007. Identification and characterization of o-xylene-degrading Rhodococcus spp. which were dominant species in the remediation of o-xylene-contaminated soils. Biodegradation 18:17–26. doi: 10.1007/s10532-005-9030-x. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson CA, Fathepure BZ. 2005. Aerobic biodegradation of benzene and toluene under hypersaline conditions at the Great Salt Plains, Oklahoma. FEMS Microbiol Lett 245:257–262. doi: 10.1016/j.femsle.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Shao Z-Z. 2006. Isolation and characterization of 4 benzene/toluene-degrading bacterial strains and detection of related degradation genes. Wei Sheng Wu Xue Bao 46:753–757. (In Chinese.) [PubMed] [Google Scholar]
- 22.Wang L, Qiao N, Sun F, Shao Z. 2008. Isolation, gene detection and solvent tolerance of benzene, toluene and xylene degrading bacteria from nearshore surface water and Pacific Ocean sediment. Extremophiles 12:335–342. doi: 10.1007/s00792-007-0136-4. [DOI] [PubMed] [Google Scholar]
- 23.Schloss PD, Handelsman J. 2004. Status of the microbial census. Microbiol Mol Biol Rev 68:686–691. doi: 10.1128/MMBR.68.4.686-691.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uhlik O, Leewis M-C, Strejcek M, Musilova L, Mackova M, Leigh MB, Macek T. 2013. Stable isotope probing in the metagenomics era: a bridge towards improved bioremediation. Biotechnol Adv 31:154–165. doi: 10.1016/j.biotechadv.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radajewski S, Ineson P, Parekh NR, Murrell JC. 2000. Stable-isotope probing as a tool in microbial ecology. Nature 403:646–649. doi: 10.1038/35001054. [DOI] [PubMed] [Google Scholar]
- 26.Dumont MG, Murrell JC. 2005. Stable isotope probing—linking microbial identity to function. Nat Rev Microbiol 3:499–504. doi: 10.1038/nrmicro1162. [DOI] [PubMed] [Google Scholar]
- 27.Cupples AM. 2016. Contaminant-degrading microorganisms identified using stable isotope probing. Chem Eng Technol 39:1593–1603. doi: 10.1002/ceat.201500479. [DOI] [Google Scholar]
- 28.Sul WJ, Park J, Quensen JF, Rodrigues JLM, Seliger L, Tsoi TV, Zylstra GJ, Tiedje JM. 2009. DNA-stable-isotope probing integrated with metagenomics for retrieval of biphenyl dioxygenase genes from polychlorinated biphenyl-contaminated river sediment. Appl Environ Microbiol 75:5501–5506. doi: 10.1128/AEM.00121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeon CO, Park W, Padmanabhan P, DeRito C, Snape JR, Madsen EL. 2003. Discovery of a bacterium, with distinctive dioxygenase, that is responsible for in situ biodegradation in contaminated sediment. Proc Natl Acad Sci U S A 100:13591–13596. doi: 10.1073/pnas.1735529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeRito CM, Pumphrey GM, Madsen EL. 2005. Use of field-based stable isotope probing to identify adapted populations and track carbon flow through a phenol-degrading soil microbial community. Appl Environ Microbiol 71:7858–7865. doi: 10.1128/AEM.71.12.7858-7865.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liou JS-C, Derito CM, Madsen EL. 2008. Field-based and laboratory stable isotope probing surveys of the identities of both aerobic and anaerobic benzene-metabolizing microorganisms in freshwater sediment. Environ Microbiol 10:1964–1977. doi: 10.1111/j.1462-2920.2008.01612.x. [DOI] [PubMed] [Google Scholar]
- 32.Cupples AM. 2011. The use of nucleic acid based stable isotope probing to identify the microorganisms responsible for anaerobic benzene and toluene biodegradation. J Microbiol Methods 85:83–91. doi: 10.1016/j.mimet.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Geyer R, Peacock AD, Miltner A, Richnow HH, White DC, Sublette KL, Kästner M. 2005. In situ assessment of biodegradation potential using biotraps amended with 13C-labeled benzene or toluene. Environ Sci Technol 39:4983–4989. doi: 10.1021/es048037x. [DOI] [PubMed] [Google Scholar]
- 34.Kästner M, Fischer A, Nijenhuis I, Geyer R, Stelzer N, Bombach P, Tebbe CC, Richnow HH. 2006. Assessment of microbial in situ activity in contaminated aquifers. Eng Life Sci 6:234–251. doi: 10.1002/elsc.200620125. [DOI] [Google Scholar]
- 35.Stelzer N, Büning C, Pfeifer F, Dohrmann AB, Tebbe CC, Nijenhuis I, Kästner M, Richnow HH. 2006. In situ microcosms to evaluate natural attenuation potentials in contaminated aquifers. Org Geochem 37:1394–1410. doi: 10.1016/j.orggeochem.2006.05.011. [DOI] [Google Scholar]
- 36.Kasai Y, Takahata Y, Manefield M, Watanabe K. 2006. RNA-based stable isotope probing and isolation of anaerobic benzene-degrading bacteria from gasoline-contaminated groundwater. Appl Environ Microbiol 72:3586–3592. doi: 10.1128/AEM.72.5.3586-3592.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie S, Sun W, Luo C, Cupples AM. 2011. Novel aerobic benzene degrading microorganisms identified in three soils by stable isotope probing. Biodegradation 22:71–81. doi: 10.1007/s10532-010-9377-5. [DOI] [PubMed] [Google Scholar]
- 38.Yagi JM, Neuhauser EF, Ripp JA, Mauro DM, Madsen EL. 2010. Subsurface ecosystem resilience: long-term attenuation of subsurface contaminants supports a dynamic microbial community. ISME J 4:131–143. doi: 10.1038/ismej.2009.101. [DOI] [PubMed] [Google Scholar]
- 39.Andreoni V, Gianfreda L. 2007. Bioremediation and monitoring of aromatic-polluted habitats. Appl Microbiol Biotechnol 76:287–308. doi: 10.1007/s00253-007-1018-5. [DOI] [PubMed] [Google Scholar]
- 40.Wilbur S, Wohlers D, Paikoff S, Keith LS, Faroon O. 2008. ATSDR evaluation of health effects of benzene and relevance to public health. Toxicol Ind Health 24:263–398. doi: 10.1177/0748233708090910. [DOI] [PubMed] [Google Scholar]
- 41.Bers K, Sniegowski K, Albers P, Breugelmans P, Hendrickx L, De Mot R, Springael D. 2011. A molecular toolbox to estimate the number and diversity of Variovorax in the environment: application in soils treated with the phenylurea herbicide linuron. FEMS Microbiol Ecol 76:14–25. doi: 10.1111/j.1574-6941.2010.01028.x. [DOI] [PubMed] [Google Scholar]
- 42.Neuhauser EF, Ripp JA, Azzolina NA, Madsen EL, Mauro DM, Taylor T. 2009. Monitored natural attenuation of manufactured gas plant tar mono- and polycyclic aromatic hydrocarbons in ground water: a 14-year field study. Ground Water Monit Remediat 29:66–76. doi: 10.1111/j.1745-6592.2009.01244.x. [DOI] [Google Scholar]
- 43.Jones SH, Alexander M. 1988. Effect of inorganic nutrients on the acclimation period preceding mineralization of organic chemicals in lake water. Appl Environ Microbiol 54:3177–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Röling WFM, Milner MG, Jones DM, Lee K, Daniel F, Swannell RJP, Head IM. 2002. Robust hydrocarbon degradation and dynamics of bacterial communities during nutrient-enhanced oil spill bioremediation. Appl Environ Microbiol 68:5537–5548. doi: 10.1128/AEM.68.11.5537-5548.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neufeld JD, Dumont MG, Vohra J, Murrell JC. 2007. Methodological considerations for the use of stable isotope probing in microbial ecology. Microb Ecol 53:435–442. doi: 10.1007/s00248-006-9125-x. [DOI] [PubMed] [Google Scholar]
- 46.Rooney-Varga JN, Anderson RT, Fraga JL, Ringelberg D, Lovley DR. 1999. Microbial communities associated with anaerobic benzene degradation in a petroleum-contaminated aquifer. Appl Environ Microbiol 65:3056–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aburto A, Fahy A, Coulon F, Lethbridge G, Timmis KN, Ball AS, McGenity TJ. 2009. Mixed aerobic and anaerobic microbial communities in benzene-contaminated groundwater. J Appl Microbiol 106:317–328. doi: 10.1111/j.1365-2672.2008.04005.x. [DOI] [PubMed] [Google Scholar]
- 48.Aburto A, Peimbert M. 2011. Degradation of a benzene-toluene mixture by hydrocarbon-adapted bacterial communities. Ann Microbiol 61:553–562. doi: 10.1007/s13213-010-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han J-I, Choi H-K, Lee S-W, Orwin PM, Kim J, Laroe SL, Kim T-G, O'Neil J, Leadbetter JR, Lee SY, Hur C-G, Spain JC, Ovchinnikova G, Goodwin L, Han C. 2011. Complete genome sequence of the metabolically versatile plant growth-promoting endophyte Variovorax paradoxus S110. J Bacteriol 193:1183–1190. doi: 10.1128/JB.00925-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miwa H, Ahmed I, Yoon J, Yokota A, Fujiwara T. 2008. Variovorax boronicumulans sp. nov., a boron-accumulating bacterium isolated from soil. Int J Syst Evol Microbiol 58:286–289. doi: 10.1099/ijs.0.65315-0. [DOI] [PubMed] [Google Scholar]
- 51.Boersma FGH, Otten R, Warmink JA, Nazir R, van Elsas JD. 2010. Selection of Variovorax paradoxus-like bacteria in the mycosphere and the role of fungal-released compounds. Soil Biol Biochem 42:2137–2145. doi: 10.1016/j.soilbio.2010.08.009. [DOI] [Google Scholar]
- 52.Kong W, Nakatsu CH. 2010. Optimization of RNA extraction for PCR quantification of aromatic compound degradation genes. Appl Environ Microbiol 76:1282–1284. doi: 10.1128/AEM.01939-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith CJ, Osborn AM. 2009. Advantages and limitations of quantitative PCR (Q-PCR)-based approaches in microbial ecology. FEMS Microbiol Ecol 67:6–20. doi: 10.1111/j.1574-6941.2008.00629.x. [DOI] [PubMed] [Google Scholar]
- 54.Madsen EL, Sinclair JL, Ghiorse WC. 1991. In situ biodegradation: microbiological patterns in a contaminated aquifer. Science 252:830–833. doi: 10.1126/science.2028258. [DOI] [PubMed] [Google Scholar]
- 55.Bakermans C, Madsen EL. 2002. Diversity of 16S rDNA and naphthalene dioxygenase genes from coal tar-waste-contaminated aquifer waters. Microb Ecol 44:95–106. doi: 10.1007/s00248-002-0005-8. [DOI] [PubMed] [Google Scholar]
- 56.Burlage RS. 1998. Molecular techniques, p 289–334. In Burlage RS, Atlas R, Stahl D, Geesey G, Sayler G (ed), Techniques in microbial ecology. Oxford University Press, New York, NY. [Google Scholar]
- 57.Kumar S, Nei M, Dudley J, Tamura K. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9:299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.