Summary
Background and aims.
Integrated vector control especially use of insecticide-treated bed nets have been reported as effective malaria preventive strategies. This study aimed at documenting factors that influence regular use of insecticide-treated nets in under-fives and impact of vector control methods on malaria outcome (severe malaria prevalence and mortality) in under-fives presenting in a tertiary health institution in Nigeria.
Methods.
Cross-sectional study carried out from June 2012 and July 2013. Data was obtained by researcher-administered questionnaire and malaria was confirmed in each child by microscopy.
Results.
329 caregiver (31.2 ± 6.0 years) /child (20.7 ± 14.0 months) pair were recruited. Netting of doors/windows (80.0%) was the most practiced vector control method. 177 (53.8%) caregivers possessed insecticide-treated bed nets, and only a quarter of their under-5s regularly sleep in these nets. Children from lower social class statistically significantly sleep in the nets (p = 0.03), however, presence of 2 or more nets in a household independently predicted its regular use for the under-5s (β = 1.09, OR = 3, p = 0.02). Prevalence of severe malaria was 36.2% and mortality was 52 per 1000. Combination of regular use of insecticide treated nets, environmental sanitation, indoor insecticide spray and netting of household doors/windows significantly predicted low prevalence of severe malaria compared to each of the malaria vector control methods used singly by the caregivers (β = 1.66, OR =5.0, p = 0.04).
Conclusions.
Integrated vector control remains the most effective method of malaria vector control at the community.
Key words: Bed-nets, Insecticide-treated, Malaria, Mortality, Strategy, Vector
Introduction
In malaria endemic areas, factors such as poverty and poor environmental sanitation allow survival and proliferation of malaria vector [1, 2]. The emergence and rapid spread of resistance both of the malaria vector (female anopheles mosquito) to insecticides and of the pathogenic plasmodia to antimalarial drugs are the major causes of increased malaria disease morbidity and mortality. The malaria vectors predominantly found in the Northern and Southern regions of Nigeria include Anopheles gambiae, Anopheles arabiensis, Anopheles funestus, and Anopheles melas [3].
Malaria is responsible for nearly half a million deaths worldwide annually [1]. With a view to reducing the malaria burden, the World Health Organization (WHO)/ Global Malaria Programme (GMP) promotes personal and communal control measures against the malaria vector known as the Integrated Vector Control (IVC). IVC comprises the use of insecticide treated nets (ITN), and other control measures such as netting of house doors/ windows, regular environmental sanitation (clearing of bushes and drains/ gutters around the house) to eliminate and reduce the burden of malaria vector [1, 2, 4].
In Nigeria, the most common methods of malaria vector control include the use of window/door mosquito screens/netting, clearing of bushes/drains, mosquito repellants/insecticides and insecticide treated-bed nets (ITN) [5, 6]. In the last decade, the ITN has been observed to be a veritable tool of malaria vector control worldwide. However, its availability and regular usage in households have been major factors against the effectiveness of this malaria vector control strategy [7]. The Nigeria Malaria Indicator Survey (MIS) report of 2010 showed an overall ITN ownership of 42.0% (75.0% ownership in areas with recent mass ITN distribution campaign) and usage of 23.0% (41.0% utilization in areas with recent mass ITN distribution campaign) [7]. Since then, there has been increased mass campaigns to improve household ownership and utilization of ITN in most countries in Africa including Nigeria [7-10].
The use of ITN has been observed to reduce malaria morbidity and mortality, however, reports from some authors in Africa showed varying results. For example Afoakwah et al. [11] observed a reduction in under-five mortality from malaria in Northern Ghana, while Loha et al. [12] did not find any influence of free mass distribution of ITNs on malaria morbidity in South Ethiopia. Although these studies assessed only ITN usage against malaria morbidity and mortality, the differences in the results have been criticized on the basis of the methods applied in establishing the association between ITN usage and the outcomes. For example, sleeping under the ITN the night before the survey was regarded as usage. This may not be an objective assessment as individual who possessed the ITN without sleeping in it until the night before a survey will be erroneously regarded as an ITN user. Again none of these studies assessed the impact of other vector control methods on malaria outcome in children.
To improve on the ITN utilization and IVC strategy in the communities, the National Malaria Elimination Programme (NMEP) in the last 5 years preceeding this study had instituted a scaled-up awareness programme on IVC strategy at all levels [13]. To provide insight into the progress made so far on malaria vector control programme especially with regards to ITN ownership and usage, this study aimed at documenting the malaria vector control practices of caregivers, ITN usage by under-fives and factors that influence the use of ITN by under-fives in households. Due to paucity of studies on the impact of vector control on malaria health indices of under-fives in the study locale, this study evaluated the impact of vector control methods by caregivers on malaria health indices (severe malaria prevalence and mortality) of their under-fives presenting in a tertiary health institution in Nigeria.
Study hypothesis
NULL HYPOTHESIS
The IVC does not have favourable impact on malaria health indices (severe malaria morbidity and mortality) in under-fives.
ALTERNATE HYPOTHESIS
The IVC have favourable impact on malaria health indices (severe malaria morbidity and mortality) in underfives.
Study participants and methods
This study was carried out in Benin City, Edo State Nigeria. The State lies within the South-south region of Nigeria and the topography is that of tropical rain forest where malaria transmission is holoendemic and stable throughout the year [1-3]. The vegetation is mainly rain forest with some regions of creeks and swamp which support the breeding of the malaria vector (anopheles mosquito) especially Anopheles gambiae and Anopheles arabiensis [3]. It is a cosmopolitan City where most inhabitants are civil servants, traders, artisans and farmers. This was a cross-sectional descriptive study carried out from June 2012 to July 2013.
The study participants included caregivers and their apparently well-nourished children (6-59 months) who presented with malaria in the index tertiary health institution. Malaria was confirmed in each child by microscopy following standard protocols [14]. Each caregiver/ child pair were recruited consecutively in the study.
Children excluded from the study were children who had clinical and/or laboratory evidence(s) of localized infection such as acute tonsillitis, otitis media, pneumonia, and urinary tract infection. These diseases are common cause of fever in children. Children who had malnutrition were also excluded from the study. The Z-scores for weight-for-age (WFA) were calculated for each child using the revised WHO growth charts from the Centre for Disease Control (CDC) as reference [15]. Children with WFA Z-score of minus 2 standard deviation of the reference median value were regarded as acute under-weight malnutrition. Malnutrition is associated with morbidities such as diarrhoea, pneumonia, urinary tract infection and sepsis; and has an increased mortality [16].
DATA COLLECTION AND SAMPLING TECHNIQUE
Data was collected by a researcher-administered semistructured questionnaire. The tool was validated by extensive literature review and was pre-tested on 20 caregiver/child pairs who were excluded from the final analysis. The questionnaire sought information on the study participants' demographic features, their knowledge and attitudes on malaria vector control practices. Children with clinical features in keeping with WHO case definition of severe malaria were classified as severe malaria while children without such features were classified as uncomplicated malaria [1]. Children with severe malaria were admitted and treated according to the national guideline for management of severe malaria while those with uncomplicated malaria received full course of artemisinin-based combination therapy (ACT). The family social class was determined as described by Olusanya et al. using mother's level of education and the father's occupation. In this method of classification of social class, information on child's mother's level of education and father's occupation is required. This is obtained by history from the child's informant. Specific score is then allotted to the father's occupations as follows: 1, 2, or 3; and mother's educational qualifications – 0, 1 or 2 as shown below. The sum of these scores i.e from father's occupation and mother's educational qualification scores describes the family social class as High –1, High Intermediate – 2, Middle – 3, Low Intermediate – 4, and Low – 5. This is then further interpreted as follows: Upper social class for scores 1 and 2, Middle social class for score 3 and Lower social class for scores 4 and 5. Households were categorized as small if they contained ≤ 5 individuals and large if they contained ≥ 6 individuals [18].
RESEARCH ETHICS
A written informed consent was obtained from the caregivers of all the children. Ethical certificate for this study was obtained from the Research and Ethics Committee of University of Benin Teaching Hospital, Benin City, Nigeria; protocol number ADM/E 22/A/ Vol.VII/ 741.
DATA HANDLING AND ANALYSIS
The data obtained in this study was analysed using the statistical package for social sciences (SPSS) version 16.0 (Chicago, Illinois, USA). Further analysis was by GraphPad InStat Software (GraphPad Software Inc, San Digeo 92130, USA) where applicable.
Malaria health indices sought for in this study were malaria morbidity at presentation (severe malaria prevalence) and mortality during the acute phase of the illness (within 72 hours of presentation). Such associations as the relationship between regular use of ITN and sociodemographic characteristics of the study participants as well as malaria vector control practices of caregivers and malaria health indices (severe malaria prevalence and mortality) of their under-fives were analyzed using Chi-square and Fisher's Exact Test where applicable. Regular use of ITN for the under-fives in this study was defined as sleeping in ITN every night for 6 weeks preceeding presentation in the health facility and not just necessarily sleeping in the ITN a night before the survey. Six weeks is the longest expected intrinsic incubation period of Plasmodium falciparum which is the commonest malaria parasite in the study locale [1, 2, 3] Sleeping in the ITN a night before the survey as it is conventionally used in some other studies [19] as demonstration of ITN usage was not employed in this study because such would not give objective assessment of impact of ITN usage on malaria health indices (severe malaria and mortality). All children were recruited in the study the same day of presentation at the health facility. Associations with p-values ≤ 0.05 were further analyzed with Binary Logistic Regression Model to identify factors that independently influenced the outcome variables – severe malaria and mortality. In order to ensure correct specification of the model and how well the model fit the data available (reliability of the model), goodness-of-fit test was performed which showed that N = 2313, correlation coefficient (R) = -0.08, 95% CI = -0.12, -0.04 and R2 (the square of the explained sum of square of test) = 0.006; p < 0.0001(severe malaria) and p = 0.000 (mortality) respectively. This showed that the model has good fitness and reliable in predicting the desired outcomes i.e that the logit model has no omitted variables in failing to accept the null hypothesis. The level of significance of each test was set at p < 0.05.
Results
Three hundred and twenty nine children/caregiver pairs were recruited for the study. Age (mean ± SD) of the children was 20.7 ± 14.0 months and that of their caregivers was 31.2 ± 6.0 years. Among the 329 children, 191/329 (58.1%) were males and 138/329 (41.9%) females. Majority of the caregivers had secondary 128/329 (38.9%) and tertiary 121/329 (36.8%) education; 111/329 (33.7%) belonged to the upper social class, 129/329 (39.2%) to the middle and 89/329 (27.1%) to the lower social class as shown in Table I.
Tab. I.
Socio-demographic characteristics of the study participants.
| Socio-demographic characteristics | N = 329 (%) |
|---|---|
| CHILDREN | |
| Gender | |
| Male | 191 (58.1) |
| Female | 138 (41.9) |
| Age group (months) | |
| < 12 | 108 (32.8) |
| 12 – 23 | 108 (32.8) |
| 24 – 35 | 50 (15.2) |
| 36 – 47 | 31 (9.4) |
| 48 – 59 | 32 (9.7) |
| Family social class | |
| Upper | 111 (33.7) |
| Middle | 129 (39.2) |
| Lower | 89 (27.1) |
| Household size | |
| Small (≤ 5) | 314 (95.4) |
| Large (≥ 6) | 15 (4.6) |
| CAREGIVER | |
| Type of caregiver | |
| Mother | 322 (97.9) |
| Grand-mother | 5 (1.5) |
| Father | 2 (0.6) |
| Age group of caregivers (years) | |
| 16 – 25 | 44 (13.4) |
| 26 – 35 | 220 (66.9) |
| 36 – 45 | 60 (18.2) |
| > 45 | 5 (1.5) |
| Level of education of caregivers/ mothers | |
| Tertiary | 121 (36.8) |
| Secondary | 128 (38.9) |
| Primary | 65 (19.7) |
| No formal | 15 (4.6) |
Three hundred and eighteen (96.7%) caregivers mentioned that malaria is caused by mosquitoes and 312/329 (94.8%) caregivers stated that the disease is preventable. The most common malaria vector control method mentioned by the caregivers was the use of ITN by 92.0%, however, only about a quarter of these caregivers regularly used the ITN for their under-fives. Other methods of malaria vector control mentioned and practiced by the caregivers is shown in Figure 1.
Fig. 1.
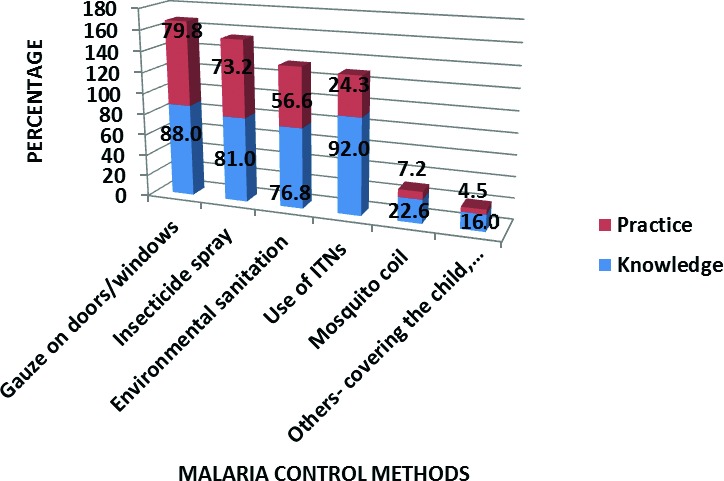
Malaria vector control methods mentioned and practiced by the caregivers.
Concerning ITN ownership, 177 (53.8%) of the 329 caregivers possessed at least one ITN in their homes while 152 (46.2%) did not have any ITN. Figure 2 shows the sources of ITN by the caregivers. Most of the ITNs (52.5%) were obtained during the ITN campaign programme in the State and 18.1% purchased their ITNs. The mean cost of one ITN was One Thousand, Five Hundred and thirty, 95% CI (1242.8, 1817.2) Naira (equivalent to USD 10.00).
Fig. 2.
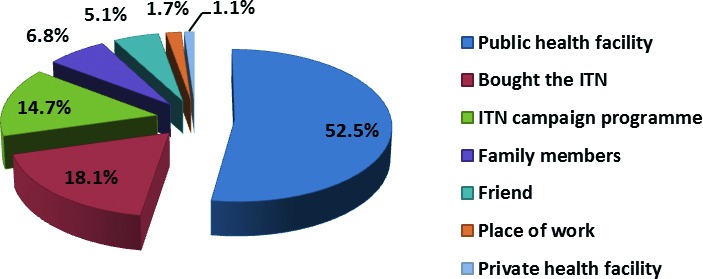
Sources of insecticide treated nets.
Forty-three (24.3%) of the 177 ITN owners stated that their children regularly sleep in it, while 134 (75.7%) did not regularly use the ITN. The mean duration of use of ITN by the children from time of acquisition of the ITN to the time of this study was 13.5, (95% CI 11.7- 15.3) months. Of those who did not regularly use the ITN, 86/134 (64.0%) gave no reasons for not using ITNs while 16 (12.0%), said that it was too hot to sleep in. Reasons for not sleeping in ITN regularly are shown in Figure 3.
Fig. 3.
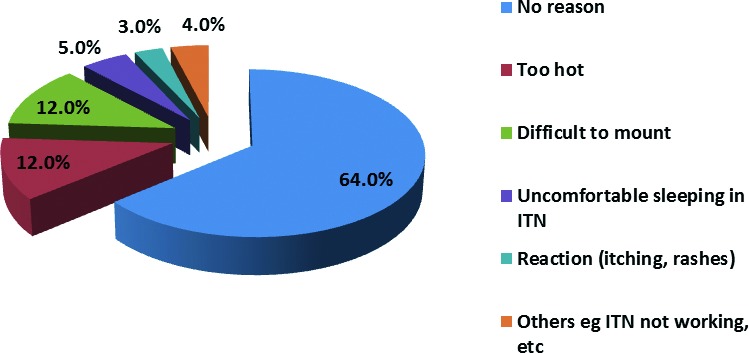
Reasons given by caregivers for not using the insecticide treated nets for their under-fives.
Table II shows factors associated with ITN regular use for the children by 177 caregivers that owned the ITNs. Significantly more caregivers from the lower social class regularly used ITN when compared with caregivers from the middle and upper social classes (χ2 = 7.09, df = 2, p = 0.03). Also households that owned two or more ITNs were statistically significantly more likely to use ITN regularly when compared with households that owned only one ITN (χ2 = 9.61, df = 2, p = 0.01).
Tab. II.
Factors associated with regular use of insecticide treated nets for the children by 177 ITN owners.
| Regular use of insecticide treated nets | |||||
|---|---|---|---|---|---|
| Socio-demographic characteristics | Yes n = 43 (%) |
No n = 134 (%) |
χ2 | df | p |
| Age Group of children (Months) | |||||
| < 12 | 15 (34.9) | 46 (34.3) | |||
| 12 – 23 | 10 (23.3) | 44 (32.8) | |||
| 24 – 35 | 9 (20.9) | 19 (14.2) | 4.68 | 4 | 0.32 |
| 36 – 47 | 6 (14.0) | 9 (6.7) | |||
| 48 – 59 | 3 (6.9) | 16 (12.0) | |||
| Social class | |||||
| Upper | 13 (30.2) | 58 (43.3) | |||
| Middle | 14 (32.6) | 52 (38.8) | 7.09 | 2 | 0.03 |
| Lower | 16 (37.2) | 24 (17.9) | |||
| Household size | |||||
| Small | 40 (93.0) | 130 (97.0) | |||
| Large | 3 (7.0) | 4 (3.0) | * | ** | 0.36 |
| Number of ITN in household | |||||
| One | 13 (30.2) | 67 (50.0) | |||
| Two or more | 30 (69.8) | 67 (50.0) | 4.37 | ** | 0.04 |
| Age group of caregivers (years) | |||||
| 16 – 25 | 8 (18.6) | 16 (12.0) | |||
| 26 – 35 | 24 (55.8) | 94 (70.1) | |||
| 36 – 45 | 11 (25.6) | 22 (16.4) | 4.18 | 3 | 0.24 |
| > 45 | 0 (0.0) | 2 (1.5) | |||
| Level of education | |||||
| Tertiary | 3 (7.0) | 7 (5.2) | |||
| Secondary | 12 (27.9) | 16 (11.9) | 7.72 | 3 | 0.05 |
| Primary | 15 (34.9) | 47 (35.1) | |||
| No formal | 13 (30.2) | 64 (47.8) | |||
Fisher's Exact Test
odds ratio = 0.4, ITN = insecticide treated nets
Prevalence of severe malaria in this study was 36.2%. Of the 329 children, 17 (5.2%) children died; a mortality rate of 52 per 1000.
Table III shows the relationship between malaria vector control methods utilized by the 329 caregivers and the study outcomes. Significantly more children whose caregivers did not use in-door insecticide spray (46.0%) (χ2 = 4.93, p = 0.03), nor had nets on the doors/windows of their houses (47.0%) (χ2 = 4.17, p = 0.04) and, who did not practice regular environmental sanitation (44.0%) (χ2 = 6.51, p = 0.01) presented with severe malaria when compared to children whose caregivers practiced these control methods. Mortality was significantly lower in children who used ITN regularly (p = 0.01) as well as in children whose caregivers used indoor insecticide spray (χ2 = 3.92, p = 0.048), used nets on the doors/windows of their houses (χ2 = 5.00, p = 0.03), and whose caregivers practiced regular environmental sanitation (p = 0.02)]. The duration of use of ITN was not significantly associated with severe malaria (χ2 = 5.64, p = 0.65, 95%CL = 0.58, 0.72), and, all the children that died had never slept in ITN.
Tab. III.
Malaria prevention methods used by the 329 caregivers and their association with study outcomes.
| Prevention Methods | Severe malaria | Mortality | ||
|---|---|---|---|---|
| Yes (%) | No (%) | Yes (%) | No (%) | |
| Insecticide spray | ||||
| Yes (n = 242) | 79 (32.6) | 163 (67.4) | 9 (3.7) | 233 (96.3) |
| No (n = 87) | 40 (46.0) | 47 (54.0) | 8 (9.2) | 79 (90.2) |
| χ2 = 4.93, OR = 0.6, p = 0.03 | χ2 = 3.92, OR = 0.4, p = 0.048 | |||
| Nets on doors/windows | ||||
| Yes (n = 263) | 88 (33.5) | 175 (66.5) | 10 (3.8) | 253 (96.2) |
| No (n = 66) | 31 (47.0) | 35 (53.0) | 7 (10.6) | 59 (89.4) |
| χ2 = 4.17, OR = 0.6, p = 0.04 | χ2 = 5.00, OR = 0.3, p = 0.03 | |||
| Environmental sanitation | ||||
| Yes (n = 188) | 57 (30.3) | 131 (69.7) | 5 (2.7) | 183 (97.3) |
| No (n = 141) | 62 (44.0) | 79 (56.0) | 12 (8.5) | 129 (91.5) |
| χ2 = 6.51, OR = 0.6, p = 0.01 | Fisher's Exact: OR = 0.3, p = 0.02 | |||
| Mosquito coil | ||||
| Yes (n = 24) | 12 (50.0) | 12 (50.0) | 1 (4.2) | 23 (95.8) |
| No (n = 305) | 107 (35.1) | 198 (64.9) | 16 (5.2) | 289 (94.8) |
| χ2 = 2.15, OR = 1.8, p = 0.14 | Fisher's Exact: OR = 0.8, p = 1.00 | |||
| Regular use of ITNs | ||||
| Yes (n = 43) | 20 (46.5) | 23 (53.5) | 0 (0.0) | 43 (100.0) |
| No (n = 134) | 41 (30.6) | 93 (69.4) | 17 (12.7) | 117 (87.3) |
| χ2 = 3.65, OR = 2.0, p = 0.06 | Fisher's Exact: OR = 0.1, p = 0.01 | |||
The Logistic Regression Model using the combined malaria vector control practices of caregivers as dependent variables and the study outcomes (severe malaria and mortality) as independent is shown in Table IV. The model showed that sleeping in ITN regularly, use of insecticide spray, regular environmental sanitation and netting of doors/windows used in combination by caregivers significantly predicted low prevalence of severe malaria.
Tab. IV.
The final Logistic regression model of malaria vector control methods utilized by the 329 caregivers and their predictor on the study outcomes (adjusting for demographic factors).
| Prevention methods | Severe malaria | Mortality |
|---|---|---|
| β (OR) p-value | β (OR) p-value | |
| ITN (n = 43) | 0.20 (1.2) 0.560 | 18.50 (1.1) 1.00 |
| IS (n = 242) | 0.51 (1.7) 0.30 | 1.51 (4.5) 0.18 |
| NDW (n = 263) | 0.43 (1.5) 0.34 | 1.06 (2.9) 0.23 |
| RES (n = 188) | -0.41 (0.7) 0.29 | 18.88 (1.6) 1.00 |
| ITN+IS+NDW+RES (n = 9) | 1.66 (5.2) 0.04 | *-0.65 (0.5) 1.00 |
| IS+NDW+RES (n = 141) | -0.19 (0.8) 0.69 | -18.58 (0.0) 1.00 |
| IS+NDW (n = 212) | -1.24 (0.3) 0.05 | -0.81 (0.4) 0.58 |
p < 0.05
OR = odds ratio, β = measure of how strongly each predictor variable influences the outcome variables. ITN – Regular use of ITN, IS – Use of insecticide spray, NDW – Netting of doors/windows and RES – Regular environmental sanitation (clearing bushes and drainages around the house); Constant for the model was -0.28 (0.8) 0.41 for severe malaria and 1.15 (3.1) 0.01 for mortality.
Discussion
The majority of the caregivers in this study were aware that malaria is preventable. Most of the malaria vector control methods mentioned and practiced by the caregivers was similar to previous documentation by some authors in Nigeria [5, 6]. These included netting of house doors and windows, use of insecticide sprays and environmental sanitation. Knowledge of ITN as a malaria vector control method was high (92.0%). The high knowledge of malaria vector control methods especially of the use of ITN could be attributed to the intensified ITN campaign programme by the NMEP at the Local, State and Federal levels in Nigeria [7, 10]. In 5 years preceeding this study, over 10 million ITN including the Long Lasting Insecticide-treated Nets (LLIN) had been distributed to different households in Nigeria through the house-to-house distribution campaign and the various antenatal/ immunization clinics in the communities [7, 10].
Despite this positive finding on knowledge of malaria vector control methods, there is still a huge gap between knowledge and practice of these methods especially as regards to the use of ITN. In this present study, there was a gap of over 50.0% between ITN ownership (53.8%) and regular usage of 24.3% in under-5s. Hot weather condition was a major factor against regular use of ITNs. Nigeria is a tropical country and the environmental temperature in the study locale usually ranged between 28oC and 38oC [3, 18]. Such hot weather could deter many households from sleeping in the ITN. This is compounded by lack of basic amenities such as electric power and good housing [18]. Nigeria is currently characterized by erratic power supply and poor housing. Sleeping in ITN is usually uncomfortable in hot weather especially in absence of fans and air-conditioners as well as in overcrowded houses with its antecedent poor ventilation.
The number of ITN available in each household has been found to correlate positively with its use in under- 5s [20, 21]. Although effort has been made by the NMEP and other Malaria Control Partners to improve on ITN coverage within the communities, but two ITNs given to each household during the distribution campaign is grossly inadequate[7, 10, 19]. The current Malaria Indicator Survey (MIS) Report still stated that the average number of LLIN per household in Nigeria was 1.6 [19]. This value grossly falls short of the ITN universal distribution goal of at least one net for every two persons [21]. Households that wished to purchase their own LLIN were unable to afford it due to financial constraints. This is because the mean cost of one ITN observed in this study was USD 10.00 which was far beyond the reach of many of the study participants. Most of the study participants were from the lower social class; and these groups also had the lowest ITN ownership rate when compared with those from middle and upper classes. These also were unlikely to access health faciies where they could obtain the LLIN during antenatal and immunization clinics. Therefore, for effective malaria control, universal coverage of ITN and intensified education on its usage for all individuals at risk of malaria in endemic regions remains the goal.
Extrinsic incubation period i.e parasite incubation period in the vector mosquito is temperature dependent.[3] The higher the environmental temperature the shorter the extrinsic incubation period. It has been described in literature that P. falciparum takes 8-11 days to complete the mosquito phase at an optimal ambient temperature of 28°C and 22 days at 20°C. It suffices to say that the extrinsic incubation period of P. falciparum may be shorter at the environmental temperature of the study locale which ranged between 28.0° C and 38.0°C. This assertion coupled with poor environmental sanitation and poverty enhance mosquito breeding sites, allow survival and proliferation of malaria vector and malaria parasite [1, 3, 4]. The implication of these include increased malaria morbidity and antecedent mortality. Most children who presented with severe malaria as well as those who died from the disease in this study were from the lower social class. Despite the beneficial effect of ITN as a veritable tool for malaria vector control, a multi-prong approach to addressing other factors such as basic amenities, environmental sanitation and poverty should be employed in process of eliminating malaria in the country.
The IVC/M advocated by the WHO includes environmental management strategies in view of eliminating mosquito breeding sites (regular environmental sanitation), use of chemicals such as use of in-door insecticide spray, physical barrier methods such as netting of doors/ windows in houses and regular use of ITN [1, 3, 4]. These combined malaria vector control methods significantly predicted low incidence of severe malaria when compared with single vector control methods. Although there is paucity of studies on relationship between IVC/M and malaria outcomes (morbidity and mortality), the observation in this study that combined malaria vector control predicted low incidence of severe malaria is in keeping with the WHO assertion that IVC/M truly reduces malaria outcomes (morbidity and mortality).
LIMITATIONS OF STUDY
Physical verification of ITN ownership and usage was not carried out. This could limit the strength of inferences drawn from the study.
Conclusions and recommendation
Although, most caregivers had good knowledge of malaria vector control, their proportion that possessed and used ITN for malaria control was still low. IVC/M is a veritable tool for malaria vector control, there should be intensified advocacy for scaling-up of ITN distribution and education on utilization of ITN and these integrated malaria vector control methods.
Acknowledgments
The authors thank Miss RoseMary Okonkwo who assisted in data collection, Mr Osas the microscopist, and Mrs Adaeze Nwaneri who edited the manuscript. The authors also thank the nursing staff and resident doctors in children emergency room University of Benin Teaching Hospital Benin City, Nigeria for providing the enabling environment for this study. The authors declare no competing interest.
References
- 1. World Health Organization. World malaria report 2012. Available at http://malaria.who.int/wmr2012/malaria2012 pdf. Accessed on 31/01/2015.
- 2. Malaria: prompt treatment saves lives. USAID Technical Brief Principal Preparer. Available at www.childsurvival.com/documents/trms/tech.cfm. Accessed on 02/07/2014.
- 3.The Federal Ministry of Health, National Malaria and Vector Control Division, Abuja Nigeria. 2015. Case management participant training manual; pp. S1–S134. [Google Scholar]
- 4. Federal Ministry of Health , author. National anti-malaria treatment policy. National Malaria and Vector Control Division, Abuja Nigeria; 2008. pp. 28–31. [Google Scholar]
- 5.Oyewole IO, Ibidapo AC. Attitudes to malaria prevention, treatment and management strategies associated with the prevalence of malaria in a Nigerian urban centre. Afr J Biotech. 2007;6:2424–2427. [Google Scholar]
- 6.Isah EC, Ofili AN, Ogbebor CE, Obahiagbon I, Isah AO. Knowledge of malaria and the practices towards its control among urban dwellers in Benin City. Niger Postgrad Med J. 2007;14(2):125–128. [PubMed] [Google Scholar]
- 7.Kilian A, Koenker H, Baba E, Onyefunafoa EO, Selby RA, Lokko K, Lynch M. Universal coverage with insecticide-treated nets- applying the revised indicators for ownership and use to the Nigeria 2010 malaria indicator survey data. Malar J. 2013;12:314–314. doi: 10.1186/1475-2875-12-314. (S1-S24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Killeen GF, Smith TA, Ferguson HM, Mshinda H, Abdulla S, Lengeler C, Kachur SP. Preventing childhood malaria in Africa by protecting adults from mosquitoes with insecticide-treated nets. PLoS Med. 2007;4:e229–e229. doi: 10.1371/journal.pmed.0040229. (S1-S20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baume CA, Marin MC. Gains in awareness, ownership and use of insecticide-treated nets in Nigeria, Senegal, Uganda, and Zambia. Malar J. 2008;7:153–153. doi: 10.1186/1475-2875-7-153. (S1-S10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Nigeria distributes 10 million ITNs in five years. World Health Organization Bulletin 2007. Available at http://www3.alliance-hpst.org/en. Accessed on 24/05/2014. [Google Scholar]
- 11.Afoakwah C, Nunoo J, Andoh FK. Effect of insecticide-treated bed net usage on under-five mortality in northern Gnaha. Malar J. 2015;14:309–309. doi: 10.1186/s12936-015-0827-8. (S1-S6) DOI 10.1186/s12936-015-0827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loha E, Lunde TM, Lindtjorn B. Effect of bed nets and indoor residual spraying on spatio-temporal clustering of malaria in a village in south Ethiopia: a longitudinal study. PLoS One. 2012;7:e47354–e47354. doi: 10.1371/journal.pone.0047354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Federal Ministry of Health, National Malaria Control Programme , author. Advocacy, communication, and social mobilization strategic framework and implementation plan. 2010. Jun, pp. 1–80.
- 14.Cheesebrough M. Examination of blood for malaria parasite in district laboratory practice in tropical countries. Part 1. second edition. Cambridge University Press; 2005. [Google Scholar]
- 15. NCHS. Clinical growth charts (black and white). CDC Growth Chart, USA. Available at http://www.cdc.gov/growthcharts. Accessed on 13/04/2015.
- 16.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al. Child Health Epidemiological Reference Group of WHO and UNICEF, author. Global, Regional and National causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 17.Olusanya O, Okpere E, Ezimokhai M. The importance of social class in voluntary fertility control in a developing country. W Afr J Med. 1985;4:205–212. [Google Scholar]
- 18. Federal Republic of Nigeria , author. 2006 population and housing census of Nigeria. Lagos: Federal Republic of Nigeria official gazette; 2007. pp. 94–94. [Google Scholar]
- 19. Nigeria Malaria Indicator Survey 2015. National Malaria Elimination Programme Federal Republic of Nigeria March 2016. Available at www.nmisurvey2015-keyindicatorsreport[PR70]. Accessed on 29/04/2016.
- 20.Buchwald AG, Walldorf JA, Cohee LM, Coalson JE, Chimbiya N, Bauleni A, Nkanaunena K, Ngwira A, Kapito-Tembo A, Mathanga DP, et al. Bed nets use among school-aged children after a universal bed net campaign in Malawi. Malar J. 2016;15:127–127. doi: 10.1186/s12936-016-1178-9. (S1-S8) doi: 10.11186/s12936-016- 1178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst KC, Hayden MH, Olsen H, Cavanaugh JI, Ruberto I, Agawo M, Munga S. Comparing ownership and use of bed nets at two sites with differential malaria transmission in Western Kenya. Malar J. 2016;15:217–217. doi: 10.1186/s12936-016-1262-1. (S1-S16). doi: 10.1186/s12936- 016-1262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


