Abstract
Background:
This paper presents the methodology and early findings of the fifth survey of a school-based surveillance program in Iran.
Methods:
This nationwide study was conducted in 2015 as the fifth survey of a surveillance program entitled “Childhood and Adolescence Surveillance and PreventIon of Adult Non- communicable disease” (CASPIAN-V) study. The protocol was mainly based on the World Health Organization-Global School student Health Survey. We studied 14400 students, aged 7-18 years, and their parents living in 30 provinces in Iran. Fasting blood was obtained from a sub-sample of 4200 randomly selected students.
Results:
The participation rate for the whole study and for blood sampling were 99% and 91.5%, respectively. The mean (SD) age of participants was 12.3 (3.2) years, consisting of 49.4% girls and 71.4% urban residents. Overall, 16.1% were underweight (17.4% of boys and 14.8% of girls), and 20.8% had excess weight consisting of 9.4% (8.7% of boys and 10.2% of girls) of overweight and 11.4% (12.5% of boys and 10.3% of girls) of obesity. Abdominal obesity was documented in 21.1% of students (21.6% of boys and 20.5% of girls). Low HDL-C was the most prevalent abnormality of the lipid profile (29.5%) followed by high serum triglycerides (27.7%). Of students, 59.9% consumed whole wheat bread; and 57% reported that they never or rarely added salt to table. The reported daily consumption of fresh fruits, vegetables, and milk was about 60%, 32% and 40%, respectively. 13.7% of participants had at least 30-min daily leisure-time physical activity.
Conclusions:
The current findings provide an overview of the current health status and lifestyle habits of children and adolescents. This surveillance program would help planning preventive programs at individual and community levels.
Keywords: Children and adolescents, methodology, prevention, surveillance
INTRODUCTION
Chronic, noncommunicable diseases (NCDs) act as an undeniable threat to global health, as well as socioeconomic development.[1,2] Defined by the World Health Organization (WHO) as cardiovascular diseases, cancers, diabetes, and chronic respiratory diseases, NCDs represent a group of preventable diseases with complex social, economic, and environmental etiologies.[1,3] Considering their significant role in the global burden of death and disability, NCDs are believed to be the most serious world health challenge.[4] NCDs account for 63% of mortality at global level, with 80% of NCD-related deaths are occurring in low- and middle-income countries.[5] Although all countries are experiencing an increase in the rate of NCDs - affecting both genders and various socioeconomic groups[6] - with more rapid escalating trend in low- and middle-income than in high-income and industrialized countries.[7]
Although NCDs become symptomatic in adulthood, they originate from early life because of the persistence of lifestyle behaviors and tracking of biological risk factors from childhood into adulthood.[8,9] This is of special concern for the pediatric population of developing countries, who are facing rapid changes in lifestyle habits.[10]
In 2001, the WHO, in collaboration with The Joint United Nations Program on HIV/AIDS, The United Nations Educational, Scientific and Cultural Organization, and The United Nations Children's Fund, and with technical assistance from the US Centers for Disease Control and Prevention, initiated development of the Global school-based student health survey (GSHS). Since 2003, Ministries of Health and Education of many countries are using the GSHS to periodically monitor the prevalence of important health risk behaviors and protective factors among students. The 10 core questionnaire modules included alcohol use, tobacco use, drug use, sexual behaviors, hygiene, dietary behaviors, mental health, physical activity, protective factors, and violence and unintentional injury.[11]
Iranian children and adolescents alike the pediatric population of many other developing countries, are experiencing lifestyle change, and are being affected by risk factors of NCDs.[10] From 2003 to 2004, a national school-based surveillance system has been implemented in Iran for health risk behaviors and risk factors.[10,12,13,14,15] This program is entitled “Childhood and Adolescence Surveillance and PreventIon of Adult Noncommunicable disease (CASPIAN) study.” The surveys were repeated every 2 years, with blood sampling for biochemical factors every 4 years.
This paper presents the methods and primary findings of the fifth survey of this school-based surveillance program, i.e., the CASPIAN-V study.
METHODS
The CASPIAN-V study was conducted in 2015 in urban and rural areas of thirty provinces of Iran.
Study population and sampling framework
The study population consisted of students aged 7–18 years in primary and secondary schools in urban and rural areas across the country. They were selected using multistage, stratified cluster sampling method.
Sampling within each province was conducted according to the student's place of residence (urban or rural) and level of education (primary and secondary) using the proportional to size method and with equal sex ratio. This means that the number of boys and girls was the same in each province, and their ratio in urban and rural areas was proportional to the number of students in urban and rural areas. Similarly, the number of samples between different grades in urban and rural areas was divided according to the number of students in each grade. Achieving the desired number of samples was obtained using cluster sampling in each province with equal cluster sizes.
Clusters were determined at school levels. The size of each cluster was ten students, which means that a total of 10 statistical units (including ten student and their parents) would be considered in each cluster. The sample size was 480 students in each province (48 clusters of 10 students), i.e., a total of 14,400 students at national level. This was the maximum sample size that helped achieving a good estimate of all risk factors of interest. In each province, 14 clusters out of 48 clusters were randomly selected for biochemical test, i.e., a total of 4200 students.
Procedure and measurements
Questionnaires
0Two sets of questionnaires were considered for students and their parents. The student's questionnaire was obtained from the WHO-GSHS that was translated into Persian. The validity and reliability of questionnaires has been assessed previously.[15,16] An expert panel approved the face validity and in the phase of content validity assessment, the questions getting a score of more than 0.75 were affirmed to have appropriate content validity. Cronbach's alpha coefficient of the whole questionnaires was 0.97 and Pearson's correlation coefficient of the test–retest phase was 0.94, which confirmed the reliability of questionnaires.
After identifying eligible students, the mission and purpose of the interview was explained. It was explained that questions are related to their health status and health-related behaviors. The students were asked to tell what they know, and easily express if they do not know something.
Interviews were conducted in a peaceful environment, away from busy classrooms. Questions were read to students with simple words, and they could not see the questions. The whole process was supervised and controlled by a team of health-care professionals.
The questionnaire includes the following sections:
Relationship with friends
Body size
Students’ schools
Life satisfaction
Dietary habits
Health behaviors
Physical activity
Leisure time activities
Injuries
Violence
Bullying
Exposure to tobacco smoke
Counseling with family members
Perceived health status
Tobacco smoking
Academic performance
Sunlight exposure
Exposure to the green space and environmental pollution.
After identifying eligible students, their parents were also invited to complete the parent's questionnaire. The presence of at least one of the parents was necessary and sufficient. Parents were informed that questions are about health status and health-related behaviors in the students’ families.
Parents’ questionnaires included the following sections:
Family characteristics (household size, order of students, socioeconomic variables)
Past medical history of the student
Family medical history
Dietary habits
Leisure time activities
History of injuries to the students
Parents’ sleep pattern
Parents’ anthropometric measures.
Physical measurements
A team of trained health-care experts recorded information based on approved checklists; they performed the examinations under standard protocols using calibrated instruments. Weight was measured on a scale placed on a flat ground to the nearest 0.1 kg while participants wearing a light cloth, and height was measured without shoes to the nearest 0.1 cm.[16] Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). We used the WHO growth charts to categorize BMI.[17] Waist circumference was measured using a nonelastic tape at a point midway between the lower border of the rib cage and the iliac crest at the end of normal expiration to the nearest 0.1 cm. Hip circumference was measured at the widest part of the hip at the level of the greater trochanter to the nearest 0.1 cm.[18]
Wrist circumference was measured to the nearest 0.1 cm on the dominant arm using a tape meter. Participans were asked to hold their arm on a flat surface as a table. The superior border of the tape measure was placed just distal to the prominences of radial and ulnar bones. The wrist circumference was measured without the tape was too tight or too loose and with lying flat on the skin. Neck circumference was measured with an accuracy of 0.1 cm with the most prominent portion of the thyroid cartilage taken as a landmark. Blood pressure (BP) was measured in the sitting position on the right arm using a mercury sphygmomanometer with an appropriate cuff size. It was measured 2 times at 5 min intervals, and the average was registered.[19]
Physical activity and leisure-time screen time
To assess the screen time (ST) behaviors, the number of hours per day that participants spent watching television (TV) and/or videos, personal computer, or electronic games were asked, and then the total cumulative spent time for ST was calculated.
Through a validated questionnaire, information of past week weekly frequency of leisure time physical activity outside the school was collected.[15] Having enough physical activity was considered as at least 30 min duration of exercises per day that led to sweating and large increases in breathing or heart rate.
Blood sampling
Eligible students were referred to the laboratory, while one of the parents accompanied him/her. Then, 6 mL venous blood sample was collected after 12 h overnight fasting. All collection tubes were centrifuged at 2500–3000 ×g for 10 min. Immediately after centrifugation, serum samples were aliquot into 200 μL tubes and stored at −70°C. All samples were transferred by cold chain to Isfahan Mahdieh Laboratory. Fasting blood glucose, triglycerides (TGs), total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C) and high-density lipoprotein-cholesterol (HDL-C), alanine aminotransferase, and creatinine were measured enzymatically by Hitachi Auto Analyzer (Tokyo, Japan).[20,21]
Definitions
We used the WHO growth curves to define BMI categories, i.e., underweight as age- and sex-specific BMI <5th, overweight as sex-specific BMI for age of 85th–95th, and obesity as sex-specific BMI for >95th . Abdominal obesity was defined as waist-to-height ratio equal to or more than 0.5.[22]
Fasting blood sugar ≥100 mg/dl, serum TGs ≥100 mg/dl, TC ≥200 mg/dl, LDL-C ≥110 mg/dl, and HDL-C <40 mg/dl (except in boy 15–18 years mg/dl <45 mg/dl) were considered as abnormal.[23] Elevated BP was defined as either high systolic BP (≥90th percentile for age, sex, and height) or high diastolic BP (≥90th percentile for age, sex, and height).[19]
Ethical concerns
Study protocols were reviewed and approved by ethical committees and other relevant national regulatory organizations. The Research and Ethics Council of Isfahan University of Medical Sciences approved the study (Project Number: 194049). After complete explanation of the study objectives and protocols, written informed consent and verbal consent were obtained from the parents and students, respectively.
Statistical analysis
Data were analyzed using STATA package version 11.0 (Stata Statistical Software: Release 11. StataCorp LP. Package, College Station, TX, USA), and P < 0.05 was considered statistically significant. Data are expressed as the mean and standard deviation (SD) for continuous variables and as number (percentage) for categorical variables. The Student's t-test was used to compare mean differences between quantitative variables. Association between qualitative variables was assessed by the Pearson's Chi-square test. All statistical measures were estimated using survey data analysis methods.
RESULTS
Overall, 14274 students and one of their parents (out of 14,440) completed the survey (participation rate: 99%); for blood sampling from students, the participation rate was 91.5% (3843 out of 4200 students selected for blood sampling). The students consisted of 7046 (49.4%) girls and 7228 (50.6%) boys; 71.4% of students were from urban and 28.6% from rural areas. The mean (SD) age of participants was 12.3 (3.2) years. In total, 12.3% of fathers and 17.6% of mothers were illiterate. The large number of students’ father was worker or employee (44.8%), whereas 86.8% of mothers were homemakers. The characteristics of participants are presented in Tables 1 and 2 according to gender and living area, respectively.
Table 1.
Characteristics of participants according to gender: The CASPIAN-V study
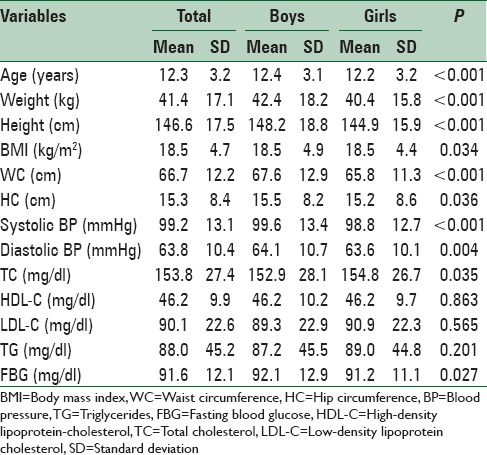
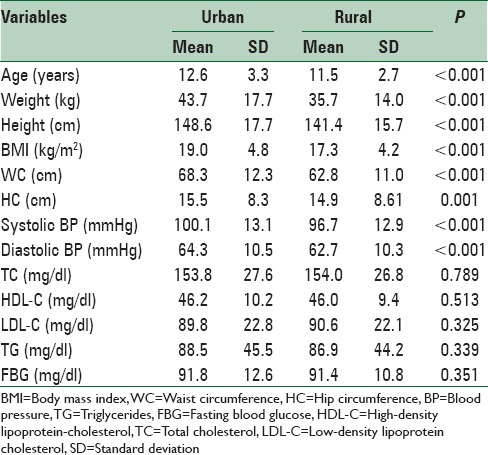
Table 2.
Characteristics of participants according to living area: The CASPIAN-V study
Overall, 16.1% (17.4% of boys and 14.8% of girls) were underweight, and 20.8% had excess weight consisting of 9.4% (8.7% of boys and 10.2% of girls) of overweight and 11.4% (12.5% of boys and 10.3% of girls) of obesity. Abdominal obesity was documented in 21.1% of students (21.6% of boys and 20.5% of girls). Low HDL-C was the most prevalent abnormality of lipid profile (29.5%) followed by high serum TGs (27.7%) [Table 3].
Table 3.
Prevalence of cardiometabolic risk factors in Iranian children and adolescents: The CASPIAN-V study
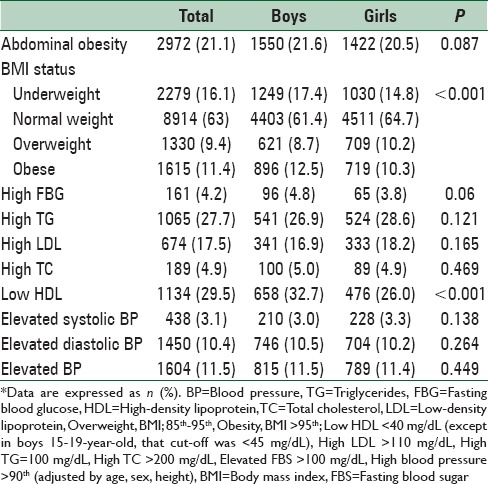
Table 4 shows some health and dietary behaviors reported by students or their parents. A total of 52% of students reported to brush their teeth once a day and 23.1% did more than once a day. Most families (59.9%) reported to consume whole wheat bread and the rest consumed refined bread. Hydrogenated solid oil and liquid oil were the most prevalent types of cooking oil in families (37.9% and 37.4%, respectively). Moreover, 57% of students reported that they never or rarely added salt to table food and the rest used it always or sometimes. Overall, 3.6% and 7.7% of students skipped breakfast during weekdays and weekends, respectively. Nearly 27.5% of students reported that they consumed junk foods every week, 25.7% consumed soft drinks every week, and 15.5% ate fast foods every week. Daily consumption of fresh fruits, vegetables, and milk among students was about 60%, 32%, and 40%, respectively [Table 5].
Table 4.
Some dietary behaviors in Iranian children and adolescents: CASPIAN-V study
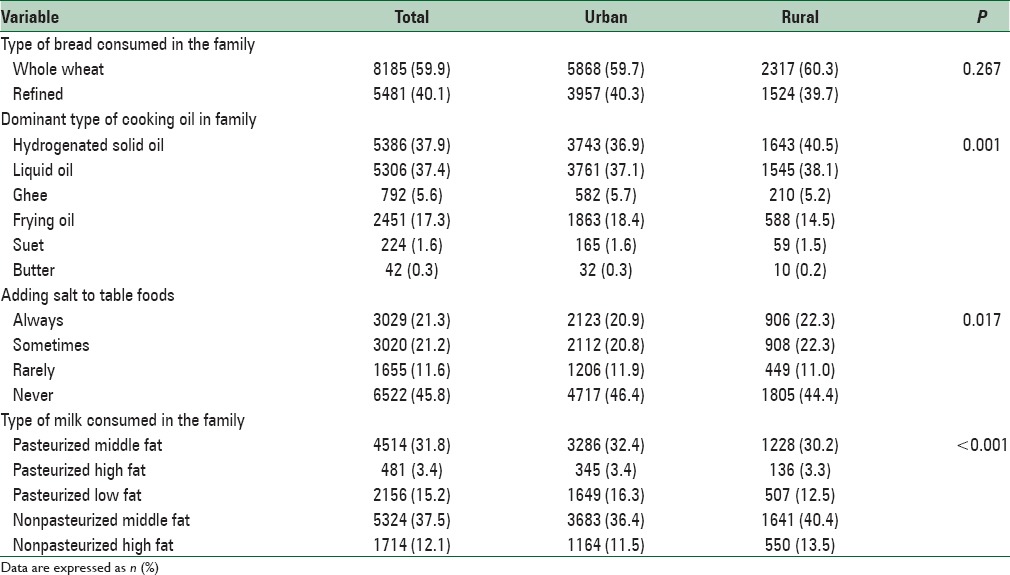
Table 5.
Consumption frequency of some healthy and junk foods in Iranian children and adolescents: CASPIAN-V study
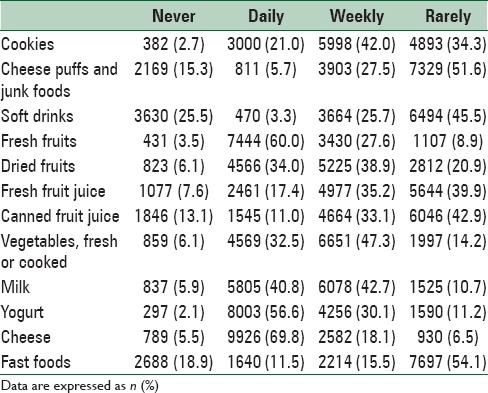
Table 6 shows physical activity and leisure time activities among students. According to reports by students, 13.7% had at least 30 min daily leisure time physical activity; however, 7.4% reported not to have any leisure time physical activity. Most students reported that they watched TV for 1 h during weekdays and 2 h during weekends (39% and 30%, respectively).
Table 6.
Physical activity and screen time pattern of study participants: The CASPIAN-V study
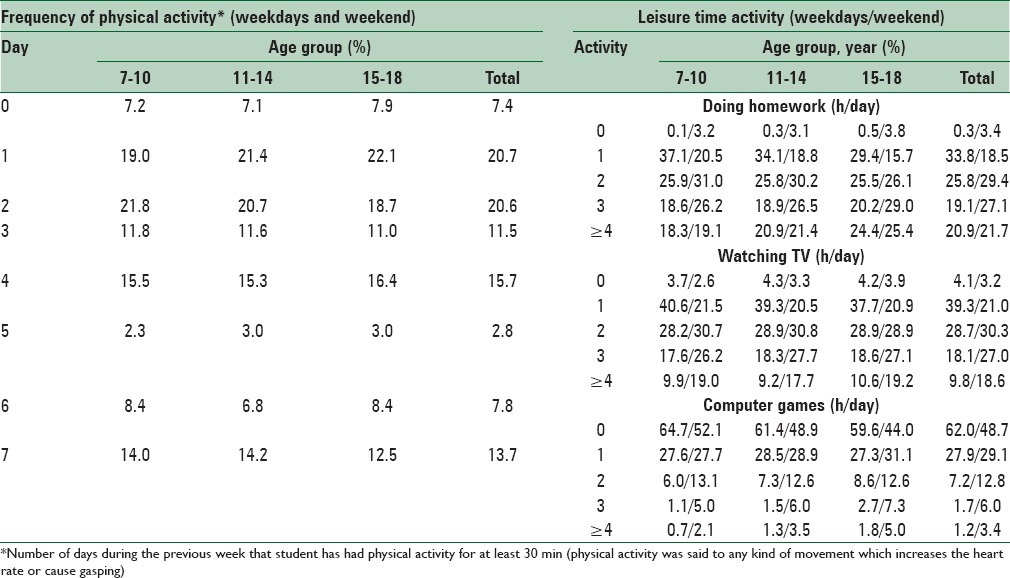
A total of 62% and 48% of students reported that they did not use personal computer during weekdays and weekends, respectively. Most students (about 50%) attended sports sessions in the school for 2 h/week. Use of school service and walking to school was approximately equal among students (39% and 38%, respectively). Mean (SD) of sleep duration was 8.4 (1.3) h in weekdays and 9.6 (1.6) h in weekends.
DISCUSSION
The current survey provided a general insight on some health-related risk behaviors and biological risk factors in a nationally representative sample of Iranian children and adolescents. The general health status was appropriate; however, some unhealthy lifestyle habits, in terms of junk food consumption and sedentary leisure time activities, as well as relatively high prevalence of excess weight and dyslipidemia deserve more attention.
Now, at the beginning of the third millennium, NCDs are encompassing populations all around the world. By 2020, NCDs will be the cause seven out of every ten deaths in developing countries.[24] This fact has led to introducing significant methods and surveys to assess the prevalence of NCDs and their risk factors. Because of the importance of primordial/primary prevention of NCDs and their risk factors, especially in developing countries, the surveys of the CASPIAN study have been performed in Iran since 2003.[11,12,13,14,15] The current study is the fifth phase of these studies which was done with the objective of obtaining new data regarding NCDs and their risk factors among Iranian students and also to compare our findings with the former CASPIAN studies and with existing literature on this matter. This national study will give us new insight and data to take necessary actions and interventions required, regarding the improvement of health, as well as preventing chronic and noncommunicable diseases.
One of the factors investigated by the current study was the weight status of children and adolescents. Our findings revealed a double burden of nutritional disorders in terms of underweight and excess weight. The prevalence of overweight and obesity was higher in urban regions compared to rural regions, which may indicate having a high-calorie low-nutrient diet such as fast foods in urban areas. Although this shows that policies concerning reducing being overweight and obese seem to have worked out well, still more than 20% of participants reported to be either overweight or obese. Actually, many countries around the world are facing a marked increase in the prevalence of childhood obesity.[25,26,27,28,29,30,31] A multinational study from 34 countries surveyed 162,305 school-aged participants, aged 10–16 years. They showed that national overweight and obesity differ from one region to another. The rate of overweight and obesity among youth was more than 15% in North America, Britain, and Southern portion of Western Europe. The prevalence of being overweight varied from 5.1% to 25.4% in different countries.[30] Another multinational study among 21 countries reported that the prevalence of overweight in adolescents, aged 14–17 years varied from 8% in Slovenia to 23% in Greece.[32] In a study performed in Saudi Arabia among 13–18-year-old adolescents, the mean BMI was 27.43 kg/m2 and 44.6% of the adolescents were reported to be overweight .[33] In a review on childhood obesity in the Middle-East, Iran is considered among countries had the lowest rate of childhood obesity in this region. It was also stated that high prevalence of childhood obesity in the Middle-East should be considered by policymakers in the region to set up effective national and regional surveillance systems.[34] Although the prevalence of childhood obesity in Iran is not as high as some other Middle-Eastern countries, its escalating trend, notably in young children, is of great concern.[29] It also should be noted that the BMI of about 16% of participants was lower than normal limit. The coexistence of relatively high prevalence of underweight and overweight is consistent with other Middle-Eastern pediatric population.[35]
The dietary behaviors of participants revealed some healthy and some unhealthy habits. Change of dietary habits from traditional foods to Western foods and tendency to junk foods are frequently reported from developing countries.[36,37] In the current study, the consumption of was higher than the previous surveys of the CASPIAN-IV and III studies which was reported to be refined bread. In CASPIAN-III and IV studies, more than 50% of families reported to use hydrogenated solid fat, which is considerably lower (around 40%) in the current study which may indicate improving dietary habits. Moreover, 21% of participants reported that they always added salt to the table food, which is considerably lower than CASPIAN-III (33%) and CASPIAN-IV (31%) studies. These improvements in dietary habits might suggest the effectiveness of community-based interventions on increasing the public awareness regarding the adverse health effects of excess salt intake. Food composition in the diet of some Middle-Eastern population contains high amounts of sodium,[37] and reducing salt intake in various age groups including children and adolescents should be considered in health programs. Moreover, in the current survey, the use of fresh fruits was higher than the CASPIAN-IV study, even though the same could not be said about the daily use of fresh vegetables. Overall, it seems that families tend to use healthier diets compared to previous years.
By deploying of technology and rapid changes in leisure time activities, the time spent on sedentary entertainments has increased in children and adolescents of different populations.[30,38] In the current study, 7.4% of participants did not have any kind of physical activity, neither on weekdays nor the weekends, which was lower than CASPIAN-IV and much lower than CASPIAN-III studies. Most participants reported that they had once and twice a week physical activity (about 20% for each group). The pattern of physical activity was nearly similar in different age groups albeit high school students seemed to be less physically active compared to elementary students, which might be due to the fact that high school students have less free time considering the difficulty of their studies. About leisure time activities, most participants were in the 1 h/day groups for both doing homework and watching TV, whereas more than 60% of participants reported a negative history of playing computer games. In most countries, reduced physical activity and watching more TV were associated with being overweight and obese.[30,39,40] Hong et al.[41] indicated that physical activity lowered the risk of obesity and that obese had functional limitations in comparison with normal-weight children.
In our study, mean (SD) of sleep duration was 8.4 (1.3) h in weekdays and 9.6 (1.6) h in weekends, which could be considered sufficient. This is of high significance as children who have prolonged ST such as watching TV and playing computer games are more likely to have poor sleep and problem behaviors.[42]
Similar to previous surveys of the CASPIAN study, we found the considerably high prevalence of dyslipidemia, mainly low HDL-C and hypertriglyceridemia, i.e., the components of metabolic syndrome, in children and adolescents. In addition to genetic predisposition, this kind of dyslipidemia might be a result of unhealthy lifestyle habits.
Study limitations and strengths
The cross-sectional design of this study is its’ main limitation by which causality cannot be determined. The second limitation is that our survey questionnaire-based study. Despite these limitations, the major strengths of this survey are the large sample size and the nationwide design of the study, which ensures the representativeness of the findings and providing the possibility of making comparisons with previous phases of the CASPIAN study. Finally, a high-quality control of data collection was the other strength of this study.
CONCLUSIONS
Considering the importance of preventable lifestyle behaviors and risk factors resulting of as well as the preventable nature of NCDs, the CASPIAN-V study gives us new insight and data to improve public health and making national policies to do proper interventions for reducing NCDs. Furthermore, a society will be healthy only if the young has a healthy lifestyle and behavior.
Financial support and sponsorship
The study was conducted as a national school-based surveillance program.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Demaio AR, Kragelund Nielsen K, Pinkowski Tersbøl B, Kallestrup P, Meyrowitsch DW. Primary health care: A strategic framework for the prevention and control of chronic non-communicable disease. Glob Health Action. 2014;7:24504. doi: 10.3402/gha.v7.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray C. Global Burden of Disease and Risk Factors. Washington, DC: World Bank, Disease Control Priorities Project; 2006. [PubMed] [Google Scholar]
- 3.World Health Organization. Noncommunicable Diseases and Mental Health. Geneva: World Health Organization; 2011. [Google Scholar]
- 4.Magnusson RS. Global health governance and the challenge of chronic, non-communicable disease. J Law Med Ethics. 2010;38:490–507. doi: 10.1111/j.1748-720X.2010.00508.x. [DOI] [PubMed] [Google Scholar]
- 5.Juma PA, Mohamed SF, Wisdom J, Kyobutungi C, Oti S. Analysis of Non-communicable disease prevention policies in five Sub-Saharan African countries: Study protocol. Arch Public Health. 2016;74:25. doi: 10.1186/s13690-016-0137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maimela E, Alberts M, Modjadji SE, Choma SS, Dikotope SA, Ntuli TS, et al. The prevalence and determinants of chronic non-communicable disease risk factors amongst adults in the Dikgale health demographic and surveillance system (HDSS) site, Limpopo Province of South Africa. PLoS One. 2016;11:e0147926. doi: 10.1371/journal.pone.0147926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelishadi R, Ardalan G, Adeli K, Motaghian M, Majdzadeh R, Mahmood-Arabi MS, et al. Factor analysis of cardiovascular risk clustering in pediatric metabolic syndrome: CASPIAN study. Ann Nutr Metab. 2007;51:208–15. doi: 10.1159/000104139. [DOI] [PubMed] [Google Scholar]
- 8.Kelishadi R, Ardalan G, Gheiratmand R, Gouya MM, Razaghi EM, Delavari A, et al. Association of physical activity and dietary behaviours in relation to the body mass index in a national sample of Iranian children and adolescents: CASPIAN study. Bull World Health Organ. 2007;85:19–26. doi: 10.2471/BLT.06.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molnár D, Livingstone B. Physical activity in relation to overweight and obesity in children and adolescents. Eur J Pediatr. 2000;159(Suppl 1):S45–55. doi: 10.1007/pl00014365. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Global School-based student Health Survey (GSHS) Purpose and Methodology. Geneva: WHO; 2009. [Google Scholar]
- 11.Motlagh ME, Kelishadi R, Ardalan G, Gheiratmand R, Majdzadeh R, Heidarzadeh A CASPIAN Study Group. Rationale, methods andfirst results of the Iranian national programme for prevention of chronic diseases from childhood: CASPIAN study. East Mediterr Health J. 2009;15:302–14. [PubMed] [Google Scholar]
- 12.Kelishadi R, Heshmat R, Motlagh ME, Majdzadeh R, Keramatian K, Qorbani M, et al. Methodology and early findings of the third survey of CASPIAN study: A national school-based surveillance of students’ high risk behaviors. Int J Prev Med. 2012;3:394–401. [PMC free article] [PubMed] [Google Scholar]
- 13.Kelishadi R, Majdzadeh R, Motlagh ME, Heshmat R, Aminaee T, Ardalan G, et al. Development and evaluation of a questionnaire for assessment of determinants of weight disorders among children and adolescents: The CASPIAN-IV study. Int J Prev Med. 2012;3:699–705. [PMC free article] [PubMed] [Google Scholar]
- 14.Kelishadi R, Ardalan G, Qorbani M, Ataie-Jafari A, Bahreynian M, Taslimi M, et al. Methodology and early findings of the fourth survey of Childhood and Adolescence Surveillance and Prevention of Adult Non-communicable disease in Iran: The CASPIAN-IV study. Int J Prev Med. 2013;4:1451–60. [PMC free article] [PubMed] [Google Scholar]
- 15.Kelishadi R, Motlagh ME, Roomizadeh P, Abtahi SH, Qorbani M, Taslimi M, et al. First report on path analysis for cardiometabolic components in a nationally representative sample of pediatric population in the Middle East and North Africa (MENA): The CASPIAN-III study. Ann Nutr Metab. 2013;62:257–65. doi: 10.1159/000346489. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Expert committee on physical status. Physical Status: The use and interpretation of anthropometry. Geneva: WHO; 1995. [PubMed] [Google Scholar]
- 17.WHO Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 18.Knowles KM, Paiva LL, Sanchez SE, Revilla L, Lopez T, Yasuda MB, et al. Waist circumference, body mass index, and other measures of adiposity in predicting cardiovascular disease risk factors among peruvian adults. Int J Hypertens. 2011;2011:931402. doi: 10.4061/2011/931402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Academy of Pediatrics. National high blood pressure education program working group on high blood pressure in children and adolescents. Pediatrics. 2004;114(Suppl 2):iv. [Google Scholar]
- 20.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 22.Li C, Ford ES, Mokdad AH, Cook S. Recent trends in waist circumference and waist-height ratio among US children and adolescents. Pediatrics. 2006;118:e1390–8. doi: 10.1542/peds.2006-1062. [DOI] [PubMed] [Google Scholar]
- 23.Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–61. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 24.Boutayeb A. The double burden of communicable and non-communicable diseases in developing countries. Trans R Soc Trop Med Hyg. 2006;100:191–9. doi: 10.1016/j.trstmh.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Tremblay MS, Katzmarzyk PT, Willms JD. Temporal trends in overweight and obesity in Canada, 1981-1996. Int J Obes Relat Metab Disord. 2002;26:538–43. [PubMed] [Google Scholar]
- 26.Booth ML, Chey T, Wake M, Norton K, Hesketh K, Dollman J, et al. Change in the prevalence of overweight and obesity among young Australians, 1969-1997. Am J Clin Nutr. 2003;77:29–36. doi: 10.1093/ajcn/77.1.29. [DOI] [PubMed] [Google Scholar]
- 27.Kelishadi R. Childhood overweight, obesity, and the metabolic syndrome in developing countries. Epidemiol Rev. 2007;29:62–76. doi: 10.1093/epirev/mxm003. [DOI] [PubMed] [Google Scholar]
- 28.de Onis M, Blössner M. Prevalence and trends of overweight among preschool children in developing countries. Am J Clin Nutr. 2000;72:1032–9. doi: 10.1093/ajcn/72.4.1032. [DOI] [PubMed] [Google Scholar]
- 29.Kelishadi R, Haghdoost AA, Sadeghirad B, Khajehkazemi R. Trend in the prevalence of obesity and overweight among Iranian children and adolescents: A systematic review and meta-analysis. Nutrition. 2014;30:393–400. doi: 10.1016/j.nut.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Janssen I, Katzmarzyk PT, Boyce WF, Vereecken C, Mulvihill C, Roberts C, et al. Comparison of overweight and obesity prevalence in school-aged youth from 34 countries and their relationships with physical activity and dietary patterns. Obes Rev. 2005;6:123–32. doi: 10.1111/j.1467-789X.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 31.Strauss RS, Pollack HA. Epidemic increase in childhood overweight, 1986-1998. JAMA. 2001;286:2845–8. doi: 10.1001/jama.286.22.2845. [DOI] [PubMed] [Google Scholar]
- 32.Lobstein T, Frelut ML. Prevalence of overweight among children in Europe. Obes Rev. 2003;4:195–200. doi: 10.1046/j.1467-789x.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 33.Washi SA, Ageib MB. Poor diet quality and food habits are related to impaired nutritional status in 13-to 18-year-old adolescents in Jeddah. Nutr Res. 2010;30:527–34. doi: 10.1016/j.nutres.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Mirmiran P, Sherafat-Kazemzadeh R, Jalali-Farahani S, Azizi F. Childhood obesity in the Middle East: A review. East Mediterr Health J. 2010;16:1009. [PubMed] [Google Scholar]
- 35.Galal O. Nutrition-related health patterns in the Middle East. Asia Pac J Clin Nutr. 2003;12:337–43. [PubMed] [Google Scholar]
- 36.Aounallah-Skhiri H, Traissac P, El Ati J, Eymard-Duvernay S, Landais E, Achour N, et al. Nutrition transition among adolescents of a South-Mediterranean country: Dietary patterns, association with socio-economic factors, overweight and blood pressure. A cross-sectional study in Tunisia. Nutr J. 2011;10:1. doi: 10.1186/1475-2891-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musaiger AO, Bader Z, Al-Roomi K, D'souza R. Dietary and lifestyle habits amongst adolescents in Bahrain. Food Nutr Res. 2011:55. doi: 10.3402/fnr.v55i0.7122. doi: 10.3402/fnr.v55i0.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annu Rev Public Health. 2001;22:309–35. doi: 10.1146/annurev.publhealth.22.1.309. [DOI] [PubMed] [Google Scholar]
- 39.Robinson TN. Reducing children's television viewing to prevent obesity: A randomized controlled trial. JAMA. 1999;282:1561–7. doi: 10.1001/jama.282.16.1561. [DOI] [PubMed] [Google Scholar]
- 40.Robinson TN. Television viewing and childhood obesity. Pediatr Clin North Am. 2001;48:1017–25. doi: 10.1016/s0031-3955(05)70354-0. [DOI] [PubMed] [Google Scholar]
- 41.Hong I, Coker-Bolt P, Anderson KR, Lee D, Velozo CA. Relationship between physical activity and overweight and obesity in children: Findings from the 2012 National Health and Nutrition Examination Survey National Youth Fitness Survey. Am J Occup Ther. 2016;70(7005180060):p1–8. doi: 10.5014/ajot.2016.021212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parent J, Sanders W, Forehand R. Youth screen time and behavioral health problems: The role of sleep duration and disturbances. J Dev Behav Pediatr. 2016;37:277–84. doi: 10.1097/DBP.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]


