Abstract
The authors conducted a retrospective cohort study of unplanned care interruption (UCI) among adults initiating antiretroviral therapy (ART) from 2009 to 2011 in a Nigerian clinic. The authors used repeated measures regression to model the impact of UCI on CD4 count upon return to care and rate of CD4 change on ART. Among 2496 patients, 83% had 0, 15% had 1, and 2% had ≥2 UCIs. Mean baseline CD4 for those with 0, 1, or ≥2 UCIs was 228/cells/mm3, 355/cells/mm3, and 392/cells/mm3 (P < .0001), respectively. The UCI was associated with a 62 CD4 cells/mm3 decrease (95% confidence interval [CI]: −78 to −45) at next measurement. In months 1 to 6 on ART, patients with 0 UCI gained 10 cells/μL/mo (95% CI: 7–4). Those with 1 and ≥2 UCIs lost 2 and 5 cells/μL/mo (95% CI: −18 to 13 and −26 to 16). Patients with UCI did not recover from early CD4 losses associated with UCI. Preventing UCI is critical to maximize benefits of ART.
Keywords: care interruption, CD4, immunologic recovery, adherence, HIV infection
Introduction
Nigeria, the most populous country in Africa, has an estimated 3.5 million people living with HIV, the second largest number in the world.1,2 Patient retention in care remains a major challenge to successful antiretroviral therapy (ART) administration globally as well as in Nigeria.3 With 82% of Nigerians living on less than US$2 per day, those with HIV face substantial competing demands for basic subsistence and health-care needs.4
Retention in HIV care requires commitment to health-related visits, including clinical evaluations and laboratory testing as well as medication adherence. Studies from sub-Saharan Africa demonstrate decreasing average retention rates for people living with HIV after starting ART, from 80% in the first year to 70% in the third year.3 Outcomes among patients not retained in care are often difficult to obtain. Tracking studies suggest that, at one extreme, some have transferred but remained engaged in care and others have died.5–7 Between these extremes, some patients become lost to care, whereas others interrupt but later return to care.8–11 Whether short- or long-term, care interruptions represent an obstacle to effective care.12–14 Multiple studies in the mid-2000s established that planned treatment interruptions increase both morbidity and mortality.15,16
Despite the clear evidence against planned care interruption, unplanned care interruption (UCI) is common in both resource-limited and resource-rich environments, occurring in 14% to 60% of all patients.14,17–19 Like loss to follow-up, the highest UCI rates are in the first year after ART initiation, when the most robust CD4 count response is anticipated.19–21 In Nigeria and other resource-limited settings (RLS), high baseline CD4 count may predict subsequent UCI.19 We sought to determine the immunologic consequence of UCI in the first year after ART initiation in a Nigerian cohort.
Methods
Setting
This study was conducted at the Ahmadu Bello University Teaching Hospital’s (ABUTH) HIV clinic in Kaduna, Nigeria, a state in northern Nigeria with a local HIV prevalence of 5.1%. The ABUTH HIV clinic is located in a semiurban community and began providing HIV care in 2006 with President’s Emergency Plan for AIDS Relief support. ABUTH offers comprehensive HIV testing and treatment to nearly 4000 patients. During the study period, the clinic was managed by AIDS Prevention Initiative in Nigeria (APIN), one of the largest HIV care and treatment programs in Nigeria.22 Upon clinic enrollment, patients completed clinical evaluation, tuberculosis screening, adherence counseling, and CD4 count and viral load testing. The World Health Organization (WHO) and Nigerian National guidelines recommended ART initiation for all patients with CD4 <350 cells/μL in 2009. After enrollment, patients were scheduled to be seen at 2, 4, 8, and 12 weeks for adherence counseling and clinical examination. Subsequently, they were seen for ART pickup every 4 weeks, clinical examination and adherence counseling every 12 weeks, and laboratory testing (including CD4 count and HIV viral load) every 24 weeks.23
Care Status Definitions
We conducted a retrospective cohort study of patients ≥15 years of age who initiated ART at the ABUTH clinic between January 1, 2009, and December 31, 2011. Data were censored at 1 year of follow-up, and clinical, laboratory, or pharmacy visits were assessed to determine care status. The UCI was defined as a period in which the time between any 2 consecutive visits was >90 days, followed by return to clinic (before the censor date). Patients were defined as having 0, 1, or ≥2 UCIs in the first year on ART. Patients known to have transferred care or died during the follow-up period were categorized based on their visit patterns prior to transfer or death.
Data Collection and Analysis
All data were collected in structured data entry forms that were entered into APIN’s central electronic clinical database. Patients with an available baseline CD4 count and at least 1 follow-up value within the first 12 months of initiating ART were included in the analysis. We summarized the proportion of patients with 0, 1, or ≥2 UCIs in the first year on ART. We used 1-way χ2 tests (for categorical variables) and Kruskal-Wallis tests (for ordinal variables) to assess whether the baseline characteristics differed by UCI status. We used a locally weighted smoothed scatterplot (LOWESS) curve function to visually assess the relationship between time on ART and observed CD4 count for those with 0, 1, or ≥2 UCIs in our aggregate data. The LOWESS curve illustrated the expected sharp, early (phase 1), and blunted later (phase 2) responses to ART, which occurred in this cohort between months 1 to 6 and months 7 to 12 on ART.21
To measure the effect of UCI status on CD4 response after ART initiation, we used 2 repeated measures linear regression models. The first allowed us to determine the short-term impact of any UCI on subsequent CD4 count upon return to care, and the second allowed us to estimate the longer-term impact by estimating the rate of CD4 change in the first year on ART for patients with 0, 1, or ≥2 UCIs. In the first model, we estimated the effect of having any UCI on the next measured CD4 count upon return to care. In this model, each patient’s total observation time during the first 12 months after ART initiation was divided into 1 or more intervals based on the CD4 counts obtained during the period. Each interval was a separate observation in the model, which had the change in CD4 count during the interval as its dependent variable, and included the patient as a random effect. The primary covariate of interest was UCI status, which was assessed as a binary variable indicating whether 0 or any UCI occurred since the previous CD4 count. In the second model, we used aggregate CD4 data to estimate the average rate of CD4 change for patients with 0, 1, or ≥2 UCIs in the first year on ART. A spline term was introduced at 6 months after ART initiation to contrast early (1–6 months) versus later (7–12 months) effects of UCIs on CD4 count. We also estimated the marginal mean CD4 count after 1 year on ART for patients with 0, 1, or ≥2 UCIs, holding all other covariates at their mean values. Both multivariate models included potential confounders including age, sex, baseline CD4 count, time since the previous CD4 count, and tuberculosis diagnosis at ART initiation.
Statistical analysis was conducted with Stata Statistical Software (Release 13; StataCorp 2013, College Station, Texas).
Institutional Review Board Approval
Institutional review board approval was obtained from Partners HealthCare (Protocol # 2013P000219), the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, and the Nigerian Institute for Medical Research in Lagos, Nigeria.
Results
Frequency of UCI
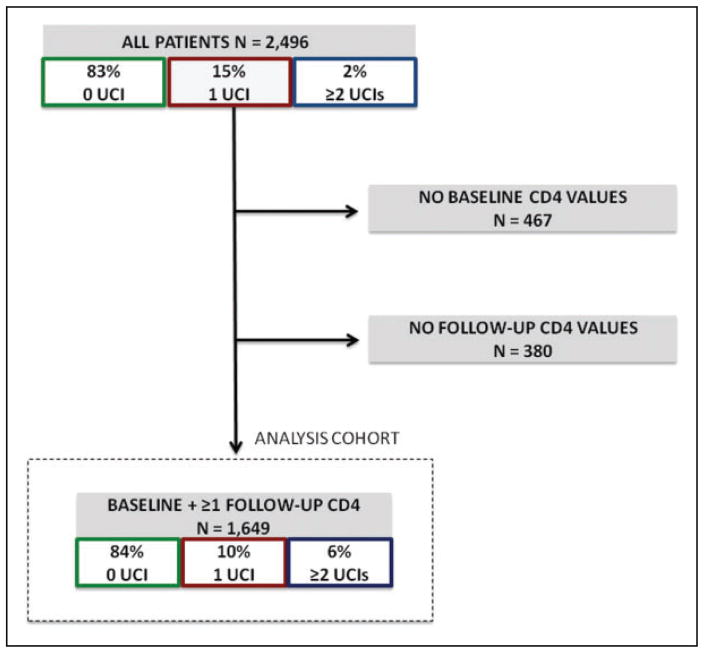
The cohort was composed of 2496 patients who initiated ART at the ABUTH clinic during the study period. Among all patients, 83% (n = 2058) had no UCI, 15% (n = 376) had 1 UCI, and 2% (n = 59) had ≥2 UCIs within the first year on ART. Baseline CD4 count at ART initiation was not available for 467 patients. Follow-up CD4 counts were not available for an additional 380 patients. The remaining 1649 patients who had both a baseline and at least 1 follow-up CD4 count in the first year on ART available in the electronic database were retained for analysis (the analysis cohort, Figure 1).
Figure 1.
Cohort summary and frequency of unplanned care interruption (UCI) during first year on antiretroviral therapy (ART).
Baseline Demographics
The majority of patients in the analysis cohort were female (70%; n = 1163), and the mean age was 33 years (standard deviation [SD]: 9.2). Overall, 61% (n = 1004) of patients were married and 63% (n = 1027) were employed. Forty-four percent of patients in the cohort (n = 734) had primary or secondary education and 34% (n = 559) had tertiary-level education (Table 1). The mean baseline CD4 count was 251/μL (SD: 193/μL). Those with ≥2 UCIs had a higher mean baseline CD4 of 392/μL (SD: 241/μL) than those with 1 UCI (355/μL; SD: 228/μL) or no UCI (228/μL; SD: 176/μL; P < .0001). Sixty-three percent (n = 61) of those with ≥2 UCIs presented to care with baseline CD4 ≥350/μL compared to 49% of those with 1 UCI (n = 83), and 18% of those with no UCI (n = 254; P = .0001).
Table 1.
Demographic and Clinical Parameters in a Cohort of HIV-Infected Persons Initiating ART in Nigeria Between 2009 and 2011.
| All (N = 1649)
|
No UCI (n = 1384)
|
1 UCI (n = 168)
|
≥2 UCIs (n = 97)
|
P Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| % | n | % | n | % | n | % | n | ||
| Sex | |||||||||
| Male | 30 | 486 | 30 | 411 | 26 | 44 | 32 | 31 | .5510 |
| Female | 70 | 1163 | 70 | 973 | 74 | 124 | 68 | 66 | |
| Mean age (SD), years | 33 (9.2) | 34 (9.2) | 32 (9.0) | 32 (8.5) | .0381 | ||||
| Marital Status | .2605 | ||||||||
| Married | 61 | 1004 | 61 | 840 | 58 | 97 | 68 | 65 | |
| Single | 25 | 411 | 25 | 345 | 27 | 45 | 22 | 21 | |
| Widowed | 14 | 234 | 14 | 199 | 15 | 25 | 10 | 10 | |
| Employment | .2447 | ||||||||
| Employed | 63 | 1027 | 64 | 873 | 55 | 91 | 65 | 63 | |
| Unemployed/retired | 29 | 469 | 29 | 396 | 30 | 49 | 25 | 24 | |
| Student | 8 | 137 | 7 | 101 | 16 | 26 | 10 | 10 | |
| Education | .5746 | ||||||||
| Tertiary | 34 | 559 | 34 | 473 | 35 | 59 | 28 | 27 | |
| Primary/secondary | 45 | 734 | 44 | 613 | 43 | 73 | 50 | 48 | |
| None | 22 | 356 | 22 | 298 | 21 | 35 | 23 | 23 | |
| Baseline CD4, cells/mm3 | |||||||||
| Mean (SD) | 251 | (193) | 228 (176) | 355 (228) | 392 (240) | <.0001 | |||
| <50 | 12 | 196 | 13 | 174 | 8 | 14 | 8 | 8 | .0001 |
| 50–100 | 12 | 190 | 13 | 175 | 7 | 11 | 4 | 4 | |
| 101–200 | 23 | 380 | 25 | 352 | 11 | 19 | 9 | 9 | |
| 200–350 | 30 | 487 | 31 | 431 | 24 | 41 | 15 | 15 | |
| >350 | 24 | 396 | 18 | 252 | 49 | 83 | 63 | 61 | |
| Baseline tuberculosis | |||||||||
| Yes | 4 | 65 | 4 | 59 | 3 | 5 | 1 | 1 | .2276 |
Abbreviations: ART, antiretroviral therapy; SD, standard deviation; UCI, unplanned care interruption.
Twenty-four percent of patients in the cohort enrolled and initiated ART with CD4 >350 cells/μL. We conducted a chart review to determine the reasons for ART initiation in this group. All were documented to have met national guideline criteria for starting ART. The indication for treatment was advanced WHO stage (3 or 4) in 36%, resumption of ART for patients who had discontinued in 20%, concomitant tuberculosis (TB) in 5%, and coinfection with hepatitis B virus in 3% of patients. The indication for ART initiation was not documented in 36% of patients.
The CD4 Count Assessments
The number of CD4 measurements per patient in the first year on ART ranged from 2 to 7. Nineteen percent of patients (n = 314) had 2, 80% (n = 1312) had 3 to 5, and 1% (n = 23) had 6 or 7 CD4 measurements. The median time between CD4 measurements was 105 days [interquartile range: 88–140].
Impact of UCI on CD4 Count
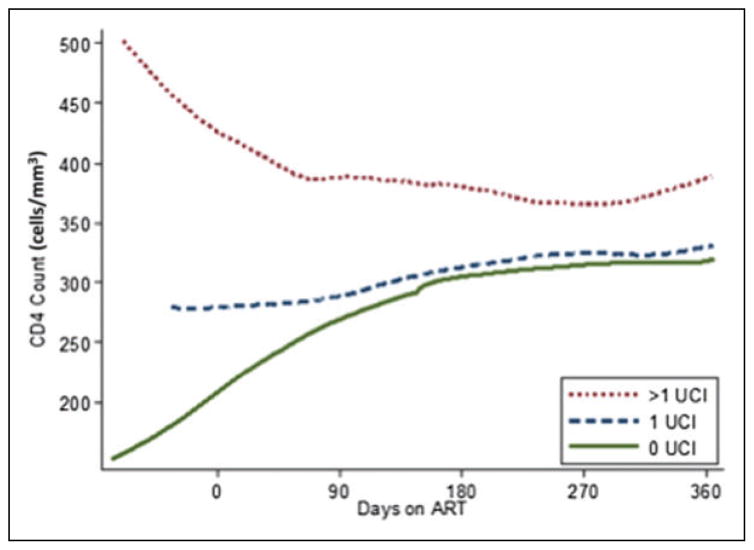
We plotted CD4 counts over time since ART initiation during the first year on ART using LOWESS smoothing with separate curves for those with 0, 1, and ≥2 UCIs during the year (Figure 2). Figure 2 illustrates that patients with no UCI had lower baseline CD4 counts but the greatest early rate of CD4 increase in the first 6 months on ART. In contrast, those with ≥2 UCIs had higher baseline CD4 counts, but early CD4 decrease in the same period.
Figure 2.
CD4 response by number of unplanned care interruptions in the first year on ART, smoothed LOWESS curve. ART, antiretro-viral therapy; LOWESS, locally weighted smoothed scatterplot; UCI, unplanned care interruption.
We assessed the effect of an UCI on the next observed CD4 count in multivariate analysis adjusted for patient age, gender, TB coinfection, baseline CD4 count (before ART initiation), days since ART initiation, previous CD4 count, and the days since the previous CD4 assessment. The CD4 counts increased by an estimated 51 cells/μL (95% confidence interval [CI]: 47–55) for each 100 cells/μL increase in baseline CD4 count and decreased by 11 cells/μL (95% CI: −17 to −5) for each decade increase in patient age. Patients with any UCI had 62 fewer CD4 cells at the next CD4 assessment compared to those with no UCI (95% CI: −78 to −45).
Impact of 0, 1, or ≥2 UCIs in the First Year on ART on Rate of CD4 Change
In multivariate analysis (adjusted for age, sex, baseline CD4 count, and TB coinfection), statistically significant increases in CD4 count were seen only for those with 0 UCI—10 cells/μL/mo (95% CI: 7–14) in the first 6 months on ART and 4 cells/μL/mo (95% CI: 2–6) in months 7 to 12. Patients with UCI did not experience statistically significant gains in the first year on ART. Estimated monthly rate of CD4 change in months 1 to 6 was −2 cells/μL/mo (95% CI: −18 to 13) and −5 cells/μL/mo (95% CI: −26 to 16) for patients with 1 and ≥2 UCIs, respectively. In months 7 to 12, the estimated rate of CD4 change UCI was 2 cells/μL/mo (95% CI: −6 to 11) and 8 cells/μL/mo (95% CI: −2 to 7) for patients with 1 and ≥2 UCIs, respectively (Table 2).
Table 2.
Predicted Rate of CD4 Change in the First Year on ART for Patients with 0, 1, or ≥2 UCIs.a
| No UCI | 30-Day CD4 Change | 95% CI | 30-Day CD4 Change | 95% CI |
|---|---|---|---|---|
| Months 0–6 on ART | Months 7–12 on ART | |||
| 0 | 10 | 7 to 14 | 4 | 2 to 6 |
| 1 | −2 | −18 to 13 | 2 | −6 to 11 |
| ≥2 | −5 | −26 to 16 | 8 | −2 to 17 |
Model adjusted for age, gender, baseline CD4 count, and tuberculosis (TB) coinfection.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval.
Estimated CD4 Gains After 1 Year on ART
Predicted CD4 count after 1 year on ART was higher for patients with ≥2 UCIs (435 cells/μL, 95% CI: 347–522) than those with 1 UCI (381 cells/μL, 95% CI: 298–464) or no UCI (354 cells/μL, 95% CI: 334–374). However, a comparison of observed baseline CD4 count to predicted 12-month CD4 count (holding all other variables in the model at their average) showed that patients with no UCI had the greatest CD4 gains at 126 cells/μL (from 228 cells/μL at baseline). Patients with 1 UCI gained an estimated 26 cells/μL (from 355 cells/μL at baseline). Patients with ≥2 UCIs gained 43 CD4 cells/μL (from 392 cells/μL at baseline).
Discussion
We found that unplanned HIV care interruption is common in a large Nigerian treatment program. Seventeen percent of patients had at least 1 UCI in their first year on ART. Although there is variability in the definition and rates of UCI in the literature, our results are consistent with studies conducted in resource-limited and resource-rich settings.10,18,19,24–27 We used several methods to quantify the impact of UCI on immune response to ART, all of which demonstrated deleterious effects of interruptions from care. The immediate consequence of any UCI was a gain of 62 CD4 cells/μL fewer than those who remained in care on the next measured CD4 count. Assessing the longer-term consequence of UCI over the first year on ART allowed us to determine whether patients with UCI were later able to recover from these early CD4 losses; importantly, they were not. Although patients without UCI had substantial CD4 gains, 126 cells/μL over the first year on ART, those with 1 or ≥2 UCIs had only one-third of these gains or less. The UCI was among the strongest predictors of immunologic recovery after ART initiation, along with baseline CD4 count and time on ART.
The first months after starting ART are critical for immune recovery. With effective ART, the CD4 response is biphasic. It is characterized by an initial CD4 increase of 50 to 120 cells/μL in the first 3 months and a sustained, gradual increase of 2 to 7 cells/μL monthly thereafter, which may continue for years.20,28,29 Many factors influence the CD4 response to ART, but baseline CD4 count and time on ART are among the most robust predictors; this was also illustrated in our analysis.28–31 Studies of patients with chronic HIV infection coinfected with TB have shown blunted CD4 responses.32 In our cohort, where only 4% of patients were diagnosed with TB upon presentation, baseline TB diagnosis was not a significant predictor of CD4 response. Studies on the impact of age on CD4 response have been mixed.33–35 We found that older patients exhibited poorer immune recovery. Similarly, data on the influence of gender are mixed, and in our cohort, gender did not predict CD4 response.30,35–37 Even after adjusting for all of these factors (baseline CD4 count, time on ART, TB, age, and gender), UCI remained a strong, significant, independent predictor of early CD4 response to ART.
We estimated the monthly rate of CD4 increase to be 10 cells/μL/mo in months 1 to 6 after starting ART for patients with no UCI. During this period in which patients without UCI were seeing their greatest gains, CD4 counts remained stagnant for those with UCI. Our findings are consistent with reports from 2 large multicenter cohorts in RLS where monthly CD4 increase in the first 6 months on ART ranged from lower estimates of 10 to 16 cells/μL to upper estimates of 22 to 23 cells/μL.30,38 Both studies adjust for baseline CD4 and gender. Of these 2 studies, only 1 aimed to characterize expected immunologic recovery after ART initiation and neither accounted for care interruption.30 Our analysis has allowed us to quantify the critical impact of interruptions from care on immune reconstitution.
Our data suggest that patients with higher baseline CD4 counts exhibited more UCIs after initiating ART. Patients with ≥2 UCIs started ART with an average of 392 CD4 cells/μL, nearly double the average baseline CD4 count of patients with no UCI. However, even when adjusting for baseline CD4 count, patients with any UCI had lower CD4 gains than those without UCI by the end of their first year in care. As a result, patients with any UCI lost the immunologic benefit of both ART and earlier presentation to care. Since patients with UCI are partially but not fully engaged in care, they still require the financial and clinical investment and commitment of care teams. Ensuring that this investment is not lost is crucial not only to individual patients but also for the sustainability of programs as global funding for HIV care levels off in Nigeria and other RLS.39,40 Moreover, strategies focusing on increased adherence and linkage to care among patients initiating ART with higher CD4 counts in Nigeria are even more important considering the recent adoption of WHO guidelines recommending immediate ART diagnosis.41
The risk of morbidity and mortality is widely appreciated for patients presenting to care with advanced immune suppression and CD4 counts <200 cells/μL.42,43 But the risk of AIDS and death persist at higher CD4 strata (as high as 500–650 cells/μL).30,44 This knowledge has driven higher global ART initiation thresholds, which increased first to 350 cells/μL then 500 cells/μL and now to immediate ART.41,45 Furthermore, the absolute risk of AIDS and death is higher in RLS regardless of CD4 count, likely due to the higher burden of TB and invasive bacterial disease.46 These findings underscore the importance of maximizing the immune response to ART and adherence to care irrespective of the baseline CD4 counts.
Our study has important limitations. Since it was conducted in an urban environment at 1 university-affiliated clinic, it may not be fully generalizable to other settings. Nonetheless, our estimates of CD4 gains in the first year after starting ART are consistent with those from other RLS. In addition, our analysis of the impact of UCI on CD4 response to ART was restricted to patients who had available baseline and at least 1 follow-up CD4 result. Missing CD4 values could have biased our estimates in either direction, but since more patients excluded from the analysis had UCI than those included, our estimates are likely to be conservative. Finally, our estimates of CD4 recovery may have been biased by the finding that patients with more UCI had higher median CD4 counts at ART initiation and thus had less capacity for immunologic recovery as assessed by CD4 gains on ART. Our models did adjust for baseline CD4 count to address this potential bias.
Despite these limitations, this analysis also has several strengths. Our robust electronic health record allowed us to quantify the frequency of UCI by relying on a range of health-care visits (clinical, laboratory, and pharmacy) over the first year on ART. With nearly one-tenth of the global population of people living with HIV in Nigeria, this setting is critical for assessing the impact of ineffective retention in care. Our analysis is one of the few providing adjusted estimates of the expected immunologic recovery on ART in RLS, where background rates of other infections may affect these estimates. As a result, our analysis allowed us to illustrate and quantify the immunologic impact of UCI for patients initiated on ART. This information can be readily provided at the point of care as a clinical tool to reinforce the importance of adherence to care and to demonstrate the impact of care interruption on CD4 recovery.
This study underscores that UCI from HIV services is common in RLS and is accompanied by a loss in expected therapeutic benefit of ART. Patients with any UCI have both a slower rate of CD4 gain and an early loss of CD4 cells that they do not regain over the first year on ART. Interventions to prevent UCI, and ensure effective retention in care, are critical to optimize clinical outcomes and maximize the health benefit that patients receive from HIV care.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers: K23 AI106406, R01 AI058736-09S1, R01 MH090326, and P30AI060354. This study was also supported in part by cooperative agreement number 5U2GPS001058 from the US Centers for Disease Control and Prevention and the Massachusetts General Hospital Executive Committee on Research.
Footnotes
Authors’ Note
These data were presented in part at the Conference on Retroviruses and Opportunistic Infections (CROI) 2015, Seattle, WA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.President’s Emergency Plan for AIDS Relief. [Accessed September 30, 2016];Partnership to fight HIV/AIDS in Nigeria. 2011 http://www.pepfar.gov/documents/organization/199599.pdf.
- 2.World Health Organization. [Accessed September 30, 2016];A Guide to Indicators for Monitoring and Evaluating National Antiretroviral Programmes. 2005 http://www.who.int/hiv/pub/me/en/naparv.pdf.
- 3.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15(suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The World Bank. [Accessed September 30, 2016];World Development Indicators. 2011 http://data.worldbank.org/data-catalog/world-development-indicators?cid=GPD_WDI.
- 5.McMahon JH, Elliott JH, Hong SY, Bertagnolio S, Jordan MR. Effects of physical tracing on estimates of loss to follow-up, mortality and retention in low and middle income country anti-retroviral therapy programs: a systematic review. PLoS One. 2013;8(2):e56047. doi: 10.1371/journal.pone.0056047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisson GP, Gaolathe T, Gross R, et al. Overestimates of survival after HAART: implications for global scale-up efforts. PLoS One. 2008;3(3):e1725. doi: 10.1371/journal.pone.0001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox MP, Brennan A, Maskew M, MacPhail P, Sanne I. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010;15(4):405–413. doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.d’arminio Monforte A, Cozzi-Lepri A, Phillips A, et al. Italian Cohort of Antiretroviral-Naive Patients Study Group. Interruption of highly active antiretroviral therapy in HIV clinical practice: results from the Italian Cohort of Antiretroviral-Naive Patients. J Acquir Immune Defic Syndr. 2005;38(4):407–416. doi: 10.1097/01.qai.0000147529.57240.b0. [DOI] [PubMed] [Google Scholar]
- 10.Brennan AT, Maskew M, Sanne I, Fox MP. The importance of clinic attendance in the first six months on antiretroviral treatment: a retrospective analysis at a large public sector HIV clinic in South Africa. J Int AIDS Soc. 2010;13:49. doi: 10.1186/1758-2652-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore DM, Zhang W, Yip B, et al. Non-medically supervised treatment interruptions among participants in a universally accessible antiretroviral therapy programme. HIV Med. 2010;11(5):299–307. doi: 10.1111/j.1468-1293.2009.00779.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahonkhai AA, Noubary F, Munro A, et al. Not all are lost: interrupted laboratory monitoring, early death, and loss to follow-up (LTFU) in a large South African treatment program. PLoS One. 2012;7(3):e32993. doi: 10.1371/journal.pone.0032993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geng EH, Nash D, Kambugu A, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep. 2010;7(4):234–244. doi: 10.1007/s11904-010-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kranzer K, Ford N. Unstructured treatment interruption of anti-retroviral therapy in clinical practice: a systematic review. Trop Med Int Health. 2011;16(10):1297–1313. doi: 10.1111/j.1365-3156.2011.02828.x. [DOI] [PubMed] [Google Scholar]
- 15.Danel C, Moh R, Minga A, et al. Trivacan ANRS 1269 trial group. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367(9527):1981–1989. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 16.Ananworanich J, Gayet-Ageron A, Le Braz M, et al. Staccato Study Group; Swiss HIV Cohort Study. CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: results of the Staccato randomised trial. Lancet. 2006;368(9534):459–465. doi: 10.1016/S0140-6736(06)69153-8. [DOI] [PubMed] [Google Scholar]
- 17.Kiguba R, Byakika-Tusiime J, Karamagi C, Ssali F, Mugyenyi P, Katabira E. Discontinuation and modification of highly active antiretroviral therapy in HIV-infected Ugandans: prevalence and associated factors. J Acquir Immune Defic Syndr. 2007;45(2):218–223. doi: 10.1097/QAI.0b013e31805d8ae3. [DOI] [PubMed] [Google Scholar]
- 18.Mugavero MJ, Lin HY, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–256. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahonkhai AA, Banigbe B, Adeola J, et al. High rates of unplanned interruptions from HIV care early after antiretroviral therapy initiation in Nigeria. BMC Infect Dis. 2015;15(1):397. doi: 10.1186/s12879-015-1137-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44(3):441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 21.da Costa TM, Barbosa BJ, Gomes e Costa DA, et al. Results of a randomized controlled trial to assess the effects of a mobile SMS-based intervention on treatment adherence in HIV/AIDS-infected Brazilian women and impressions and satisfaction with respect to incoming messages. Int J Med Inform. 2012;81(4):257–269. doi: 10.1016/j.ijmedinf.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [Accessed January 26, 2016];AIDS Prevention Initiative Nigeria. http://apin.org.ng.
- 23.Federal Ministry of Health, Nigeria. [Accessed December 21, 2015];National Guidelines for HIV and AIDS Treatment and Care in Adolescents and Adults. 2010 http://www.who.int/hiv/pub/guidelines/nigeria_art.pdf.
- 24.Boyer S, Clerc I, Bonono CR, Marcellin F, Bile PC, Ventelou B. Non-adherence to antiretroviral treatment and unplanned treatment interruption among people living with HIV/AIDS in Cameroon: individual and healthcare supply-related factors. Soc Sci Med. 2011;72(8):1383–1392. doi: 10.1016/j.socscimed.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Mann M, Diero L, Kemboi E, et al. Antiretroviral treatment interruptions induced by the Kenyan postelection crisis are associated with virological failure. J Acquir Immune Defic Syndr. 2013;64(2):220–224. doi: 10.1097/QAI.0b013e31829ec485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luebbert J, Tweya H, Phiri S, et al. Virological failure and drug resistance in patients on antiretroviral therapy after treatment interruption in Lilongwe, Malawi. Clin Infect Dis. 2012;55(3):441–448. doi: 10.1093/cid/cis438. [DOI] [PubMed] [Google Scholar]
- 27.Kavasery R, Galai N, Astemborski J, et al. Nonstructured treatment interruptions among injection drug users in Baltimore, MD. J Acquir Immune Defic Syndr. 2009;50(4):360–366. doi: 10.1097/QAI.0b013e318198a800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrogoua DP, Kablan BJ, Kamenan BA, Aulagner G, N’Guessan K, Zohore C. Assessment of the impact of adherence and other predictors during HAART on various CD4 cell responses in resource-limited settings. Patient Prefer Adherence. 2012;6:227–237. doi: 10.2147/PPA.S26507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufmann GR, Perrin L, Pantaleo G, et al. Swiss HIV Cohort Study Group. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163(18):2187–2195. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 30.Nash D, Katyal M, Brinkhof MW, et al. ART-LINC Collaboration of IeDEA. Long-term immunologic response to antiretroviral therapy in low-income countries: a collaborative analysis of prospective studies. AIDS. 2008;22(17):2291–2302. doi: 10.1097/QAD.0b013e3283121ca9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michael CG, Kirk O, Mathiesen L, Nielsen SD. The naive CD4+ count in HIV-1-infected patients at time of initiation of highly active antiretroviral therapy is strongly associated with the level of immunological recovery. Scand J Infect Dis. 2002;34(1):45–49. doi: 10.1080/00365540110076930. [DOI] [PubMed] [Google Scholar]
- 32.Cingolani A, Cozzi Lepri A, Castagna A, et al. Impaired CD4 T-cell count response to combined antiretroviral therapy in antiretroviral-naive HIV-infected patients presenting with tuberculosis as AIDS-defining condition. Clin Infect Dis. 2012;54(6):853–861. doi: 10.1093/cid/cir900. [DOI] [PubMed] [Google Scholar]
- 33.Teixeira L, Valdez H, McCune JM, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS. 2001;15(14):1749–1756. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 34.Aiuti F, Mezzaroma I. Failure to reconstitute CD4+ T-cells despite suppression of HIV replication under HAART. AIDS Rev. 2006;8(2):88–97. [PubMed] [Google Scholar]
- 35.Patterson K, Napravnik S, Eron J, Keruly J, Moore R. Effects of age and sex on immunological and virological responses to initial highly active antiretroviral therapy. HIV Med. 2007;8(6):406–410. doi: 10.1111/j.1468-1293.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 36.Collazos J, Asensi V, Carton JA. Sex differences in the clinical, immunological and virological parameters of HIV-infected patients treated with HAART. AIDS. 2007;21(7):835–843. doi: 10.1097/QAD.0b013e3280b0774a. [DOI] [PubMed] [Google Scholar]
- 37.Moore AL, Kirk O, Johnson AM, et al. EuroSIDA group. Virologic, immunologic, and clinical response to highly active anti-retroviral therapy: the gender issue revisited. J Acquir Immune Defic Syndr. 2003;32(4):452–461. doi: 10.1097/00126334-200304010-00017. [DOI] [PubMed] [Google Scholar]
- 38.Keiser O, Chi BH, Gsponer T, et al. IeDEA Southern Africa Collaboration. Outcomes of antiretroviral treatment in programmes with and without routine viral load monitoring in Southern Africa. AIDS. 2011;25(14):1761–1769. doi: 10.1097/QAD.0b013e328349822f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.President’s Emergency Plan for AIDS Relief. [Accessed January 10, 2016];Congressional budget justification supplement: fiscal year. 2014 http://www.pepfar.gov/reports/progress/c61745.htm. Published August 2013.
- 40.Kaiser Family Foundation. [Accessed November 1, 2015];Financing the Response to HIV in Low- and Middle-Income Countries: International Assistance from Donor Governments in 2013. http://kff.org/global-health-policy/report/financing-the-response-to-aids-in-low/. Published July 2015.
- 41.World Health Organization. [Accessed September 30, 2016];Treat all people living with HIV, offer antiretrovirals as additional prevention choice for people at “substantial” risk. http://www.who.int/mediacentre/news/releases/2015/hiv-treat-all-recommendation/en/. Published 2016.
- 42.Abo Y, Minga A, Menan H, et al. Incidence of serious morbidity in HIV-infected adults on antiretroviral therapy in a West African care centre, 2003–2008. BMC Infect Dis. 2013;13:607. doi: 10.1186/1471-2334-13-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368(9543):1254–1259. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 44.Gabillard D, Lewden C, Ndoye I, et al. ANRS 12222 Morbidity Mortality Study Group. Mortality, AIDS-morbidity, and loss to follow-up by current CD4 cell count among HIV-1-infected adults receiving antiretroviral therapy in Africa and Asia: data from the ANRS 12222 collaboration. J Acquir Immune Defic Syndr. 2013;62(5):555–561. doi: 10.1097/QAI.0b013e3182821821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirnschall G, Harries AD, Easterbrook PJ, Doherty MC, Ball A. The next generation of the World Health Organization’s global antiretroviral guidance. J Int AIDS Soc. 2013;16:18757. doi: 10.7448/IAS.16.1.18757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anglaret X, Minga A, Gabillard D, et al. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d’Ivoire. Clin Infect Dis. 2012;54(5):714–723. doi: 10.1093/cid/cir898. [DOI] [PMC free article] [PubMed] [Google Scholar]