Abstract
Transient elevations of cytosolic Ca2+ are a common mechanism of cellular signaling. In striated muscle, the sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) plays an important role in terminating Ca2+ transients by returning cytosolic Ca2+ to intracellular stores. Stored Ca2+can then be released again for subsequent signaling. We down-regulated SERCA2 gene expression in cultured cardiac myocytes by means of endogenous transcription of small interfering RNA encoded by an exogenous cDNA template. The cDNA template was delivered by adenovirus vector. Reduction of SERCA expression in all myocytes in culture was documented by immunochemistry, real-time RT-PCR, and determination of ATP-dependent Ca2+ transport. The reduction of SERCA2 expression was associated with the up-regulation of transient receptor potential (TRP) channel proteins (TRPC4 and TRPC5) and Na+/Ca2+ exchanger, indicating that intracellular store deficiency was compensated for by Ca2+ fluxes through the plasma membrane. In fact, SERCA silencing was followed by increased transcription of Na+/Ca2+ exchanger, TRPC4, TRPC5, and related transcriptional factors, such as stimulating protein 1, myocyte enhancer factor 2, and nuclear factor of activated cells 4, through activation of calcineurin. This finding demonstrates that the observed compensation occurs through transcriptional crosstalk and the remodeling of Ca2+ signaling pathways. The wide significance of this regulatory mechanism is related to its general involvement in Ca2+ signaling dynamics and in cardiac development and hypertrophy.
Keywords: Na+/Ca2+ exchanger, transient receptor potential channel, calcineurin, small interfering RNA
Numerous cellular functions, including motility, secretion, and gene activation, are triggered by a rise of cytosolic Ca2+ after electrical or chemical excitation of the plasma membrane (1, 2). Trigger Ca2+ derives from intracellular stores and/or extracellular fluids and reaches the cytosol by diffusion through gated channels as permitted by large concentration gradients. When membrane excitation ceases, Ca2+ is returned to intracellular stores by the sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) or to extracellular fluid by the plasma membrane Ca2+ ATPase and the Na+/Ca2+ exchanger (NCX).
In cardiac muscle, intracellular Ca2+ stores and the Ca2+ ATPase (SERCA2 isoform) of sarcoplasmic reticulum play a prominent role in contractile activation and relaxation (3–5). In fact, SERCA overexpression in transgenic animals (6, 7) and in cultured myocytes (8–11) improves the signaling of Ca2+ spikes under limiting conditions. On the other hand, SERCA2 gene-targeting does not allow development of homozygous mice with SERCA2-null mutations, whereas heterozygous mutants manifest altered Ca2+ homeostasis in cardiac muscle (12, 13). We report here that SERCA2 gene expression can be strongly reduced in cultured cardiac myocytes by endogenous production of small interfering RNA (siRNA) encoded by an exogenous cDNA template. Adenovirus vectors ensure homogeneous delivery of the cDNA template, resulting in the down-regulation of SERCA2 expression in all cells in culture. We were then able to study the primary effects of SERCA down-regulation as well as the secondary effects on expression of functionally related genes and remodeling of the Ca2+ signaling pathways.
Methods
siRNA Construct and Adenoviral Vectors. DNA templates for the synthesis of silencing RNA were cloned into a pSilencer plasmid under the control of the U6 RNA Polymerase III promoter (–315 to +1) (Ambion, Austin, Tx). The selection of the coding sequence for targeting rat Ca2+ ATPase mRNA was done by using the siRNA Target Finder and Design tool from Ambion. The potential siRNA target sequence was subjected to a blast search against EST libraries of rat to ensure that no other gene(s) was targeted. The target sequence for rat SERCA2a mRNA was 5′-A AGACT TACTAGT TAGA AT T T-3′. The sequence started at position 173 and had a GC content of 23.8%. To obtain transcription of a complementary sequence to the target, we designed the following sequence, where the segment in italic type indicates the loop: sense, 5′-GACTTACTAGTTAGAATTTGGCTAAGAGCAAATTCTAACTAGTAAGTCTTTTT-3′; antisense, 3′-CCGGCTGAATGATCAATCTTAAACCGATTCTCGTTTAAGATTGATCATTCAGAAAAATTAA-5′. The plasmid and the oligonucleotides were digested at the ApaI and EcoRI sites and then ligated together. The position of the DNA oligonucleotide was such that it was immediately preceded by the U6 promoter. The ligated DNA was transformed into competent DH5α cells, and the cells were selected for ampicillin resistance. The diagram of the template construct is presented in Fig. 6, which is published as supporting information on the PNAS web site.
Adenoviral vectors were constructed from a pAd-lox plasmid with a simian virus 40 polyadenylation signal (14). The U6 promoter and siRNA construct were subcloned into the pAd-lox plasmid. Both the silencing construct and the control construct with just the promoter were cotransfected separately with purified ψ5 adenovirus genome into CRE8 cells (15).
Cell Culture and Infections. Cultures of HEK-293 and CRE8 cells for recombination and amplification of adenoviral preparations were maintained according to ref. 14. DC3F cells (Chinese hamster lung fibroblasts) were maintained as described in ref. 16. Primary myocyte cultures were prepared as described for embryonic chicken (17) and neonatal rat (18). Infections with adenovirus vectors, empty or containing cDNA templates, were performed by using viral titers of either 2–4 plaque-forming units per cell for rat or 10 plaque-forming units per cell for chicken myocytes (14). Cells were analyzed 48–72 h after infection.
Western Blots and in Situ Immunostaining. Protocols described in ref. 14 were followed. The primary monoclonal antibodies that were used were CaS-3H2 for chicken SERCA2a (1:1,000) (17), MA3–919 (1:250) (Affinity BioReagents, Golden, CO), 2A7-A1 (kindly supplied by Larry Jones, Indiana University, Indianapolis) for rat SERCA2a, actin (Ab-1) (1:2,000) (Oncogene Research Products, San Diego) for actin in both chicken and rat cardiac myocytes, NCX antibody (1:500) from Swant (Bellinzona, Switzerland), and antibodies to canonical transient receptor potential channel (TRPC) proteins TRPC4 and TRPC5 were obtained from Sigma and Alomone Labs (Jerusalem, Israel), respectively.
Microarray Analysis, Real-Time RT-PCR, and Ca2+ Transport Studies. Total RNA was isolated by using TRIzol-LS reagent and the protocol from Invitrogen. The isolated RNA was subjected to microarray analysis by using a Ca2+ signaling nuclear factor of activated T cells (NFAT) chip constructed by SuperArray Bioscience (Frederick, MD; catalog no. MM-022-2). The microarray chip includes 96 genes related to Ca2+ signaling. Genes showing significant change were further evaluated by time-resolved RT-PCR (see Table 1, which is published as supporting information on the PNAS web site, for list of primers). Functional Ca2+ uptake measurements were performed by using whole-myocyte homogenates as described in ref. 17.
Voltage Clamp Experiments. Myocytes grown on microscope coverslips were voltage-clamped in the whole-cell configuration (19) by using a Heka EPC-10 amplifier and patchmaster software. Pipettes had resistances of 1.5–3 MÙ. Values for series resistances were 3–8 MÙ, and were compensated up to 60%. The bathing solution contained (): 135 mM NaCl, 5 mM KCl, 20 mM Hepes, 2 mM CaCl2, 10 mM Glucose, and 0.8 mM MgSO4, pH 7.4. Na+-free solutions contained equimolar amounts of N-methyl-d-glucamine. The internal solution contained 100 mM Cs glutamate, 30 mM CsCl, 20 mM Hepes, 20 mM tetraethylammonium-Cl, 4 mM Mg-ATP, 3 mM K-phosphocreatine, 0.33 mM MgCl2, 0.1 mM EGTA, and 0.05 mM fluo-4, pH 7.2. The temperature was maintained at 30°C by a regulated solution heater. The holding potential was –80 mV.
Internal Ca2+ Imaging and Calcineurin Activity. Cytosolic Ca2+ was determined by the fluorescence of fluo-4 with a Bio-Rad Radiance confocal scanner operating in line-scan mode mounted on an Olympus IX-70 inverted microscope. Measured fluorescence (F) was converted to Ca2+ by Ca2+ = F Kd/(((CaR + Kd)/CaR)FR – F) (20), where Kd is the dissociation constant of Ca-fluo-4 (0.8 μM), CaR is the assumed value of resting Ca2+ (100 nM), and FR is the fluorescence measured at CaR. Alternatively, cytosolic Ca2+ or Ba2+ were determined in myocytes grown on cover slips by using fura-2 acetoxymethyl ester as the indicator, monitored in an InCyt dual-wavelength fluorescence imaging system (Intracellular Imaging, Cincinnati) as described in ref. 21. Calcineurin activity was estimated by using the protocol recommended for a PathDetect NFAT cis-reporting system (Stratagene) with pNFAT-luciferase as the reporter plasmid.
Results
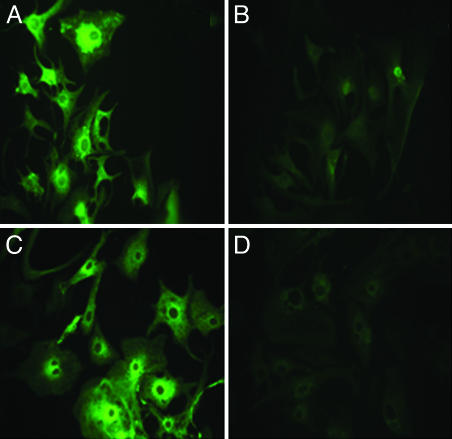
SERCA2 Gene Expression in Cultured Cardiac Myocytes Is Down-Regulated After Endogenous Transcription of siRNA Encoded by Exogenous cDNA Template. Reduction of SERCA2 expression in cardiac myocytes after delivery of cDNA templates for endogenous expression of siRNA was documented by immunostaining and fluorescence microscopy, real-time RT-PCR, Western blots, and measurements of Ca2+ transport. Wide microscopic fields of neonatal rat and chicken embryo cardiac myocytes, stained with primary SERCA monoclonal and secondary fluorescent antibodies, are shown in Fig. 1. Staining is noted in the control myocytes, with perinuclear prominence likely caused by active SERCA expression as is often observed in proliferating cells or after exogenous SERCA gene transfer. However, it is clear that most myocytes exposed to the cDNA templates exhibit reduction of SERCA levels. We attribute such a reduction to posttranscriptional siRNA interference with SERCA expression in developing and proliferating myocytes. SERCA expression is reduced in all myocytes visible in the field, indicating a very efficient delivery of cDNA templates by the adenovirus vector.
Fig. 1.
Immunostaining of endogenous SERCA2a in neonatal rat (A and B) or embryonic chicken (C and D) myocytes. The myocytes were infected with adenoviral vectors containing just the U6 promoter (A and C) or the U6 promoter followed by the RNAi template (B and D). Forty-eight hours after infection, the cells were harvested for staining. Expression of endogenous SERCA2a is significantly decreased by infection with the siRNA-containing vector in both rat and chicken cardiac myocytes.
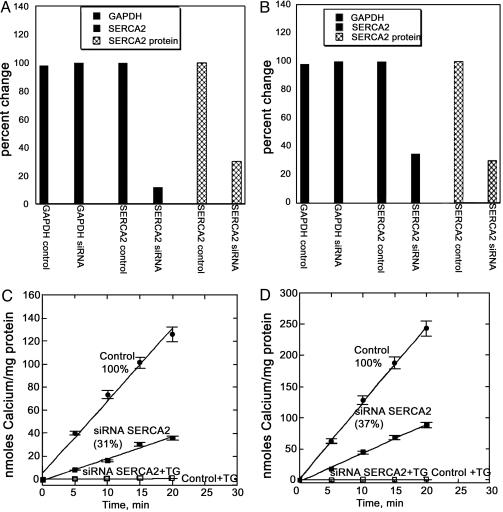
It should be noted that the rat SERCA2 cDNA 173–193 segment was selected for design of the template, resulting in a 21-bp match. This template finds a continuous 20-bp match in the 173–192 chicken SERCA2 cDNA segment. On the other hand, a corresponding 21-bp segment, with a mismatch at position 17, is present in the 173–193 segment of the SERCA2 cDNA in DC3F cells. We found that this mismatch in the middle of the template interfered with SERCA-silencing in DC3F cells, underscoring the specificity of the procedure. Consistent with immunostaining in situ, we found that the silencing procedure reduced ATP-dependent Ca2+ transport activity by 70% in rat and 67% in chicken myocytes (Fig. 2 C and D). Interestingly, RT-PCR shows a more pronounced reduction of SERCA mRNA compared to Western blotting and Ca2+ transport. This finding is likely due to drastic interference and specific degradation of mRNA by siRNA, whereby expression of SERCA protein is prevented in the developing myocytes. On the other hand, the low levels of residual SERCA protein and transport activity correspond to enzyme already present at the time of exposure to the cDNA templates. It is noteworthy that high titers of adenovirus vector carrying the cDNA templates did not produce any reduction of SERCA protein and transport activity in adult rat cardiac myocytes (not shown), indicating that our procedure of RNA interference (RNAi) is effective only in myocytes undergoing development.
Fig. 2.
Real-time RT-PCR, Western blot, and functional analysis of endogenous SERCA2a in neonatal rat (A and C) or embryonic chicken (B and D) myocytes. RT-PCR and Western blots (A and B) as well as Ca2+ transport (C and D) measurements demonstrate down-regulation of endogenous SERCA2a expression in both rat and chicken cardiac myocytes after infection with adenoviral vectors containing the U6 promoter and the RNAi template. Control myocytes were infected with vectors containing the U6 promoter only. GAPDH and actin (data not shown) were used as standards for analysis of the total RNA (RT-PCR) or protein (Western blot) samples, respectively.
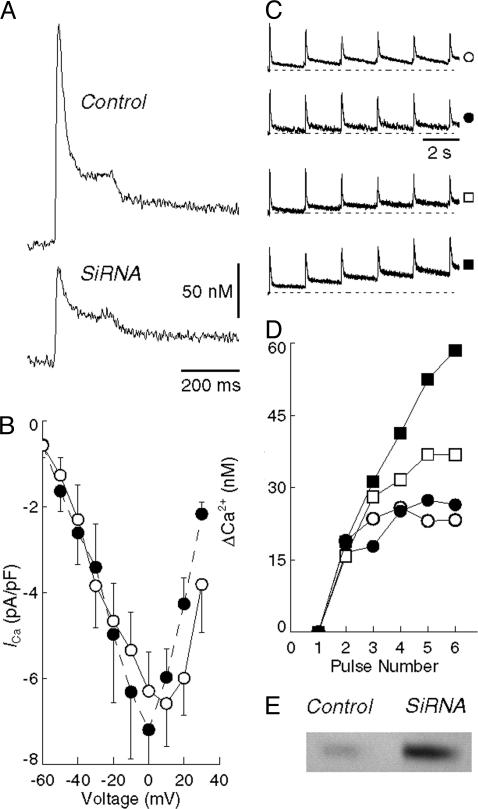
After SERCA Down-Regulation, the Contribution of Internal Stores to Signaling-Spikes of Cytosolic Ca2+ Is Reduced, Whereas Entry of External Ca2+ Through TRPC Channels and Exit Through NCX Are Increased. We assessed the effects of reduced SERCA2-expression levels on Ca2+ signaling in voltage-clamped neonatal rat myocytes while monitoring the fluorescence of the indicator fluo-4 and assuming a 100 nM value for the resting (i.e., diastolic) Ca2+ for all experimental groups (20). It is apparent in Fig. 3A that the Ca2+ transients, after step depolarizations, have lower amplitude in the myocytes subjected to SERCA RNA interference, demonstrating a reduced sarcoplasmic reticulum Ca2+ load. In fact, statistical analysis shows lower values for peak Ca2+ (0.08 ± 0.02 μM, mean ± SEM) and full-width at half maximum (73.8 ± 15.4 ms) in the silenced myocytes compared with control myocytes (0.22 ± 0.04 μM and 131.8 ms). These differences are unambiguously significant (P < 0.05; Student's two-tailed t test).Furthermore, we derived two exponential rate constants showing a systematic reduction of the rate of decline of Ca2+ for both the fast and slower phases in the siRNA cells (51.6 ± 6.7 ms and 641 ± 49.5 ms) as compared with control cells (36.5 ± 7.5 ms and 566 ± 222.8 ms). However, the difference in rate constants is not quite statistically significant, possibly because of the smaller elevation of Ca2+ in the siRNA myocytes.
Fig. 3.
Myoplasmic Ca2+ and l-type Ca2+ currents in neonatal rat myocytes. (A) Average myoplasmic Ca2+ transients recorded during a step depolarization to 0 mV in control (Upper, n = 8) and siRNA (Lower, n = 5) myocytes. (B) Current–voltage relationship for control (○, n = 7) and siRNA (•, n = 4) myocytes. Points represent the mean ± SEM peak inward current (200 ms, P/4 leak correction) with a Na+-free bathing solution. (C) Ca2+ transients in response to a series of six voltage-clamp depolarizations (data not shown) to –10 mV at 2-s intervals. The top two records are from control myocytes in Na+-containing (○, n = 6) and Na+-free (•, n = 3) bathing solution, respectively. The two lowermost records are from siRNA myocytes in Na+-containing (□, n = 5) and Na+-free (▪, n = 4) solution. The symbols to the right of each record indicate the corresponding trace in D. Each record is normalized to the peak of its first transient. (D) The elevation of myoplasmic Ca2+ before each of six test pulses from records shown in C, plotted as the difference (ΔCa2+) from the resting level (before the first transient). Note that the Ca2+ does not recover completely by the time of the next pulse, with the result that diastolic Ca2+ slowly accumulates. The degree of accumulation is greater in siRNA myocytes (□) than in controls (○). Also, functional suppression of NCX by Na+-free bathing solution results in a further increase in the accumulation of diastolic Ca2+ of siRNA myocytes (compare □ and ▪) but not in control cells (compare ○ and •). The accumulation of diastolic Ca2+ reflects the compromised ability of the siRNA cells to remove myoplasmic Ca2+ after a transient, which is further slowed in Na+-free bathing solution by suppression of NCX. (E) Western blot of the expression of NCX (see Methods) in control and siRNA myocytes, showing that NCX expression is 5-fold greater in siRNA cells compared with controls.
With regard to possible sarcolemmal contribution to Ca2+ signaling, it is shown in Fig. 3B that the function of l-type Ca2+ channels was not significantly different at any voltage as the result of the silencing procedure. We then tested the ability of the myocytes to remove cytosolic Ca2+ in response to a series of voltage-clamp depolarizations. These experiments were conducted in the presence and absence of Na+ to evaluate the contribution of NCX (22) to cytosolic Ca2+ removal. In the absence of Na+, the ability to remove cytosolic Ca2+ is significantly reduced in myocytes subjected to SERCA RNAi (Fig. 3 C and D), indicating that removal of cytosolic Ca2+ by NCX acquires a greater role after exposure to SERCA RNAi. In fact, marked up-regulation of NCX protein was demonstrated by Western blotting (Fig. 3E).
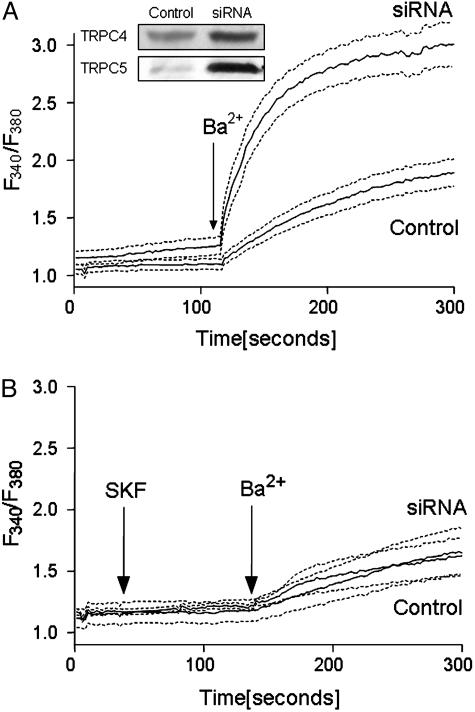
In additional experiments, we measured Ba2+ influx in fura-2-loaded myocytes to evaluate the possible effect of SERCA silencing on TRPC channels. To this aim, we used myocytes treated with thapsigargin to block SERCA pump activity. The use of thapsigargin-treated myocytes has the combined effect of eliminating RNAi-induced differences in SERCA pumping and providing store-dependent activation of TRPC channels (23). In addition, we included nifedipine to inhibit l-type Ca2+ channels, and we replaced Na+ in the medium with N-methyl-d-glucamine to prevent changes in membrane potential and possible Ba2+ exchange with Na+ through the NCX. In these experiments, the myocytes did not manifest contractile activity and, because of the medium composition, did not undergo membrane depolarization or display l-type Ca2+ channel activity. After depletion of intracellular stores, in the absence of extracellular Ca2+, we added Ba2+ (which cannot be pumped by the plasmalemmal Ca2+ ATPase) to assess divalent cation entry through the plasma membrane. We observed Ba2+ fluxes that were strongly reduced by the TRPC inhibitor SKF96365 (24). It is shown in Fig. 4 that the well resolved initial rates of Ba2+ influx are greater in the silenced cells than in the control cells, which is consistent with the up-regulation of TRPC4 and TRPC5 (25–29), as shown directly by Western blots (Fig. 4) and RT-PCR (Fig. 5). It is important to note, however, that significant influx through TRPCs occurs even in control myocytes (Fig. 4). This may then play a role in transcriptional activation following SERCA silencing and Ca2+ depletion of internal stores, as described below.
Fig. 4.
Silencing the SERCA2a gene in neonatal rat cardiac myocytes results in increased Ba2+ entry through TRPCs. The myocytes were infected with empty or RNAi template vectors as described in Fig. 1. Seventy-two hours after infection, the cells, which were grown on coverslips, were loaded with fura-2 acetoxymethyl ester, and the medium was changed to one that was Ca2+-free and in which Na+ was replaced by N-methyl-d-glucamine. (A) After 8 min of exposure to 2 μM thapsigargin and 2 min of exposure to 10 μM nifedipine, 1.0 mM BaCl2 was added by medium replacement, and the fura-2 fluorescence was monitored with an InCyt imaging system. (B) The experiments were repeated in the presence of 10 μM SKF96365. Each fluorescence trace is the average derived from 10–15 cells in each of three different experiments. The dashed lines delimit the SE for each condition. The Western blots in A demonstrate the increased expression of TRPC4 and TRPC5 protein after SERCA silencing.
Fig. 5.
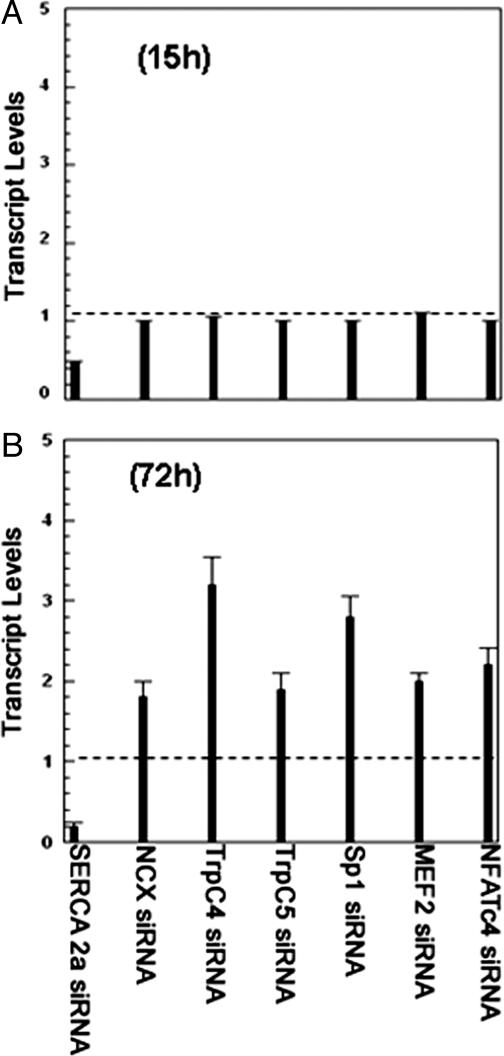
Effects of silencing the SERCA2a gene in neonatal rat cardiac myocytes on various Ca2+ signaling genes as tested by real-time RT-PCR. (A) Infection of myocytes with an RNAi template leads to reduced SERCA transcription within 15 h after infection, with no change in the transcriptional level of other genes. (B) Seventy-two hours after infection, SERCA transcription is further reduced, whereas increased transcription of NCX (1.7-fold) and TRPCs (2- to 3-fold) is noted. Transcription levels of the Sp1 promoter (2.8-fold); in addition, MEF2 and NFATc4 transcription factors are also increased (∼2-fold). Note that the dashed lines indicate normal transcript levels for these genes. In all of the experiments, GAPDH served as an internal control for all of the PCRs carried out, and the threshold count values were normalized to GAPDH before calculating the fold change for all of the genes. The data sets for each of the genes shown were subjected to unpaired two-tailed t test. Confidence limits: NCX, 0.02; MEF2, 0.034; Sp1, 0.035; TRPC4 and TRPC5, 0.049; NFATc4, 0.055.
Reduced SERCA Levels and Function Lead to Transcriptional Crosstalk and Remodeling of Ca2+ Signaling Pathways. Considering the lower contribution of intracellular Ca2+ stores after reduction of SERCA by RNAi and the compensatory increase in Ca2+ fluxes through the plasmalemmal proteins, we extracted total RNA to survey the behavior of several genes associated with signal transduction through Ca2+, calcineurin and the NFAT transcription complex. After a preliminary microarray analysis, we pursued more quantitatively, by real-time RT-PCR (Table 1), specific variations suggested by the initial survey. It is shown in Fig. 5A that at an early time point (15 h) after infection, the SERCA transcript was reduced to less than half, whereas transcription of all other genes remained normal. At a later time point (72 h after infection), the reduction of SERCA2 transcription was accompanied by increased transcription of NCX, TRPC4, TRPC5, stimulating protein 1 (Sp1), myocyte enhancer factor 2 (MEF2), and NFATc4 (Fig. 5). The greatest variation was observed for TRPC4 and Sp1. No change was observed in the transcription of plasma membrane Ca2+ ATPase 1, l-type Ca2+ channel, phospholamban, calcineurin, calmodulins 1 and 3, casein kinase 2, natriuretic peptide precursor type B, or protein phosphatase 3 catalytic subunit beta isoform. Consistent with the effects on rat, a 55–60% reduction in SERCA transcription and a 2-fold increase in NCX and MEF2 transcription were detected in chicken myocytes. The time-dependence of transcriptional changes of specific genes, as well as the lack of effect on several other genes, indicates that the observed effects are related primarily to SERCA down-regulation to provide alternate pathways for Ca2+ signaling. It is apparent that as a consequence of SERCA down-regulation, the calcineurin-dependent complex is activated, leading to increased expression of Sp1, MEF2, NFATc4 transcriptional factors, and, in turn, Ca2+-handling proteins. We then obtained estimates of calcineurin activity by enzymatic assay of luciferase in the myocytes after luciferase gene transfections under the control of calcineurin-dependent NFAT promoter. We found that the luciferase level was 30–40% higher in the silenced myocytes compared with control myocytes, indicating activation of calcineurin by SERCA2 silencing. It is interesting that calcineurin transcription was not increased as a consequence of SERCA silencing, but its function was activated.
Discussion
RNAi is a mechanism of posttranscriptional control whereby siRNAs induce specific degradation of mRNA with complementary sequence by acting as guides for enzymes that cleave the target mRNA (30). However, the poor efficiency (i.e., percentage of cells affected) of cardiac myocyte transfection with siRNA has thwarted previous attempts at gene silencing in these cells. We report here that effective reduction of SERCA2 gene expression can be obtained by the endogenous production of siRNA after introduction of a cDNA template with the aid of adenovirus vectors. Transcription of fold-back stem-loop structures yielding siRNAs occurs through intracellular processing; the adenovirus vector ensures efficient delivery of the cDNA template to all cells in culture. Endogenous production of siRNA, encoded by exogenous cDNA templates, is a highly efficient method of regulating specific genes in cultured cardiac myocytes and is likely to become a useful and convenient complement to gene targeting in whole animals.
The information gained with our experiments on SERCA2 down-regulation was used to clarify the mechanism of compensation for Ca2+ signaling. In fact, reduced levels of SERCA2 protein were demonstrated by immunochemistry in situ (Fig. 1) as well as by Western blots and direct measurements of ATP-dependent Ca2+ transport in cell homogenates (Fig. 2). Nevertheless, the myocytes retained the ability to develop transient elevations of cytosolic Ca2+ upon membrane stimulation. The reduction of SERCA2 expression was associated with an increase in TRPC expression and activity under conditions of internal-store Ca2+ depletion (Fig. 4), which may compensate for reduced sarcoplasmic reticulum Ca2+ uptake and cycling. Likewise, the reduced SERCA2 function was associated with increased NCX expression and activity (Fig. 3), which may compensate for reduced sarcoplasmic reticulum Ca2+ cycling by enhancing transsarcolemmal Ca2+ cycling by means of NCX.
Most importantly, our study demonstrates that the mechanism of remodeling is based on transcriptional activation, which includes up-regulation of TRPC and NCX transcripts and leads to increased expression of the proteins directly involved in compensatory Ca2+ import and export, respectively. Furthermore, we demonstrate up-regulation of transcriptional factors Sp1, MEF2, and NFATc4 (Fig. 5), indicating that the mechanism of remodeling involves the calcineurin and NFAT transcriptional complex (31, 32). It is apparent that as a consequence of SERCA down-regulation and inadequate removal of cytosolic Ca2+, the calcineurin-dependent complex is activated, leading to up-regulated expression of Sp1, MEF2, NFATc4, and, in turn, Ca2+-handling proteins. Therefore, perturbation of Ca2+ homeostasis plays a direct role in evoking the transcriptional crosstalk that leads to the remodeling of Ca2+ signaling pathways (1, 33).
It is noteworthy that increased levels of NCX have been previously detected in conjunction with SERCA down-regulation in transgenic animals and in failing heart muscle (34–37). Our experiments indicate that up-regulation of NCX is likely to provide assistance in the export of cytosolic Ca2+ in the normal exchange direction (Fig. 3) and Ca2+ import by reverse exchange (38, 39). Our findings also suggest that import of extracellular Ca2+ occurs through increased expression of TRPCs. The importance of transient receptor potential-mediated capacitative Ca2+ entry was previously noted in cardiac myocytes subjected to experimental hypertrophy (40). In conclusion, our data demonstrate transcriptional crosstalk and compensatory expression of genes encoding Ca2+-handling proteins triggered by SERCA down-regulation. The information so derived may provide a better understanding of the pathogenic role played by inadequate SERCA expression in heart failure. The broad significance of this regulatory mechanism is related to its general involvement in Ca2+ signaling mechanisms not only for excitation–contraction coupling but also for cardiac development, remodeling, and hypertrophy (41–44).
Supplementary Material
Acknowledgments
We thank Drs. Derek Damron, William DuBell, and Terry Rogers for useful suggestions and help in the initial phase of this work; Dr. Sanjeev Ahuja for help in the statistical analysis; Dr. Larry Jones for the SERCA2a antibody; and Yueli Hua and students Cheng Xu and Joy Wan for technical assistance. This work was supported by the Human Frontier Science Program (G.I.) and National Institutes of Health Grants HL69830 (to G.I.) and HL55426 and AI058173 (to D.L.G.).
Author contributions: G.I. designed research; M.S., C.S., S.P.M., D.L., and M.G.K. performed research; M.G.K. and D.G. contributed new reagents/analytic tools; M.S., C.M.S., S.P.M., M.G.K., and J.S. analyzed data; G.I. wrote the paper; and A.H. provided the cDNA sequence for the DC3F line.
Abbreviations: SERCA, sarco(endo)plasmic reticulum Ca2+ ATPase; NCX, Na+/Ca2+ exchanger; siRNA, small interfering RNA; RNAi, RNA interference; TRPC, transient receptor potential channel; NFAT, nuclear factor of activated T cells; MEF2, myocyte enhancer factor 2; Sp1, stimulating protein 1.
References
- 1.Carafoli, E., Santella, L., Branca, D. & Brini, M. (2001) Crit. Rev. Biochem. Mol. Biol. 36, 107–260. [DOI] [PubMed] [Google Scholar]
- 2.Berridge, M. J., Bootman, M. D. & Roderick, H. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 517–529. [DOI] [PubMed] [Google Scholar]
- 3.Eisner, D. A., Choi, H. S., Diaz, M. E., O'Neill, S. C. & Trafford, A. W. (2000) Circ. Res. 87, 1087–1094. [DOI] [PubMed] [Google Scholar]
- 4.Bers, D. M. (2002) Nature 415, 198–205. [DOI] [PubMed] [Google Scholar]
- 5.MacLennan, D. H. & Kranias, E. G. (2003) Nat. Rev. Mol. Cell Biol. 4, 566–577. [DOI] [PubMed] [Google Scholar]
- 6.Baker, D. L., Hashimoto, K., Grupp, I. L., Ji, Y., Reed, T., Loukianov, E., Grupp, G., Bhagwhat, A., Hoit, B., Walsh, R., et al. (1998) Circ. Res. 83, 1205–1214. [DOI] [PubMed] [Google Scholar]
- 7.Loukianov, E., Ji, Y., Grupp, I. L., Kirkpatrick, D. L., Baker, D. L., Loukianova, T., Grupp, G., Lytton, J., Walsh, R. A. & Periasamy, M. (1998) Circ. Res. 83, 889–897. [DOI] [PubMed] [Google Scholar]
- 8.Hajjar, R. J., Kang, J. X., Gwathmey, J. K. & Rosenzweig, A. (1997) Circulation 95, 423–429. [DOI] [PubMed] [Google Scholar]
- 9.He, H., Giordano, F. J., Hilal-Dandan, R., Choi, D. J., Rockman, H. A., McDonough, P. M., Bluhm, W. F., Meyer, M., Sayen, M. R., Swanson, E., et al. (1997) J. Clin. Invest. 100, 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giordano, F. J., He, H., McDonough, P., Meyer, M., Sayen, M. R. & Dillmann, W. H. (1997) Circulation 96, 400–403. [DOI] [PubMed] [Google Scholar]
- 11.Inesi, G., Lewis, D., Sumbilla, C., Nandi, A., Strock, C., Huff, K. W., Rogers, T. B., Johns, D. C., Kessler, P. D. & Ordahl, C. P. (1998) Am. J. Physiol. 274, C645–C653. [DOI] [PubMed] [Google Scholar]
- 12.Periasamy, M., Reed, T. D., Liu, L. H., Ji, Y., Loukianov, E., Paul, R. J., Nieman, M. L., Riddle, T., Duffy, J. J., Doetschman, T., et al. (1999) J. Biol. Chem. 274, 2556–2562. [DOI] [PubMed] [Google Scholar]
- 13.Ji, Y., Lalli, M. J., Babu, G. J., Xu, Y., Kirkpatrick, D. L., Liu, L. H., Chiamvimonvat, N., Walsh, R. A., Shull, G. E. & Periasamy, M. (2000) J. Biol. Chem. 275, 38073–38080. [DOI] [PubMed] [Google Scholar]
- 14.Ma, H., Sumbilla, C. M., Farrance, I. K., Klein, M. G. & Inesi, G. (2004) Am. J. Physiol. 286, C556–C564. [DOI] [PubMed] [Google Scholar]
- 15.Hardy, S., Kitamura, M., Harris-Stansil, T., Dai, Y. & Phipps, M. L. (1997) J. Virol. 71, 1842–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu, M., Zhang, L., Rishi, A., Khaedeer, M., Inesi, G. and Hussain, A. (1998) J. Biol. Chem. 273, 3542–3546. [DOI] [PubMed] [Google Scholar]
- 17.Cavagna, M., O'Donnell, J. M., Sumbilla, C., Inesi, G. & Klein, M. G. (2000) J. Physiol. (London) 528, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright, G., Singh, I. S., Hasday, J. D., Farrance, I. K., Hall, G., Cross, A. S. & Rogers, T. B. (2002) Am. J. Physiol. 282, H872–H879. [DOI] [PubMed] [Google Scholar]
- 19.Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. (1981) Pflügers Arch. 391, 85–100. [DOI] [PubMed] [Google Scholar]
- 20.Cannell, M. B., Cheng, H. & Lederer, W. J. (1994) Biophys. J. 67, 1942–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatachalam, K., Zheng, F. & Gill, D. L. (2003) J. Biol. Chem. 278, 29031–29040. [DOI] [PubMed] [Google Scholar]
- 22.Philipson, K. D., Nicoll, D. A., Ottolia, M., Quednan, B. D., Reuter, H., John, S. & Qiu, Z. (2002) Ann. N.Y. Acad. Sci. 976, 1–10. [DOI] [PubMed] [Google Scholar]
- 23.Zeng, F., Xu, S. Z., Jackson, P. K., McHugh, D., Kumar, B., Fountain, S. J. & Beech, D. J. (2004) J. Physiol. (London) 559, 739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boulay, G., Zhu, X., Peyton, M., Jang, M., Hurst, R., Stefani, E. & Birnbaumer, L. (1997) J. Biol. Chem. 272, 29672–29680. [DOI] [PubMed] [Google Scholar]
- 25.Clapham, D. E., Runnels, L. W. & Strubing, C. (2001) Nat. Rev. Neurosci. 2, 387–396. [DOI] [PubMed] [Google Scholar]
- 26.Minke, B. & Cook, B. (2002) Physiol. Rev. 82, 429–472. [DOI] [PubMed] [Google Scholar]
- 27.Montell, C., Birnbaumer, L. & Flockerzi, V. (2002) Cell 108, 595–598. [DOI] [PubMed] [Google Scholar]
- 28.Vennekens, R., Voets, T., Bindels, R. J., Droogmans, G. & Nilius, B. (2002) Cell Calcium 31, 253–264. [DOI] [PubMed] [Google Scholar]
- 29.Venkatachalam, K., van Rossum, D. B., Patterson, R. L., Ma, H. T. & Gill, D. L. (2002) Nat. Cell Biol. 4, E263–E272. [DOI] [PubMed] [Google Scholar]
- 30.Nykanen, A., Haley, B. & Zamore, P. D. (2001) Cell 107, 309–321. [DOI] [PubMed] [Google Scholar]
- 31.Crabtree, G. R. (1999) Cell 96, 611–614. [DOI] [PubMed] [Google Scholar]
- 32.Mellstrom, B. & Naranjo, J. R. (2001) Curr. Opin. Neurobiol. 11, 312–319. [DOI] [PubMed] [Google Scholar]
- 33.Carafoli, E., Genazzani, A. & Guerini, D. (1999) Biochem. Biophys. Res. Commun. 266, 624–632. [DOI] [PubMed] [Google Scholar]
- 34.Hasenfuss, G. (1998) Cardiovasc. Res. 37, 279–289. [DOI] [PubMed] [Google Scholar]
- 35.Reed, T. D., Babu, G. J., Ji, Y., Zilberman, A., Ver, H. M., Wuytack, F. & Periasamy, M. (2000) J. Mol. Cell Cardiol. 32, 453–464. [DOI] [PubMed] [Google Scholar]
- 36.Terracciano, C. M., Philipson, K. D. & MacLeod, K. T. (2001) Cardiovasc. Res. 49, 38–44. [DOI] [PubMed] [Google Scholar]
- 37.Plank, D. M., Yatani, A., Ritsu, H., Witt, S., Glascock, B., Lalli, M. J., Periasamy, M., Fiset, C., Benkusky, N., Valdivia, H. H., et al. (2003) Am. J. Physiol. 285, H305–H315. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, X. Q., Song, J., Rothblum, L. I., Lun, M., Wang, X., Ding, F., Dunn, J., Lytton, J., McDermott, P. J. & Cheung, J. Y. (2001) Am. J. Physiol. 281, H2079–H2088. [DOI] [PubMed] [Google Scholar]
- 39.Piacentino, V., III, Margulies, K. B. & Houser, S. R. (2002) Ann. N.Y. Acad. Sci. 976, 476–477. [DOI] [PubMed] [Google Scholar]
- 40.Hunton, D. L., Lucchesi, P. A., Pang, Y., Cheng, X., Dell'Italia, L. J. & Marchase, R. B. (2002) J. Biol. Chem. 277, 14266–14273. [DOI] [PubMed] [Google Scholar]
- 41.Chien, K. R., Zhu, H., Knowlton, K. U., Miller-Hance, W., van Bilsen, M., O'Brien, T. X. & Evans, S. M. (1993) Annu. Rev. Physiol. 55, 77–95. [DOI] [PubMed] [Google Scholar]
- 42.Sugden, P. H. & Clerk, A. (1998) J. Mol. Med. 76, 725–746. [DOI] [PubMed] [Google Scholar]
- 43.Olson, E. N. & Williams, R. S. (2000) Cell 101, 689–692. [DOI] [PubMed] [Google Scholar]
- 44.Vega, R. B., Bassel-Duby, R. & Olson, E. N. (2003) J. Biol. Chem. 278, 36981–36984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.