Abstract
Human epidemiological and animal studies have shown the beneficial effect of zinc supplementation on mitigating diabetic nephropathy. However, the mechanism by which zinc protects the kidney from diabetes remains unknown. Here we demonstrate the therapeutic effects of zinc on diabetes-induced renal pathological and functional changes. These abnormalities were found in both transgenic OVE26 and Akt2-KO diabetic mouse models, accompanied by significant changes in glucose-metabolism-related regulators. The changes included significantly decreased phosphorylation of Akt and GSK-3β, increased phosphorylation of renal glycogen synthase, decreased expression of hexokinase II and PGC-1α, and increased expression of the Akt negative regulators PTEN, PTP1B, and TRB3. All of these were significantly prevented by zinc treatment for 3 months. Furthermore, zinc-stimulated changes in glucose metabolism mediated by Akt were actually found to be metallothionein dependent, but not Akt2 dependent. These results suggest that the therapeutic effects of zinc in diabetic nephropathy are mediated, in part, by the preservation of glucose-metabolism-related pathways via the prevention of diabetes-induced upregulation of Akt negative regulators. Given that zinc deficiency is very common in diabetics, this finding implies that regularly monitoring zinc levels in diabetic patients, as well as supplementing if low, is important in mitigating the development of diabetic nephropathy.
Keywords: Diabetic nephropathy; Zinc; Akt, GSK-3β, Akt2 gene defect; Metallothionein; Free radicals
Diabetic nephropathy (DN) is the most common cause of end-stage renal disease in developed countries and is associated with significantly increased mortality in diabetic patients [1]. Albuminuria, a common characteristic of DN, is associated with a higher incidence of progression toward renal failure [1]; therefore, reducing urine albumin is an important goal toward preventing renal functional decline [2]. To date, there remains no completely effective approach to prevent the development and/or progression of DN in diabetic patients. Several new drugs had been developed and approved by the FDA, but were withdrawn because of unexpected toxic side effects. One option is to apply drugs that are currently used in clinics for other, nondiabetic, diseases and that are without side effects to diabetic patients [2].
Zinc (Zn) is a trace element that plays a pivotal role in the proper functioning of many enzymes and transcription factors [3]. Zn has been used clinically in the treatment of several diseases. Its low toxicity profile makes it possible to use in pediatric patients [4,5]. Recently, meta-analysis and systematic review of clinical data showed the beneficial effects of Zn supplementation for diabetic patients, which included the following findings [6,7]. Zn supplementation has a hypoglycemic effect and improves lipid profiles [6,7]. Human study proves that Zn supplementation reduces albumin excretion in microalbuminuric type 2 diabetic patients [8,9]. These authors stated the need for further studies to identify the exact mechanisms responsible for these Zn-mediated beneficial effects [6–9]. Using animal models, we have demonstrated that Zn supplementation provides renal protection against diabetes-induced pathological changes [10], which has already been supported by subsequent studies [11,12]. However, the underlying mechanism has yet to be established.
We have reported that renal pathological changes such as inflammation, cell death, and remodeling, occurring in the late stage of diabetes, were associated with decreased Akt (protein kinase B) function in the kidney [13]. The Akt family of serine–threonine kinases participates in diverse cellular processes, including the promotion of cell survival, glucose metabolism, and cellular protein synthesis. Among the three isoforms of Akt, each has its own predominant function [14–18]: Akt1 is involved in cellular survival pathways by inhibiting apoptotic processes; Akt1 is also able to induce protein synthesis, making it a key signaling protein in the cellular pathways that lead to skeletal muscle hypertrophy and general tissue growth. Akt2 is an important molecule in the insulin-signaling pathway. Mice with Akt1 gene deletion show normal glucose metabolism [16], whereas mice with Akt2 gene deletion (Akt2-KO) or humans with an Akt2 gene mutation develop insulin resistance and a type 2 diabetes-like phenotype [15,16,19]. The role of Akt3 is less clear, though it seems to be important for the brain [17,18]. Reportedly, Zn has an insulin-like function allowing it to stimulate Akt phosphorylation and activate glucose metabolism [20–22]. Therefore, because of the important role of Akt2 in insulin-mediated glucose metabolism, we assume that Zn-mediated protection from diabetes-induced renal damage may be predominantly dependent on Akt2.
Another potential mechanism responsible for Zn-induced renal protection may be the induction of metallothionein (MT). MTs are a group of intracellular metal-binding proteins characterized by low molecular mass (6–7 kDa) and high cysteine content (20 of the 61 or 62 amino acids). Although four isoforms of MTs have been characterized, MT-I and MT-II are the major isoforms in most human and animal organs. Intracellular Zn is regulated by binding to MT and by compartmentalization through the activities of Zn transporters. MTs have high binding affinity for Zn and play a central role in maintaining intracellular Zn availability through sequestration or release of Zn [23,24]. However, it is unclear whether Zn protection of the diabetic kidney is dependent on MT. It is also unclear whether there is any cross talk between Zn-induced Akt/glycogen synthase kinase-3β (GSK-3β) activation and MT induction.
In this study we examined whether Zn can provide a therapeutic effect against DN using the OVE26 type 1 diabetic mouse model. We then examined whether the therapeutic effect of Zn on the diabetic kidney is dependent on Akt2 using an Akt2-KO mouse model. Finally, we examined if MT is required for Zn stimulation of Akt and its downstream pathways by using mice with an MT gene deletion (MT-KO).
Materials and methods
Animals
OVE26 mice with an FVB background were developed by Dr. Paul N. Epstein and characterized before [25]. Akt2-KO mice with C57BL/ 6J background were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and have been cross-bred with FVB mice for more than 12 generations. MT-KO mice (MT-I and MT-II knockout mice) and wild-type (129S1) control mice were also obtained from The Jackson Laboratory. Mice were housed at 22 °C with a 12:12-h light: dark cycle and free access to rodent chow and tap water. All of the aforementioned studies were performed with male mice to be consistent with our previous studies [25–30]. All animal procedures were approved by the Institutional Animal Care and Use Committee.
Type 1 diabetes model
Male transgenic type 1 diabetic OVE26 mice typically develop severe hyperglycemia before 3 weeks of age and albuminuria by 2–3 months of the age [25,26]. Therefore, male OVE26 mice at 3 months of age were randomly divided into two groups: diabetes (DM, n = 6) and diabetes with Zn treatment (DM/Zn, n = 6). Age-matched FVB mice were also randomly divided into two groups: nondiabetic control (control, n = 6) and Zn control (Zn, n = 6). Zn supplementation was given by gavage at 5 mg Zn/kg (ZnSO4) every other day for 3 months. Control and DM group mice were administered equal amounts of saline. The volume of ZnSO4 solution was calculated based on individual mouse body weight (0.1 ml ZnSO4/g body wt). All mice were sacrificed at 6 months of age (i.e., at the end of 3 months of Zn supplementation).
Akt2-KO mouse model
Two-month-old male Akt2-KO mice were randomly divided into two groups: diabetes (Akt2-KO, n = 6) and diabetes supplemented with Zn (Akt2-KO/Zn, n = 6). Age-matched FVB mice were also randomly divided into two groups: control (control, n = 6) and Zn control (Zn, n = 6). For Zn supplementation and control mice, Zn and saline were given by the same methods described above for the type 1 diabetes model. All mice were sacrificed at 5 months of age.
MT-KO and FVB mouse model
Two-month-old male MT-KO mice were randomly divided into two groups: control group (MT-KO, n = 6) and Zn supplementation group (MT-KO/Zn, n = 6). Age-matched 129S1 mice and FVB mice were also randomly divided into two groups: control (n = 6) and Zn supplementation (Zn, n = 6). Inclusion of FVB mice was to ensure Zn-induced Akt function in both 129S1 and FVB mice. All mice were sacrificed at the end of 1 month of Zn supplementation.
Biochemical assays
Plasma glucose and insulin levels were assayed according to the manufacturer’s procedures described in the corresponding kits (Crystal Chemical, Downer Grove, IL, USA). Urinary albumin-to-creatinine ratio (ACR) was calculated as ACR = urine albumin/ urine creatinine (μg/mg).
Histopathological examination, immunohistochemical and fluorescent staining
Kidney tissue was fixed overnight in 10% phosphate-buffered formalin, dehydrated in a graded alcohol series, cleared with xylene, embedded in paraffin, and sectioned at 5 μm thickness for pathological exam, immunohistochemical staining, and immunofluorescent staining. Paraffin sections were dewaxed and incubated with 1 × target retrieval solution (Dako, Carpinteria, CA, USA) for 15 min at 98 °C for antigen retrieval and then treated with 3% hydrogen peroxide for 15 min at room temperature, followed by blocking with 5% bovine serum albumin (BSA) for 30 min.
Kidney sections were stained with hematoxylin and eosin (H&E) and Sirius red as previously described [27,28]. For immunohistochemical staining, sections were incubated overnight at 4 °C with the following primary antibodies: 4-hydroxy-2-nonenal (4-HNE; 1:400 dilution; Alpha Diagnostic International, San Antonio, TX, USA), TNF-α (1:100 dilution; Abcam, Cambridge, MA, USA), plasminogen activator inhibitor-1 (PAI-1; 1:400 dilution; BD Biosciences, San Jose, CA, USA), TGF-β1 (1:100 dilution; Santa Cruz Biotechnologies, Dallas, TX, USA), and CTGF (1:100 dilution; Santa Cruz Biotechnologies). Sections were then washed with phosphate-buffered saline (PBS) and incubated with horseradish peroxidase-conjugated secondary antibodies (1:300–400 dilutions in PBS) for 2 h at room temperature. For the development of color, sections were treated with peroxidase substrate 3,3-diaminobenzidine in the developing system (Vector Laboratories, Burlingame, CA, USA).
For immunofluorescent staining, sections were incubated with anti-Akt (1:100; Cell Signaling Technology, Danvers, MA, USA) or anti-phospho-Akt (1:100; Cell Signaling Technology) antibody. Then, secondary antibodies of Cy3-conjugated IgG (1:200; Cell Signaling Technology) and FITC-conjugated IgG (1:100; Abcam) were applied for 1 h at room temperature. Slides were counter-stained with DAPI (Sigma–Aldrich, St. Louis, MO, USA), covered with aqueous mounting medium (Sigma–Aldrich), and analyzed under a fluorescence microscope (Nikon, Tokyo, Japan).
Western blotting
MT expression was detected using a modified Western blot protocol [30]. Kidney proteins were treated with dithiothreitol at a final concentration of 20 mM at 56 °C for 30 min. This was followed by the addition of iodoacetamide (Sigma–Aldrich) at 50 mM at room temperature for 1 h in the dark and then centrifugation for collecting the protein suspension. In addition, proteins were transferred to a nitrocellulose membrane with transfer buffer including 2 mM CaCl2. The monoclonal antibody against human MT (Dako North America, Carpinteria, CA, USA) was used at 1:1000 dilutions in 3% BSA at 4 °C overnight. Because the transfer buffer contains CaCl2, these blots could not be stripped for reprobing β-actin. Therefore, a parallel gel was used for β-actin analysis using the method described below.
Regular Western blot protocol was performed as described in our previous studies [29,30]. Primary antibodies used included 4-HNE (1:1000), TNF-α (1:500), intercellular adhesion molecule 1 (ICAM-1; 1:500), CTGF (1:500), TGF-β1 (1:500), hexokinase II (HKII; 1:1000), and β-actin (1:2000), all of which were purchased from Santa Cruz Biotechnologies. Phospho-Akt (Ser473, 1:1000), Akt (1:1000), phospho-PTEN (Ser380/Thr382/383, 1:1000), PTEN (1:1000), phospho-GSK-3β (Ser9, 1:1000), GSK-3β (1:1000), phosphoglycogen synthase (GS) (Ser641, 1:1000), GS (1:1000), phospho-Akt1 (Ser473, 1:1000), Akt1 (1:1000), phospho-Akt2 (Ser474, 1:1000), Akt2 (1:1000), and PPAR-γ coactivator 1α (PGC-1α; 1:1000) were purchased from Cell Signaling. PAI-1 (1:2000) and protein tyrosine phosphatase 1B (PTP1B; 1:2000) were purchased from BD Biosciences. TNF-α (1:1000) and TRB3 (1:1000) were purchased from Abcam and Calbiochem (San Diego, CA, USA), respectively.
Statistical analysis
Data were collected from six animals for each group and presented as the mean ± SD. Comparisons between groups were performed by one-way ANOVA, followed by a Tukey’s post hoc test. Statistical analysis was performed with Origin 7.5 Laboratory Data Analysis and Graphing software. Statistical significance was considered as p < 0.05.
Results
Therapeutic effect of Zn on the functional and pathogenic changes in the kidney of OVE26 type 1 diabetic mice
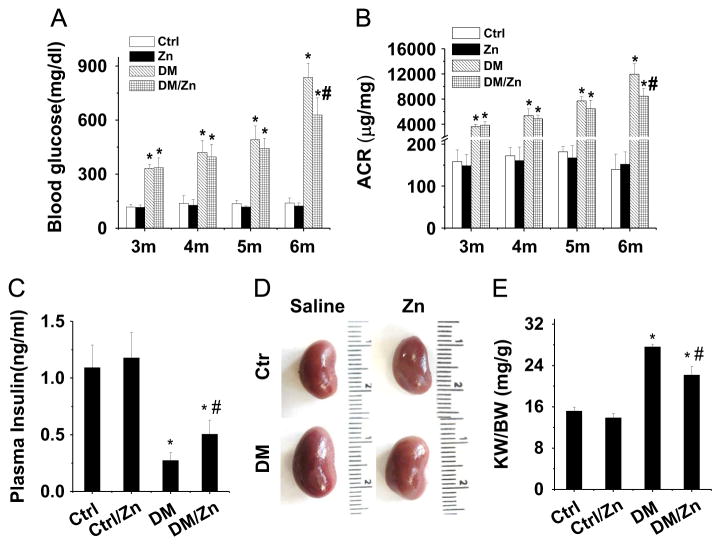
Significant albuminuria occurs in 2- to 3-month-old OVE26 (DM) mice [25]. To explore the therapeutic effect of Zn on diabetes-induced renal changes, we gave Zn to 3-month-old OVE26 diabetic and age-matched FVB control (Ctrl) mice. Zn was given by gavage at 5 mg/kg every other day for 3 months based on our previous studies [10]. Blood glucose levels, measured before and after Zn treatment (Fig. 1A), were found to continually increase in both the DM group and the DM/Zn group, but they were slightly lower in DM/Zn mice than in DM mice at 6 months (Fig. 1A, p < 0.05). Similarly, the increased ACR in 6-month Zn/DM mice was also slightly lower than in 6-month DM mice (Fig. 1B, p < 0.05).
Fig. 1.
Therapeutic effects of Zn on general changes and renal function in OVE26 type 1 diabetes mouse model. Three-month-old male OVE26 mice and FVB control mice were given Zn (5 mg/kg) or physiological saline every other day for 3 months. (A) Blood glucose, (B) urinary ACR, (C) plasma insulin concentration, (D) kidney size, and (E) kidney weight (KW) to body weight (BW) ratio were measured. Data are presented as the mean ± SD (n = 6). *p < 0.05 vs control (Ctrl); #p < 0.05 vs corresponding DM group.
There was no significant difference in body weight among groups at the termination of the study (31.68±1.89, 30.88±1.67, 29.21±2.80, and 30.87±3.27 g for Ctrl, Ctrl/Zn, DM, and DM/Zn, respectively). There was no significant difference in blood triglyceride levels between Ctrl (89.67±26.35 mg/dl) and Ctrl/Zn mice (103.72±13.02 mg/dl) or between DM (190.23±34.66 mg/dl) and DM/Zn mice (204.88±31.34 mg/dl). There was no significant effect of Zn on BUN between Ctrl (25.85±5.39 mg/dl) and Ctrl/ Zn mice (24.56±4.43 mg/dl) or between DM (43.05±6.05 mg/dl) and DM/Zn mice (38.65±4.78 mg/dl). However, serum insulin levels were significantly increased in DM/Zn mice compared to DM mice. Both DM groups showed much lower serum insulin levels than Ctrl and Ctrl/Zn mice (Fig. 1C).
Kidney size was larger in DM mice than in Ctrl mice. This was slightly attenuated by Zn treatment (Fig. 1D). Consistent with kidney size, the ratio of kidney weight to body weight in DM mice was also significantly increased compared to that in Ctrl mice. This was also slightly attenuated by Zn treatment (Fig. 1E, p < 0.05).
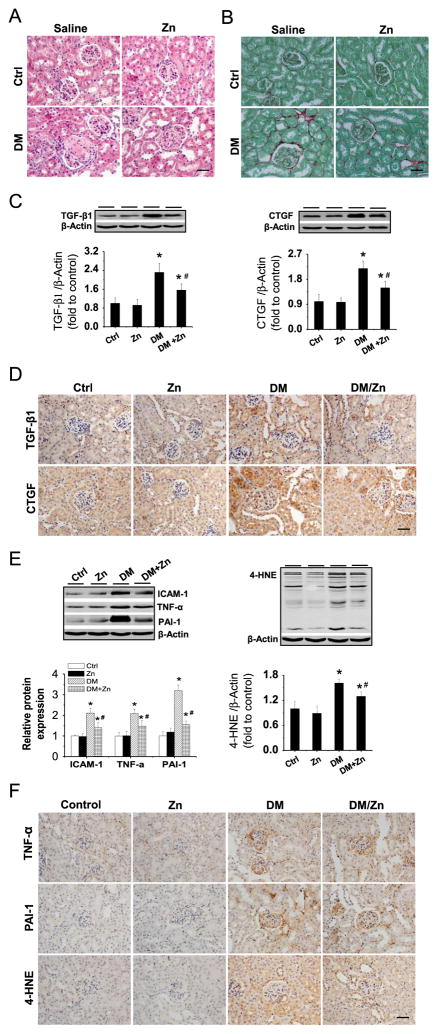
Histological examination with H&E staining showed that the kidneys from DM mice exhibited segmental glomerulosclerosis and excessive accumulation of extracellular matrix, resulting in glomerular enlargement (Fig. 2A). Zn treatment significantly decreased these pathological changes in the DM/Zn group. Sirius red staining revealed significantly increased collagen accumulation in the kidneys of DM mice, but not in DM/Zn mice. To further examine the profibrotic response, Western blot analysis (Fig. 2C) and immunohistochemical staining (Fig. 2D) were performed for TGF-β1 and CTGF. The renal fibrotic response was significantly increased in DM mice, but was significantly prevented by Zn treatment.
Fig. 2.
Therapeutic effects of Zn on renal remodeling, inflammation, and oxidative damage in OVE26 type 1 diabetes mice. Renal structural changes were examined with (A) H&E and (B) Sirius red staining. Renal expression of TGF-β1 and CTGF, as indices of profibrotic mediators, was examined by (C) Western blot and (D) immunohistochemical staining. Renal expression of TNF-α, ICAM-1, and PAI-1, as indices of inflammation, and 4-HNE, as an index of lipid oxidation, was also detected by (E) Western blot assay and (F) immunohistochemical staining. Data are presented as the mean ± SD (n = 6). *p < 0.05 vs control (Ctrl); #p < 0.05 vs DM group. Bar, 50 μm.
Because the fibrotic response is often induced by inflammation and/or associated oxidative damage, we examined renal expression and location of ICAM-1, TNF-α, PAI-1, and 4-HNE via Western blot analysis (Fig. 2E) and immunohistochemical staining (Fig. 2F). Renal expression of ICAM-1, TNF-α, and PAI-1 was significantly increased in DM mice, but was significantly attenuated by Zn treatment. The attenuation of renal inflammation by Zn treatment in diabetic mice was also accompanied by a reduction in renal oxidative damage, demonstrated by decreased expression of 4-HNE.
Effects of Zn treatment on Akt-mediated molecules related to metabolism and Akt negative regulators in the kidney of OVE26 type 1 diabetic mice
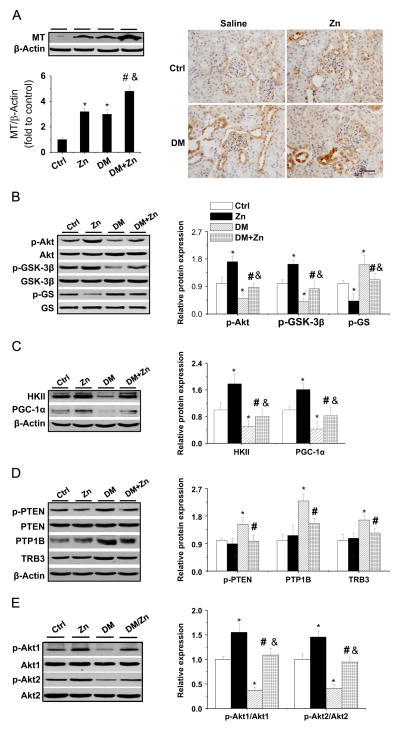
To mechanistically study the therapeutic effects of Zn on the diabetic kidney, renal MT was measured. Consistent with previous results [10], Zn treatment increased renal MT expression in control and diabetic mice; however, diabetes also increased renal MT expression (Fig. 3A). Thus, MT elevation alone cannot explain the renal protection gained from Zn against diabetic kidney damage.
Fig. 3.
Effects of Zn on the expression of renal MT, Akt, and Akt’s downstream effectors and negative regulators in OVE26 type 1 diabetes mice. (A) Zn-induced renal MT expression was detected by Western blot assay and immunohistochemical staining. (B) Phosphorylation of Akt, GSK-3β, and GS, as well as (C) the expression of HKII and PGC-1α, was examined by Western blot assay. (D) The phosphorylation of PTEN and the expression of PTP1B and TRB3 along with (E) the phosphorylation of Akt1 and Akt2 were examined by Western blotting. Data are presented as the mean ± SD (n = 6). *p < 0.05 vs control (Ctrl); #p < 0.05 vs DM. Bar, 50 μm; &p<0.05 vs the Zn supplementation (Zn).
Next, we explored whether Zn supplementation in DM mice stimulates renal Akt-mediated molecules involved in glucose metabolism. This was done by examining the phosphorylation of Akt(1,2,3), GSK-3β, and GS (Fig. 3B). In DM mice, the phosphorylation of Akt and GSK-3β was significantly decreased, whereas the phosphorylation of GS was significantly increased, both of which were completely prevented by Zn treatment. The renal expression of HKII and PGC-1α was also significantly decreased in DM mice, but not DM/Zn mice (Fig. 3C). The expression of the Akt negative regulators p-PTEN, PTP1B, and TRB3 was also significantly increased in the kidney of DM mice, but not DM/Zn mice (Fig. 3D).
Further analysis of Akt changes in its various isoforms revealed that both Akt1 and Akt2 phosphorylation was decreased in DM mice. Furthermore, Zn treatment can prevent both of these changes from occurring because of DM (Fig. 3E).
Therapeutic effects of Zn on the functional and pathogenic changes in the kidney of Akt2-KO diabetic mice
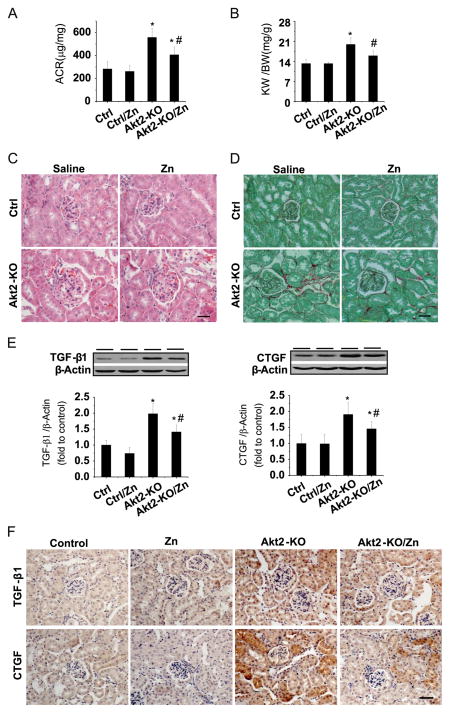
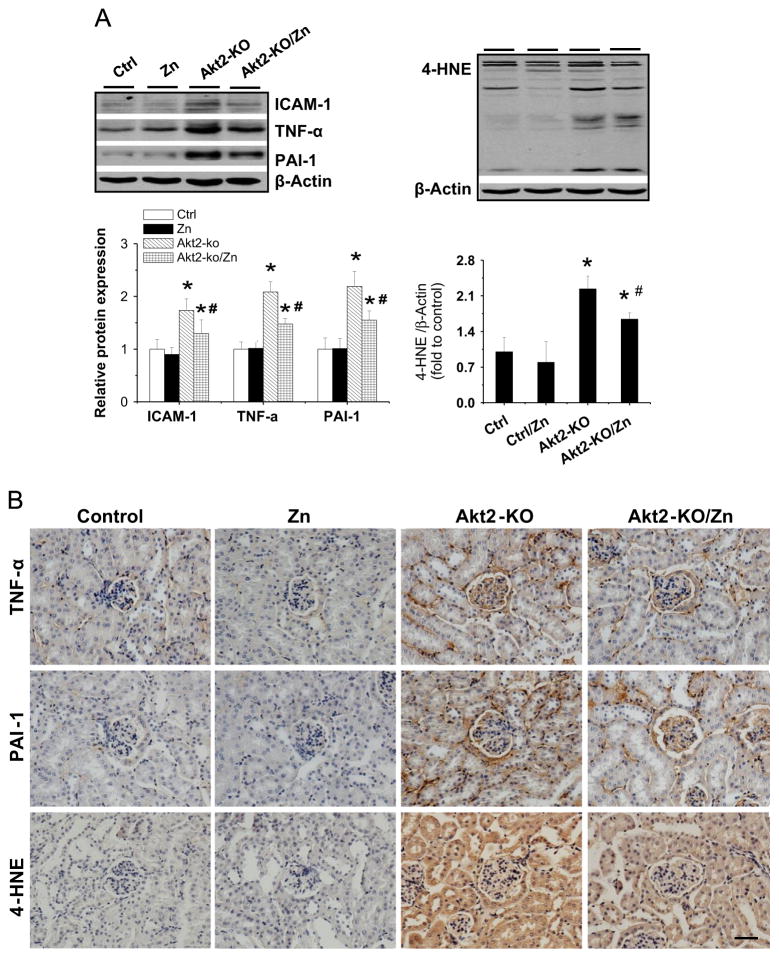
Because Akt2 is considered a major player in energy metabolism, the following studies were done to define whether Zn’s renal protection from diabetes is Akt2-dependent using Akt2-KO mice. Consistent with previous studies in which Akt2-KO mice spontaneously developed a type 2 diabetes-like phenotype [15,16,19], we also found that 2-month-old male Akt2-KO mice developed hyperglycemia (Akt2-KO mice, 253.5±41.7, p < 0.05, vs FVB mice, 98.5±5.9). These mice were randomly divided into two groups (n = 6), DM (Akt2-KO) and Zn-treated DM (Akt2-KO/Zn). Age-matched male FVB mice were also randomly divided into two groups (n = 6): Ctrl and Ctrl/Zn. Therapeutic effects of Zn, 5 mg Zn/kg administered every other day for 3 months, on diabetes-induced renal changes were evaluated by examining the urinary ACR (Fig. 4A), the ratio of kidney weight to body weight (Fig. 4B), and renal histology (Fig. 4C and 4D). ACR and kidney weight were significantly increased in DM mice, effects that were significantly attenuated by Zn treatment. H&E (Fig. 4C) and Sirius red (Fig. 4D) staining showed characteristic hypertrophy of glomerular structures, accumulation of extracellular matrix, and interstitial fibrosis in the kidney of DM mice, but not in DM/Zn mice. To further support Zn’s role in attenuating the renal fibrotic response, Western blot analysis (Fig. 4E) and immunohistochemical staining (Fig. 4F) also showed a significant increase in TGF-β1 and CTGF expression in the Akt2-KO group, but not in the Akt2-KO/Zn group.
Fig. 4.
Therapeutic effects of Zn on renal functional and pathological changes in Akt2-KO diabetic mice. Two-month-old male Akt2-KO mice and FVB control mice were given Zn (5 mg/kg) or physiological saline every other day for 3 months. (A) Urinary ACR and (B) kidney weight (KW) to body weight (BW) ratio were measured. Renal pathology was examined with (C) H&E and (D) Sirius red staining. Renal expression of TGF-β1 and CTGF was tested by (E) Western blot assay and (F) immunohistochemical staining. Data are presented as the mean ± SD (n = 6). *p < 0.05 vs control (Ctrl); #p < 0.05 vs Akt2-KO. Bar, 50 μm.
As measurements of inflammatory and oxidative damage, renal expression of TNF-α, ICAM-1, and PAI-1, as well as renal accumulation of 4-HNE, examined by Western blot analysis (Fig. 5A) was significantly increased in the kidneys of Akt2-KO mice. These effects were significantly attenuated by Zn treatment. Furthermore, increased expression of TNF-α, PAI-1, and 4-HNE was found in both glomeruli and tubules via immunohistochemical staining (Fig. 5B).
Fig. 5.
Therapeutic effects of Zn on renal inflammation and oxidative damage in Akt2-KO diabetic mice. (A) Renal expression of ICAM-1 was detected by Western blot assay. Renal expression of TNF-α, PAI-1, and 4-HNE was detected by (A) Western blot assay and (B) immunohistochemical staining. Data are presented as the mean ± SD (n = 6). *p < 0.05 vs control (Ctrl); #p < 0.05 vs Akt2-KO. Bar, 50 μm.
Effects of Zn on Akt-mediated molecules related to metabolism and Akt negative regulators in the kidney of Akt2-KO diabetic mice
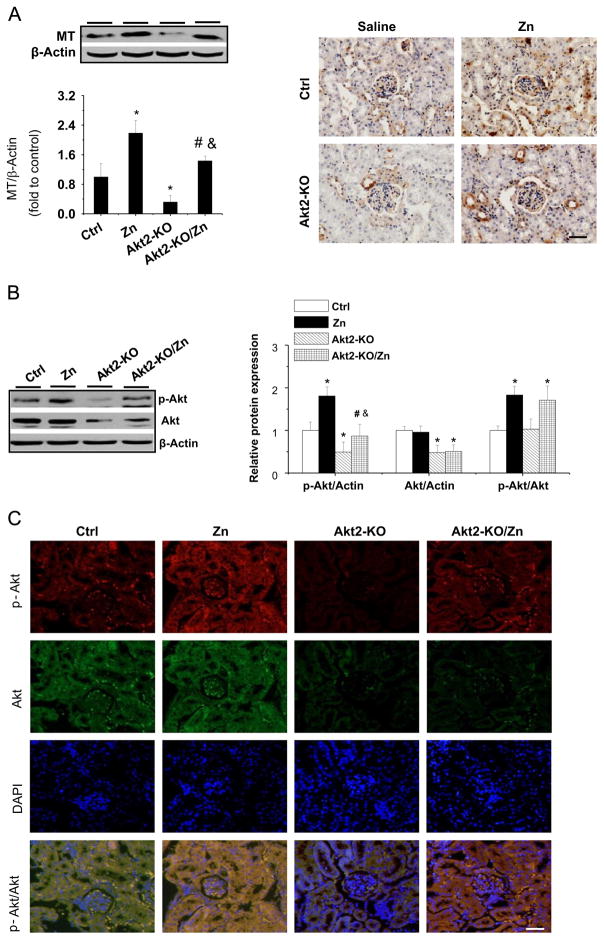
As in the OVE26 diabetic mice, we examined renal expression of MT among the groups. Both Western blot analysis and immunohistochemical staining showed that renal expression of MT was decreased in Akt2-KO mice compared to control mice. Zn treatment increased renal MT expression in both control and diabetic mice (Fig. 6A).
Fig. 6.
Effects of Zn on renal MT expression and Akt expression and phosphorylation in Akt2-KO diabetic mice. (A) Renal MT expression was detected using Western blot assay and by immunohistochemical staining. Akt expression and phosphorylation was examined by (B) Western blot assay and (C) double-immunofluorescent staining. In (C), Akt is shown by green fluorescence with goat anti-rabbit IgG secondary antibody, phospho-Akt is shown by red fluorescence with Cy3-conjugated Affinipure donkey anti-rabbit IgG (H+L), and nuclei are shown by blue fluorescence with DAPI (original magnification ×200). Data are presented as the mean ± SD (n = 6). *p < 0.05 vs control (Ctrl); #p < 0.05 vs Akt2-KO. Bar, 50 μm; &p<0.05 vs the Zn supplementation (Zn).
Next we examined the expression and phosphorylation of Akt (1,2,3) by Western bot analysis. Total Akt expression was significantly decreased in Akt2-KO diabetic mice, which was not affected by Zn treatment (Fig. 6B). The Akt(1,2,3) phosphorylation was significantly decreased in the kidney of Akt2-KO diabetic mice compared to control mice, but Zn treatment stimulated Akt phosphorylation in both groups. The Western blot finding was confirmed by double-immunofluorescent staining (Fig. 6C).
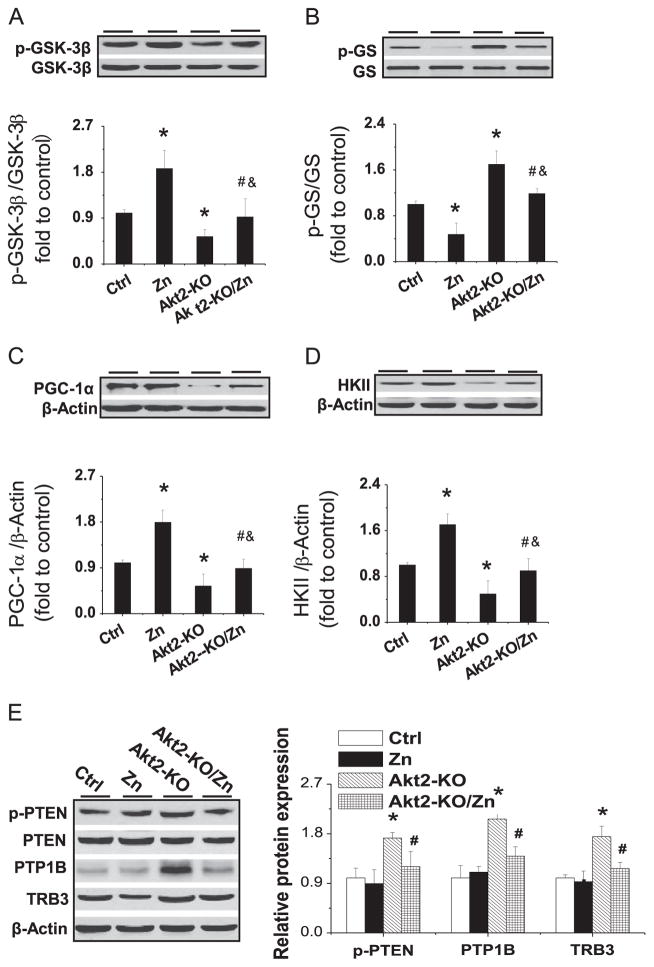
In control mice, phosphorylation of Akt’s downstream targets, GSK-3β (Fig. 7A) and GS (Fig. 7B), was increased and decreased, respectively, by Zn treatment. Deletion of the Akt2 gene significantly decreased GSK-3β phosphorylation and significantly increased GS phosphorylation (Fig. 7A and 7B), both of which could be completely prevented by Zn treatment.
Fig. 7.
Effects of Zn on the expression of renal expression and phosphorylation of Akt downstream effectors and its negative regulators in Akt2-KO diabetic mice. Expression and phosphorylation of (A) GSK-3β and (B) GS, as well as expression of (C) HKII and (D) PGC-1α, were examined by Western blotting. Renal PTEN expression and phosphorylation, along with PTP1B and TRB3 expression, were measured by (E) Western blot. Data are presented as the mean ± SD (n = 6). *p < 0.05 vs control (Ctrl); #p < 0.05 vs Akt2-KO; &p<0.05 vs the Zn supplementation (Zn).
The expression of HKII (Fig. 7C) and PGC-1α (Fig. 7D) was decreased in Akt2-KO DM mice. Zn treatment significantly increased the expression of HKII and PGC-1α in control mice and also completely reversed DM-induced reduction of these proteins in Akt2-KO mice. Fig. 7E shows renal expression of three Akt negative regulators, p-PTEN, PTP1B, and TRB3, all of which were significantly elevated in Akt2-KO diabetic mice, but not in Zn-treated Akt2-KO mice.
MT is required for Zn to stimulate the phosphorylation of renal Akt and GSK-3β as well as downregulation of GS phosphorylation
The above studies have shown that Zn treatment significantly improved renal pathological changes in both OVE26 and Akt2-KO diabetic mice, indicating that the therapeutic effect was not dependent on Akt2, which was previously assumed to play a critical role in Zn-induced Akt-mediated renal glucose metabolic pathways. In Akt2-KO mice, Zn treatment maintained GSK-3β phosphorylation and associated GS dephosphorylation and induced HKII expression, as observed in OVE26 mice. In contrast to MT expression in OVE26 mice, MT expression in Akt2-KO mice was decreased; the expression of MT remained inducible by Zn treatment. Thus, the next study was to define whether the stimulating effects of Zn treatment on renal Akt-mediated glucose metabolic signaling are dependent on MT expression. To address this issue, we used MT-KO mice under nondiabetic conditions in order to avoid diabetic confounding effects.
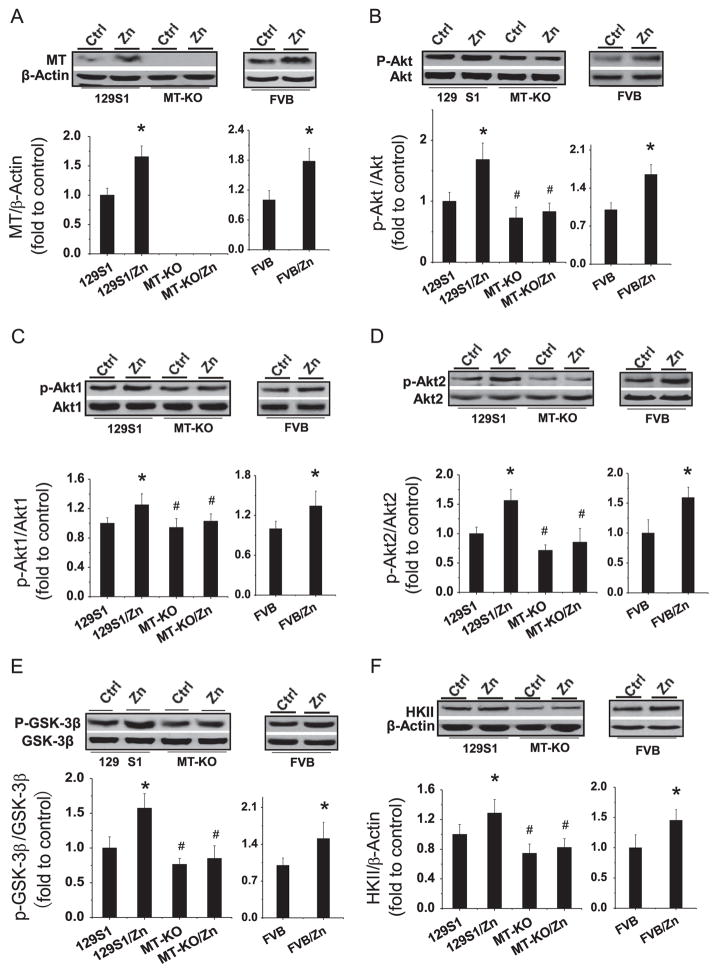
Two-month MT-KO mice, their age-matched control (129S1 strain) mice, and FVB strain mice (control for OVE26 and Akt2-KO mice) were given either Zn, at 5 mg/kg, or saline every other day for 1 month to examine the effects of Zn on renal MT expression, Akt and GSK phosphorylation, and HKII expression. As shown in Fig. 8A, Zn treatment significantly increased renal MT expression in both 129S1 and FVB mice, but not in MT-KO mice.
Fig. 8.
Effects of Zn on renal MT expression, Akt1 and Akt2 expression and phosphorylation, and HKII expression in MT-KO mice. Two-month-old male MT-KO (129S1 background) mice, 129S1 control mice, and FVB mice were given Zn (5 mg/kg) or physiological saline every other day for 1 month. (A) MT expression was detected by Western blot with antibody against MT1/2 isoforms. Akt expression and phosphorylation were detected by Western blot with antibodies against (B) Akt(1,2,3), (C) Akt1, and (D) Akt2. (E) GSK-3β expression and phosphorylation, as well as (F) HKII expression, was detected by Western blot assay. Data are presented as the mean ± SD (n = 6). *p < 0.05 vs corresponding controls (129S1 or FVB); #p < 0.05 vs 129S1/Zn group.
It was noticed that deletion of MT-I/II genes did not significantly affect basal Akt(1,2,3) phosphorylation (Fig. 8B) in MT-KO mice. Interestingly, Zn treatment significantly increased Akt(1,2,3) phosphorylation in both 129S1 and FVB wild-type mice, but not in MT-KO mice (Fig. 8B). To further define the isoform of Akt phosphorylation that is MT dependent, Western blot analysis performed with specific Akt1 and Akt2 phosphorylation antibodies demonstrated that Zn treatment significantly increased both Akt1 and Akt2 phosphorylation in 129S1 and FVB strain mice, but not in MT-KO mice (Fig. 8C and 8D). Concordantly, Zn treatment also stimulated GSK-3β phosphorylation and increased HKII expression in 129S1 and FVB mice, but not in MT-KO mice (Fig. 8E and 8F).
Discussion
This study has demonstrated the following novel findings: (1) Zn treatment can significantly improve diabetes-induced renal functional and pathological changes in OVE26 and Akt2-KO diabetic models; (2) the therapeutic effect of Zn treatment on the diabetic kidney is associated with the stimulation of MT expression and Akt-mediated metabolic signaling; (3) preservation of Akt-mediated metabolic signaling by Zn treatment may be associated with Zn-mediated suppression of Akt negative regulators PTEN phosphorylation and PTP1B and TRB3 expression, under diabetic conditions; (4) deletion of the Akt2 gene had no effect on Zn’s ability to provide renal protection against diabetes-induced functional and pathologic changes; furthermore, deletion of the Akt2 gene had no effect on Zn-induced stimulation of Akt-mediated metabolic signaling; (5) however, Zn-induced stimulation of Akt-related metabolic signaling is MT dependent, at least under normal physiological conditions.
Zn plays an important role in maintaining the normal physiological function of many different organs, including the kidney [31–33]. For example, only moderate Zn deficiency caused a decrease in glomerular filtration rate, a decrease in NADPH diaphorase activity in glomeruli and tubular nephron segments, and a decrease in nitric oxide synthase activity in the renal medulla and cortex [31]. Zn-deficient rats showed a reduction in nephron number, decreased glomerular capillary area, a reduction in the total number of glomerular nuclei in cortical and juxtamedullary areas, and an increase in the number of apoptotic cells present in distal renal tubules, cortical collecting ducts, and glomeruli [31]. Diabetes-induced pathologic changes were much further advanced in the kidneys of Zn-deficient rats [34]. In contrast, Zn supplementation was able to protect the kidney from those same DM-induced changes [10,11] along with some other renal pathologies unrelated to DM [31]. Consistent with previous animal studies from our own [10] and other labs [11,12] as well as human data [8,9,35], we extend the earlier findings to show the therapeutic effects of Zn on diabetic kidneys in OVE26 type 1 and Akt2-KO type 2-like diabetic models.
Our first novel finding is that renal improvement by Zn treatment is associated with activation of Akt-mediated metabolic pathways. In our previous study [13], we found that Akt phosphorylation was increased in the early stage of diabetes, but significantly decreased in later stages, coinciding with renal pathological damage. This was consistent with an earlier study showing a significant decrease in renal Akt and GSK-3β phosphorylation in type 1 diabetic rats [36]. Here we demonstrated that decreased renal Akt phosphorylation along with diabetes induced pathological changes in the kidney, which were significantly attenuated by Zn treatment, such that Akt phosphorylation in both OVE26 and Akt-KO diabetic mouse models was almost completely preserved.
Preservation of renal Akt phosphorylation by Zn treatment was associated with normal GSK-3β phosphorylation and HKII expression in both OVE26 and Akt2-KO diabetic mice. GSK-3β phosphorylation not only plays a critical role in renal glucose metabolism, but also has a preventive effect on pathogenic fibrosis of the kidney [37,38]. A study showed that high-glucose-treated glomerular mesangial cells exhibited elevated TGF-β1 expression, elevated fibronectin expression, and increased GSK-3β activation (reduced GSK-3β phosphorylation). Renal mesangial cells may therefore actively respond to high glucose by increasing TGF-β1 and fibronectin expression through GSK-3β-dependent profibrogenic signaling [39]. This suggests that suppression of GSK-3β activation with pharmacological GSK-3β inhibitors or genetic GSK-3β dominant negative mutations could prevent the aforementioned hyperglycemic-induced adverse effects on glomerular mesangial cells. Consistent with this finding, we and other researchers have demonstrated that inhibition of GSK-3β activity may also offer cardiac and pulmonary protection against pathogenic fibrosis of the heart and lungs, respectively [40,41].
HKII controls cell survival by promoting metabolism and/or inhibiting apoptosis. Involvement of HKII in renal glucose metabolism requires its attachment to the mitochondrial membrane, which is inhibited by activated GSK-3β [42]. Renal HKII activity was significantly reduced in diabetic animal models [43]. Resveratrol administration reversed renal pathological changes and produced associated improvement in renal HK activity [43]. Therefore, the present study also suggests that Zn’s improvement of renal function in diabetic conditions may also be associated with the preservation of normal HKII glucose metabolism due to GSK-3β inactivation by Zn treatment.
Another novel finding of this study is that Zn protection against diabetes-induced renal damage is independent of Akt2. This is an unexpected result because Akt2 has been shown to play an important role in insulin-stimulated glucose uptake and glycogen synthesis [15,16,19]. Animal studies also showed the renal pathological abnormalities in Akt2-KO mice [44,45]. A more recent study has defined the important role of Akt2 in protecting podocytes from damage induced by chronic kidney disease [46]. However, the following results from a recent study may provide some new clues to help us better understand our finding. Akt2 gene deletion does not affect basal or exercise-stimulated glucose uptake or intracellular glycogen content in the soleus muscle. Akt2 gene deletion does not result in alterations in basal Akt phosphorylation, basal and contraction-stimulated GSK-3β phosphorylation, GS phosphorylation, or GS activity [47]. These recent findings along with our current data suggest that under normal conditions Akt2 plays a critical role in glucose metabolism. However, in the absence of Akt2, other Akt isoforms such as Akt1 and/or Akt3 may compensate for the absence of Akt2. These specific issues need to be further investigated.
In this study we demonstrated that Zn treatment prevents diabetic upregulation of the Akt negative mediators PTEN, PTP1B, and TRB3 in OVE26 and Akt2-KO diabetic models, but had no effect under normal conditions. Zn-induced prevention of the diabetic upregulation of PTEN and PTP1B is most likely due to Zn’s ability to negatively regulate them both [48–51] However, we do not have the answer as to why Zn supplementation had no effect on PTEN and PTP1B regulation under normal conditions. Although both are important Akt negative mediators, Zn-induced Akt and GSK-3β activation is not always mediated by Zn inhibition of PTEN and PTPs [22,52]. Consistent with these findings, here Zn stimulated Akt and GSK-3β phosphorylation not only in diabetic conditions, but also in normal kidney tissue. This is not in parallel with the PTEN and PTP1B results, suggesting that Zn regulation of these metabolic molecules may be mediated by multiple mechanisms, not only via Akt negative regulators.
The last novel finding of this study is that MT is required for Zn treatment to be therapeutically effective in its renal protection. MT stimulates the phosphorylation of Akt and GSK-3β and increases HKII expression under normal conditions. We do not have an explanation for this unexpected result. We assume that without MT there is no Zn chaperone to store and release Zn to target molecules, which may be needed to stimulate phosphorylation of Akt and GSK-3β and promote expression of HKII. There is indirect evidence to support our idea, which shows that Akt phosphorylation is reduced in aged FVB mouse cardiac tissue, but not in aged MT-overexpressing transgenic mouse cardiac tissue [53]. This may be because 60-fold MT overexpression releases excess Zn under aged oxidative stress conditions, which provides the Zn resource for Akt phosphorylation.
In summary, this study shows that Zn stimulation of Akt-mediated renal glucose metabolism improves renal function in OVE26 and Akt2-KO diabetic models. We further showed that the preservation of Akt-mediated metabolic signaling by Zn was not dependent on Akt2, but was dependent on MT. The preservation of Akt-mediated metabolic signaling under diabetic conditions may be associated with Zn suppression of Akt negative regulators, PTEN phosphorylation, and PTP1B, as well as TRB3, which needs further investigation. Considering that diabetic patients are often Zn deficient owing to elevated urinary Zn excretion, decreased intestinal Zn absorption, and a restricted diet [3,54–56], this study suggests that regularly monitoring Zn levels in diabetic patients, as well as adequate Zn supplementation in patients whose Zn levels are low, would be very important in mitigating the development of diabetic nephropathy.
Acknowledgments
The authors acknowledge the editorial assistance of Dr. Kristen A. McClung, Kosair Children’s Hospital and Department of Pediatrics of the University of Louisville. This study was supported in part by a Basic Research Award from the American Diabetes Association (1-11-BA-17, to L.C.), a regular grant from the National Natural Science Foundation of China (81070189, to Y.W.), a grant from the Jilin University Bethune Foundation (2012221, to Y.W.), a grant from the National Institutes of Health (1 R01 DK091338-01A1, to L.C.), and the 4th Youth Foundation from the First Hospital of the Jilin University (JDYY42013009, to W.S.).
Footnotes
Authors’ contributions
W.S., X.M., S.Z., Y.W., and Y.X. performed the experiments; Y.W., L.Z., P.N.E., Y.F., and L.C. designed the experiments; L.C. wrote the manuscript. All the authors contributed to the preparation of the manuscript and reviewed the final version of the manuscript.
References
- 1.Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444–452. doi: 10.1038/ncpendmet0894. [DOI] [PubMed] [Google Scholar]
- 2.Brosius FC, 3rd, Alpers CE. New targets for treatment of diabetic nephropathy: what we have learned from animal models. Curr Opin Nephrol Hypertens. 2013;22:17–25. doi: 10.1097/MNH.0b013e32835b3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miao X, Sun W, Fu Y, Miao L, Cai L. Zinc homeostasis in the metabolic syndrome and diabetes. Front Med. 2013;7:31–52. doi: 10.1007/s11684-013-0251-9. [DOI] [PubMed] [Google Scholar]
- 4.Lassi ZS, Haider BA, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2010;12:CD005978. doi: 10.1002/14651858.CD005978.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth DE, Richard SA, Black RE. Zinc supplementation for the prevention of acute lower respiratory infection in children in developing countries: meta-analysis and meta-regression of randomized trials. Int J Epidemiol. 2010;39:795–808. doi: 10.1093/ije/dyp391. [DOI] [PubMed] [Google Scholar]
- 6.Capdor J, Foster M, Petocz P, Samman S. Zinc and glycemic control: a meta-analysis of randomised placebo controlled supplementation trials in humans. J Trace Elem Med Biol. 2013;27:137–142. doi: 10.1016/j.jtemb.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Jayawardena R, Ranasinghe P, Galappatthy P, Malkanthi R, Constantine G, Katulanda P. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. 2012;4:13. doi: 10.1186/1758-5996-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parham M, Amini M, Aminorroaya A, Heidarian E. Effect of zinc supplementation on microalbuminuria in patients with type 2 diabetes: a double blind, randomized, placebo-controlled, cross-over trial. Rev Diabetic Stud. 2008;5:102–109. doi: 10.1900/RDS.2008.5.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan MI, Siddique KU, Ashfaq F, Ali W, Reddy HD, Mishra A. Effect of high-dose zinc supplementation with oral hypoglycemic agents on glycemic control and inflammation in type-2 diabetic nephropathy patients. J Nat Sci Biol Med. 2013;4:336–340. doi: 10.4103/0976-9668.117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Y, Yang Q, Lu J, Zhang X, Suen D, Tan Y, Jin L, Xiao J, Xie R, Rane M, Li X, Cai L. Zinc supplementation partially prevents renal pathological changes in diabetic rats. J Nutr Biochem. 2010;21:237–246. doi: 10.1016/j.jnutbio.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Karatug A, Kaptan E, Bolkent S, Mutlu O, Yanardag R. Alterations in kidney tissue following zinc supplementation to STZ-induced diabetic rats. J Trace Elem Med Biol. 2013;27:52–57. doi: 10.1016/j.jtemb.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Ozcelik D, Naziroglu M, Tuncdemir M, Celik O, Ozturk M, Flores-Arce MF. Zinc supplementation attenuates metallothionein and oxidative stress changes in kidney of streptozotocin-induced diabetic rats. Biol Trace Elem Res. 2012;150:342–349. doi: 10.1007/s12011-012-9508-4. [DOI] [PubMed] [Google Scholar]
- 13.Rane MJ, Song Y, Jin S, Barati MT, Wu R, Kausar H, Tan Y, Wang Y, Zhou G, Klein JB, Li X, Cai L. Interplay between Akt and p38 MAPK pathways in the regulation of renal tubular cell apoptosis associated with diabetic nephropathy. Am J Physiol Renal Physiol. 2010;298:F49–F61. doi: 10.1152/ajprenal.00032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 15.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 17.Dummler B, Tschopp O, Hynx D, Yang ZZ, Dirnhofer S, Hemmings BA. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol. 2006;26:8042–8051. doi: 10.1128/MCB.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J, Hemmings BA. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–2954. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- 19.George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL, Dunger DB, Barford D, Umpleby AM, Wareham NJ, Davies HA, Schafer AJ, Stoffel M, O’Rahilly S, Barroso I. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang X, Shay NF. Zinc has an insulin-like effect on glucose transport mediated by phosphoinositol-3-kinase and Akt in 3T3-L1 fibroblasts and adipocytes. J Nutr. 2001;131:1414–1420. doi: 10.1093/jn/131.5.1414. [DOI] [PubMed] [Google Scholar]
- 21.Ilouz R, Kaidanovich O, Gurwitz D, Eldar-Finkelman H. Inhibition of glycogen synthase kinase-3β by bivalent zinc ions: insight into the insulin-mimetic action of zinc. Biochem Biophys Res Commun. 2002;295:102–106. doi: 10.1016/s0006-291x(02)00636-8. [DOI] [PubMed] [Google Scholar]
- 22.Georgiades SN, Mak LH, Angurell I, Rosivatz E, Firouz Mohd Mustapa M, Polychroni C, Woscholski R, Vilar R. Identification of a potent activator of Akt phosphorylation from a novel series of phenolic, picolinic, pyridino, and hydroxamic zinc(II) complexes. J Biol Inorg Chem. 2011;16:195–208. doi: 10.1007/s00775-010-0716-0. [DOI] [PubMed] [Google Scholar]
- 23.Cai L, Satoh M, Tohyama C, Cherian MG. Metallothionein in radiation exposure: its induction and protective role. Toxicology. 1999;132:85–98. doi: 10.1016/s0300-483x(98)00150-4. [DOI] [PubMed] [Google Scholar]
- 24.Cai L. Diabetic cardiomyopathy and its prevention by metallothionein: experimental evidence, possible mechanisms and clinical implications. Curr Med Chem. 2007;14:2193–2203. doi: 10.2174/092986707781389646. [DOI] [PubMed] [Google Scholar]
- 25.Zheng S, Noonan WT, Metreveli NS, Coventry S, Kralik PM, Carlson EC, Epstein PN. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes. 2004;53:3248–3257. doi: 10.2337/diabetes.53.12.3248. [DOI] [PubMed] [Google Scholar]
- 26.Epstein PN, Overbeek PA, Means AR. Calmodulin-induced early-onset diabetes in transgenic mice. Cell. 1989;58:1067–1073. doi: 10.1016/0092-8674(89)90505-9. [DOI] [PubMed] [Google Scholar]
- 27.Miao X, Bai Y, Sun W, Cui W, Xin Y, Wang Y, Tan Y, Miao L, Fu Y, Su G, Cai L. Sulforaphane prevention of diabetes-induced aortic damage was associated with the upregulation of Nrf2 and its down-stream antioxidants. Nutr Metab (London) 2012;9:84. doi: 10.1186/1743-7075-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miao X, Wang Y, Sun J, Sun W, Tan Y, Cai L, Zheng Y, Su G, Liu Q, Wang Y. Zinc protects against diabetes-induced pathogenic changes in the aorta: roles of metallothionein and nuclear factor (erythroid-derived 2)-like 2. Cardiovasc Diabetol. 2013;12:54. doi: 10.1186/1475-2840-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Sun W, Du B, Miao X, Bai Y, Xin Y, Tan Y, Cui W, Liu B, Cui T, Epstein PN, Fu Y, Cai L. Therapeutic effect of MG-132 on diabetic cardiomyopathy is associated with its suppression of proteasomal activities: roles of Nrf2 and NF-kappaB. Am J Physiol Heart Circ Physiol. 2013;304:H567–H578. doi: 10.1152/ajpheart.00650.2012. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, Saari JT, Cai L. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation. 2006;113:544–554. doi: 10.1161/CIRCULATIONAHA.105.537894. [DOI] [PubMed] [Google Scholar]
- 31.Tomat AL, Costa MA, Girgulsky LC, Veiras L, Weisstaub AR, Inserra F, Balaszczuk AM, Arranz CT. Zinc deficiency during growth: influence on renal function and morphology. Life Sci. 2007;80:1292–1302. doi: 10.1016/j.lfs.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Lobo JC, Torres JP, Fouque D, Mafra D. Zinc deficiency in chronic kidney disease: is there a relationship with adipose tissue and atherosclerosis? Biol Trace Elem Res. 2010;135:16–21. doi: 10.1007/s12011-009-8504-9. [DOI] [PubMed] [Google Scholar]
- 33.Baltaci AK, Sunar F, Mogulkoc R, Oztekin E. Effect of zinc deficiency and supplementation on lipid peroxidation of renal tissue in ovariectomized rats. Biol Trace Elem Res. 2004;101:231–239. doi: 10.1385/BTER:101:3:231. [DOI] [PubMed] [Google Scholar]
- 34.Minami T, Ichii M, Okazaki Y, Kubo M, Kadota E, Inoue T, Yamada Y, Fushimi H. Renal changes of streptozotocin-induced diabetic rats fed a low-zinc diet. Renal Fail. 1995;17:349–363. doi: 10.3109/08860229509037601. [DOI] [PubMed] [Google Scholar]
- 35.Farvid MS, Jalali M, Siassi F, Hosseini M. Comparison of the effects of vitamins and/or mineral supplementation on glomerular and tubular dysfunction in type 2 diabetes. Diabetes Care. 2005;28:2458–2464. doi: 10.2337/diacare.28.10.2458. [DOI] [PubMed] [Google Scholar]
- 36.Zdychova J, Vesela J, Kazdova L, Komers R. Renal activity of Akt kinase in experimental Type 1 diabetes. Physiol Res. 2008;57:709–715. doi: 10.33549/physiolres.931337. [DOI] [PubMed] [Google Scholar]
- 37.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 38.Howard C, Tao S, Yang HC, Fogo AB, Woodgett JR, Harris RC, Rao R. Specific deletion of glycogen synthase kinase-3beta in the renal proximal tubule protects against acute nephrotoxic injury in mice. Kidney Int. 2012;82:1000–1009. doi: 10.1038/ki.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho C, Lee PH, Hsu YC, Wang FS, Huang YT, Lin CL. Sustained Wnt/ beta-catenin signaling rescues high glucose induction of transforming growth factor-beta1-mediated renal fibrosis. Am J Med Sci. 2012;344:374–382. doi: 10.1097/MAJ.0b013e31824369c5. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Feng W, Xue W, Tan Y, Hein DW, Li XK, Cai L. Inactivation of GSK-3beta by metallothionein prevents diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Diabetes. 2009;58:1391–1402. doi: 10.2337/db08-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baarsma HA, Engelbertink LH, van Hees LJ, Menzen MH, Meurs H, Timens W, Postma DS, Kerstjens HA, Gosens R. Glycogen synthase kinase-3 (GSK-3) regulates TGF-β1-induced differentiation of pulmonary fibroblasts. Br J Pharmacol. 2013;169:590–603. doi: 10.1111/bph.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65:10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- 43.Palsamy P, Subramanian S. Modulatory effects of resveratrol on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin–nicotinamide-induced diabetic rats. Chem Biol Interact. 2009;179:356–362. doi: 10.1016/j.cbi.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Kempe DS, Ackermann TF, Boini KM, Klaus F, Umbach AT, Dermaku-Sopjani M, Judenhofer MS, Pichler BJ, Capuano P, Stange G, Wagner CA, Birnbaum MJ, Pearce D, Foller M, Lang F. Akt2/PKBbeta-sensitive regulation of renal phosphate transport. Acta Physiol (Oxford) 2010;200:75–85. doi: 10.1111/j.1748-1716.2010.02109.x. [DOI] [PubMed] [Google Scholar]
- 45.Kempe DS, Siraskar G, Frohlich H, Umbach AT, Stubs M, Weiss F, Ackermann TF, Volkl H, Birnbaum MJ, Pearce D, Foller M, Lang F. Regulation of renal tubular glucose reabsorption by Akt2/PKBbeta. Am J Physiol Renal Physiol. 2010;298:F1113–F1117. doi: 10.1152/ajprenal.00592.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canaud G, Bienaime F, Viau A, Treins C, Baron W, Nguyen C, Burtin M, Berissi S, Giannakakis K, Muda AO, Zschiedrich S, Huber TB, Friedlander G, Legendre C, Pontoglio M, Pende M, Terzi F. AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med. 2013;19:1288–1296. doi: 10.1038/nm.3313. [DOI] [PubMed] [Google Scholar]
- 47.Sakamoto K, Arnolds DE, Fujii N, Kramer HF, Hirshman MF, Goodyear LJ. Role of Akt2 in contraction-stimulated cell signaling and glucose uptake in skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291:E1031–E1037. doi: 10.1152/ajpendo.00204.2006. [DOI] [PubMed] [Google Scholar]
- 48.Haase H, Maret W. Intracellular zinc fluctuations modulate protein tyrosine phosphatase activity in insulin/insulin-like growth factor-1 signaling. Exp Cell Res. 2003;291:289–298. doi: 10.1016/s0014-4827(03)00406-3. [DOI] [PubMed] [Google Scholar]
- 49.Wu W, Wang X, Zhang W, Reed W, Samet JM, Whang YE, Ghio AJ. Zinc-induced PTEN protein degradation through the proteasome pathway in human airway epithelial cells. J Biol Chem. 2003;278:28258–28263. doi: 10.1074/jbc.M303318200. [DOI] [PubMed] [Google Scholar]
- 50.Haase H, Maret W. Fluctuations of cellular, available zinc modulate insulin signaling via inhibition of protein tyrosine phosphatases. J Trace Elem Med Biol. 2005;19:37–42. doi: 10.1016/j.jtemb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Mocanu MM, Yellon DM. Letter to the editor: “Zinc and cardioprotection: the missing link”. Am J Physiol Heart Circ Physiol. 2009;296:H233–H234. doi: 10.1152/ajpheart.01193.2008. (author reply H235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S, Chanoit G, McIntosh R, Zvara DA, Xu Z. Molecular mechanism underlying Akt activation in zinc-induced cardioprotection. Am J Physiol Heart Circ Physiol. 2009;297:H569–H575. doi: 10.1152/ajpheart.00293.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fang CX, Doser TA, Yang X, Sreejayan N, Ren J. Metallothionein antagonizes aging-induced cardiac contractile dysfunction: role of PTP1B, insulin receptor tyrosine phosphorylation and Akt. Aging Cell. 2006;5:177–185. doi: 10.1111/j.1474-9726.2006.00201.x. [DOI] [PubMed] [Google Scholar]
- 54.Kinlaw WB, Levine AS, Morley JE, Silvis SE, McClain CJ. Abnormal zinc metabolism in type II diabetes mellitus. Am J Med. 1983;75:273–277. doi: 10.1016/0002-9343(83)91205-6. [DOI] [PubMed] [Google Scholar]
- 55.Bideci A, Camurdan MO, Cinaz P, Dursun H, Demirel F. Serum zinc, insulin-like growth factor-I and insulin-like growth factor binding protein-3 levels in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2005;18:1007–1011. doi: 10.1515/jpem.2005.18.10.1007. [DOI] [PubMed] [Google Scholar]
- 56.Jansen J, Rosenkranz E, Overbeck S, Warmuth S, Mocchegiani E, Giacconi R, Weiskirchen R, Karges W, Rink L. Disturbed zinc homeostasis in diabetic patients by in vitro and in vivo analysis of insulinomimetic activity of zinc. J Nutr Biochem. 2012;23:1458–1466. doi: 10.1016/j.jnutbio.2011.09.008. [DOI] [PubMed] [Google Scholar]