Abstract
Diabetes and cancer are a major global public health problem. Plant-derived agents with undesirable side-effects were required. This study aimed to evaluate antidiabetic and anticancer activities of the ethanolic leaf extract of Mangifera indica cv. Okrong and its active phytochemical compound, mangiferin. Antidiabetic activities against yeast α-glucosidase and rat intestinal α-glucosidase were determined using 1 mM of p-nitro phenyl-α-D-glucopyranoside as substrate. Inhibitory activity against porcine pancreatic α-amylase was performed using 1 mM of 2-chloro-4 nitrophenol-α-D-maltotroside-3 as substrate. Nitrophenol product was spectrophotometrically measured at 405 nm. Anticancer activity was evaluated against five human cancer cell lines compared to two human normal cell lines using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Mango leaf extract and mangiferin exhibited dose-dependent inhibition against yeast α-glucosidase with the IC50 of 0.0503 and 0.5813 mg/ml, respectively, against rat α-glucosidase with the IC50 of 1.4528 and 0.4333 mg/ml, respectively, compared to acarbose with the IC50 of 11.9285 and 0.4493 mg/ml, respectively. For anticancer activity, mango leaf extract, at ≥200 μg/ml showed cytotoxic potential against all tested cancer cell lines. In conclusion, mango leaf possessed antidiabetic and anticancer potential in vitro.
Key words: Anticancer, antidiabetic, Mangifera indica L., mangiferin
INTRODUCTION
Diabetes mellitus is a chronic metabolic disorder characterized by an uncontrolled increase in blood glucose level.[1,2,3] Key enzymes for hydrolysis of carbohydrates are α-amylase, which is participating in hydrolysis of polysaccharides and oligosaccharides and α-glucosidase which further hydrolysis di-and tri-saccharides to glucose and other monosaccharides.[4] Cancer is a group of diseases differentiated by the uncontrolled growth and spread of abnormal cells. There are many occurrences common cancer types, that is, breast, lung, liver and colorectal cancers.[5] Currently, chemical agents such as acarbose and doxorubicin are available for treatment of diabetes and cancer, respectively. However, all of these treatments are related with undesirable side effect[1,2,3,4,6,7] leading to increase in ethnobotanical use of medicinal plants which may be safer and less destructive to human body.
Mango (Mangifera indica L.), a long living large evergreen tree, belong to the Anacardiaceae family.[8] It possesses pharmacological effects, that is, antidiabetic, antioxidant, antimicrobial, anticancer, and anti-inflammatory properties.[9] Mango is a rich source of various polyphenolic compounds, especially mangiferin [Figure 1], which is the major component that can be detected in all parts of the mango. This compound is a xanthone derivative that referred as super antioxidant. It also has been found for pharmacological effects including antioxidant, radioprotective, antiallergic, antidiabetic, anticancer, antimicrobial, immunomodulatory, and anti-inflammatory activities.[10,11] This study aimed to evaluate antidiabetic activity of mango leaf extract and mangiferin on in vitro inhibition of α-amylase and α-glucosidase enzymes and to demonstrate inhibitory activity against selected cancer cell lines.
Figure 1.

Chemical structure of mangiferin (1,3,6,7-Tetrahydroxyxanthone C2-ß-D-glucoside)
MATERIALS AND METHODS
Materials and chemicals
M. indica “Okrong” leaves were collected in Thailand. They were authenticated by Assoc. Prof. Dr. Nijsiri Ruangrungsi. Voucher specimens were deposited at College of Public Health Sciences, Chulalongkorn University, Thailand. Leaf samples were washed with water and dried in hot air oven at 50°C. The dried leaves were pulverized and exhaustively extracted with ethanol by Soxhlet apparatus. The extract was filtered through Whatman number 1 filter paper and evaporated to dryness in vacuo. The yield was recorded and the extract was stored at −20°C.
Antidiabetic activities
Inhibition of yeast alpha-glucosidase activity
The enzyme inhibition activity against yeast α-glucosidase (Sigma-Aldrich, USA) were determined using 1 mM of p-nitrophenyl-α-D-glucopyranoside (PNPG) as substrate according to Wan et al.[12] with minor modifications. In 96 well plate, 30 μl of enzyme solution (0.5 U/ml), 30 μl of 0.1 M sodium phosphate buffer (pH 6.9) and 30 μl of tested inhibitors (mango, mangiferin [MIRA, China], or acarbose [Sigma-Aldrich, USA]) in dimethyl sulfoxide (DMSO) were mixed and incubated at 37°C for 10 min. Next, 30 μl of substrate were added and incubated again at 37°C for 20 min. After incubation, 80 μL of 0.2 μM Na2 CO3 was added to stop the reaction. The absorbance was measured at 405 nm using Anthos Zenyth 200 RT microplate reader (Biochrom, England). All tested inhibitors were analyzed in triplicate. The percent inhibition was calculated by the following formula:
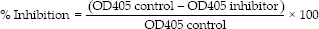
Inhibition of rat alpha-glucosidase activity
The enzyme inhibition activity against intestinal acetone powders from rat (Sigma-Aldrich, USA) were determined using 1 mM of PNPG as substrate, according to Lordan et al.[13] and Hemalatha et al.[14] with minor modifications. Intestinal acetone powders from rat (30 mg/ml) in 0.1 M sodium phosphate buffer (pH 6.9) was sonicated for 20 min. The suspension was centrifuged at 3500 rpm for 30 min to remove particulate matter. In 96 well plate, 50 μl of tested inhibitors in DMSO, 100 μl of substrate and 50 μl of enzyme solution (0.5 U/ml) were mixed and incubated at 37°C for 30 min. The absorbance was measured at 405 nm using microplate reader. All tested inhibitors were analyzed in triplicate. The percent inhibition was calculated as aforementioned formula.
Inhibition of pancreatic alpha-amylase activity
The enzyme inhibition activity against α-amylase from porcine pancreas (Sigma-Aldrich, USA) were determined using 1 mM of 2-chloro-4 nitrophenol-α-D-maltotroside-3 as substrate, following a method as described previously by Yonemoto et al.[15] with modifications. In 96 well plate, 30 μl of enzyme solution (25 U/ml) and 30 μl of tested inhibitors in DMSO were mixed and preincubated at 37°C for 10 min. Then, 30 μl of substrate were added and incubated again at 37°C for 20 min. The absorbance was measured at 405 nm using microplate reader. All tests were analyzed in triplicate. The percent inhibition was calculated as aforementioned formula.
Anticancer activity
Cell cultures
The human cancer cell lines; ductal carcinoma (BT474, ATCC HTB20), bronchogenic carcinoma (Chago K-1, ATCC HTB-168TB), liver hepatoblastoma (Hep-G2, ATCC HB8065), gastric carcinoma (Kato-III, ATCC HTB103), and colon adenocarcinoma (SW 620, ATCC CCL227); The human normal cell lines; skin fibroblast (CCD-986SK, ATCC CRL1947) and lung fibroblast (WI-38 VA-13 subline 2RA, ATCC CLS 300421) were obtained from the Institute of Biotechnology and Genetic Engineering, Chulalongkorn University. BT474, Chago K-1, Hep-G2, Kato-III, SW 620, and WI-38 cell lines were cultured in RPMI 1640 medium containing 5% fetal calf serum and CCD-986SK cell line was cultured in Dulbecco's modified Eagle's medium. They were incubated at 37°C in a 5% (v/v) CO2 atmosphere.
Colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
Cell viability using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (MTT; Sigma-Aldrich, USA) were determined as described previously by Mosmann[16] with minor modifications. In 96 well plate, 198 μl of 5,000 cells in culture medium were added and incubated at 37°C in a 5% (v/v) CO2 atmosphere for 24 h. Then, 2 μl of tested inhibitors (mango extract, mangiferin, or doxorubicin), or 2 μl of negative control (DMSO for mango extract and mangiferin; or water for doxorubicin) were added and incubated at 37°C for 48 h. Ten microliter of MTT solution (5 mg/ml) were added into each well and incubated at 37°C for 4 h. The media were removed. A mixture of 150 μl of DMSO and 25 μl of glycine (0.1 mol/l) were added into each well and mixed thoroughly to dissolve the formazan crystals. The absorbance was measured at 540 nm using microplate reader. All tests were analyzed in quadruplicate. The percent survival was calculated as follows:

RESULTS
Antidiabetic activities
Antidiabetic activities of mango leaf extract, mangiferin, and acarbose showed a dose-response relationship [Figure 2]. For yeast α-glucosidase, mango leaf extract showed the greatest inhibition with the IC50 of 0.05 mg/ml [Table 1]. Mango leaf extract, mangiferin, and acarbose revealed 86.6, 93.3, and 73.9% inhibition at the concentration of 0.156, 2.5, and 40 mg/ml, respectively [Figure 2]. For rat α-glucosidase, mangiferin showed the greatest inhibition with the IC50 of 0.43 mg/ml [Table 1]. They revealed 86.0, 92.6, and 58.7% inhibition at the concentration of 2.5, 1.25, and 1.25 mg/ml, respectively [Figure 1]. For pancreatic α-amylase, mangiferin also showed the most inhibition with the IC50 of 1.0485 mg/ml [Table 1]. They revealed 97.2, 95.6, and 73.9% inhibition at the concentration of 10, 5 and 0.156 mg/ml, respectively. Acarbose was used as a positive control in this study.
Figure 2.
Yeast α-glucosidase, rat α-glucosidase and pancreatic α-amylase inhibitions of mango leaf extract, mangiferin, and acarbose at different concentrations
Table 1.
Antidiabetic activities of mango leaf extract, mangiferin, and acarbose*

Anticancer activity
Mango leaf extract, at 200 μg/ml, showed cytotoxicity against all tested cancer cell lines. Mangiferin did not significantly affect % survival of tested cancer cells [Figure 3]. Doxorubicin was used as a positive control; normal skin fibroblast (CCD) and normal lung fibroblast (Wi-38) were comparable cell lines in this study. Mango leaf extract, at high dose, also showed toxicity on lung fibroblast. On the contrary, the extract increased %survival of skin fibroblast. At high dose, mangiferin tended to increase the survival of skin and lung fibroblasts [Figure 3]. The IC50 for cytotoxic activities of the extract, mangiferin and doxorubicin are shown in Table 2.
Figure 3.

Inhibition of cancer cell growth by mango leaf extract, mangiferin and doxorubicin
Table 2.
Cytotoxic activities of mango leaf extract, mangiferin, and doxorubicin

DISCUSSION
α-Glucosidase may be largely divided into two types due to the difference in primary structure, Types I (yeast) and II (mammals).[17] Previous studies reported that various foods were active for yeast α-glucosidase, they had the potential to inhibit yeast α-glucosidase more than rat α-glucosidase and had inhibited those α-glucosidase more than α-amylase. On the contrary, acarbose which was anti-diabetic drug, had more potential to inhibit α-amylase than α-glucosidase and had slightly or no ability to inhibit yeast α-glucosidase relative to rat α-glucosidase.[6,17] The similar results were found that both mango peels and mango seeds extracts had potential to inhibit α-glucosidase more than α-amylase with the IC50 of 3.5, 4.0 and 0.34, 0.71 μg/ml, respectively.[1,6] Their leaf extract inhibited α-glucosidase with the IC50 of 59.0 μg/ml. They were active for yeast α-glucosidase, these dose-dependent inhibitory activity were significantly higher than acarbose.[3,6] Different solvent extractions gave different inhibited potency. As an example, mango stem barks ethanolic extract showed the maximum inhibitory effects with the IC50 of 37.86 μg/ml; hexane extract showed moderate inhibitory effects with the IC50 of 114.13 μg/ml; petroleum ether, chloroform and aqueous showed no inhibitory effects on alpha-amylase activities.[18] However, the low IC50 value may be because the occurrence of other phenolic acids, flavonoids and carotenoids.[1] Previous study compared antidiabetic potential of mature and tender mango leaves aqueous methanolic extracts. Mature leaves extract inhibited α-glucosidase and α-amylase with the IC50 of 21.03 and 35.73 μg/ml, respectively due to their higher saponin, polyphenol, flavonoid contents. Tender leaves extract inhibited α-glucosidase and α-amylase with the IC50 of 27.16 and 22.01 μg/ml, respectively. They concluded that mango mature leaf had more potential to inhibit α-glucosidase; whereas, mango tender leaf had potential to inhibit α-amylase when compared to each other.[2] Mangiferin had more potent to inhibit α-glucosidase than α-amylase with the IC50 of 41.88 and 74.35 μg/ml, respectively.[19] In addition, many flavonoids were weakly inhibiting rat α-glucosidase. Our findings, mango leaf extract had strong potential to inhibit yeast α-glucosidase when compared to acarbose and mangiferin. It had the potential to inhibit α-glucosidase more than α-amylase. Mangiferin had strong potential for rat α-glucosidase when compared to acarbose and mango leaves extract. It also had more potent to inhibit α-glucosidase than α-amylase. Acarbose had strong potential to inhibit α-amylase compared to α-glucosidase.
MTT assay, an accurate and uncomplicated method, provides a useful quantitative data on the antiproliferative and anticancer potential of natural extracts.[20,21] Whole fruit, fruit juice, or fruit peel extracts from several mango cultivars showed toxic effect on cancer cell lines, including breast (MDA-MB-231 and MCF 7), cervix (HeLa cells), colon (SW-480 and SW-620), leukemia (Molt-4), lung (A-549), prostate (LnCap), and renal (786-0) cancer cell line. They had low cytotoxicity against normal cell lines, including breast (MCF-10A) and colon (CCD-18Co) normal cell lines, and no toxicity effect on lung fibroblast normal cell line (CCD-25 Lu).[7,20,21,22] In this study, mango leaf was used. The leaf extract at high dose (IC50 >200 μg/ml) possessed cytotoxic activities against all tested cancer cell lines (ductal carcinoma, bronchogenic carcinoma, liver hepatoblastoma, gastric carcinoma, and colon adenocarcinoma). However, at that high dose, the toxicity on lung fibroblast normal cell line was also shown; whereas there was no toxic effect, especially enhancing effect toward skin fibroblast normal cell line. The antiproliferative potential of mango extracts might be due to their bioactive compounds synergistic actions.[21] Mangiferin is one of the natural xanthone, which was extracted from mango tree. It was shown to inhibit cancer cell lines, including liver, breast, prostate, colon and nasopharyngeal cancer cell lines.[23,24] From the findings, mangiferin did not show significantly toxicity against all tested cancer cell lines. The previous study also reported that only high dose of mangiferin inhibited tested cancer cell lines.[23] This study found that mangiferin also had the potential on increasing the survival of skin and lung normal cell lines.
CONCLUSION
Mango leaf extract and its active compound, mangiferin showed in vitro inhibitory potential on key enzymes involving glucose metabolism, that is, α-amylase and α-glucosidase. Mango leaf extract, ≥200 μg/ml, showed cytotoxicity against tested cancer cell lines. Both mango leaf extract and mangiferin increased % survival of skin fibroblast.
Financial support and sponsorship
Chulalongkorn University fund (Ratchadaphiseksomphot Endowment Fund) was GCUGR1125592015D, and Mae Fah Luang University scholarship was MFU002/2555.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gondi M, Prasada Rao UJ. Ethanol extract of mango (Mangifera indica L.) peel inhibits a-amylase and a-glucosidase activities, and ameliorates diabetes related biochemical parameters in streptozotocin (STZ)-induced diabetic rats. J Food Sci Technol. 2015;52:7883–93. doi: 10.1007/s13197-015-1963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhuvaneshwari J, Khanam S, Devi K. In-vitro enzyme inhibition studies for antidiabetic activity of mature and tender leaves of Mangifera indica var. Totapuri. Res Rev J Microbiol Biotechnol. 2014;3:36–41. [Google Scholar]
- 3.Andrew O, Yusuf S, Jangabe LM, Lawal BS, Adamu AA. α-Glucosidase inhibitory potential of selected antidiabetic plants used in North-Western Nigeria. JMPR. 2013;7:2010–8. [Google Scholar]
- 4.Ercan P, El SN. Inhibitory effects of chickpea and Tribulus terrestris on lipase, a-amylase and a-glucosidase. Food Chem. 2016;205:163–9. doi: 10.1016/j.foodchem.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Facts and Figures 2016. Atlanta: American Cancer Society; 2016. American Cancer Society. [Google Scholar]
- 6.Irondi EA, Oboh G, Akindahunsi AA, Boligon AA, Athayde ML. Phenolic composition and inhibitory activity of Mangifera indica and Mucuna urens seeds extracts against key enzymes linked to the pathology and complications of type 2 diabetes. Asian Pac J Trop Biomed. 2014;4:903–10. [Google Scholar]
- 7.Abdullah AS, Mohammed AS, Abdullah R, Mirghani ME, Al-Qubaisi M. Cytotoxic effects of Mangifera indica L. kernel extract on human breast cancer (MCF-7 and MDA-MB-231 cell lines) and bioactive constituents in the crude extract. BMC Complement Altern Med. 2014;14:199. doi: 10.1186/1472-6882-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prakash O, Khan R. A Tryst with Mango: Retrospect, Aspects, Prospects. New Delhi: APH Publishing; 2005. [Google Scholar]
- 9.Shah KA, Patel MB, Patel RJ, Parmar PK. Mangifera indica (mango) Pharmacogn Rev. 2010;4:42–8. doi: 10.4103/0973-7847.65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Telang M, Dhulap S, Mandhare A, Hirwani R. Therapeutic and cosmetic applications of mangiferin: A patent review. Expert Opin Ther Pat. 2013;23:1561–80. doi: 10.1517/13543776.2013.836182. [DOI] [PubMed] [Google Scholar]
- 11.Sekar M. Molecules of Interest – Mangiferin – A review. Annu Res Rev Biol. 2015;5:307–20. [Google Scholar]
- 12.Wan LS, Min QX, Wang YL, Yue YD, Chen JC. Xanthone glycoside constituents of Swertia kouitchensis with a-glucosidase inhibitory activity. J Nat Prod. 2013;76:1248–53. doi: 10.1021/np400082g. [DOI] [PubMed] [Google Scholar]
- 13.Lordan S, Smyth TJ, Soler-Vila A, Stanton C, Ross RP. The a-amylase and a-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013;141:2170–6. doi: 10.1016/j.foodchem.2013.04.123. [DOI] [PubMed] [Google Scholar]
- 14.Hemalatha P, Bomzan DP, Sathyendra Rao BV, Sreerama YN. Distribution of phenolic antioxidants in whole and milled fractions of quinoa and their inhibitory effects on a-amylase and a-glucosidase activities. Food Chem. 2016;199:330–8. doi: 10.1016/j.foodchem.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Yonemoto R, Shimada M, Gunawan-Puteri MD, Kato E, Kawabata J. A-Amylase inhibitory triterpene from Abrus precatorius leaves. J Agric Food Chem. 2014;62:8411–4. doi: 10.1021/jf502667z. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;16(65):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Oki T, Matsui T, Osajima Y. Inhibitory effect of alpha-glucosidase inhibitors varies according to its origin. J Agric Food Chem. 1999;47:550–3. doi: 10.1021/jf980788t. [DOI] [PubMed] [Google Scholar]
- 18.Dineshkumar B, Mitra A, Manjunatha M. A comparative study of alpha amylase inhibitory activities of common anti-diabetic plants at Kharagpur 1 block. IJGP. 2010;4:115–21. [Google Scholar]
- 19.Dineshkumar B, Mitra A, Manjunatha M. Studies on the anti-diabetic and hypolipidemic potentials of mangiferin (Xanthone Glucoside) in streptozotocin-induced type 1 and type 2 diabetic model rats. IJAPS. 2010;1:75–85. [Google Scholar]
- 20.Noratto GD, Bertoldi MC, Krenek K, Talcott ST, Stringheta PC, Mertens-Talcott SU. Anticarcinogenic effects of polyphenolics from mango (Mangifera indica) varieties. J Agric Food Chem. 2010;58:4104–12. doi: 10.1021/jf903161g. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Moon JY, Kim H, Lee DS, Cho M, Choi HK, et al. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010;121:429–36. [Google Scholar]
- 22.Parvez GM. Pharmacological activities of mango (Mangifera Indica): A review. J Pharmacogn Phytochem. 2016;5:1–7. [Google Scholar]
- 23.Li H, Huang J, Yang B, Xiang T, Yin X, Peng W, et al. Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases, epithelial to mesenchymal transition, and ß-catenin signaling pathway. Toxicol Appl Pharmacol. 2013;272:180–90. doi: 10.1016/j.taap.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Ma H, Yang L, Li P. Mangiferin inhibition of proliferation and induction of apoptosis in human prostate cancer cells is correlated with downregulation of B-cell lymphoma-2 and upregulation of microRNA-182. Oncol Lett. 2016;11:817–22. doi: 10.3892/ol.2015.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]



