Abstract
We evaluated differences in growth between fetuses with and without congenital heart disease (CHD) and tested associations between growth and early childhood neurodevelopment (ND). In this prospective cohort study, fetuses with hypoplastic left heart syndrome (HLHS), transposition of the great arteries (TGA), and tetralogy of Fallot (TOF) and controls had biparietal diameter (BPD), head (HC) and abdominal circumference (AC), femur length (FL), and estimated fetal weight (EFW) recorded serially during pregnancy at 18and controls were assessed using–26 weeks GA (F1), at 27–33 weeks GA (F2), and at 34–40 weeks GA (F3). CHD subjects underwent Bayley Scales of Infant Development-III ND testing at 18 months. Differences between CHD fetuses and controls were assessed using t tests and generalized linear modeling. Correlations between biometry and ND informed regression modeling. We enrolled 41 controls and 68 fetuses with CHD (N = 24 HLHS, N = 21 TGA, N = 23 TOF), 46 of whom had ND scores available. At 18–26 weeks, CHD fetuses were smaller than controls in all biometric parameters. Differences in growth rates were observed for HC, BPD, and AC, but not for FL or EFW. Cognitive score correlated with HC/AC at F2 (r = −0.33, P = 0.04) and mean HC/AC across gestation (r = −0.35, P = 0.03). Language correlated with FL/BPD at F2 (r = 0.34, P = 0.04). In stepwise linear regression, mean HC/AC predicted Cognition (B = −102, P = 0.026, R2 = 0.13) and FL/BPD at F2 predicted Language score (B = 127, P = 0.03, R2 = 0.12). Differences in growth between CHD fetuses and controls can be measured early in pregnancy. In CHD fetuses, larger abdominal relative to head circumference is associated with better 18-month neurodevelopment.
Keywords: Fetal cardiology, Growth, Biometry, Congenital heart disease, Neurodevelopment
Introduction
Children with congenital heart disease (CHD) are at risk for neurodevelopmental deficits [7]. Recent research suggests potential fetal contributors to neurocognitive development including correlations between abnormal fetal cerebrovascular resistance and 14- and 18-month neurodevelopmental scores [17, 18]. CHD neonates are known to be small for gestational age, and CHD fetuses have been shown in cross-sectional analyses to be smaller than controls [10, 11]. Furthermore, smaller head circumference, lower birth weight, as well as decreased growth in the first year of life are associated with poor neurodevelopment [12, 13, 15]. How CHD affects serial growth of the fetus throughout pregnancy and whether fetal growth impacts neurodevelopment is not as well understood. We hypothesized that differences in growth between CHD and non-CHD subjects begin in fetal life and that fetal growth measures, specifically fetal head circumference and weight, would predict postnatal neurodevelopment. We aimed: (1) to evaluate differences in fetal growth rates between fetuses with and without CHD and (2) to investigate associations between fetal growth and early childhood neurodevelopmental outcome. This is the first study to report on serial growth measurements in CHD fetuses and to investigate whether differences in growth over time impact neurodevelopment.
Methods
This was a prospective, observational cohort study of fetuses with and without CHD, the aim of which was to investigate early markers of neurodevelopmental outcome. Columbia University IRB approval was obtained, and all pregnant women provided written consent prior to study enrollment. Fetuses with hypoplastic left heart syndrome (HLHS), d-transposition of the great arteries (TGA), and tetralogy of Fallot (TOF) at <26 weeks gestational age (GA) were enrolled following fetal echocardiography. Normal controls were recruited via posted advertisements throughout the medical center and underwent a complete fetal echocardiogram to ensure normal cardiac anatomy prior to enrollment. Serial biometry assessments were collected three times during pregnancy: at 18–26 weeks GA (F1), at 27–33 weeks GA (F2), and at 34–40 weeks GA (F3). These GA periods were selected to coincide with the timing of regularly scheduled appointments for the CHD patients to minimize the inconvenience of travel to the study center. All measurements were taken by trained obstetric sonographers. Head circumference (HC), biparietal diameter (BPD), abdominal girth (AG), and femur length (FL) were recorded and transformed into GA-based percentiles [5]. Estimated fetal weight (EFW) was calculated using Hadlock’s formula [6]. Rates of change over time as well as standard biometry ratios FL/AC, HC/AC, and FL/BPD ratios were calculated. Neonatal parameters including birth weight, length, and head circumference were collected and transformed into z-scores using WHO standards, and predictors of neonatal measurements were investigated [1]. Information about maternal diabetes and genetic testing was collected. Subjects with CHD returned at 18 months of age to undergo neurodevelopmental assessment with a trained psychologist using the Bayley Scales of Infant Development, Third edition (BSID-III). The BSID-III provides three summary scores: Cognitive, Language, and Motor. These scores carry a population mean and standard deviation of 100 ± 15.
Statistics
Differences in biometry measures between CHD and controls were assessed using Student’s t tests. Growth rates over time for individual subjects were calculated and compared between groups. Generalized linear modeling evaluated changes in biometry measures over time between groups using paired analysis techniques. Differences between CHD diagnosis subgroups were tested using analysis of variance. Associations between fetal biometry and neonatal measurements were tested initially with Pearson’s correlation coefficient followed by linear regression models. Correlations between biometry measures and BSID-III scores were performed using the Pearson’s correlation coefficient. Variables with correlations significant at ≤ 0.1 were entered into multivariable stepwise regression models with 18-month Cognition, Language, and Motor scores serving as the dependent variables. We also used factor analysis to assess collinearity among variables and to reduce the number of predictors used in regression modeling as an additional method to identify predictors of neurodevelopmental outcome.
Results
From November 2010 through November 2012, we enrolled 41 controls and 68 fetuses with CHD, of whom 24 had HLHS, 21 had TGA, and 23 had TOF. Genetic testing revealed one CHD patient with Noonan syndrome and one CHD patient with a 164-Kb deletion at 3q21.1 who had an unusual cardiac phenotype of HLHS with truncus arteriosus and did not survive the neonatal period. Both individuals were removed from all analyses, leaving 66 CHD fetuses and 41 controls.
Of the 66 CHD fetuses, 46 (70 %) completed neurodevelopmental testing at a mean age of 18.8 ± 0.6 months. Mean Cognition score was 95 ± 12, mean Language score was 87 ± 15, and mean Motor score was 91 ± 13. These scores, though within 1SD of the normal population mean of 100 ± 15, were all statistically significantly lower than normal (P = 0.002 for Cognition score, P < 0.001 for Language and Motor scores).
Baseline Differences
At F1, 18–26 weeks GA, CHD fetuses demonstrated smaller biometry percentiles compared with controls (Table 1). At F2, 27–33 weeks GA, AC was statistically smaller among the CHD group. At F3, 34–40 weeks GA, BPD, HC, AC, and EFW percentiles were smaller among the CHD group. Though FL/AC at 27–33 weeks and FL/BPD at 18–26 weeks differed statically by groups, the differences were small and unlikely to be of clinical significance. There was no significant difference in HC/AC ratio between groups at any GA period.
Table 1.
Differences in biometry percentiles between congenital heart disease (CHD) and control subjects at 18–26 weeks, 27–33 weeks, and 34–40 weeks gestational age (GA)
| Biometric percentile |
18–26 wks | P | 27–33 wks | P | 34–40 wks | P | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| CHD | Control | CHD | Control | CHD | Control | ||||
| GA (weeks) | 23.6 ± 2 (N = 55) |
21.0 ± 1.7 (N = 39) |
<0.001 | 30.6 ± 1.6 (N = 60) |
30 ± 1.7 (N = 16) |
NS | 36.4 ± 1 (N = 60) |
36.3 ± 1.8 (N = 23) |
NS |
| BPD (percentile) |
43 ± 21 (N = 55) |
54 ± 18 (N = 39) |
0.008 | 48 ± 24 (N = 60) |
52 ± 18 (N = 16) |
NS | 41 ± 24 (N = 60) |
56 ± 22 (N = 23) |
0.013 |
| HC (percentile) |
39 ± 17 (N = 55) |
48 ± 15 (N = 39) |
0.01 | 45 ± 23 (N = 60) |
50 ± 15 (N = 16) |
NS | 34 ± 21 (N = 60) |
55 ± 22 (N = 23) |
<0.001 |
| AC (percentile) |
47 ± 17 (N = 55) |
55 ± 15 (N = 39) |
0.036 | 48 ± 19 (N = 60) |
60 ± 23 (N = 16) |
0.031 | 54 ± 25 (N = 60) |
67 ± 22 (N = 23) |
0.032 |
| FL (percentile) |
42 ± 16 (N = 55) |
49 ± 15 (N = 39) |
0.046 | 42 ± 17 (N = 60) |
44 ± 20 (N = 16) |
NS | 41 ± 22 (N = 60) |
49 ± 23 (N = 23) |
NS |
| EFW (percentile) |
48 ± 20 (N = 55) |
57 ± 23 (N = 39) |
0.042 | 47 ± 17 (N = 60) |
56 ± 17 (N = 16) |
NS | 49 ± 18 (N = 60) |
60 ± 20 (N = 23) |
0.017 |
| HC/AC | 1.13 ± 0.05 (N = 55) |
1.15 ± 0.05 (N = 39) |
NS | 1.08 ± 0.05 (N = 60) |
1.06 ± 0.06 (N = 16) |
NS | 1.00 ± 0.04 (N = 60) |
1.00 ± 0.06 (N = 23) |
NS |
| FL/AC | 0.22 ± 0.01 (N = 55) |
0.22 ± 0.01 (N = 39) |
NS | 0.22 ± 0.01 (N = 60) |
0.21 ± 0.01 (N = 16) |
0.034 | 0.22 ± 0.01 (N = 60) |
0.21 ± 0.01 (N = 23) |
NS |
| FL/BPD | 0.73 ± 0.04 (N = 55) |
0.71 ± 0.06 (N = 39) |
0.04 | 0.75 ± 0.04 (N = 60) |
0.76 ± 0.04 (N = 16) |
NS | 0.79 ± 0.04 (N = 60) |
0.79 ± 0.04 (N = 23) |
NS |
BPD biparietal diameter, HC head circumference, AC abdominal circumference, FL femur length, EFW estimated fetal weight, HC/AC head-to-abdominal circumference ratio, FL/AC femur length-to-abdominal circumference ratio, FL/BPD femur length-to-biparietal diameter ratio, NS nonsignificant
The proportion of subjects with biometry parameters <10th percentile was small and were primarily seen in the CHD subgroup. Differences between groups were only seen for HC at F2 (1.5 vs 0 %, P = 0.04) and F3 (14.7 vs. 0 %, P = 0.01) and FL at F3 (10.2 vs. 0 %, P = 0.04).
Growth Rate Differences
Differences in growth rates between CHD and control fetuses were observed for HC, AC, and BPD (Table 2). In paired analyses, differences between growth rates from time F1 to F2 and from time F2 to F3 were seen for HC, BPD, FL, and EFW, but not for AC in both CHD and control subjects (Table 3). In order to control for the change over time that occurs in non-CHD fetuses, we created multivariable models to investigate both baseline biometry percentile and CHD status as predictors of growth rates. In these models, a diagnosis CHD was the only predictor for HC (B = −0.12, P < 0.001, R2 = 0.21), BPD (B = −0.02, P = 0.01, R2 = 0.09), and AC (B = −0.07, P = 0.01, R2 = 0.08) growth rates over time. Neither CHD status nor baseline FL percentile predicted FL growth rate. EFW baseline percentile was the only variable that predicted EFW change over time (B = 0.5, P < 0.001, R2 = 0.17).
Table 2.
Differences in biometry growth rates between congenital heart disease (CHD) and control fetuses from F1 to F2, F2 to F3, and from F1 to F3
| Growth rates |
Change from F1 to F2 | P | Change from F2 to F3 | P | Change from F1 to F3 | P | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| CHD | Control | CHD | Control | CHD | Control | ||||
| ΔBPD (cm/wk) |
0.29 ± 0.04 (N = 57) |
0.29 ± 0.03 (N = 18) |
NS | 0.19 ± 0.04 (N = 60) |
0.24 ± 0.07 (N = 14) |
0.03 | 0.24 ± 0.03 (N = 55) |
0.26 ± 0.03 (N = 23) |
0.01 |
| ΔHC (cm/ wk) |
1.02 ± 0.16 (N = 57) |
1.09 ± 0.08 (N = 18) |
0.03 | 0.61 ± 0.18 (N = 60) |
0.79 ± 0.22 (N = 14) |
0.002 | 0.82 ± 0.11 (N = 55) |
0.94 ± 0.10 (N = 23) |
<0.001 |
| ΔAC (cm/ wk) |
1.05 ± 0.19 (N = 57) |
1.17 ± 0.15 (N = 18) |
0.02 | 1.01 ± 0.25 (N = 60) |
1.08 ± 0.22 (N = 14) |
NS | 1.03 ± 0.11 (N = 55) |
1.10 ± 0.11 (N = 23) |
0.01 |
| ΔFL (cm/ wk) |
0.23 ± 0.04 (N = 57) |
0.25 ± 0.03 (N = 18) |
NS | 0.20 ± 0.05 (N = 60) |
0.20 ± 0.04 (N = 14) |
NS | 0.22 ± 0.03 (N = 55) |
0.23 ± 0.02 (N = 23) |
NS |
| ΔEFW (gm/wk) |
141 ± 28 (N = 57) |
142 ± 35 (N = 18) |
NS | 211 ± 51 (N = 60) |
240 ± 43 (N = 14) |
0.05 | 172 ± 26 (N = 55) |
174 ± 29 (N = 23) |
NS |
F1 = 18–26 weeks, F2 = 27–33 weeks, and F3 = 34–40 weeks
cm centimeter, wk weeks, BPD biparietal diameter, HC head circumference, AC abdominal circumference, FL femur length, EFW estimated fetal weight, HC/AC head-to-abdominal circumference ratio, FL/AC = femur length-to-abdominal circumference ratio, FL/BPD femur length-to-biparietal diameter ratio, NS nonsignificant
Table 3.
Paired t test analyses showing differences in growth rates from F1 to F2 and from F2 to F3 for CHD and control fetuses
| Growth Rates | Change from | P | |
|---|---|---|---|
|
|
|||
| F1 to F2 | F2 to F3 | ||
| CHD ΔBPD (N = 55) (cm/wk) | 0.28 ± 0.04 | 0.2 ± 0.05 | <0.001 |
| Control ΔBPD (N = 14) (cm/wk) | 0.29 ± 0.03 | 0.24 ± 0.07 | 0.04 |
| CHD ΔHC (N = 55) (cm/wk) | 1.02 ± 0.16 | 0.61 ± 0.18 | <0.001 |
| Control ΔHC (N = 14) (cm/wk) | 1.07 ± 0.09 | 0.8 ± 0.22 | <0.001 |
| CHD ΔAC (N = 55) (cm/wk) | 1.04 ± 0.19 | 1.01 ± 0.25 | NS |
| Control ΔAC (N = 14) (cm/wk) | 1.07 ± 0.09 | 1.08 ± 0.22 | NS |
| CHD ΔFL (N = 55) (cm/wk) | 0.23 ± 0.04 | 0.2 ± 0.06 | 0.006 |
| Control ΔFL (N = 14) (cm/wk) | 0.24 ± 0.03 | 0.2 ± 0.04 | 0.02 |
| CHD ΔEFW (N = 55) (gm/wk) | 139 ± 27 | 210 ± 51 | <0.001 |
| Control ΔEFW (N = 14) (gm/wk) | 144 ± 39 | 240 ± 43 | <0.001 |
Differences for both groups are seen for all parameters except abdominal circumference
F1 = 18–26 weeks, F2 = 27–33 weeks, and F3 = 34–40 weeks
cm centimeter, wk weeks, BPD biparietal diameter, HC head circumference, AC abdominal circumference, FL femur length, EFW estimated fetal weight, NS nonsignificant
Generalized linear modeling showed differences over time in biometry measures for HC (P < 0.001) (Fig. 1) and BPD (P = 0.015) between CHD and controls.
Fig. 1.
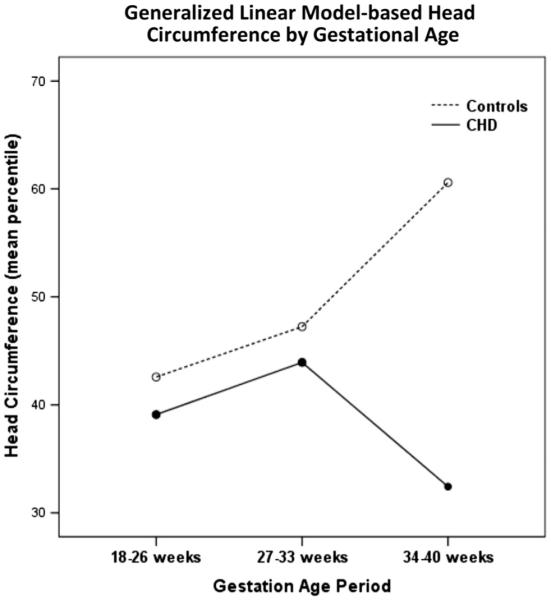
Graphical output of generalized linear modeling of change in head circumference over time by CHD versus Control. The difference in change over time is significant at P < 0.001
CHD Subgroup Analyses
Differences were seen between CHD subgroups for EFW at 18–26 weeks (Table 4). At 27–33 weeks, differences were seen for BPD, HC, EFW, and HC/AC. At 34–40 weeks, differences between CHD subgroups were only seen for HC. In all cases except for HC/AC, the TOF subgroup was the smallest. Differences in mean values over time were observed for HC, BPD, AC, EFW, as well as HC/AC and FL/BPD (data not shown). Differences in change over time were only seen for BPD for F1 to F2 with the TOF subgroup again showing the smallest increase (Table 5).
Table 4.
Differences in biometry values between CHD subgroups hypoplastic left heart syndrome (HLHS), d-transposition of the great arteries (TGA), and tetralogy of Fallot (TOF)
| Biometry | 18-26 wks | P | 27-33 wks | P | 34-40 wks | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| HLHS | TGA | TOF | HLHS | TGA | TOF | HLHS | TGA | TOF | ||||
| BPD (percentile) |
47 ± 20 (N = 19) |
46 ± 19 (N = 18) |
36 ± 22 (N = 18) |
NS | 57 ± 22 (N = 21) |
54 ± 20 (N = 18) |
33 ± 24 (N = 21) |
0.002 | 48 ± 24 (N = 21) |
44 ± 22 (N = 20) |
31 ± 24 (N = 19) |
NS |
| HC (percentile) |
41 ± 18 (N = 19) |
46 ± 19 (N = 18) |
36 ± 22 (N = 18) |
NS | 54 ± 22 (N = 21) |
47 ± 21 (N = 18) |
34 ± 22 (N = 21) |
0.02 | 44 ± 26 (N = 21) |
30 ± 16 (N = 20) |
28 ± 17 (N = 19) |
0.03 |
| AC (percentile) |
47 ± 17 (N = 19) |
53 ± 14 (N = 18) |
42 ± 17 (N = 18) |
NS | 49 ± 17 (N = 21) |
56 ± 21 (N = 18) |
40 ± 17 (N = 20) |
0.03 | 61 ± 23 (N = 21) |
53 ± 27 (N = 20) |
46 ± 25 (N = 19) |
NS |
| FL (percentile) |
45 ± 16 (N = 19) |
44 ± 16 (N = 18) |
37 ± 16 (N = 18) |
NS | 44 ± 17 (N = 21) |
42 ± 15 (N = 18) |
38 ± 19 (N = 21) |
NS | 40 ± 19 (N = 21) |
48 ± 22 (N = 20) |
34 ± 23 (N = 19) |
NS |
| EFW (percentile) |
50 ± 20 (N = 19) |
54 ± 20 (N = 18) |
38 ± 17 (N = 18) |
0.03 | 51 ± 16 (N = 21) |
52 ± 16 (N = 18) |
38 ± 16 (N = 21) |
0.01 | 54 ± 18 (N = 21) |
50 ± 18 (N = 20) |
42 ± 18 (N = 19) |
NS |
| HC/AC | 1.13 ± 0.06 (N = 19) |
1.12 ± 0.06 (N = 18) |
1.14 ± 0.05 (N = 18) |
NS | 1.10 ± 0.05 (N = 21) |
1.06 ± 0.05 (N = 18) |
1.08 ± 0.05 (N = 21) |
0.03 | 1.00 ± 0.04 (N = 21) |
0.99 ± 0.04 (N = 20) |
1.00 ± 0.03 (N = 19) |
NS |
| FL/AC | 0.22 ± 0.01 (N = 19) |
0.22 ± 0.01 (N = 18) |
0.22 ± 0.01 (N = 18) |
NS | 0.22 ± 0.01 (N = 21) |
0.22 ± 0.01 (N = 18) |
0.22 ± 0.01 (N = 21) |
NS | 0.21 ± 0.01 (N = 21) |
0.22 ± 0.01 (N = 20) |
0.22 ± 0.01 (N = 19) |
NS |
| FL/BPD | 0.73 ± 0.04 (N = 19) |
0.73 ± 0.04 (N = 18) |
0.73 ± 0.05 (N = 18) |
NS | 0.74 ± 0.04 (N = 21) |
0.75 ± 0.04 (N = 18) |
0.77 ± 0.04 (N = 21) |
NS | 0.78 ± 0.04 (N = 21) |
0.80 ± 0.04 (N = 20) |
0.79 ± 0.04 (N = 19) |
NS |
Fl = 18-26 weeks, F2 = 27-33 weeks, and F3 = 34-10 weeks
cm centimeter, wk weeks, BPD biparietal diameter, HC head circumference, AC abdominal circumference, FL femur length, EFW estimated fetal weight, HC/AC head-to-abdominal circumference ratio, FL/AC femur length-to-abdominal circumference ratio, FL/BPD femur length-to-biparietal diameter ratio, NS nonsignificant
Table 5.
Differences in biometry growth rates between congenital heart disease (CHD) subgroups hypoplastic left heart syndrome (HLHS), d-transposition of the great arteries (TGA), and tetralogy of Fallot (TOF)
| Growth rates |
Change from Fl to F2 | P | Change from F2 to F3 | P | Change from Fl to F3 | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| HLHS | TGA | TOF | HLHS | TGA | TOF | HLHS | TGA | TOF | ||||
| ΔBPD (cm/wk) |
0.30 ± 0.04 (N = 20) |
0.29 ± 0.03 (N = 18) |
0.26 ± 0.05 (N = 19) |
0.03 | 0.19 ± 0.04 (N = 21) |
0.18 ± 0.05 (N = 20) |
0.21 ± 0.04 (N = 19) |
NS | 0.24 ± 0.03 (N = 20) |
0.24 ± 0.03 (N = 18) |
0.24 ± 0.03 (N = 17) |
NS |
| ΔHC (cm/ wk) |
1.08 ± 0.18 (N = 20) |
1.00 ± 0.12 (N = 18) |
0.97 ± 0.16 (N = 19) |
NS | 0.64 ± 0.17 (N = 21) |
0.54 ± 0.17 (N = 20) |
0.65 ± 0.19 (N = 19) |
NS | 0.85 ± 0.11 (N = 20) |
0.81 ± 0.08 (N = 18) |
0.81 ± 0.13 (N = 17) |
NS |
| ΔAC (cm/ wk) |
1.05 ± 0.15 (N = 20) |
1.09 ± 0.17 (N = 18) |
1.02 ± 0.25 (N = 19) |
NS | 1.07 ± 0.22 (N = 21) |
0.92 ± 0.27 (N = 20) |
1.04 ± 0.23 (N = 19) |
NS | 1.06 ± 0.10 (N = 20) |
1.01 ± 0.11 (N = 18) |
1.00 ± 0.11 (N = 17) |
NS |
| ΔFL(cm/ wk) |
0.23 ± 0.05 (N = 20) |
0.23 ± 0.03 (N = 18) |
0.24 ± 0.04 (N = 19) |
NS | 0.19 ± 0.06 (N = 21) |
0.22 ± 0.05 (N = 20) |
0.19 ± 0.05 (N = 19) |
NS | 0.21 ± 0.03 (N = 20) |
0.22 ± 0.02 (N = 18) |
0.22 ± 0.03 (N = 17) |
NS |
| ΔEFW (gm/wk) |
140 ± 28 (N = 20) |
144 ± 28 (N = 18) |
139 ± 28 (N = 19) |
NS | 218 ± 56 (N = 21) |
208 ± 49 (N = 20) |
206 ± 47 (N = 19) |
NS | 179 ± 20 (N = 21) |
173 ± 28 (N = 18) |
163 ± 29 (N = 17) |
NS |
Fl = 18-26 weeks, F2 = 27-33 weeks, and F3 = 34-10 weeks
cm centimeter, wk weeks, BPD biparietal diameter, HC head circumference, AC abdominal circumference, FL femur length, EFW estimated fetal weight, NS nonsignificant
Associations with Neonatal Biometrics
Among the 66 CHD and 41 controls included in fetal analyses, birth measurements were available for 63 (93 %) CHD and 30 (73 %) controls. Missing data were due to birth outside our institution. There was no difference in gestational age at birth between CHD (38.6 ± 1.4 weeks) and controls (38.9 ± 1.4 weeks, P = 0.2). Differences between CHD and control newborns were seen for birth length and head circumference, but the magnitude of difference was small (Table 6). Low birth weight, defined as birth weight ≤ 2500 grams, occurred in 7 subjects, 6 of whom had CHD. Due to the small prevalence of low birth weight in our study, there was no statistical difference between groups.
Table 6.
Differences between CHD versus controls for neonatal measurements
| Congenital heart disease | N | Mean | Standard deviation | P value |
|---|---|---|---|---|
| Birth weight (g) | ||||
| YES | 63 | 3208 | 572 | 0.4 |
| No | 30 | 3306 | 406 | |
| Birth length (cm) | ||||
| YES | 63 | 49.7 | 2.9 | 0.05* |
| No | 30 | 50.8 | 2.1 | |
| Head circumference (cm) | ||||
| YES | 63 | 33.6 | 1.9 | 0.04* |
| No | 30 | 34.3 | 1.1 | |
| Weight for age z-score | ||||
| YES | 63 | −0.26 | 1.18 | 0.3 |
| No | 30 | 0.02 | 0.9 | |
| Length for age z-score | ||||
| YES | 63 | 0.04 | 1.5 | 0.04* |
| No | 30 | 0.68 | 1.13 | |
| Head circumference for age z-score | ||||
| YES | 63 | −0.51 | 1.46 | 0.016* |
| No | 30 | 0.11 | 0.95 | |
| Weight for age percentile | ||||
| YES | 63 | 45.6 | 30.4 | 0.5 |
| No | 30 | 50 | 26.4 | |
| Length for age percentile | ||||
| YES | 63 | 52.7 | 33.6 | 0.016* |
| No | 30 | 68.7 | 26.9 | |
| Head circumference for age percentile | ||||
| YES | 63 | 39.4 | 33.3 | 0.06 |
| No | 30 | 52.9 | 30.1 | |
| Gestational age at birth | ||||
| YES | 63 | 38.6 | 1.4 | 0.2 |
| No | 30 | 38.9 | 1.4 | |
Statistical significance
Fetal biometry values at all gestational age periods were highly correlated with neonatal measurements for the entire cohort as well as CHD and control groups separately. Due to the large number of available fetal variables, factor analysis was used to reduce the number of possible predictors in regression models. Fetal biometric factors remained significant predictors of birth weight, length, and head circumference, whereas assignment of a CHD diagnosis did not. A diagnosis of gestational diabetes (N = 4) also did not remain as a significant factor.
Associations with Neurodevelopmental Outcome
Fetal biometry variables that correlated with Cognitive score included AC at F1 (r = 0.32, P = 0.049), HC/AC at F1 (r = −0.39, P = 0.02), and mean HC/AC (r = −0.4, P = 0.007) (Fig. 2). Language score correlated significantly with FL/BPD at F2 (r = 0.32, P = 0.037) and mean HC/AC (r = −0.3, P = 0.045). Motor score was not correlated with any biometry variables. Neither biometry growth rates nor newborn parameters correlated with 18-month neurodevelopmental scores.
Fig. 2.
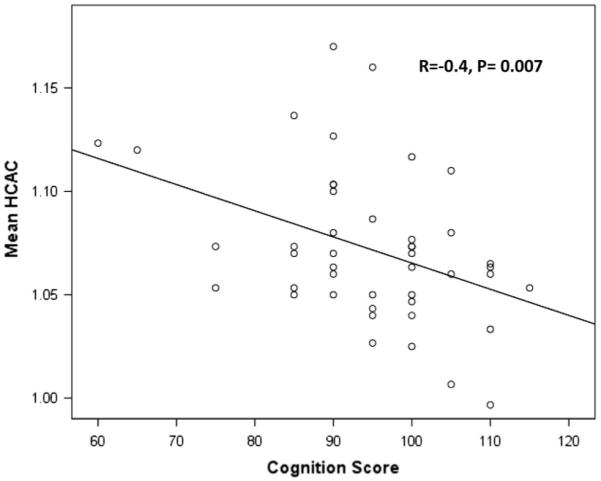
Correlation between mean head-to-abdominal circumference (HC/AC) ratio over time and 18-month Cognition score. As abdominal circumference increases relative to head circumference, Cognitive score increases
Multivariable Regression
When we added variables that correlated with scores at the 0.1 level or lower to a multivariable model predicting Cognitive score, only HC/AC at 18–26 weeks remained an independent predictor (B = −83, P = 0.01, R2 = 0.16). Language score was predicted by FL/BPD at F2, 27 to 33 weeks (B = 129, P = 0.03, R2 = 0.11).
Factor Analysis
We identified four factors that cumulatively accounted for 62 % of the variance among all biometry variables. The variables that comprised the four factors along with their loading values are listed in Table 7. Only factor 2, which contained mean HC/AC as well as HC/AC at F1, F2, and F3, mean HC, and AC at F2 and F3, correlated with Cognitive (r = 0.4, P = 0.01) and Language (r = 0.3, P = 0.03) scores. When added to regression models, factor 2, the head and abdominal circumference factor, predicted Cognition (B = 5.2, P = 0.01, R2 = 0.14) and Language (B = 5.2, P = 0.01, R2 = 0.14). We added the top three loading variables for factor 2 to regression models predicting Cognitive and Language score. Mean HC/AC remained the only independent predictor of Cognitive score (B = −145, P = 0.004, R2 = 0.19) and Language score (B = −147, P = 0.03, R2 = 0.1).
Table 7.
All biometric variables were entered into the factor analysis
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||
| Variable | Loading value |
Variable | Loading value |
Variable | Loading value |
Variable | Loading value |
| Mean BPD |
0.935 | Mean HC/AC | −0.801 | Mean FL | 0.789 | Change in HC between F3 and F1 |
0.766 |
| Mean HC |
0.874 | Mean HC | 0.762 | FL at F3 | 0.775 | Change in HC between F3 and F2 |
0.750 |
| BPD at F2 |
0.872 | AC at F3 | 0.758 | FL/BPD at F3 |
0.770 | Change in FL between F3 and F1 |
0.654 |
| HC at F2 | 0.844 | HC/AC at F3 | −0.725 | Mean FL/AC |
0.715 | Change in BPD between F3 and F1 |
0.634 |
| HC at F1 | 0.804 | AC at F2 | 0.705 | Mean FL/BPD |
0.660 | Change in EFW between F3 and F2 |
0.631 |
| BPD at F3 |
0.802 | HC/AC at F2 | −0.670 | FL at F2 | 0.651 | ||
| BPD at F1 |
0.792 | HC/AC at F1 | −0.663 | FL/AC at F3 |
0.608 | ||
| EFW at F2 |
0.769 | Change in EFW between F3 and F1 |
0.644 | ||||
| Mean EFW |
0.753 | EFW at F3 | 0.603 | ||||
| EFW at F1 |
0.721 | ||||||
| HC at F3 | 0.647 | ||||||
Variables were grouped into 4 factors based on collinearity. The 4 factors with their composite variables and loading values, or contributions to the factor, are demonstrated
F1 = 18–26 weeks, F2 = 27–33 weeks, and F3 = 34–40 weeks
cm centimeter, wk weeks, BPD biparietal diameter, HC head circumference, AC abdominal circumference, FL femur length, EFW estimated fetal weight, HC/AC head-to-abdominal circumference ratio, FL/AC femur length-to-abdominal circumference ratio, FL/BPD femur length-to-biparietal diameter ratio
Discussion
In this prospective cohort study, we demonstrated that as early as 18–26 weeks gestation, differences are observed between CHD and control fetuses using standard ultrasound biometry. Using serial fetal growth measurements over time within individual subjects, we also demonstrated that CHD fetuses display lower growth rates compared with controls, most noticeably for head circumference. Ours is the first study to show differences in fetal growth rates throughout pregnancy.
Levy et al. [9] reported lower birth weights among CHD infants compared with the population mean. Why fetal growth is impaired in the setting of CHD remains speculative. Unmeasurable genetic or environmental factors that cause CHD may also influence growth during and after pregnancy. It is also possible that the cardiac defect itself impacts growth, perhaps through altered fetal hemodynamics and impaired oxygen and substrate delivery. Recently, Masoller et al. [11], in a cross-sectional study of CHD fetuses 20–23.5 weeks GA, demonstrated lower middle cerebral artery pulsatility and cerebral-to-placental resistance along with smaller HC and BPD; however, a direct association between the two findings was not reported. Donofrio et al. [2] found that CHD fetuses with diminished cerebrovascular resistance had smaller head circumferences than expected, though this relationship was dependent on fetal weight. Further investigation is needed to understand the potential and likely complex interplay between fetal blood flow and growth.
We also found that fetal growth rates differ by CHD diagnosis, with the TOF subgroup demonstrating lower head and EFW growth rates than HLHS or TGA. In 1988, Khoury et al. [8] reported that newborns with TOF had a higher relative risk of intrauterine growth retardation compared with HLHS and TGA. We did not see a difference between TGA and HLHS fetuses, though this may be secondary to sample size constraints. Why the TOF subgroup measures smaller than others is unclear. All CHD fetuses included in this analysis underwent standard clinical genetic testing, which in most cases consisted of microarray analyses. Therefore, genetic syndromes, to the best of current detection, can be eliminated as a cause. Future studies including larger numbers of subjects within pre-specified cardiac subgroups will be necessary to address this issue. Additionally, we showed that while fetal growth appears to be affected by the presence of CHD, neonatal anthropometrics were more highly predicted by an individual’s fetal measurements than by a diagnosis of CHD itself.
Finally, we identified fetal biometric predictors of 18-month Cognitive and Language outcome using two different statistical approaches. Both approaches led us to a HC/AC ratio relationship to Cognition Score: HC/AC ratio inversely predicted outcome, meaning that a larger abdominal relative to head circumference was associated with a higher score, particularly when measured early in gestation. Using one approach, mean HC/AC also predicted Language score with the same inverse relationship. We also found that FL/BPD ratio at mid-gestation predicted Language, where a larger femur length relative to biparietal diameter predicted higher score. This is the first study to identify standard measures of fetal biometry collected during routine growth scans as predictors of neurodevelopment in the CHD population.
We were unable to identify any significant predictors of motor functioning. In CHD studies that use the Bayley Scales of Infant Development-Second edition, motor function, measured by the Psychomotor Developmental Index, is often more severely impaired than Cognitive development, measured by the Mental Developmental Index [13, 15]. While our study also demonstrated a mean Motor score below the Cognitive score, Language score, previously unavailable until the Third Edition of the Bayley, was the lowest. It is possible that the less notable impairment of the motor domain in our cohort relates to the different instrument used to measure neurodevelopment. The lesser degree of motor impairment may have impacted our ability to detect predictors of outcome, though we were able to identify predictors of Cognitive outcome despite an even more normal score. We feel it is more likely that fetal growth has less of an impact on motor development than on Cognitive or Language development. Larger study sizes are needed to pursue this hypothesis.
van Batenburg-Eddes et al. [16], reporting on the Generation R Study, a population-based sample from the Netherlands, found that higher HC/AC ratio, along with lower AC and EFW, predicted impaired infant neuromotor development, whereas HC alone did not. The association between HC/AC and neurodevelopment was independent of neonatal anthropometrics and whether the ratio was measured in mid- or late-pregnancy and was not affected by the presence of intra-uterine growth restriction, or maternal hypertension, diabetes, or preeclampsia. The authors concluded that fetal size and body symmetry were important for ultimate brain development, more so than the HC alone. Similarly, in our study of subjects with CHD, it is possible that the inverse relationship between the HC/AC and Cognitive and Language outcomes reflects the importance of overall growth of the fetus as opposed to growth of the head alone.
We did not see a direct association between head size at any time in pregnancy, or in the neonatal period, and neurologic function. Owen et al. [14] described associations between reduced gray matter volume on MRI in infants with CHD and newborn neurobehavioral assessment; however, longer-term follow-up is not yet reported in this cohort. Matos et al. [12] evaluated the neurocognitive performance of 77 CHD adolescents and found a positive correlation between Cognitive score and neonatal HC; however, the presence of cyanosis was the main predictor of later development. Fetal ultrasound has been shown to underestimate reductions in brain volumes detected by MRI [4], and it is possible that ultrasound measurement of HC and BPD may not be sensitive enough to detect diminished neuronal cell division.
Our study is limited by sample size and the single-center design. Notably, return rate for multiple testing was lower among controls than among CHD fetuses, presumably due to differences in maternal motivation. Measurements of fetal biometry and estimation of fetal weight are subject to error [3], yet both CHD and control fetuses faced this risk equally. Not all controls subjects delivered at our institution; therefore, birth weight was not always available. Due to cost constraints, neurodevelopmental testing was not performed in controls. Rather, developmental test scores of CHD subjects were compared with the normal population. Finally, not all CHD subjects obtained 18-month neurodevelopmental testing as some died and were lost to follow-up, though there was no statically significant difference in biometry measures between subjects who did and did not undergo Bayley assessment.
Nonetheless, our longitudinal prospective study is the only one to date to demonstrate that growth diminishes among CHD fetuses throughout pregnancy. Furthermore, we identified an easily measured fetal biometric parameter, the HC-to-AC ratio, which may be related to 18-month Cognitive and Language outcome. Fetal predictors of ND would inform more precise prognostication for parents and providers and would allow early intervention services to be provided within the crucial first years of life to those who need it most. Larger, multi-center prospective studies are needed to confirm these findings.
Acknowledgments
I.A. Williams received support from Grant No. 1K23HD061601 from the National Institute of Child Health & Human Development of the National Institutes of Health and from the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR000040. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Child Health & Human Development.
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest or relationships to disclose beyond those listed above.
Ethical standard All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
Contributor Information
Ismée A. Williams, Division of Pediatric Cardiology, Department of Pediatrics, Morgan Stanley Children’s Hospital of New York-Presbyterian, Columbia University Medical Center, 3959 Broadway, New York, NY 10032, USA
William P. Fifer, Department of Developmental Psychobiology, New York State Psychiatric Institute, New York, NY, USA Division of Pediatric Cardiology, Department of Pediatrics, Morgan Stanley Children’s Hospital of New York-Presbyterian, Columbia University Medical Center, 3959 Broadway, New York, NY 10032, USA.
Howard Andrews, Department of Biostatistics, Mailman School of Public Health, New York, NY, USA.
References
- 1.De Onis M. WHO Multicenter Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;450(Suppl):76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 2.Donofrio MT, Bremer YA, Schieken RM, Gennings C, Morton LD, Eidem BW, Cetta F, Falkensammer CB, Huhta JC, Kleinman CS. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol. 2003;24(5):436–443. doi: 10.1007/s00246-002-0404-0. [DOI] [PubMed] [Google Scholar]
- 3.Dudley NJ. A systematic review of the ultrasound estimation of fetal weight. Ultrasound Obstet Gynecol. 2005;25(1):80–89. doi: 10.1002/uog.1751. [DOI] [PubMed] [Google Scholar]
- 4.Duncan KR, Issa B, Moore R, Baker PN, Johnson IR, Gowland PA. A comparison of fetal organ measurements by echoplanar magnetic resonance imaging and ultrasound. BJOG. 2005;112(1):43–49. doi: 10.1111/j.1471-0528.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- 5.Hadlock FP, Deter RL, Harrist RB, Park SK. Estimating fetal age: computer-assisted analysis of multiple fetal growth parameters. Radiology. 1984;152:497–5011. doi: 10.1148/radiology.152.2.6739822. [DOI] [PubMed] [Google Scholar]
- 6.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements—a prospective study. Am J Obstet Gynecol. 1985;151(3):333–337. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 7.Khalil A, Suff N, Thilaganathan B, Hurrell A, Cooper D, Carvalho JS. Brain abnormalities and neurodevelopmental delay in congenital heart disease: systematic review and metaanalysis. Ultrasound Obstet Gynecol. 2014;43(1):14–24. doi: 10.1002/uog.12526. [DOI] [PubMed] [Google Scholar]
- 8.Khoury MJ, Erickson JD, Cordero JF, McCarthy BJ. Congenital malformations and intrauterine growth retardation: a population study. Pediatrics. 1988;82(1):83–90. [PubMed] [Google Scholar]
- 9.Levy RJ, Rosenthal A, Fyler DC, Nadas AS. Birthweight of infants with congenital heart disease. Am J Dis Child. 1978;132(3):249–254. doi: 10.1001/archpedi.1978.02120280033007. [DOI] [PubMed] [Google Scholar]
- 10.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, Jr, Guizard N, McGrath E, Geva J, Annese D, Dunbar-Masterson C, Trainor B, Laussen PC, du Plessis AJ. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121(1):26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masoller N, Martínez JM, Gómez O, Bennasar M, Crispi F, Sanz M, Egaña-Ugrinovic G, Bartrons J, Puerto B, Gratacós E. Evidence of second trimester changes in head biometry and brain perfusion in fetuses with congenital heart disease. Ultrasound Obstet Gynecol. 2014;44(2):182–187. doi: 10.1002/uog.13373. [DOI] [PubMed] [Google Scholar]
- 12.Matos SM, Sarmento S, Moreira S, Pereira MM, Quintas J, Peixoto B, Areias JC, Areias ME. Impact of fetal development on neurocognitive performance of adolescents with cyanotic and acyanotic congenital heart disease. Congenit Heart Dis. 2014;9(5):373–381. doi: 10.1111/chd.12152. [DOI] [PubMed] [Google Scholar]
- 13.Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, Mussatto KA, Williams IA, Gustafson KE, Mital S, Pike N, Sood E, Mahle WT, Cooper DS, Dunbar-Masterson C, Krawczeski CD, Lewis A, Menon SC, Pemberton VL, Ravishankar C, Atz TW, Ohye RG, Gaynor JW. Pediatric Heart Network Investigators. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation. 2012;125(17):2081–2091. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owen M, Shevell M, Donofrio M, Majnemer A, McCarter R, Vezina G, Bouyssi-Kobar M, Evangelou I, Freeman D, Weisenfeld N, Limperopoulos C. Brain volume and neurobehavior in newborns with complex congenital heart defects. J Pediatr. 2014;164(5):1121–1127. doi: 10.1016/j.jpeds.2013.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravishankar C, Zak V, Williams IA, Bellinger DC, Gaynor JW, Ghanayem NS, Krawczeski CD, Licht DJ, Mahony L, Newburger JW, Pemberton VL, Williams RV, Sananes R, Cook AL, Atz T, Khaikin S, Hsu DT. Pediatric Heart Network Investigators. Association of impaired linear growth and worse neurodevelopmental outcome in infants with single ventricle physiology: a report from the pediatric heart network infant single ventricle trial. J Pediatr. 2013;162(2):250–256. doi: 10.1016/j.jpeds.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Batenburg-Eddes T, de Groot L, Steegers EA, Hofman A, Jaddoe VW, Verhulst FC, Tiemeier H. Fetal programming of infant neuromotor development: the generation R study. Pediatr Res. 2010;67(2):132–137. doi: 10.1203/PDR.0b013e3181c2dc76. [DOI] [PubMed] [Google Scholar]
- 17.Williams IA, Tarullo AR, Grieve PG, Wilpers A, Vignola EF, Myers MM, Fifer WP. Fetal cerebrovascular resistance and neonatal EEG predict 18-month neurodevelopmental outcome in infants with congenital heart disease. Ultrasound Obstet Gynecol. 2012;40(3):304–309. doi: 10.1002/uog.11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams IA, Fifer C, Jaeggi E, Levine JC, Michelfelder EC, Szwast AL. The association of fetal cerebrovascular resistance with early neurodevelopment in single ventricle con- genital heart disease. Am Heart J. 2013;165(4):544–550. doi: 10.1016/j.ahj.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


