Abstract
The heat shock response controls levels of chaperones and proteases to ensure a proper cellular environment for protein folding. In Escherichia coli, this response is mediated by the bacterial-specific transcription factor, σ32. The DnaK chaperone machine regulates both the amount and activity of σ32, thereby coupling σ32 function to the cellular protein folding state. In this manuscript, we analyze the ability of other major chaperones in E. coli to regulate σ32, and we demonstrate that the GroEL/S chaperonin is an additional regulator of σ32. We show that increasing the level of GroEL/S leads to a decrease in σ32 activity in vivo and this effect can be eliminated by co-overexpression of a GroEL/S-specific substrate. We also show that depletion of GroEL/S in vivo leads to up-regulation of σ32 by increasing the level of σ32. In addition, we show that changing the levels of GroEL/S during stress conditions leads to measurable changes in the heat shock response. Using purified proteins, we show that that GroEL binds to σ32 and decreases σ32-dependent transcription in vitro, suggesting that this regulation is direct. We discuss why using a chaperone network to regulate σ32 results in a more sensitive and accurate detection of the protein folding environment.
Keywords: sigma32 (σ32), GroEL, chaperone, heat shock response, sigma factor (σ factor), DnaK
Cellular survival depends on maintaining an appropriate environment for protein folding. Chaperones, which assist protein folding, and proteases, which remove misfolded proteins, are among the cellular factors that influence protein folding in vivo (Horwich et al. 1999; Bukau et al. 2000). Environmental factors, including temperature and organic solvents, also influence the internal folding milieu (Hartl 1996). In order to maintain homeostasis for protein folding, cells tightly regulate the expression of chaperones and proteases to compensate for environmental perturbations (Morimoto 1998). This response, called the “heat shock response” because it was identified in relation to heat stress, leads to the induction of the almost universally conserved “heat shock genes”, which encode chaperones, proteases, and other stress-related proteins (Herman and Gross 2000). Heat shock proteins are not only important during stress conditions; many are among the most abundant proteins in the cell in all conditions because they have a general role in protein folding (Nollen and Morimoto 2002).
A complex control system regulates expression of heat shock genes, ensuring that they can respond even to small changes in intracellular folding. In Escherichia coli, regulation of heat shock genes is mediated by rpoH, which encodes the σ32 transcription factor (Connolly et al. 1999; Arsene et al. 2000). σ factors are bacterial-specific initiation factors that recruit RNA polymerase to particular classes of promoters (Gruber and Gross 2003). σ32 is regulated at multiple levels that use inputs both directly from some stresses and also from the protein folding state of the cell. Increases in temperature have been shown to directly increase translation of rpoH mRNA by destabilizing an RNA structural element overlapping the translation start point (Morita et al. 1999a,b). The protein folding state of the cell regulates both the degradation and activity of σ32. In response to an increased need for protein folding agents (e.g., immediately after temperature upshift), σ32 is transiently stabilized (Straus et al. 1987). In response to decreased need for protein folding agents (e.g., during the recovery phase following temperature upshift or immediately after temperature downshift), the activity of σ32 decreases (Straus et al. 1989). Additionally, cells use these feedback systems to constantly monitor their folding status during growth under steady-state conditions so that the cellular folding environment remains optimal.
The DnaK chaperone machine, consisting of DnaK (Hsp70 homolog), DnaJ (Hsp40 homolog), and GrpE (nucleotide exchange factor), is implicated in regulation of σ32 activity and σ32 degradation (Tilly et al. 1983, 1989). Mutations in dnaK, dnaJ, and grpE all induce the heat shock response through increases in σ32 stability and activity (Straus et al. 1990). Also, DnaK overexpression and depletion in vivo leads to changes in σ32 activity and degradation (Tomoyasu et al. 1998). Activity control has been replicated in vitro, as DnaK binds directly to σ32 and inhibits σ32-dependent transcription in vitro (Gamer et al. 1992, 1996; Liberek et al. 1992). However, the role of DnaK in the regulation of σ32 degradation is less clear because it has not been possible to replicate this control in vitro.
The current model for regulation of σ32 by the DnaK chaperone machine has been referred to as the “unfolded protein titration model” (Straus et al. 1990; Craig and Gross 1991; Bukau 1993). In this model, unfolded proteins and σ32 compete for binding to DnaK, with the DnaK–σ32 complex inactive in transcription. In addition, DnaK binding to σ32 facilitates σ32 degradation in an unknown way. When unfolded proteins are low relative to DnaK, the inactive DnaK–σ32 complex predominates, σ32 is rapidly degraded, and heat shock gene expression is low. However, when unfolded proteins are high relative to DnaK, DnaK is titrated away from σ32; the active, stable, chaperone-free state of σ32 predominates; and heat shock genes are induced.
A chaperone network controls protein folding in the cell (Buchberger et al. 1996); therefore, it would be surprising if σ32 sensed the folding state of the cell by only sampling a single chaperone. Good candidates for additional regulators of σ32 are GroEL/S (Hsp60/10 homologs) and HtpG (Hsp90 homolog) because, like DnaK, they are some of the most abundant chaperones in the cell, and they are highly conserved through evolution (Fink 1999). However, previous experiments with GroEL/S and HtpG have not provided evidence that they are involved in regulation of σ32. First, mutations in groEL and groES that are defective in bacteriophage growth are not altered in heat shock gene regulation (Tilly et al. 1983; Straus et al. 1990). Although those mutations were assumed to be generally defective in GroEL/S function, more recent work has shown that GroEL/S has multiple classes of substrates, and mutations in groELS can have differential effects on these classes (Wang et al. 2002). It is important to note that these mutations in GroEL/S did not lead to a defect in cell growth, even though GroEL/S is essential; therefore, we now know that the lack of effect of these mutations on heat shock gene expression does not resolve this question. An additional study examining the long-term effects of GroEL/S overexpression failed to provide evidence that GroEL/S regulates σ32 activity (Kanemori et al. 1994). By examining long-term GroEL overexpression, the study does not definitively rule out GroEL/S as a regulator, because it is possible that other mechanisms can compensate for long-term overexpression. Previous studies with HtpG have given evidence that it can bind to σ32 in extracts, yet no experiments have been performed to determine its role in σ32 regulation (Nadeau et al. 1993).
In this report, we investigate the role of these two chaperones, GroEL/S and HtpG, in regulation of the heat shock response. We present in vivo and in vitro evidence supporting the idea that GroEL/S, but not HtpG, is used to sense the protein folding state of the cell. We further show that GroEL/S is used together with the DnaK chaperone machine to regulate heat shock gene transcription, and we conclude that a cellular chaperone network regulates the activity of the σ32 heat shock factor.
Results
GroEL/S and σ32 show a genetic interaction
Our initial evidence that GroEL/S was a regulator of the heat shock response came from identification of a genetic interaction between σ32 and GroEL/S. Overexpression of σ32 is toxic, resulting in a dramatic reduction in efficiency of plating (EOP) on minimal medium. Toxicity can be alleviated by simultaneously overexpressing a negative regulator of σ32 (Herman et al. 1995b). We found that overexpression of σ32 alone resulted in an EOP of 1 × 10-4, whereas simultaneous overexpression of GroEL/S and σ32 restored the EOP to ∼1. Therefore, GroEL/S can alleviate the toxicity of σ32 overexpression, suggesting that it is a negative regulator of σ32.
Overexpression of GroEL/S decreases σ32-dependent transcription
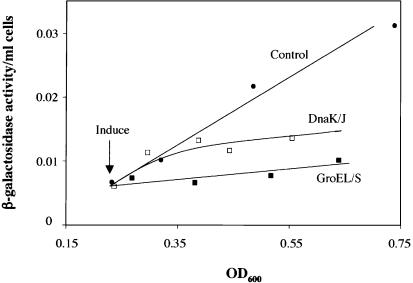
We asked whether the genetic interaction between GroEL/S and σ32 was due to the ability of GroEL/S to negatively regulate σ32-dependent transcription. In this experiment, we compared σ32-dependent transcription in cells with and without overexpression of GroEL/S, using a plasmid with the groELS operon under the control of an inducible Para promoter (Fig. 1). We assayed σ32-dependent transcription by measuring the accumulation of β-galactosidase from a chromosomal, σ32-dependent lacZ transcriptional reporter as a function of cell growth. This “differential rate of β-galactosidase synthesis” measures how σ32 activity changes in response to a signal over time, with the slope of the line reflecting the protein synthesis rate of β-galactosidase and therefore σ32 activity. Our results indicate that GroEL/S overexpression significantly decreases σ32-dependent transcription, thereby confirming the idea that GroEL/S negatively regulates σ32. Next, we directly compared GroEL/S inhibition with DnaK/J inhibition. When we overexpressed DnaK/J from the same plasmid vector used for GroEL/S overexpression, we found that the eventual extent of inhibition of σ32-dependent lacZ reporter expression was approximately the same as that mediated by GroEL/S (Fig. 1). However, whereas GroEL/S overexpression exhibited an immediate inhibitory effect, inhibition by DnaK/J was manifest more slowly. Delayed inhibition was noticed in multiple experiments (data not shown). This effect was not due to a different extent of overexpression of the two proteins. Western blot analysis indicated that both proteins were overexpressed about 10-fold after 1 h of induction (data not shown). We also tested whether HtpG participated in regulation of σ32. In contrast to GroEL/S and DnaK/J, overexpression of HtpG had no effect on σ32-dependent lacZ reporter expression (data not shown), indicating that HtpG, at least alone, does not behave as a negative regulator of σ32 in vivo.
Figure 1.
Effects of GroEL/S and DnaK/J overexpression on σ32-dependent transcription. An exponential phase culture of strain 594 with a σ32-dependent lacZ reporter and carrying either a plasmid able to overexpress GroEL/S (pGro7) or a plasmid able to overexpress DnaK/J (pKJE7) was grown at 30°C with and without 0.2% arabinose to induce GroEL/S or DnaK/J overexpression. A standard differential rate of synthesis plot is shown. The uninduced (control) strains gave identical results; therefore, for simplicity, we have included the data for only one control strain. This experiment and every other differential rate of synthesis experiment was performed at least three times with similar results.
Our results indicating that GroEL/S overexpression decreased σ32 activity differed from previous results showing no effect after long-term GroEL/S overexpression (Kanemori et al. 1994), leading us to wonder whether cells can adapt to long-term GroEL/S overexpression. To determine the effects of prolonged GroEL/S overexpression, we repeated our previous experiments and re-examined the same cultures the next day. Our results confirmed that σ32 activity is not repressed after long-term overexpression of GroEL/S (data not shown), indicating that the cell possesses a mechanism to adapt to long-term GroEL/S overexpression.
Overexpression of GroEL decreases the activity of σ32
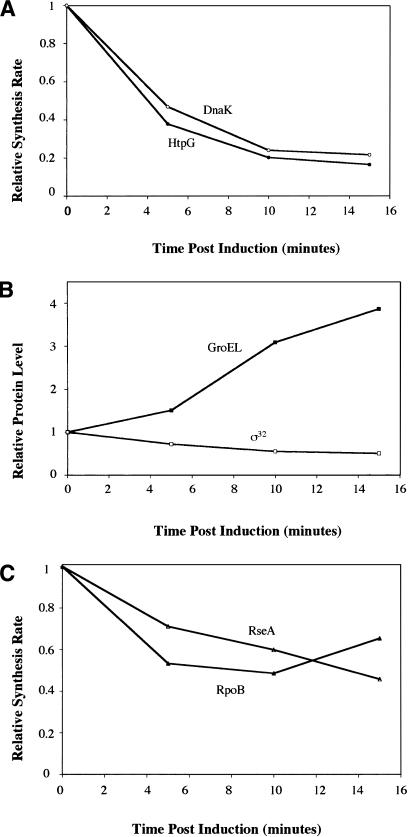
The experiments described earlier indicate that overexpression of GroEL/S results in decreased transcription from σ32-dependent promoters in vivo. This decrease could result from a change in the amount or activity of σ32, or from a combination of these two effects. To investigate this issue, we examined the synthesis rate of σ32-dependent proteins, which gives us an instantaneous indication of σ32 function. We also determined protein levels using Western analysis, in order to correlate σ32-dependent protein synthesis with GroEL and σ32 levels. We overexpressed the groELS operon on a plasmid using an inducible Ptet promoter and analyzed the synthesis of two σ32-dependent proteins, HtpG and DnaK (Fig. 2A; Zhou et al. 1988). Synthesis of both HtpG and DnaK begins to decrease immediately, exhibiting a four- to fivefold reduction by 10 min following overexpression of GroEL/S. By 10 min after GroEL/S induction, the concentration of σ32 had declined less than twofold as measured by Western analysis (Fig. 2B), indicating that GroEL/S must be inhibiting the activity of σ32, as the decrease in its level is insufficient to explain the drop in σ32-dependent protein synthesis. To determine if these inhibitory effects occurred at physiologically relevant levels of GroEL/S, we measured GroEL levels. Western blotting revealed that the amount of GroEL increased approximately twofold at 5 min and fourfold over the course of our experiments (Fig. 2B). We confirmed this result by showing that the synthesis rate of GroEL increases ∼16-fold (data not shown), which, when combined with our observed doubling time of 1 h, allows the accumulation of GroEL to be calculated, assuming that it is a stable protein. This estimation of GroEL/S levels is in good agreement with our observed changes in GroEL with Western blotting. A two- to fourfold accumulation of GroEL/S is physiologically relevant, as cells growing at 42°C have two to three times as much GroEL/S as those growing at 30°C (Straus et al. 1990). In summary, GroEL is able to repress σ32 activity with an efficiency similar to that of DnaK overexpression in vivo (Tomoyasu et al. 1998). Moreover, the negative regulation of σ32 activity following GroEL/S overproduction occurs at amounts corresponding to its normal physiological variation within the cell.
Figure 2.
GroEL/S overexpression results in inhibition of σ32 activity in vivo. An exponential phase culture of strain C600 carrying plasmid able to overexpress GroEL/S (pGro11) was grown in M9 minimal media containing all amino acids except for methionine and cysteine at 30°C. Anhydrotetracycline was added at time 0 to induce GroEL/S overexpression. The rate of synthesis of two σ32-dependent proteins, HtpG and DnaK (A), and a σE-dependent protein, RseA, and a σ70-dependent protein, RpoB (C), were measured. (B) The level of GroEL and σ32 were measured using Western analysis. All protein synthesis and Western data shown are the average from at least two independent experiments.
As specificity controls for this experiment, we analyzed the synthesis of RseA, a σE-dependent protein (De Las Penas et al. 1997), and RpoB, a σ70-dependent protein (Fig. 2C; Barry et al. 1979). The synthesis of these proteins declined only twofold by 10 min after overexpression of GroE/S from the Ptet promoter, as compared with the four- to fivefold repression of HtpG synthesis, indicating that GroEL/S has a specific repression component for σ32-mediated transcription. To ensure that the repression of σ32 activity was not an artifact of the induction system, we tested two additional induction systems, Para and Plac. For each system, overexpression of GroEL/S repressed HtpG synthesis several fold more than RseA synthesis (data not shown), indicating that there is σ32-specific repression of gene expression regardless of the promoter used to overexpress GroEL/S.
The ratio of GroEL/S to substrates is important for regulation of σ32
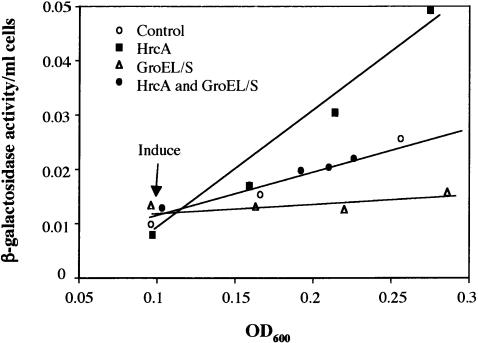
We showed that increasing the levels of GroEL/S leads to down-regulation of σ32 activity; however, the unfolded protein titration model suggests that it is not the total level of chaperone that is important for determining σ32 activity, but the ratio of chaperone to substrate. To determine what population of GroEL/S was important for regulation of σ32, we asked whether simultaneous induction of HrcA, a GroEL/S-specific substrate, would reverse the effects of GroEL/S overexpression (Mogk et al. 1997; Reischl et al. 2002; Wang et al. 2002). We found that simultaneous induction of HrcA and GroEL/S led to a reversal of the effects of GroEL/S overexpression alone (Fig. 3). This indicates that the population of GroEL/S that regulates σ32 is most likely free GroEL/S not associated with substrates, confirming an important prediction of the unfolded protein titration model.
Figure 3.
The ratio of GroEL/S to substrates in vivo is important for determining the activity of σ32. An exponential phase culture of strain 594 carrying a plasmid able to overexpress GroEL/S (pGro7) and one able to overexpress HrcA (pJDW39) was grown in LB at 30°C in the absence of inducers (control) or in the presence of 0.2% arabinose to induce GroEL/S overexpression and/or 1 mM IPTG to induce HrcA expression. A standard differential rate of synthesis plot is shown.
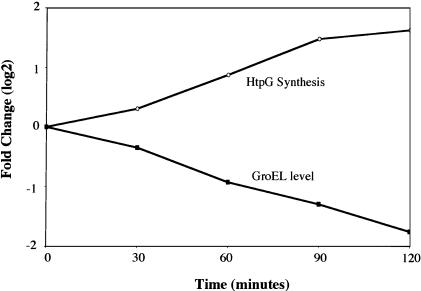
Depletion of GroEL increases σ32 levels
The experiments described earlier show that overexpression of GroEL/S results in decreases in σ32 activity; however, if GroEL/S is a regulator of σ32, then decreases in the level of GroEL/S should lead to an increase in σ32 activity. A previous report indicated that depleting GroEL/S resulted in stabilization of σ32 and therefore increased σ32-dependent transcription (Kanemori et al. 1994). We validated that the activity of σ32 increases when GroEL/S is depleted using a strain with the chromosomal copy of groEL/S driven by the Para promoter (Fig. 4). Also, we examined whether decreasing the amount of free GroEL/S by induction of a GroEL/S-specific substrate increases σ32 activity and whether this depletion also works by increasing the concentration of σ32. We found that induction of HrcA increased both σ32 activity and the amount of σ32 (Fig. 3; data not shown). By analogy with the effects of GroEL/S depletion, this increase in amount is likely to reflect a decrease in degradation of σ32. Thus, whether the concentration of free GroEL/S is decreased by depletion or titration with an unfolded protein substrate, the result is the same: The amount and activity of σ32 increases.
Figure 4.
Depletion of GroEL/S in vivo increases σ32 activity. Strain CAG48176, whose chromosomal groELS gene is driven by the inducible Para promoter, was grown in exponential phase at 30°C in M9 minimal media containing all amino acids except methionine and cysteine with 0.2% fructose as the main carbon source and 0.1% arabinose to maintain near wild-type levels of GroEL/S. Depletion of GroEL/S was initiated at time 0 by removing arabinose from the media and adding 0.2% glucose. HtpG synthesis and GroEL levels were analyzed as in Figure 2.
GroEL regulates σ32 during stress conditions
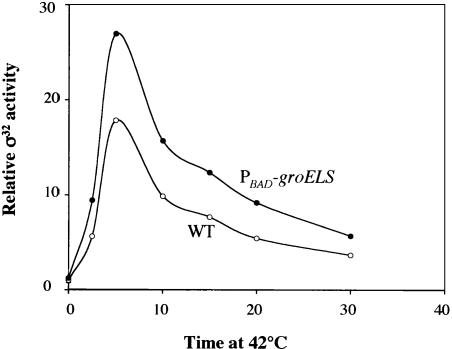
Our results with steady-state growth predict that the changes in GroEL/S levels during temperature upshift play a regulatory role in the heat shock response. We tested this idea by comparing the heat shock response of cells whose chromosomal copy of groEL/S is driven by the Para promoter with wild-type cells containing σ32-mediated transcription of groE/S. Previous work established that Para groELS cells have about 80% as much GroEL as wild-type cells growing at 30°C (McLennan and Masters 1998). We validated that number by determining the rate of GroEL synthesis in the two strain backgrounds and showed further that after a shift to 42°C, there is less than a twofold increase in GroEL synthesis from Para (data not shown), indicating that over the short 30 min window of a temperature upshift experiment, Para groELS cells will experience little change in GroEL level. In contrast, the wild-type cells are able to quickly increase the level of GroEL in response to a temperature upshift. We find that the heat shock response in Para groELS cells is altered in two respects (Fig. 5). First, the heat shock response is greater, showing twice as much induction at 2.5 min as wild-type cells, and higher peak expression at 5 min. Second, the shut-off response is delayed compared with wild-type cells, even though all other hsps are present at higher-than-normal levels. This experiment, which has been replicated several times, indicates that even a very small (20%) reduction in the level of GroEL prior to upshift, combined with prevention of GroEL/S accumulation during upshift, leads to a demonstrable increase in the extent and duration of the heat shock response. Together, these effects establish that GroEL/S is involved in negatively regulating heat-shock gene expression during stress conditions.
Figure 5.
Changing the levels of GroEL/S increases the magnitude and duration of the heat shock response. An exponential phase culture of strain C600 or a derivative having the chromosomal groELS gene under control of the inducible Para promoter (CAG48176) was grown at 30°C and subjected to heat shock by increasing the temperature to 42°C. σ32 activity was measured by examining the rate of synthesis of HtpG.
The data presented thus far argue that the level of GroEL/S is important for σ32 regulation in vivo but does not reveal whether this regulation is direct or indirect. This issue is particularly important for chaperones, which may be expected to have indirect effects. We addressed this issue in the next two sections by using in vitro experiments to determine whether GroEL/S, in the absence of other molecules, can bind to σ32 and alter its transcriptional activity.
GroEL binds to native σ32
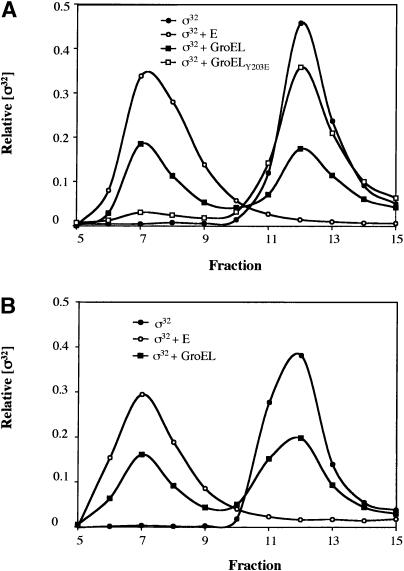
We examined whether the GroEL subunit of the GroEL/S chaperone machine could bind directly to σ32 in vitro using gel filtration (Fig. 6A). In this and all other in vitro experiments performed with chaperones, the chaperone preparation was first cleaned of peptides by incubation with Affi-gel blue beads (see Materials and Methods). When GroEL and σ32 were incubated together and then separated on a gel filtration column, approximately one-half of the σ32 eluted in a peak coincident with free σ32 and the remainder eluted in a higher molecular weight fraction, indicating binding to GroEL. This experiment shows that GroEL can bind directly to σ32 in vitro. Two additional experiments indicated that GroEL binds to native rather than misfolded σ32. First, 95% of the σ32 preparation was able to bind to RNA polymerase (E), indicating that our preparation was almost completely active. Second, after our initial gel filtration with σ32 and GroEL, we isolated the peak containing unbound, free σ32 and reanalyzed GroEL binding (Fig. 6B). Analysis of this σ32 fraction revealed that its binding characteristics were identical to those of unfractionated σ32, exhibiting partial binding to GroEL and almost complete binding to RNA polymerase. This indicates that our σ32 does not exist in two distinct populations, for example, one misfolded and one native, and further, that passage through the gel filtration column did not lead to inactivation or misfolding of our σ32 preparation. To determine whether σ32 binding to GroEL was similar to misfolded protein binding to GroEL, we tested whether a substrate-binding mutation in GroEL, GroELY203E, prevented σ32 binding. This mutant is believed to affect the normal protein-binding site of GroEL, as it is defective in the binding of several unfolded proteins in vitro and cannot complement a temperature-sensitive groEL mutant in vivo (Fenton et al. 1994). We found that GroELY203E binds very poorly to σ32 in our gel filtration assay, suggesting that the normal unfolded substrate-binding site on GroEL is used for σ32 binding. Taken together, these experiments show that GroEL is able to directly bind to native σ32 using its normal substrate-binding site. Assuming that the binding is in equilibrium and that our GroEL preparation is mostly active, the binding constant for this reaction is ∼1 μM.
Figure 6.
GroEL interacts directly with active σ32 in vitro. (A) Purified 35S-labeled σ32 (500 nM) was incubated with GroEL (2 μM), core RNAP (2 μM), or a GroEL-binding mutant, GroELY203E (2 μM), for 30 min at 20°C in protein-binding buffer (PBB). The proteins were then fractionated on a Superose 12 gel filtration column with PBB at 4°C. Fractions were collected and counted on a scintillation counter to determine the level of σ32 in each fraction. (B) The free σ32 peaks from the GroEL and σ32-binding reaction in A were pooled and additional unlabeled σ32 was added to bring the concentration to 500 nM as in A. This σ32 was then incubated with 2 μM GroEL or 2 μM core RNAP and analyzed as in A.
The GroEL/S chaperone machine inhibits σ32-dependent transcription in vitro
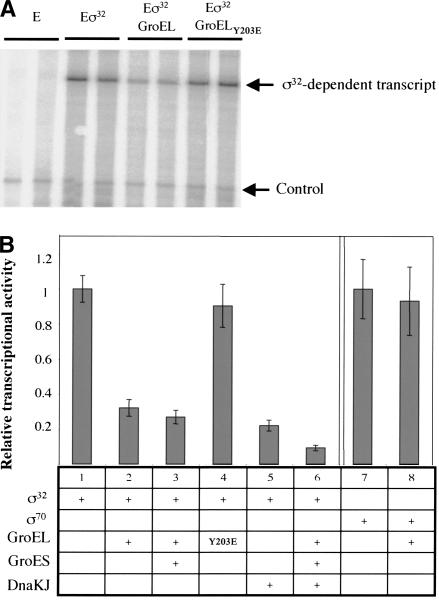
GroEL binds directly to σ32 in vitro, suggesting that this interaction may be sufficient to inhibit σ32 function. We therefore tested whether addition of GroEL to a σ32-dependent in vitro transcription reaction decreased the activity of σ32. Results of a representative transcription reaction are shown in Figure 7A, and the quantified and summarized data for all experiments are shown in Figure 7B. We found that a fivefold molar excess of either GroEL or GroEL/S over σ32 inhibits σ32-dependent transcription in vitro approximately threefold (Fig. 7B, cf. columns 1 and 2,3, respectively). Moreover, this inhibition is specific to σ32, as GroEL has no effect on σ70-dependent transcription (Fig. 7B, cf. columns 7 and 8). Inhibition requires binding to σ32, as the GroELY203E-binding mutant does not inhibit σ32-dependent transcription (Fig. 7B, cf. columns 1 and 4). These two controls allow us to rule out that the ATPase activity of GroEL is causing the inhibition; however, we did an additional experiment to further rule out the possibility that the GroEL ATPase activity was contributing to inhibition of σ32. We showed that GroEL inhibits σ32 activity even when reactions are performed with 1.2 mM ATP (six times higher than the ATP concentration normally present in the transcription reaction; data not shown). We conclude that direct binding of GroEL/S to σ32 inhibits its transcription activity.
Figure 7.
GroEL inhibits σ32-dependent in vitro transcription. Multiround in vitro transcription was performed with holoenzyme containing either σ32 (Eσ32) or σ70 (Eσ70) (100 nM) incubated with GroEL (500 nM), GroES (1 μM), GroELY203E (500 nM), DnaK (2 μM), DnaJ (400 nM), or combinations thereof. An end-labeled oligo was added to each reaction as an internal control. Transcription reactions were phenol-chloroform extracted, ethanol precipitated, and analyzed on a 6% polyacrylamide gel. (A) Representative transcription gel showing duplicate reactions documenting GroEL inhibition of Eσ32. (B) Quantification and summary of transcription results from A as well as from additional transcription experiments.
Previous work has shown that the DnaK/J chaperone machine inhibits σ32 activity in an in vitro transcription reaction (Gamer et al. 1996). We replicated that result in our system using DnaK/J cleaned of peptides by passage through an Affi-gel blue column (see Materials and Methods; Fig. 7B, cf. columns 1 and 5). We then asked whether the DnaK/J and GroEL/S together further inhibited transcription. We added sufficient DnaK/J and GroEL/S to result in approximately threefold inhibition of activity by each separately and found that, together, they result in close to a ninefold decrease in σ32 activity, indicating that these two chaperone machines independently inhibit σ32 (Fig. 7B, cf. columns 6 and 5,3).
Discussion
A major challenge for all organisms is to maintain a constant intracellular folding environment. This requires a robust and sensitive stress response that is capable of responding to small changes in the level of misfolded proteins. Until now, it was believed that the DnaK chaperone machine was the sole sensor of the folding environment in E. coli. In this work, we show that every important criterion used to establish the regulatory role of DnaK also establishes a similar role for the GroEL/S chaperone machine. The use of a chaperone network to detect unfolded proteins allows the cellular folding status to be sensed more completely, and, in addition, provides a more sensitive indicator of folding state than is possible by a single chaperone machine.
GroEL/S, like DnaK, is involved in the regulation of both σ32 activity and σ32 stability. Overexpression of GroEL/S inhibits the activity of σ32 in vivo, with efficiency approximately comparable to inhibition resulting from DnaK overexpression. In both cases, inactivation of σ32 is direct, as either protein can selectively inhibit σ32 transcription in vitro with comparable efficiency. There is also a link between chaperone level and the stability of σ32, with underproduction of either the DnaK or GroEL/S chaperone machines stabilizing σ32. However, the mechanism of this effect is unclear because it cannot be reconstituted in vitro. σ32 is degraded very rapidly in vivo (T 1/2 ∼ 1 min) by the FtsH protease (Herman et al. 1995b; Tomoyasu et al. 1995). FtsH degrades σ32 very slowly in vitro, and neither the addition of DnaK or GroEL/S separately or together facilitates proteolysis (Blaszczak et al. 1999; C. Herman, unpubl.). Recent evidence indicates that FtsH lacks a robust unfoldase activity, and an additional unidentified factor may allow FtsH to proteolyze σ32 by acting as an unfoldase (Herman et al. 2003). It remains to be seen whether the chaperones work together with this factor to promote degradation, or whether they influence degradation by a more indirect pathway. Interestingly, we further show that cells can adapt to long-term overexpression of GroEL/S. This suggests a new layer of complexity in the regulation of the heat shock response. We show that, in addition to its role in the regulation of σ32 under steady-state conditions, GroEL/S is important for proper regulation of σ32 during stress conditions.
To our knowledge, this is the first reported example of GroEL/S binding to a folded, active protein. Moreover, our studies indicate that the normal substrate-binding site on GroEL/S mediates interaction between the two proteins. Together, these observations suggest that GroEL/S is able to bind to σ32 because some aspect of the structure of native σ32 mimics that of an unfolded protein. Although there are no structures of intact, uncomplexed σ factors, a combination of high-resolution structures of individual domains and a low-resolution structure of σ bound to RNA polymerase has led to the generally accepted notion that all σ factors consist of domains separated by flexible linkers (Campbell et al. 2002; Murakami et al. 2002). The flexible linkers, a portion of one of the domains or the aspects of σ32 that are not conserved among other σs, may be specialized to exist as an unfolded segment to allow σ32 binding to GroEL/S and possibly to DnaK. In support of this idea, deuterium/hydrogen exchange followed by rapid proteolysis and mass spectrometry analysis has shown that a large portion of the C terminus of σ32 undergoes an unusually fast exchange with the solvent, suggesting that it is either highly flexible or poorly structured (Rist et al. 2003). Such regions could serve as chaperone binding sites. As σ32 is specialized to transcribe at lethal temperatures and has been shown to maintain active transcription in vitro at such temperatures (Blaszczak et al. 1995), such unstructured regions would have to exist in concert with a core folded region that allows the protein to maintain activity.
The mechanism by which binding to GroEL inhibits σ32 transcriptional activity is unknown. The simplest model is that GroEL binding simply sequesters σ32 in its central cavity, thereby preventing it from binding to RNA polymerase. However, the simple sequestration model seems inconsistent with our current data. First, GroEL binding to σ32 appears to be much weaker than RNA polymerase binding to σ32, and in the concentrations used in the in vitro transcription experiments, we would not expect GroEL to be able to compete with RNA polymerase for σ32 binding. Moreover, sequestration of σ32 by GroEL/S may be expected to prevent rapid σ32 degradation in vivo, as GroEL normally binds proteins inside its central cavity. However, σ32 does not accumulate on GroEL/S overproduction. An alternative model is that inactivation of σ32 involves two processes: sequestration followed by release in a different conformation that is transiently unable to bind RNA polymerase but can still be degraded. This model may also help to explain the role of GroEL in regulation of both σ32 activity and σ32 stability.
The use of two independent sensors of the intracellular folding state has important consequences for the cell. First, the use of a chaperone network is expected to increase the accuracy of the surveillance for this signal-transduction pathway. Even though a single chaperone can sense a significant number of the proteins in the cell, use of both DnaK and GroEL/S allows accurate counting of the folding state for the many substrates that interact preferentially or solely with one of the two machines. In this regard, it is particularly important to sense GroEL/S occupancy, as it is the only essential chaperone and therefore folds dedicated substrates important to cellular viability. Second, the use of a chaperone network is expected to increase the sensitivity of the signal-transduction pathway. Global changes in protein folding, such as the increased misfolding during heat shock, leads to changes in protein folding for both GroEL-dependent and DnaK-dependent proteins. Sensing the levels of both classes of proteins leads to a much larger signal from a given stress, and, therefore, a much smaller change in global protein folding can be sensed.
Interestingly, there is emerging evidence in eukaryotic cells that a chaperone network may be used to regulate the heat shock response. For example, in Saccharomyces cerevisiae, the heat shock response is regulated by the transcription factor Hsf1. Hsf1 has long been known to be under the control of Hsp70 (Baler et al. 1992; Halladay and Craig 1995; Shi et al. 1998), but recent evidence also implicates Hsp90 (Zou et al. 1998). This interesting parallel lead us to analyze the ability of the E. coli homolog of Hsp90, HtpG, to regulate σ32, but we observed no regulation in our experiments. This difference between prokaryotes and eukaryotes may be correlated with the physiology of these chaperone systems. In prokaryotes, GroEL/S is one of the most important general protein folding machines, and HtpG plays a poorly understood but presumably less critical role. In contrast, eukaryotes use Hsp90 in many important physiological pathways, whereas CCT/TriC, the eukaryotic cytoplasmic chaperonin, appears to be more specialized, although the extent of specialization is controversial (Feldman and Frydman 2000; Young et al. 2001; Hartl and Hayer-Hartl 2002). Although the two specific sensors of the protein folding environment may be different in E. coli and S. cerevisiae, it seems likely that the rationale behind having multiple sensors of protein folding has been conserved.
Materials and methods
Strains
Strains used in this study are all derivatives of K-12. All strains were isogenic with JM105, genotype supE endA sbcB15 hsdR4 rpsL thi Δ(lac-proAB) (Herman et al. 1995b); C600, genotype supE44 hsdR thi-1 thr-1 leuB6 lacY1 tonA21 (Sambrook et al. 1989); or 594, genotype lacZ-350 galK2 galT22 rpsL179 lacIpoZΔ(Mlu) λJW2(PhtpG::lacZ) (Herman et al. 1995a). Strain CAG48176 was made by standard P1 transduction (Miller 1972) of a Para-groELS (McLennan and Masters 1998; Nielsen et al. 1999) into C600. Transductants were selected for resistance to kanamycin and confirmed by ensuring arabinose-dependent growth.
Media and antibiotics
LB rich medium and M9 minimal medium were prepared as described (Sambrook et al. 1989). M9 medium was supplemented with 0.2% glucose (unless otherwise noted), 1 mM MgSO4, and 2 μg/mL thiamine. Complete M9 minimal medium was also supplemented with all amino acids (40 μg/mL) except methionine and cysteine. When required, media was supplemented with the following antibiotics: 30 μg/mL kanamycin; 20 μg/mL chloramphenicol; 100 μg/mL ampicillin, 50 μg/mL spectinomycin. A final concentration of 0.2% L-(+)-arabinose, 25 ng/mL anhydrotetracycline, and 1 mM IPTG were used as inducers for Para, Ptet, Plac, and Ptac promoters.
Efficiency of plating
Overnight cultures of strain JM105 carrying either pDS1 (Ptac-rpoH) (Bahl et al. 1987) or pDS1 and pKV1561 (Plac-groELS) (Kanemori et al. 1994) were grown at 30°C in M9 minimal media. Serial dilutions were made and plated on M9 minimal media with and without 1 mM IPTG at 30°C. EOP values were calculated by dividing the number of colony forming units (cfu) in the presence of IPTG by the number of cfu in the absence of IPTG.
β-Galactosidase assays
Overnight cultures of strain 594 carrying either plasmid pGro7 (Para-groELS) (Nishihara et al. 1998), or plasmids pGro7, pJDW39 (PT5/lac-hrcA) (Wang et al. 2002), and pJM100 (lacIq) (McCarty and Walker 1994) in LB media at 30°C were diluted 1:100 and grown until they reached exponential phase. Cultures were then either used as a control or induced with 0.2% arabinose, 1 mM IPTG, or both. σ32 activity was assayed by monitoring β-galactosidase activity from a chromosomal σ32-dependent promoter in strain 594 (Herman et al. 1995b). Samples were taken at various time points to determine σ32 activity, and assays were performed as described (Miller 1972).
Pulse-labeling
For GroEL/S overexpression, saturated overnight cultures of strain C600 carrying plasmids pGro7 (Para-groELS), pKV1561 (Plac-groELS), or pGro11 (Ptet-groELS) (Nishihara et al. 1998) grown in M9 minimal media with all amino acids except methionine and cysteine at 30°C were diluted 1:100 and then grown until they reached exponential phase. For GroEL/S depletion, an overnight culture of strain CAG48176, with the chromosomal groELS promoter replaced with Para, grown at 30°Cin M9 minimal media containing all amino acids except methionine and cysteine and having 0.2% fructose as the carbon source and 0.1% arabinose to maintain near wild-type levels of GroEL/S, was diluted 1:100 and then grown until it reached exponential phase. For each time point, an 800-μL aliquot of cells was pulse-labeled for 1 min with EasyTag Expre35S35S protein labeling mix (NEN) followed by a chase with unlabeled methionine and cysteine. Extracts were then TCA precipitated as described in the Western blotting section. Samples were resuspended in 50 μL of 2% SDS and 50 mM Tris (pH 7.5). The extracts were diluted in 750 μL RIPA (50 mM Tris at pH 7.5, 500 mM NaCl, 0.1% SDS, 1% NP-40, and 0.5% sodium deoxycholate) and an aliquot was counted in a scintillation counter. To normalize the samples, we used equal numbers of counts per minute. Immunoprecipitation was done in a total volume of 750 μL containing extract, polyclonal antibodies, 25 μL of a 1:1 suspension of protein A-conjugated Sepharose beads, and RIPA buffer. For HtpG, DnaK, and RseA synthesis, we added an extract containing a labeled truncated version of the protein to use as an internal control prior to immunoprecipitation. The samples were rocked at 4°C for at least 1 h, and the beads were washed three times with 900 μL RIPA. Immunoprecipitated proteins were eluted from the beads with Laemmli sample buffer and boiling. The entire sample was then loaded onto an acrylamide gel, and the proteins were visualized using a Molecular Dynamics Storm 560 PhosphorImager scanning system.
Western blotting
Samples for Westerns (900 μL) were collected and ice-cold TCA was added to a final concentration of 5%. Samples were precipitated on ice for at least 30 min, followed by centrifugation. After TCA was removed, the samples were resuspended directly in Laemmli sample buffer. An equal number of cells were loaded in each lane of the polyacrylamide gels and the proteins were transferred to nitrocellulose. The blots were probed with 1:10,000 dilutions of polyclonal rabbit antibodies, and then probed with 1:10,000 dilution of anti-rabbit horseradish peroxidase-conjugated secondary antibody. Western blots were developed with chemiluminescence and exposed to film. Bands were scanned and analyzed using Alpha Innotech densitometry software (Alpha Innotech).
Gel filtration
The following proteins were purified essentially as described: GroEL and GroELY203E (Fenton et al. 1994), core RNA polymerase (Sharp et al. 1999), and σ32 (Gamer et al. 1996). All chaperone preparations were cleaned of misfolded proteins by incubation with Affi-Gel Blue beads overnight at 4°C in the presence of 5 mM ATP and 10 mM MgCl2. Proteins were diluted to the appropriate concentrations in protein-binding buffer (PBB) in a final volume of 500 μL and incubated at 20°C for at least 30 min. PBB contains 100 mM KCl, 0.01% NP-40, 20 mM Tris (pH 7.5), 5 mM MgCl, and 10% glycerol. Samples were then loaded onto a Superose 12 gel filtration column and run in PBB. For σ32, a fraction of the protein used was whole-cell labeled with 35S before the purification. This was added to the cold σ32 before the mixture was added to the binding reaction. Thirty fractions, 1 mL each, were collected and the fractions were counted in a scintillation counter.
In vitro transcription
The following proteins were purified essentially as described: GroES (Fenton et al. 1994), σ70 (Sharp et al. 1999), DnaK, DnaJ, and GrpE (Suh et al. 1998). All chaperone preparations were cleaned of misfolded proteins by incubation with Affi-Gel Blue beads overnight at 4°C in the presence of 5 mM ATP and 10 mM MgCl2. Holoenzyme was reconstituted by incubation of core RNA polymerase and σ32 or σ70 in protein dilution buffer (PDB) containing 20 mM HEPES (pH 7.9), 100 mM KCl, 10 mM MgCl2, 0.1% BME, 10% glycerol, 12 μg/mL BSA, and 0.1% Tween. Additional proteins were added as required and samples were incubated at least 10 min on ice. Transcription was initiated by adding an equal volume of transcription mix containing 20 mM HEPES (pH 7.9), 100 mM KCl, 10 mM MgCl2, 0.1% BME, 50 nM template, 2 mM ATP, 2 mM GTP, 2 mM UTP, 0.1 mM CTP, and 0.4 μL 32P α-CTP (3000 Ci/mmol), and samples were incubated 10 min at 30°C. Linear DNA templates were generated using PCR, for Eσ32 transcription, the promoter was PhtpG, and for Eσ70, PT7A1 was used. Reactions were stopped by the addition of 10 volumes of transcription stop mix (TSM) containing 20 mM EDTA, 250 mM NaCl, 1% SDS, and 200 μg/mL glycogen. Samples were then phenol extracted to remove proteins from the reaction mixture and the RNA was ethanol precipitated and loaded onto a 6% polyacrylamide gel. As an internal control, a 60-nucleotide, 32P end-labeled oligomer was added to each reaction. The transcripts were visualized using a Molecular Dynamics Storm 560 PhosphorImager scanning system.
Acknowledgments
We thank B. Bukau for σ32 antibodies, M. Masters for strain containing Para-groELS, T. Yura for plasmids, and T. Yura, K. Kim and members of the Gross lab for discussion. This work was supported by NIH Grant number GM36278, Human Frontier Program Organization Fellowship (C.H.), and NSF Graduate Research Fellowship (E.G.).
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1219204.
Corresponding authors.
References
- Arsene F., Tomoyasu, T., and Bukau, B. 2000. The heat shock response of Escherichia coli. Int. J. Food Microbiol. 55: 3-9. [DOI] [PubMed] [Google Scholar]
- Bahl H., Echols, H., Straus, D.B., Court, D., Crowl, R., and Georgopoulos, C.P. 1987. Induction of the heat shock response of E. coli through stabilization of σ32 by the phage lambda cIII protein. Genes & Dev. 1: 57-64. [DOI] [PubMed] [Google Scholar]
- Baler R., Welch, W.J., and Voellmy, R. 1992. Heat shock gene regulation by nascent polypeptides and denatured proteins: hsp70 as a potential autoregulatory factor. J. Cell Biol. 117: 1151-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G., Squires, C.L., and Squires, C. 1979. Control features within the rplJL–rpoBC transcription unit of Escherichia coli. Proc. Natl. Acad. Sci. 76: 4922-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaszczak A., Zylicz, M., Georgopoulos, C., and Liberek, K. 1995. Both ambient temperature and the DnaK chaperone machine modulate the heat shock response in Escherichia coli by regulating the switch between σ70 and σ32 factors assembled with RNA polymerase. EMBO J. 14: 5085-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaszczak A., Georgopoulos, C., and Liberek, K. 1999. On the mechanism of FtsH-dependent degradation of the σ32 transcriptional regulator of Escherichia coli and the role of the Dnak chaperone machine. Mol. Microbiol. 31: 157-166. [DOI] [PubMed] [Google Scholar]
- Buchberger A., Schroder, H., Hesterkamp, T., Schonfeld, H.J., and Bukau, B. 1996. Substrate shuttling between the DnaK and GroEL systems indicates a chaperone network promoting protein folding. J. Mol. Biol. 261: 328-333. [DOI] [PubMed] [Google Scholar]
- Bukau B. 1993. Regulation of the Escherichia coli heat-shock response. Mol. Microbiol. 9: 671-680. [DOI] [PubMed] [Google Scholar]
- Bukau B., Deuerling, E., Pfund, C., and Craig, E.A. 2000. Getting newly synthesized proteins into shape. Cell 101: 119-122. [DOI] [PubMed] [Google Scholar]
- Campbell E.A., Muzzin, O., Chlenov, M., Sun, J.L., Olson, C.A., Weinman, O., Trester-Zedlitz, M.L., and Darst, S.A. 2002. Structure of the bacterial RNA polymerase promoter specificity σ subunit. Mol. Cell 9: 527-539. [DOI] [PubMed] [Google Scholar]
- Connolly L., Yura, T., and Gross, C.A. 1999. Autoregulation of the heat shock response in procaryotes. In Molecular chaperones and folding catalysts. Regulation, cellular function and mechanism (ed. B. Bukau), pp. 13-33. Harwood Academic Publishers, Amsterdam.
- Craig E.A. and Gross, C.A. 1991. Is hsp70 the cellular thermometer? Trends Biochem. Sci. 16: 135-140. [DOI] [PubMed] [Google Scholar]
- De Las Penas A., Connolly, L., and Gross, C.A. 1997. The σE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of σE. Mol. Microbiol. 24: 373-385. [DOI] [PubMed] [Google Scholar]
- Feldman D.E. and Frydman, J. 2000. Protein folding in vivo: The importance of molecular chaperones. Curr. Opin. Struct. Biol. 10: 26-33. [DOI] [PubMed] [Google Scholar]
- Fenton W.A., Kashi, Y., Furtak, K., and Horwich, A.L. 1994. Residues in chaperonin GroEL required for polypeptide binding and release. Nature 371: 614-619. [DOI] [PubMed] [Google Scholar]
- Fink A.L. 1999. Chaperone-mediated protein folding. Physiol. Rev. 79: 425-449. [DOI] [PubMed] [Google Scholar]
- Gamer J., Bujard, H., and Bukau, B. 1992. Physical interaction between heat shock proteins DnaK, DnaJ, and GrpE and the bacterial heat shock transcription factor σ32. Cell 69: 833-842. [DOI] [PubMed] [Google Scholar]
- Gamer J., Multhaup, G., Tomoyasu, T., McCarty, J.S., Rudiger, S., Schonfeld, H.J., Schirra, C., Bujard, H., and Bukau, B. 1996. A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulates activity of the Escherichia coli heat shock transcription factor σ32. EMBO J. 15: 607-617. [PMC free article] [PubMed] [Google Scholar]
- Gruber T.M. and Gross, C.A. 2003. Multiple σ subunits and the partitioning of bacterial transcription space. Annu. Rev. Microbiol. 57: 441-466. [DOI] [PubMed] [Google Scholar]
- Halladay J.T. and Craig, E.A. 1995. A heat shock transcription factor with reduced activity suppresses a yeast HSP70 mutant. Mol. Cell. Biol. 15: 4890-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F.U. 1996. Molecular chaperones in cellular protein folding. Nature 381: 571-579. [DOI] [PubMed] [Google Scholar]
- Hartl F.U. and Hayer-Hartl, M. 2002. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science 295: 1852-1858. [DOI] [PubMed] [Google Scholar]
- Herman C. and Gross, C.A. 2000. Heat stress. In Encyclopedia of microbiology (ed. J. Lederberg), pp. 598-606. Academic Press.
- Herman C., Lecat, S., D'Ari, R., and Bouloc, P. 1995a. Regulation of the heat-shock response depends on divalent metal ions in an hflB mutant of Escherichia coli. Mol. Microbiol. 18: 247-255. [DOI] [PubMed] [Google Scholar]
- Herman C., Thevenet, D., D'Ari, R., and Bouloc, P. 1995b. Degradation of σ32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc. Natl. Acad. Sci. 92: 3516-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman C., Prakash, S., Lu, C.Z., Matouschek, A., and Gross, C.A. 2003. Lack of a robust unfoldase activity confers a unique level of substrate specificity to the universal AAA protease FtsH. Mol. Cell 11: 659-669. [DOI] [PubMed] [Google Scholar]
- Horwich A.L., Weber-Ban, E.U., and Finley, D. 1999. Chaperone rings in protein folding and degradation. Proc. Natl. Acad. Sci. 96: 11033-11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemori M., Mori, H., and Yura, T. 1994. Effects of reduced levels of GroE chaperones on protein metabolism: Enhanced synthesis of heat shock proteins during steady-state growth of Escherichia coli. J. Bacteriol. 176: 4235-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberek K., Galitski, T.P., Zylicz, M., and Georgopoulos, C. 1992. The DnaK chaperone modulates the heat shock response of Escherichia coli by binding to the σ32 transcription factor. Proc. Natl. Acad. Sci. 89: 3516-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty J.S. and Walker, G.C. 1994. DnaK mutants defective in ATPase activity are defective in negative regulation of the heat shock response: Expression of mutant DnaK proteins results in filamentation. J. Bacteriol. 176: 764-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan N. and Masters, M. 1998. GroE is vital for cell-wall synthesis. Nature 392: 139. [DOI] [PubMed] [Google Scholar]
- Miller J.H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Mogk A., Homuth, G., Scholz, C., Kim, L., Schmid, F.X., and Schumann, W. 1997. The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J. 16: 4579-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R.I. 1998. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes & Dev. 12: 3788-3796. [DOI] [PubMed] [Google Scholar]
- Morita M., Kanemori, M., Yanagi, H., and Yura, T. 1999a. Heat-induced synthesis of σ32 in Escherichia coli: Structural and functional dissection of rpoH mRNA secondary structure. J. Bacteriol. 181: 401-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M.T., Tanaka, Y., Kodama, T.S., Kyogoku, Y., Yanagi, H., and Yura, T. 1999b. Translational induction of heat shock transcription factor σ32: Evidence for a built-in RNA thermosensor. Genes & Dev. 13: 655-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K.S., Masuda, S., and Darst, S.A. 2002. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 Å resolution. Science 296: 1280-1284. [DOI] [PubMed] [Google Scholar]
- Nadeau K., Das, A., and Walsh, C.T. 1993. Hsp90 chaperonins possess ATPase activity and bind heat shock transcription factors and peptidyl prolyl isomerases. J. Biol. Chem. 268: 1479-1487. [PubMed] [Google Scholar]
- Nielsen K.L., McLennan, N., Masters, M., and Cowan, N.J. 1999. A single-ring mitochondrial chaperonin (Hsp60–Hsp10) can substitute for GroEL–GroES in vivo. J. Bacteriol. 181: 5871-5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara K., Kanemori, M., Kitagawa, M., Yanagi, H., and Yura, T. 1998. Chaperone coexpression plasmids: Differential and synergistic roles of DnaK–DnaJ–GrpE and GroEL–GroES in assisting folding of an allergen of Japanese cedar pollen, Cryj2, in Escherichia coli. Appl. Environ. Microbiol. 64: 1694-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen E.A. and Morimoto, R.I. 2002. Chaperoning signaling pathways: Molecular chaperones as stress-sensing `heat shock' proteins. J. Cell Sci. 115: 2809-2816. [DOI] [PubMed] [Google Scholar]
- Reischl S., Wiegert, T., and Schumann, W. 2002. Isolation and analysis of mutant alleles of the Bacillus subtilis HrcA repressor with reduced dependency on GroE function. J. Biol. Chem. 277: 32659-32667. [DOI] [PubMed] [Google Scholar]
- Rist W., Jorgensen, T.J., Roepstorff, P., Bukau, B., and Mayer, M.P. 2003. Mapping temperature-induced conformational changes in the Escherichia coli heat shock transcription factor σ32 by amide hydrogen exchange. J. Biol. Chem. 278: 51415-51421. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch, E., and Maniatis, T. 1989. Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sharp M.M., Chan, C.L., Lu, C.Z., Marr, M.T., Nechaev, S., Merritt, E.W., Severinov, K., Roberts, J.W., and Gross, C.A. 1999. The interface of σ with core RNA polymerase is extensive, conserved, and functionally specialized. Genes & Dev. 13: 3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Mosser, D.D., and Morimoto, R.I. 1998. Molecular chaperones as HSF1-specific transcriptional repressors. Genes & Dev. 12: 654-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D.B., Walter, W.A., and Gross, C.A. 1987. The heat shock response of E. coli is regulated by changes in the concentration of σ32. Nature 329: 348-351. [DOI] [PubMed] [Google Scholar]
- ____. 1989. The activity of σ32 is reduced under conditions of excess heat shock protein production in Escherichia coli. Genes & Dev. 3: 2003-2010. [DOI] [PubMed] [Google Scholar]
- ____. 1990. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of σ32. Genes & Dev. 4: 2202-2209. [DOI] [PubMed] [Google Scholar]
- Suh W.C., Burkholder, W.F., Lu, C.Z., Zhao, X., Gottesman, M.E., and Gross, C.A. 1998. Interaction of the Hsp70 molecular chaperone, DnaK, with its cochaperone DnaJ. Proc. Natl. Acad. Sci. 95: 15223-15228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., McKittrick, N., Zylicz, M., and Georgopoulos, C. 1983. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell 34: 641-646. [DOI] [PubMed] [Google Scholar]
- Tilly K., Spence, J., and Georgopoulos, C. 1989. Modulation of stability of the Escherichia coli heat shock regulatory factor σ. J. Bacteriol. 171: 1585-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoyasu T., Gamer, J., Bukau, B., Kanemori, M., Mori, H., Rutman, A.J., Oppenheim, A.B., Yura, T., Yamanaka, K., Niki, H., et al. 1995. Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor σ32. EMBO J. 14: 2551-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoyasu T., Ogura, T., Tatsuta, T., and Bukau, B. 1998. Levels of DnaK and DnaJ provide tight control of heat shock gene expression and protein repair in Escherichia coli. Mol. Microbiol. 30: 567-581. [DOI] [PubMed] [Google Scholar]
- Wang J.D., Herman, C., Tipton, K.A., Gross, C.A., and Weissman, J.S. 2002. Directed evolution of substrate-optimized GroEL/S chaperonins. Cell 111: 1027-1039. [DOI] [PubMed] [Google Scholar]
- Young J.C., Moarefi, I., and Hartl, F.U. 2001. Hsp90: A specialized but essential protein-folding tool. J. Cell Biol. 154: 267-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.N., Kusukawa, N., Erickson, J.W., Gross, C.A., and Yura, T. 1988. Isolation and characterization of Escherichia coli mutants that lack the heat shock σ factor σ32. J. Bacteriol. 170: 3640-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Guo, Y., Guettouche, T., Smith, D.F., and Voellmy, R. 1998. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94: 471-480. [DOI] [PubMed] [Google Scholar]