Abstract
Systemic lupus erythematous (SLE) is an autoimmune disease with a strong female predilection. Pregnancy remains a commonly encountered yet high risk situation in this setting. Both maternal and fetal mortality and morbidity are still significantly increased despite improvements in outcomes. Maternal morbidity includes higher risk of disease flares, pre-eclampsia and other pregnancy-related complications. Fetal issues include higher rates of pre-term birth, intra-uterine growth restriction and neonatal lupus syndromes. Treatment options during pregnancy are also limited and maternal benefit has to be weighed against fetal risk. A coordinated approach, with close monitoring by a multidisciplinary team, is essential for optimal outcomes.
Keywords: Systemic lupus erythematosus, antibodies, pregnancy, fetal loss, pre-eclampsia, neonatal lupus syndromes
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease with a strong female predilection. Disease onset in younger age group, coupled with improved survival, makes pregnancy a likely occurrence in the setting of SLE. Although outcomes have improved over time and successful live births can now be achieved in a large majority, pregnancy still remains a high risk situation in SLE1-3. Both maternal and fetal mortality and morbidity are significantly increased, along with health care utilization and costs2-5. A multidisciplinary coordinated approach with involvement of appropriate specialists, and close monitoring is essential for optimal outcomes. This review will discuss major issues and the management principles to guide the clinician caring for the pregnant women with SLE.
Effects of pregnancy on SLE disease activity
Although opinions differ, most of the studies have shown that risk of SLE flare is higher during pregnancy. Variable flare rates of between 25-65% have been reported, likely attributable to different study designs, patient populations and assessment tools utilized6-8. Multiple predictors for flares have been identified including disease activity at the time of conception, lupus nephritis, and discontinuation of medications such as hydroxychloroquine9,10. The majority of these flares are mild to moderate in severity and involve renal, musculoskeletal, and hematological systems11. Recognition and management of the flares during pregnancy can be challenging as features may be altered and therapeutic options limited.
Recognition of disease activity during pregnancy
Recognition of disease activity and flare in pregnancy can be a difficult as physiological changes of pregnancy may overlap with features of active disease (Table 1). Investigations have to be interpreted with caution: mild degrees of anemia, thrombocytopenia, proteinuria, and raised erythrocyte sedimentation rate are common during pregnancy. Complement levels become less informative with the rise in levels during normal pregnancy. The trend becomes more important, and decline in levels of complement during pregnancy has been associated with poor pregnancy outcomes 12,13. The use of SLE disease activity indices face similar issues, as physiologic pregnancy changes were not accounted in these tools. Pregnancy-specific disease activity scales have been developed but utility remain limited. Clinical judgment of an experienced physician may the best tool to evaluate disease activity in some scenarios.
Table 1.
Overlapping features of pregnancy and SLE
| Pregnancy changes | SLE activity | |
|---|---|---|
| Clinical Features | Facial flush Palmar erythema Arthralgias Fatigue Mild edema Mild resting dyspnea |
Photosensitive rash Oral or nasal ulcers Inflammatory arthritis Fatigue, lethargy Moderate to s evere edema Pleuritis |
| Laboratory Features | Mild anemia, Mild thrombocytopenia Mildly raised ESR Physiologic proteinuria <300mg/day |
Immune hemolytic anemia Thrombocytopenia Leucopenia, lymphopenia Raised Inflammatory markers Proteinuria >300mg/day Active urinary sediment |
Management of disease activity pregnancy
Treatment of disease activity and flares during pregnancy require use of medications that are effective yet safe for the growing fetus. Unfortunately, patients and sometimes even physicians discontinue medications due to concerns over presumed toxicity, resulting in avoidable disease flares and associated consequences. The fear is compounded by lack of information as the data on safety of drugs during pregnancy is generally limited to registries, case reports or animal studies. However, although choices are limited and maternal benefit has to be weighed against fetal toxicity, multiple effective options exist and should be utilized 14,15(Table 2).
Table 2.
Immunosuppressant use during SLE Pregnancy
| Drugs | Comments |
|---|---|
|
Corticosteroids • Prednisolone/ Pulse methyl prednisolone • Flourinated compounds (Betamethasone/ dexamethasone) |
Use lowest possible dose Higher doses can lead to maternal complications Pulse therapy can be used for acute flares Limit to one course, for fetal lung maturation Repeated use associated with impaired neuropsychological development of the child with |
|
Antimalarials • Hydroxychloroquine |
Reduced risk of disease flares, CHB and NLS Should be continued in all SLE pregnancies |
|
Immunosuppressants • Azathioprine • Calcineurin inhibitors (cyclosporine/tacrolimus) |
Limit azathioprine dose to 2mg/kg/day |
| Drugs | Comments |
|---|---|
|
Corticosteroids • Prednisolone/ Pulse methyl prednisolone • Flourinated compounds (Betamethasone/ dexamethasone) |
Use lowest possible dose Higher doses can lead to maternal complications Pulse therapy can be used for acute flares Limit to one course, for fetal lung maturation Repeated use associated with impaired neuropsychological development of the child with |
|
Antimalarials • Hydroxychloroquine |
Reduced risk of disease flares, CHB and NLS Should be continued in all SLE pregnancies |
|
Immunosuppressants • Azathioprine • Calcineurin inhibitors (cyclosporine/tacrolimus) |
Limit azathioprine dose to 2mg/kg/day |
Steroids can be continued during pregnancy for optimal disease control but attempts should be made to minimize the exposure. High doses of steroids are associated with an increased risk of diabetes, hypertension, pre-eclampsia and premature rupture of membranes, but short-term use for flares and disease control is permissible 14. Similarly, use of fluorinated compounds, such as dexamethasone and betamethasone should be limited to single course for fetal lung maturity in cases of premature delivery. Repeated use should be avoided in view of association with impaired neuro-psychological development of the offspring in later life 16.
Hydroxychloroquine (HCQ) has multiple proven benefits in SLE and continued use throughout pregnancy is strongly recommended. Pregnancy specific benefits include reduction in disease activity, lower risk of flares, and reduced risk of heart block in at risk pregnancies 17-20. HCQ discontinuation was shown to increase disease flares during pregnancy and should be discouraged 17.
Commonly used immunosuppressive agents such as cyclophosphamide, methotrexate, and mycophenolate have teratogenic potential and ideally should be discontinued before conception. Safe immunosuppressants for pregnancy use include azathioprine and calcineurin inhibitors, tacrolimus and cyclosporine 14,15. Multiple studies have shown them to be safe and effective therapies for use during pregnancy. An association between maternal azathioprine therapy and late developmental delays (specifically, increased utilization of special education services) in offspring was suggested by one study but remains to be confirmed21. Some risk of fetal cytopenias and immune suppression has been reported with higher doses and it is recommended to limit the dose to maximum of 2mg/kg/day14. Although safety of inadvertent exposure to leflunomide (usually followed by cholestyramine washout) has been reported, data is limited22,23. It should be discontinued before pregnancy with consideration of wash out procedure15. Use of biologic drugs during pregnancy is increasing but is still limited to anti TNF agents which are not an option for SLE 15,24. Data on other biologic agents such as rituximab and belimumab are very limited, and use should be limited to situations where no other pregnancy safe option is viable 15. Intravenous immunoglobulin (IVIG) and plasmapheresis remain alternative options in selected situations 15,25.
Effect of SLE on pregnancy outcomes
The interaction of SLE, an immune mediated disease, and immunological adaptations of pregnancy lead to unique challenges in this setting. Both mother and the baby are at high risk of adverse pregnancy outcomes (APO) including pre-eclampsia, pre-term delivery, pregnancy loss, and intra-uterine growth restriction (IUGR). The predictors of APO include active maternal disease, nephritis, proteinuria, hypertension, thrombocytopenia, and presence of anti-phospholipid antibodies (aPLs), especially lupus anticoagulant (LAC)10,26-30. Ethnic differences have also been reported, likely reflective of racial differences in disease and access to health care 10,31.
Pregnancy loss has declined significantly over the decades and live birth rates of 80-90% have been reported 1,25. Pre-term births are now the most frequent problem, occurring in up to half of the pregnancies with poor prognostic markers listed above. Additionally, thyroid disease is associated with pre-term birth in SLE pregnancy 32.
Higher rates of maternal death, thrombosis, infection, and hematologic complications during SLE pregnancy have been reported, although non- pregnant SLE patients also have higher risks of these medical complications and mortality 2,3. Neurodevelopmental disorders in offspring of mothers with SLE represent an emerging concern that requires further study.33,34.
Pre-eclampsia in SLE pregnancy
Pre-eclampsia affects 16-30% of SLE pregnancies compared to 5-7% in healthy women. In addition to the general predisposing factors (advanced maternal age, previous personal or family history of preeclampsia, pre-existing hypertension or diabetes mellitus, obesity), SLE specific predictors for pre-eclampsia include active or history of lupus nephritis, presence of anti-phospholipid antibodies, thrombocytopenia, declining complement levels, and mutations in complement regulatory proteins 35-37.
The high risk of pre-eclampsia in SLE pregnancy is compounded by the difficulty in differentiating it from lupus nephritis. Both conditions can manifest with increasing proteinuria, deteriorating renal function, hypertension, and thrombocytopenia, and can even co-exist. Guidelines and biomarkers have been proposed but have limited utility. Ultrasound findings such as abnormal uterine artery waveforms have shown good utility as diagnostic tools, and predictive modeling has been attempted for early recognition 38-40. However, all these measures have limitations and differentiation may be extremely difficult. Renal biopsy could guide management in selected cases and is safe in experienced hands 41. However, at times, delivery of the baby may be the only definitive answer.
Management guidelines for pregnancy in SLE
Ideally, pregnancy should be timed during period of disease quiescence as active disease at the time of conception is known to be one of the strongest predictor of APO. In reality, unplanned pregnancies are common, highlighting the often neglected need for contraceptive counseling in this group of women 42. Effective contraceptive choices include combined oral contraceptives in women with stable disease and negative aPL, progesterone only contraceptives, and intra-uterine devices, while barrier methods are ineffective with high failure rate 43. (See Chapter 1). Caution is required when making decisions regarding contraception in women with aPL and active disease in view of limited data.
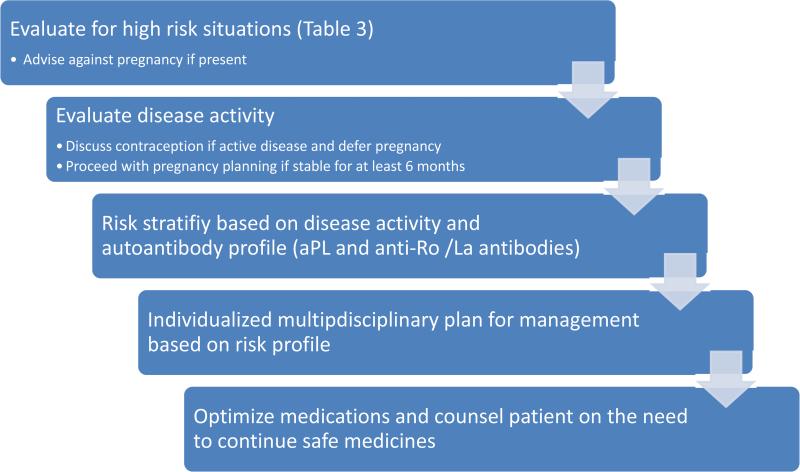
Pregnancy may carry a very high maternal risk in a subset of SLE patients, and should be avoided in these women (Table 3). However, successful pregnancy is possible for a large majority of women with SLE, albeit with a higher risk. Pre-pregnancy evaluation with assessment of auto-antibody profile, end organ function, disease activity, and medication use helps to risk stratify, identify optimal timing and plan the management strategy for each pregnancy (Figure 1).
Table 3.
High maternal risk situations for pregnancy in SLE
| Avoid pregnancy if: |
|---|
| Severe pulmonary hypertension (systolic pulmonary artery pressure > 50mm Hg) |
| Severe restrictive lung disease (Forced vital capacity <1 L) |
| Advanced renal insufficiency (creatinine >2.8 mg/dL) |
| Advance heart failure |
| Previous severe preeclampsia or HELLP despite therapy |
| Stroke within the previous 6 months |
| Severe disease flare within last 6 months |
Figure 1.
Pre-pregnancy evaluation for SLE patients
All pregnant women with SLE should be closely monitored during pregnancy, preferably by a multidisciplinary team of appropriate specialists. A recent large study showed reduction in immunosuppression use and rheumatologist visits despite overall increased health care utilization during SLE pregnancies 4. This again emphasizes the need for team-based approach in care of these high risk pregnancies. Ante-natal monitoring should be tailored to the individual needs of the particular patient but generally requires frequent review, especially in the presence of poor prognostic markers. Presence of certain specific antibodies poses special risk and deserves closer attention.
Anti-phospholipid antibodies in SLE pregnancy
Anti-phospholipid antibodies (aPL) are present in a quarter to half of SLE patients. Some of these patients are asymptomatic, while others develop thrombotic or obstetric complications, termed the antiphospholipid syndrome (APS). The presence of aPL significantly increases the risk of APO, even in asymptomatic women.
Management of exposed pregnancies depends on the risk profile and can be categorized into 3 main groups. Asymptomatic carriers are women with positive aPL but no prior clinical event. Low dose aspirin has been recommended but multiple studies have failed to show the benefit of this approach44-46. However, use of prophylactic aspirin in this setting remains common. The second group includes women with recurrent pregnancy losses but no systemic thrombosis, termed obstetric anti-phospholipid syndrome (OB-APS). Combination therapy with aspirin and prophylactic doses of heparin significantly reduces the risk of pregnancy loss in this group 46,47. The third group is comprised of patients with APS and prior systemic thrombosis. These women should receive full therapeutic doses of heparin throughout pregnancy. Heparin should be continued for 6 weeks post-partum. Low molecular weight heparin (LMWH) can be used in place of unfractionated heparin as it has comparable efficacy but less adverse effects with easier monitoring.
The outcomes of aPL-exposed pregnancies have significantly improved with current therapies, and live birth rates of over 80% can be achieved. However, some patients remain refractory and continue to have recurrent losses. Management of this group remains challenging; steroids, IVIG, and plasmapheresis have been tried with some benefit, but data are limited 48-50. Therapy has to be individualized and the patient counseled accordingly.
Anti Ro/La antibodies and Neonatal Lupus Syndromes
Pregnancies exposed to anti-Ro and anti-La antibodies have higher risk of developing neonatal lupus syndromes (NLS), a form of passively acquired fetal autoimmunity from maternal antibodies that cross the placenta. The majority of the manifestations, such as rash, hematologic and hepatic abnormalities, tend to resolve with clearance of the maternal antibodies by six to eight months of life51. However, injury to the developing fetal cardiac conduction pathway by these antibodies can lead to permanent damage. The cardiac manifestations include conduction defects, structural abnormalities, cardiomyopathy and congestive cardiac failure but the most feared complication is development of complete heart block (CHB)52.
Affecting up to 2% of exposed pregnancies, but with recurrence rates of 16-20% after the first event, CHB is associated with high fetal mortality of 20%. The majority (up to 70%) of survivors require pacemaker insertion53. CHB development is generally preceded by lower degrees of conduction delays although rapid development without warning signs has been described53. The majority of the events occur between 16-26 weeks of gestation but late cases do occur and even post-partum development of CHB has been reported. Early detection and treatment initiation might halt this progression but reversal of established CHB has not been reported. Multiple monitoring tools have been proposed for early detection of milder forms of conduction defects including doppler echocardiography, tissue-velocity based fetal kinetocardiogram, and trans-abdominal fetal electrocardiogram54.
Fetal doppler echocardiography remains the most commonly used method. Based on the most vulnerable period, recommended approach is to monitor all exposed fetuses weekly between 16–26 weeks of gestation, and bi-weekly thereafter52,55,56. Detection of an early conduction defect such as prolonged PR interval should prompt a discussion about prophylactic therapy. Although results have not been consistent, maternal administration of fluorinated corticosteroids and beta agonists has shown fetal survival benefit in some studies55,57-59. In absence of any other therapy of known benefit, this remains the treatment of choice but any expected benefit has to be weighed against the risk of IUGR and preterm birth. Treatment of established CHB remains an unresolved issue with minimal benefit with any available approach.
The high risk of recurrence in subsequent pregnancies has prompted the quest for prophylactic therapy for at risk pregnancies. Beneficial effects of IVIG were reported in open label studies, but two large randomized controlled trials were negative60,61. Both trials have been criticized for methodology, and use of IVIG in this setting can still be considered as an option. However, the patient should be informed about the limited data and involved in the decision-making process.
HCQ again deserves special mention. Multiple studies have shown that HCQ reduces the risk of cardiac NLS in at-risk fetuses and possible recurrences62. In view of multiple beneficial effects of HCQ, need for continued use in all and especially at-risk pregnancies cannot be over-emphasized.
Medication use during pregnancy
An important aspect of pregnancy management in SLE is optimization of medication use during this period. The choices of effective yet safe immunosuppressants have been discussed above.
The management of blood pressure can also become quite challenging as most of the antihypertensive drugs are contraindicated during pregnancy. The safe options include hydralazine, methyl-dopa, nifedipine, and labetalol 63,64. Beta-blocker use has been associated with IUGR and fetal bradycardia, and caution is required. Angiotensin-converting-enzyme (ACE) inhibitors and angiotensin II receptor blockers are associated with specific malformations, neonatal arterial hypotension, and renal failure, and should be avoided 63,65.
Non-steroidal anti-inflammatory drugs (NSAIDS) are generally considered safe during the first and second trimesters 14. Recently, associations between NSAID use in first trimester and specific birth defects were reported, along with potential risk of impaired fetal renal function with use after 20 weeks of gestation66. Hence, caution is required for use during early pregnancy. NSAIDSs should be discontinued by 32 week of gestation in view of the significantly higher risk of premature closure of the ductus arteriosus. Cyclo-oxygenase 2 inhibitors should be avoided during pregnancy as data are very limited for safety evaluation14.
Antiplatelet agents considered safe for use during pregnancy include aspirin and clopidegrol 67. However, clopidegrol has to be discontinued at least 7 days prior to delivery to avoid the increased risk of excessive hemorrhage. Heparin remains the anticoagulant of choice during pregnancy with data emerging on safety of direct factor Xa inhibitor, fondoparinux 67. LMWH is easier to use and has similar efficacy and safety to unfractionated heparin 68. Warfarin is teratogenic and should be avoided during pregnancy, especially during the first trimester.
Calcium supplementation is mandatory for all pregnant women with SLE, especially those receiving corticosteroids and heparin. Although Low vitamin D levels during pregnancy have been associated with poor outcomes, supplemental vitamin D during pregnancy did not reduce the risk 69,70. Bisphosphonates have long half-lives and use in women with reproductive potential should be carefully considered.
Summary
Pregnancy in women with SLE remains a high risk condition despite considerable improvement in outcomes. Disease flares may occur during the pregnancy, recognition and effective treatment is difficult but a realistic goal. High maternal and fetal mortality and morbidity are related to higher incidence of complications such as pre-eclampsia, pregnancy loss, pre-term births, IUGR, and neonatal lupus syndromes including CHB. Close monitoring, tailored approach according to specific risks involved, and judicious use of appropriate therapies are the key to achieve optimal outcomes.
Key points.
Outcomes for pregnancy in the setting of SLE have considerably improved but the maternal and fetal risks still remain high
Disease flares, pre-eclampsia, pregnancy loss, pre-term births, intra-uterine growth restriction and neonatal lupus syndromes (especially heart block) remain the main complications
Specific monitoring and treatment protocols need to be employed for situations such as presence of specific antibodies (aPL and anti-Ro/La)
Safe and effective treatment options exist and should be used as appropriate to control disease activity during pregnancy
Close monitoring, tailored multidisciplinary care, and judicious use of medications are the key to achieve optimal outcomes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Aisha Lateef: No commercial or financial conflict of interest. No funding source for this work.
Michelle Petri: No commercial or financial conflict of interest. No funding source for this work.
References
- 1.Clark CA, Spitzer KA, Laskin CA. Decrease in pregnancy loss rates in patients with systemic lupus erythematosus over a 40-year period. J Rheumatol. 2005;32(9):1709–1712. [PubMed] [Google Scholar]
- 2.Clowse ME, Jamison M, Myers E, James AH. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol. 2008;199(2):127, e121–126. doi: 10.1016/j.ajog.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakravarty EF, Nelson L, Krishnan E. Obstetric hospitalizations in the United States for women with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54(3):899–907. doi: 10.1002/art.21663. [DOI] [PubMed] [Google Scholar]
- 4.Petri M, Daly RP, Pushparajah DS. Healthcare costs of pregnancy in systemic lupus erythematosus: retrospective observational analysis from a US health claims database. J Med Econ. 2015;18(11):967–973. doi: 10.3111/13696998.2015.1066796. [DOI] [PubMed] [Google Scholar]
- 5.Lateef A, Petri M. Management of pregnancy in systemic lupus erythematosus. Nat Rev Rheumatol. 2012;8(12):710–718. doi: 10.1038/nrrheum.2012.133. [DOI] [PubMed] [Google Scholar]
- 6.Cortes-Hernandez J, Ordi-Ros J, Paredes F, Casellas M, Castillo F, Vilardell-Tarres M. Clinical predictors of fetal and maternal outcome in systemic lupus erythematosus: a prospective study of 103 pregnancies. Rheumatology (Oxford) 2002;41(6):643–650. doi: 10.1093/rheumatology/41.6.643. [DOI] [PubMed] [Google Scholar]
- 7.Gladman DD, Tandon A, Ibanez D, Urowitz MB. The effect of lupus nephritis on pregnancy outcome and fetal and maternal complications. J Rheumatol. 2010;37(4):754–758. doi: 10.3899/jrheum.090872. [DOI] [PubMed] [Google Scholar]
- 8.Imbasciati E, Tincani A, Gregorini G, et al. Pregnancy in women with pre-existing lupus nephritis: predictors of fetal and maternal outcome. Nephrol Dial Transplant. 2009;24(2):519–525. doi: 10.1093/ndt/gfn348. [DOI] [PubMed] [Google Scholar]
- 9.Jara LJ, Medina G, Cruz-Dominguez P, Navarro C, Vera-Lastra O, Saavedra MA. Risk factors of systemic lupus erythematosus flares during pregnancy. Immunol Res. 2014;60(2-3):184–192. doi: 10.1007/s12026-014-8577-1. [DOI] [PubMed] [Google Scholar]
- 10.Buyon JP, Kim MY, Salmon JE. Predictors of Pregnancy Outcomes in Patients With Lupus. Ann Intern Med. 2016;164(2):131. doi: 10.7326/L15-0500. [DOI] [PubMed] [Google Scholar]
- 11.Petri M. The Hopkins Lupus Pregnancy Center: ten key issues in management. Rheum Dis Clin North Am. 2007;33(2):227–235, v. doi: 10.1016/j.rdc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clowse ME, Magder LS, Petri M. The clinical utility of measuring complement and anti-dsDNA antibodies during pregnancy in patients with systemic lupus erythematosus. J Rheumatol. 2011;38(6):1012–1016. doi: 10.3899/jrheum.100746. [DOI] [PubMed] [Google Scholar]
- 13.Lateef A, Magder L, Petri M. Decrease in Complement (C3) Levels during Systemic Lupus Erythematosus Pregnancy is Associated with Higher Rates of Pre-eclampsia. Arthritis Rheum. 2010;62(10):S193. [Google Scholar]
- 14.Ostensen M, Khamashta M, Lockshin M, et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther. 2006;8(3):209. doi: 10.1186/ar1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gotestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75(5):795–810. doi: 10.1136/annrheumdis-2015-208840. [DOI] [PubMed] [Google Scholar]
- 16.Wapner RJ, Sorokin Y, Mele L, et al. Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357(12):1190–1198. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- 17.Clowse ME, Magder L, Witter F, Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum. 2006;54(11):3640–3647. doi: 10.1002/art.22159. [DOI] [PubMed] [Google Scholar]
- 18.Levy RA, Vilela VS, Cataldo MJ, et al. Hydroxychloroquine (HCQ) in lupus pregnancy: double-blind and placebo-controlled study. Lupus. 2001;10(6):401–404. doi: 10.1191/096120301678646137. [DOI] [PubMed] [Google Scholar]
- 19.Izmirly PM, Costedoat-Chalumeau N, Pisoni CN, et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation. 2012;126(1):76–82. doi: 10.1161/CIRCULATIONAHA.111.089268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izmirly PM, Kim MY, Llanos C, et al. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann Rheum Dis. 2010;69(10):1827–1830. doi: 10.1136/ard.2009.119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marder W, Ganser MA, Romero V, et al. In utero azathioprine exposure and increased utilization of special educational services in children born to mothers with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2013;65(5):759–766. doi: 10.1002/acr.21888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassina M, Johnson DL, Robinson LK, et al. Pregnancy outcome in women exposed to leflunomide before or during pregnancy. Arthritis Rheum. 2012;64(7):2085–2094. doi: 10.1002/art.34419. [DOI] [PubMed] [Google Scholar]
- 23.Chambers CD, Johnson DL, Robinson LK, et al. Birth outcomes in women who have taken leflunomide during pregnancy. Arthritis Rheum. 2010;62(5):1494–1503. doi: 10.1002/art.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai RJ, Huybrechts KF, Bateman BT, et al. Brief Report: Patterns and Secular Trends in Use of Immunomodulatory Agents During Pregnancy in Women With Rheumatic Conditions. Arthritis Rheumatol. 2016;68(5):1183–1189. doi: 10.1002/art.39521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lateef A, Petri M. Managing lupus patients during pregnancy. Best Pract Res Clin Rheumatol. 2013;27(3):435–447. doi: 10.1016/j.berh.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clowse ME, Magder LS, Witter F, Petri M. The impact of increased lupus activity on obstetric outcomes. Arthritis Rheum. 2005;52(2):514–521. doi: 10.1002/art.20864. [DOI] [PubMed] [Google Scholar]
- 27.Clowse ME, Magder LS, Witter F, Petri M. Early risk factors for pregnancy loss in lupus. Obstet Gynecol. 2006;107(2 Pt 1):293–299. doi: 10.1097/01.AOG.0000194205.95870.86. [DOI] [PubMed] [Google Scholar]
- 28.Lockshin MD, Kim M, Laskin CA, et al. Lupus anticoagulant, but not anticardiolipin antibody, predicts adverse pregnancy outcome in patients with antiphospholipid antibodies. Arthritis Rheum. 2012 doi: 10.1002/art.34402. doi: 10.1002/art.34402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mankee A, Petri M, Magder LS. Lupus anticoagulant, disease activity and low complement in the first trimester are predictive of pregnancy loss. Lupus Sci Med. 2015;2(1):e000095. doi: 10.1136/lupus-2015-000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yelnik CM, Laskin CA, Porter TF, et al. Lupus anticoagulant is the main predictor of adverse pregnancy outcomes in aPL-positive patients: validation of PROMISSE study results. Lupus Sci Med. 2016;3(1):e000131. doi: 10.1136/lupus-2015-000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clowse ME, Grotegut C. Racial and Ethnic Disparities in the Pregnancies of Women with Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.22847. [DOI] [PubMed] [Google Scholar]
- 32.Stagnaro-Green A, Akhter E, Yim C, Davies TF, Magder L, Petri M. Thyroid disease in pregnant women with systemic lupus erythematosus: increased preterm delivery. Lupus. 2011;20(7):690–699. doi: 10.1177/0961203310394894. [DOI] [PubMed] [Google Scholar]
- 33.Vinet E, Pineau CA, Clarke AE, et al. Increased Risk of Autism Spectrum Disorders in Children Born to Women With Systemic Lupus Erythematosus: Results From a Large Population-Based Cohort. Arthritis Rheumatol. 2015;67(12):3201–3208. doi: 10.1002/art.39320. [DOI] [PubMed] [Google Scholar]
- 34.Vinet E, Pineau CA, Clarke AE, Fombonne E, Platt RW, Bernatsky S. Neurodevelopmental disorders in children born to mothers with systemic lupus erythematosus. Lupus. 2014;23(11):1099–1104. doi: 10.1177/0961203314541691. [DOI] [PubMed] [Google Scholar]
- 35.Chakravarty EF, Colon I, Langen ES, et al. Factors that predict prematurity and preeclampsia in pregnancies that are complicated by systemic lupus erythematosus. Am J Obstet Gynecol. 2005;192(6):1897–1904. doi: 10.1016/j.ajog.2005.02.063. [DOI] [PubMed] [Google Scholar]
- 36.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Salmon JE, Heuser C, Triebwasser M, et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 2011;8(3):e1001013. doi: 10.1371/journal.pmed.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim MY, Buyon JP, Guerra MM, et al. Angiogenic factor imbalance early in pregnancy predicts adverse outcomes in patients with lupus and antiphospholipid antibodies: results of the PROMISSE study. Am J Obstet Gynecol. 2016;214(1):108 e101–108 e114. doi: 10.1016/j.ajog.2015.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuc S, Wortelboer EJ, van Rijn BB, Franx A, Visser GH, Schielen PC. Evaluation of 7 serum biomarkers and uterine artery Doppler ultrasound for first-trimester prediction of preeclampsia: a systematic review. Obstet Gynecol Surv. 2011;66(4):225–239. doi: 10.1097/OGX.0b013e3182227027. [DOI] [PubMed] [Google Scholar]
- 40.Kleinrouweler C, Wiegerinck M, Ris-Stalpers C, et al. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: a systematic review and meta-analysis. BJOG. 2012;119(7):778–787. doi: 10.1111/j.1471-0528.2012.03311.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen TK, Gelber AC, Witter FR, Petri M, Fine DM. Renal biopsy in the management of lupus nephritis during pregnancy. Lupus. 2015;24(2):147–154. doi: 10.1177/0961203314551812. [DOI] [PubMed] [Google Scholar]
- 42.Yazdany J, Trupin L, Kaiser R, et al. Contraceptive counseling and use among women with systemic lupus erythematosus: a gap in health care quality? Arthritis Care Res (Hoboken) 2011;63(3):358–365. doi: 10.1002/acr.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lateef A, Petri M. Hormone replacement and contraceptive therapy in autoimmune diseases. J Autoimmun. 2012;38(2-3):J170–176. doi: 10.1016/j.jaut.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Amengual O, Fujita D, Ota E, et al. Primary prophylaxis to prevent obstetric complications in asymptomatic women with antiphospholipid antibodies: a systematic review. Lupus. 2015;24(11):1135–1142. doi: 10.1177/0961203315578765. [DOI] [PubMed] [Google Scholar]
- 45.Del Ross T, Ruffatti A, Visentin MS, et al. Treatment of 139 pregnancies in antiphospholipid-positive women not fulfilling criteria for antiphospholipid syndrome: a retrospective study. J Rheumatol. 2013;40(4):425–429. doi: 10.3899/jrheum.120576. [DOI] [PubMed] [Google Scholar]
- 46.Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev. 2005;(2):CD002859. doi: 10.1002/14651858.CD002859.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mak A, Cheung MW, Cheak AA, Ho RC. Combination of heparin and aspirin is superior to aspirin alone in enhancing live births in patients with recurrent pregnancy loss and positive anti-phospholipid antibodies: a meta-analysis of randomized controlled trials and meta-regression. Rheumatology (Oxford) 2010;49(2):281–288. doi: 10.1093/rheumatology/kep373. [DOI] [PubMed] [Google Scholar]
- 48.Bramham K, Thomas M, Nelson-Piercy C, Khamashta M, Hunt BJ. First-trimester low-dose prednisolone in refractory antiphospholipid antibody-related pregnancy loss. Blood. 2011;117(25):6948–6951. doi: 10.1182/blood-2011-02-339234. [DOI] [PubMed] [Google Scholar]
- 49.Branch DW, Peaceman AM, Druzin M, et al. A multicenter, placebo-controlled pilot study of intravenous immune globulin treatment of antiphospholipid syndrome during pregnancy. The Pregnancy Loss Study Group. Am J Obstet Gynecol. 2000;182:122–127. doi: 10.1016/s0002-9378(00)70500-x. [DOI] [PubMed] [Google Scholar]
- 50.El-Haieg DO, Zanati MF, El-Foual FM. Plasmapheresis and pregnancy outcome in patients with antiphospholipid syndrome. Int J Gynaecol Obstet. 2007;99(3):236–241. doi: 10.1016/j.ijgo.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 51.Izmirly PM, Rivera TL, Buyon JP. Neonatal lupus syndromes. Rheum Dis Clin North Am. 2007;33(2):267–285, vi. doi: 10.1016/j.rdc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 52.Brito-Zeron P, Izmirly PM, Ramos-Casals M, Buyon JP, Khamashta MA. The clinical spectrum of autoimmune congenital heart block. Nat Rev Rheumatol. 2015;11(5):301–312. doi: 10.1038/nrrheum.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brito-Zeron P, Izmirly PM, Ramos-Casals M, Buyon JP, Khamashta MA. Autoimmune congenital heart block: complex and unusual situations. Lupus. 2016;25(2):116–128. doi: 10.1177/0961203315624024. [DOI] [PubMed] [Google Scholar]
- 54.Sonesson SE. Diagnosing foetal atrioventricular heart blocks. Scand J Immunol. 2010;72(3):205–212. doi: 10.1111/j.1365-3083.2010.02434.x. [DOI] [PubMed] [Google Scholar]
- 55.Buyon JP, Clancy RM, Friedman DM. Cardiac manifestations of neonatal lupus erythematosus: guidelines to management, integrating clues from the bench and bedside. Nat Clin Pract Rheumatol. 2009;5(3):139–148. doi: 10.1038/ncprheum1018. [DOI] [PubMed] [Google Scholar]
- 56.Friedman DM, Kim MY, Copel JA, Llanos C, Davis C, Buyon JP. Prospective evaluation of fetuses with autoimmune-associated congenital heart block followed in the PR Interval and Dexamethasone Evaluation (PRIDE) Study. Am J Cardiol. 2009;103(8):1102–1106. doi: 10.1016/j.amjcard.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levesque K, Morel N, Maltret A, et al. Description of 214 cases of autoimmune congenital heart block: Results of the French neonatal lupus syndrome. Autoimmun Rev. 2015;14(12):1154–1160. doi: 10.1016/j.autrev.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Izmirly PM, Saxena A, Sahl SK, et al. Assessment of fluorinated steroids to avert progression and mortality in anti-SSA/Ro-associated cardiac injury limited to the fetal conduction system. Ann Rheum Dis. 2016;75(6):1161–1165. doi: 10.1136/annrheumdis-2015-208311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jaeggi ET, Fouron JC, Silverman ED, Ryan G, Smallhorn J, Hornberger LK. Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation. 2004;110(12):1542–1548. doi: 10.1161/01.CIR.0000142046.58632.3A. [DOI] [PubMed] [Google Scholar]
- 60.Friedman DM, Llanos C, Izmirly PM, et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: Results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum. 2010;62(4):1138–1146. doi: 10.1002/art.27308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pisoni CN, Brucato A, Ruffatti A, et al. Failure of intravenous immunoglobulin to prevent congenital heart block: Findings of a multicenter, prospective, observational study. Arthritis Rheum. 2010;62(4):1147–1152. doi: 10.1002/art.27350. [DOI] [PubMed] [Google Scholar]
- 62.Peart E, Clowse ME. Systemic lupus erythematosus and pregnancy outcomes: an update and review of the literature. Curr Opin Rheumatol. 2014;26(2):118–123. doi: 10.1097/BOR.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 63.ACOG Practice Bulletin No. 125: Chronic hypertension in pregnancy. Obstet Gynecol. 2012;119(2 Pt 1):396–407. doi: 10.1097/AOG.0b013e318249ff06. [DOI] [PubMed] [Google Scholar]
- 64.Mustafa R, Ahmed S, Gupta A, Venuto RC. A comprehensive review of hypertension in pregnancy. J Pregnancy. 2012;2012:105918. doi: 10.1155/2012/105918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354(23):2443–2451. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 66.Adams K, Bombardier C, van der Heijde DM. Safety of pain therapy during pregnancy and lactation in patients with inflammatory arthritis: a systematic literature review. J Rheumatol Suppl. 2012;90:59–61. doi: 10.3899/jrheum.120344. [DOI] [PubMed] [Google Scholar]
- 67.Yarrington CD, Valente AM, Economy KE. Cardiovascular Management in Pregnancy: Antithrombotic Agents and Antiplatelet Agents. Circulation. 2015;132(14):1354–1364. doi: 10.1161/CIRCULATIONAHA.114.003902. [DOI] [PubMed] [Google Scholar]
- 68.Giannubilo SR, Tranquilli AL. Anticoagulant therapy during pregnancy for maternal and fetal acquired and inherited thrombophilia. Curr Med Chem. 2012;19(27):4562–4571. doi: 10.2174/092986712803306466. [DOI] [PubMed] [Google Scholar]
- 69.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O'Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 70.De-Regil LM, Palacios C, Ansary A, Kulier R, Pena-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2012;2:CD008873. doi: 10.1002/14651858.CD008873.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]