Abstract
Background
Cannabis has been shown to affect sleep in humans. Findings from animal studies indicate that higher endocannabinoid levels promote sleep, suggesting that chronic use of cannabis, which downregulates endocannabinoid activity, may disrupt sleep.
Objectives
This study sought to determine if past year cannabis use and genes that regulate endocannabinoid signaling, FAAH rs324420 and CNR1 rs2180619, predicted sleep quality. As depression has been previously associated with both cannabis and sleep, the secondary aim was to determine if depressive symptoms moderated or mediated these relationships.
Methods
Data were collected from 41 emerging adult (ages 18–25) cannabis users. Exclusion criteria included Axis I disorders (besides SUD) and medical and neurologic disorders. Relationships were tested using multiple regressions, controlling for demographic variables, past year substance use, and length of cannabis abstinence.
Results
Greater past year cannabis use and FAAH C/C genotype were associated with poorer sleep quality. CNR1 genotype did not significantly predict sleep quality. Depressive symptoms moderated the relationship between cannabis use and sleep at a non-significant trend level, such that participants with the greatest cannabis use and most depressive symptoms reported the most impaired sleep. Depressive symptoms mediated the relationship between FAAH genotype and sleep quality.
Conclusions
This study demonstrates a dose-dependent relationship between chronic cannabis use and reported sleep quality, independent of abstinence length. Furthermore, it provides novel evidence that depressive symptoms mediate the relationship between FAAH genotype and sleep quality in humans. These findings suggest potential targets to impact sleep disruptions in cannabis users.
Introduction
Cannabis is the most commonly used illicit substance among emerging adults, with approximately one third of U.S. 19–24 year olds reporting use within the past year (1). Many young people report using cannabis to help them initiate sleep (2). However, cannabis and two of its major components, Δ-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), are associated with disrupted sleep using objective measures (3). THC exerts its effects on the brain and body by binding to CB1 and CB2 receptors in the endocannabinoid system, which modulates the sleep-wake cycle (4). CB1 receptor antagonists increase wakefulness and reduce rapid eye movement (REM) time in animal studies (5–8). In addition, CB1 knockout mice experience increased wakefulness (9). The relationship between CB1 receptor inactivation and wakefulness is likely related to anandamide (AEA), an endogenous cannabinoid that, like THC, binds to the CB1 receptor and promotes sleep, including increased slow wave sleep (SWS) and REM time (6, 10–13). AEA is degraded by the enzyme, fatty acid amide hydrolase (FAAH). FAAH and its associated gene have also been linked with aspects of sleep. FAAH knockout mice experience longer and more intense SWS compared to controls (14). This body of literature indicates that due to the sleep-promoting properties of AEA, increases in CB1 activity and decreases in FAAH activity may induce sleep while increasing time spent in SWS and REM.
Acute THC and CBD administration, as well as chronic cannabis use, have been associated with sleep alterations. However, the literature regarding the effects of THC and CBD on sleep is mixed. Acute cannabis or THC administration has been shown to promote sleep onset (15–18) and increase REM sleep (19), which is important for memory consolidation (20). However, other studies have demonstrated a reduction in REM sleep following THC administration (21–23). Similarly, while in some cases CBD has demonstrated hypnotic properties (24, 25), it has alternatively been associated with increased wakefulness (26). Furthermore, chronic cannabis users exhibit decreased SWS compared to controls immediately following termination of use, with recovery after fours weeks of abstinence (27). Reductions in SWS are associated with daytime sleepiness (28). Similarly, on a self-report measure, current adolescent cannabis users reported increased daytime drowsiness (29). Taken together, the evidence suggests that although using cannabis may decrease time to sleep onset in youth, the quality of their sleep is often diminished.
In addition to sleep quality, research has also linked cannabinoids with depression and mood. Chronic cannabis use is associated with both depressive disorders (eg, 30–37) as well as subclinical depressive symptoms in adolescents and adults (38–40). This aligns with findings that the endocannabinoid system regulates mood (41–46). Furthermore, CNR1 (which codes for the human CB1 receptor) and FAAH genes have been associated with risk for mood disorders in humans (47–49). Thus, many findings suggest the endocannabinoid system modulates mood, which has been linked with sleep problems (50).
To our knowledge, no study has investigated the relationships between cannabis use, endocannabinoid genes, and sleep in a human sample. The primary aim of the present study is to determine if past year cannabis use, FAAH genotype, and CNR1 genotype predict past month sleep quality in a group of cannabis-using emerging adults. Specifically, we examine the FAAH rs324420 C to A single nucleotide polymorphism (SNP), which results in diminished stability of the FAAH enzyme (51), and the CNR1 rs2180619 A to G SNP, which decreases CNR1 expression (52). We predict that greater past year cannabis use will be associated with increased sleep problems. Furthermore, we expect that genotypes (FAAH A carriers and CNR1 A/A genotype) with higher endocannabinoid levels will be related to increased sleep quality compared to genotypes (FAAH C/C genotype and CNR1 G carriers) with lower endocannabinoid levels. We also explore whether these genotypes moderate the relationship between cannabis use and sleep quality. Finally, our secondary aim is to determine if depressive symptoms moderate or mediate the relationships between cannabis use, FAAH, CNR1, and sleep quality.
Methods
Participants
Participants included 41 cannabis using right-handed emerging adults, ages 18–25 (26 M/15 F) from a larger imaging genetics study (NIH R03 DA027457). Eligible participants included in the current analysis met minimum cannabis use requirements (>10 joints in past year). Individuals were excluded for MRI contraindications; history of chronic medical or neurologic illness or injury (see 53 for more details); history of a learning disability; substantial birth complications or premature birth; known prenatal alcohol (>4 drinks/day or >7 drinks/week) or illicit drug (>10 uses) exposure; >14 standard alcoholic drinks per week (5 included participants exceeded these criteria); excessive illicit drug use besides cannabis (> 25 lifetime uses of any drug class); current use of psychotropic medication; past year DSM-IV-TR Axis I diagnosis, aside from substance abuse/dependence; failure to remain abstinent for six days prior to the study; and pregnancy.
Materials and Procedure
The Institutional Review Board at the University of Cincinnati approved all aspects of this study. Participants were recruited through advertisements and fliers distributed throughout the community. Interested participants completed a phone screen to determine eligibility. This included a semi-structured interview to screen for DSM-IV-TR anxiety, mood, attentional, and psychotic disorders (determined by KML; 54).
Prior to the study, participants provided informed consent. All participants completed a brief background questionnaire, psychological questionnaires, and a drug use interview. Participants were paid $160 for completing two sessions. Participants were required to remain abstinent from all substances for 7 days prior to the study sessions (two participants were abstinent for six days). Cannabis abstinence was ensured by examining the difference in THC metabolite ratio totals (while controlling for creatinine) between sessions 1 and 2 to verify that these values did not increase during study participation (55). No participants failed to meet these abstinence requirements. All participants received parking reimbursement, local substance use treatment resources, and images of their brain.
Demographic Information
Participants completed a background questionnaire outlining demographic variables including age, gender, ethnicity, self and biological parents’ educations, incomes, and employments, marital status, history of medical or neurological illness, psychological disorders or use of psychiatric medication, and learning disability.
Substance use
Past year alcohol and other drug use frequency was assessed using a modified version of the Time-Line Follow-Back (TLFB) (56) interview, which uses memory cues to evaluate frequency of drug use over the past year. Substance use was measured in standard units (e.g., joints [1 joint= 0.5 grams] for cannabis). Lifetime and past 3-month substance use was measured using the Customary Drinking and Drug Use Record (CDDR), which also assessed withdrawal symptoms, DSM-IV abuse and dependence criteria, and substance-related difficulties (57, 58).
Self-reported Mood
Participants completed the Beck Depression Inventory-II (BDI-II; 59), which contains 21 items measuring past two-week depressive symptoms on a 0–3 point scale. Total scores indicate either “minimal” depression (0–13), “mild” depression (14–19), “moderate” depression (20–28), or “severe” depression (29–63). Independent depressive disorders were excluded as part of the larger imaging study and no participants reported currently experiencing “severe” depression as classified by the BDI-II.
Sleep Quality
The Pittsburgh Sleep Quality Index (PSQI) (60) contains 19 items measuring aspects of sleep. Items 1–4 determine length of sleep, while Items 5–19 use a 0–3 point scale to assess sleep quality. PSQI global scores range from 0–21, with scores ≤5 associated with good sleep quality and >5 associated with poor sleep quality.
Biological Samples
Participants underwent a breathalyzer test, a pregnancy test, and urine toxicology screening using the One Step Drug Screen Test. Participants testing positive for substances except cannabis and nicotine were excluded. For those testing positive for cannabis, metabolite levels were examined via mass spectrometry.
Genotyping
The FAAH rs324420 and CNR1 rs2180619 variants were sequenced using TaqMan (fluorogenic 5’ nuclease) assay by a trained geneticist. The primers and probes were manufactured by Applied Biosystems, and PCR was conducted via an ABI 9700 thermocycler. Endpoint results were scored using the 7900HT Sequence Detection System.
Data Analysis
Preliminary Analysis
All analyses were conducted using SPSS. Additionally, ANOVA and Chi-square tests were run to determine if differences in demographic variables, or past year/lifetime drug use patterns were present. Variables that either differed between groups or that are known to affect sleep quality were entered as covariates; these included age, gender, ethnicity, past year alcohol and nicotine use, and number of days abstinent from cannabis use. All substance use variables were continuous.
Primary Analysis
General linear modeling (GLM) in SPSS was used to examine whether past year cannabis use, FAAH genotype, and CNR1 genotype were associated with sleep problems after controlling for demographics and other drug use. To test the mediation model (61), regression one established whether cannabis use, FAAH or CNR1 genotypes were associated with depressive symptoms. Regression two tested whether cannabis and depressive symptoms predicted sleep quality. Then regression three tested the main effects (cannabis, FAAH, CNR1), mediation model, and moderation of depressive symptoms. Block One tested the main effects of cannabis use, FAAH and CNR1 genotype on sleep quality; Block Two tested the impact of depressive symptoms; Block Three tested whether depressive symptoms or genotype moderated the effects of cannabis on sleep quality. Significance was determined if p<.05.
Results
Since one question on the BDI-II assesses sleep quality, we conducted the analyses both with and without the sleep question included in the BDI-II total score. The p<.05 significance of variables in the regression models did not change as a function of including or excluding the sleep question from the BDI-II. Regression results are reported with the sleep question removed from the BDI-II.
Demographic Information (see Table 1)
Table 1.
Participant Demographics by Genotype
| FAAH (N=41) | CNR1 (N=41) | |||
|---|---|---|---|---|
| C/C (n=20) | A/C and A/A (n=21) | A/A (n=11) | A/G and G/G (n=30) | |
| % or M ± SD (range) |
% or M ± SD (range) | % or M ± SD (range) |
% or M ± SD (range) | |
| Gender (% female) | 25% | 47.6% | 36.4% | 36.7% |
| Ethnicity (Caucasian) | 85%* | 33%* | 81.8% | 50% |
| Age (years) | 20.7 ± 2.0 (18–25)* | 22.3 ± 2.3 (18–25)* | 21.1 ± 2.3 (18–25) | 21.6 ± 2.3 (18–25) |
| Education (years) | 13.4 ± 1.4 (11–16) | 12.9 ± 1.7 (9–17) | 13.0 ± 1.5 (11–16) | 13.2 ± 1.6 (9–17) |
| Beck Depression Inventory-II (BDI-II) |
6.9 ± 5.3 (0–20) | 4.1 ± 5.1 (0–21) | 4.4 ± 3.2 (0–11) | 5.9 ± 5.9 (0–21) |
| BDI-II (sleep question removed) |
6.0 ± 4.9 (0–19) | 3.7 ± 4.8 (0–20) | 3.8 ± 3.2 (0–11) | 5.2 ± 5.4 (0–20) |
| Past year nicotine use (cigarettes) |
1490.8 ± 2580.0 (0–7350.0) | 645.95 ± 791.9 (0–2399.0) | 2184.6 ± 3246.7 (4–7350.0)* | 645.0 ± 867.8 (0–2890.0)* |
| Past year cannabis use (joints) |
1000.2 ± 1751.5 (13–7343) | 579.9 ± 940.6 (12–4179) | 885.6 ± 1148.4 (42–3895) | 748.0 ± 1490.7 (12–7343) |
| Length of cannabis abstinence (days) |
13.2 ± 7.5 (6–36) | 15.9 ± 13.7 (6–53) | 12.9 ± 8.9 (7–36) | 15.2 ± 11.2 (6–53) |
| Past year alcohol use (standard drinks) |
357.8 ± 379.2 (29–1156) | 188.9 ± 367.2 (2–1724) | 293.2 ± 513.3 (19–1724) | 263.2 ± 325.9 (2–1156) |
| PSQI global score | 7.3 ± 2.0 (5–11) | 6.1 ± 2.0 (4–11) | 6.1 ± 1.7 (4–10) | 6.9 ± 2.2 (4–11) |
| % Disturbed sleep (PSQI global score >5) |
85% | 52.4% | 54.5% | 73.3% |
Note.
Group differences: p< .05. PSQI= Pittsburgh Sleep Quality Index. FAAH= Fatty acid amide hydrolase rs324420 C to A single nucleotide polymorphism (SNP); CNR1= CNR1 rs2180619 A to G SNP.
Genotype Differences
A chi-square test revealed no significant relationship between participants’ FAAH genotype and CNR1 genotype (χ2(1)= 1.33, p=0.25).
FAAH Genotype & Demographics
ANOVAs and chi-square tests yielded significant differences between those with the C/C genotype and A carriers in ethnicity (85.0% of C/C genotype and 33.3% of A carriers were Caucasian; previous studies have reported African Americans to have increased FAAH rs324420 diversity compared to Caucasians (62)) (χ2(4)= 15.55, p<0.01) and age (F(1,39)= 5.98, p= 0.02) (Age and ethnicity were controlled for in the regressions). No differences were found between genotypes in gender (χ2(1)= 2.26, p=0.13), education (F(1,39)= 1.21, p= 0.28), or depressive symptoms (F(1,39)= 2.80, p= 0.10). Additionally, there was no difference in depressive symptoms between the genotype groups when the sleep question was removed from the BDI-II total score (F(1,39)= 2.17, p= 0.15).
CNR1 Genotype & Demographics
ANOVAs and chi-square tests revealed no significant differences between those with the A/A genotype and G carriers in age (F(1,39)= 0.45, p= 0.51) gender (χ2(1)= 0.00, p=0.99), ethnicity (χ2(4)=8.53, p=0.07), education (F(1,39)= 0.09, p= 0.77), or depressive symptoms (F(1,39)= 0.64, p= 0.43). There was no difference in depressive symptoms between the genotype groups when the sleep question was removed from the BDI-II total score (F(1,40)=.60, p= 0.45).
Drug Use Information
FAAH Genotype & Drug Use
C/C genotype and A carriers did not differ on any drug use variable, including past year cannabis (F(1,39)= .93, p= 0.34), nicotine (F(1,39)= 2.05, p= 0.16), or alcohol (F(1,39)= 2.10, p= 0.16) use. Additionally, there was no difference in length of cannabis abstinence (F(1,39)= .61, p= 0.44) between the two genotype groups.
CNR1 Genotype & Drug Use
A/A genotype and G carriers did not differ on measures of past year cannabis (F(1,39)= 0.08, p= 0.78) or alcohol (F(1,39)= 0.05, p= 0.83) use. However, those with the A/A genotype reported significantly greater past year nicotine use (F(1,39)= 5.85, p= 0.02) compared to G carriers. No significant difference in length of cannabis abstinence was found between genotype groups (F(1,39)= .34, p= 0.56).
Sleep Changes Associated with Drug Withdrawal
Drug (any substance, including cannabis, besides alcohol) withdrawal, including sleep symptoms, was assessed using the CDDR (57, 58). Out of 41 participants, 24.4% reported that in the past, within two days of ceasing drug use, they had “difficulty sleeping (such as taking >30 minutes to fall asleep, waking during the night and taking >30 minutes to fall back asleep, waking up earlier than usual and not being able to fall back asleep)” and 29.3% of the sample reported increased dreaming after stopping their drug use.
Primary Results (see Table 3)
Table 3.
Regression Results Testing Mediation Model
| Regression Model | ||||
|---|---|---|---|---|
| Predictors: | ||||
| Regression 1 | Outcome Variable | Beta | t-value | Significance (p) |
| Age | BDI-II Total (no sleep) | .27 | 1.40 | .17 |
| Ethnicity | .03 | .17 | .87 | |
| Gender | .32 | 1.74 | .09 | |
| Past year nicotine use | .06 | .36 | .72 | |
| Past year alcohol use | .07 | .40 | .70 | |
| Past year cannabis use | .04 | .26 | .80 | |
| FAAH genotype | −.42 | −2.41 | .02 | |
| CNR1 genotype | .18 | 1.20 | .28 | |
| Length of cannabis abstinence | .21 | 1.15 | .26 | |
| Regression 2 |
||||
| Age | PSQI | −.04 | −.40 | .69 |
| Ethnicity | −.10 | −1.08 | .29 | |
| Gender | −.15 | −1.36 | .18 | |
| Past year nicotine use | −.19 | −1.86 | .07 | |
| Past year alcohol use | −.03 | −.31 | .76 | |
| Past year cannabis use | .43 | 4.12 | .00 | |
| BDI-II Total (no sleep) | .76 | 7.44 | .00 | |
| Length of cannabis abstinence | −.09 | −.76 | .45 | |
| Regression 3: Block 2 |
||||
| Age | PSQI | .17 | .93 | .36 |
| Ethnicity | −.08 | −.51 | .61 | |
| Gender | .11 | .60 | .55 | |
| Past year nicotine use | −.13 | −.78 | .44 | |
| Past year alcohol use | .01 | .08 | .94 | |
| Past year cannabis use | .46 | 2.83 | .01 | |
| FAAH genotype | −.36 | −2.09 | <.05 | |
| CNR1 genotype | .17 | 1.08 | .29 | |
| Length of cannabis abstinence | .06 | .36 | .72 | |
| Regression 3: Block 3 |
||||
| Age | PSQI | −.03 | −.20 | .84 |
| Ethnicity | −.10 | −.97 | .34 | |
| Gender | −.13 | −1.04 | .31 | |
| Past year nicotine use | −.18 | −1.59 | .12 | |
| Past year alcohol use | −.04 | −.33 | .75 | |
| Past year cannabis use | .43 | 3.98 | .00 | |
| FAAH genotype | −.05 | −.37 | .72 | |
| CNR1 genotype | .04 | .37 | .71 | |
| Length of cannabis abstinence | −.09 | −.77 | .49 | |
| BDI-II Total (no sleep) | .74 | 6.39 | .00 | |
Note. This table includes the regression blocks testing the mediation model. Bolded and italicized text denotes relationships significant at the p < .05 level. For added rigor, regression results are reported with the sleep questions removed from the BDI-II. Significance values reported as p= .00 are equivalent to values p< 0.005.
Regression One (Cannabis & Genotypes Predicting Depressive Symptoms)
Cannabis Use: Past year cannabis use was not significantly associated with depressive symptoms [t (31) = .26, beta=.04, p=.80]. FAAH Genotype: FAAH genotype was significantly associated with depressive symptoms [t (31) = −2.41, beta=−.42, p=.02], such that C/C genotypes reported greater depressive symptomatology compared to A carriers. CNR1 Genotype: CNR1 genotype did not significantly predict depressive symptoms [t (31) = 1.10, beta=.18, p=.28]. No other covariates significantly predicted depressive symptoms.
Regression Two (Cannabis & Depressive Symptoms Predicting Sleep Quality)
Cannabis Use: Higher levels of past year cannabis use significantly predicted poorer sleep quality [t (32) = 4.12, beta=.43, p<.001] (Figure 1). Depressive Symptoms: Greater depressive symptoms were significantly related to poorer sleep quality [t (32) = 7.44, beta=.76, p<.001]. No additional covariates demonstrated a significant relationship with sleep quality.
Figure 1.
After controlling for age, gender, ethnicity, depressive symptoms, length of cannabis abstinence, and past year alcohol and nicotine use, a dose-dependent relationship between increased past month sleep problems and greater past year cannabis use was revealed.
Regression Three: Block One (Cannabis Use Alone)
Past year cannabis use was significantly associated with greater sleep problems [t (31) = 2.93, beta=.42, p< .006].
Regression Three: Block Two (Cannabis Use & Genotypes Predicting Sleep Quality)
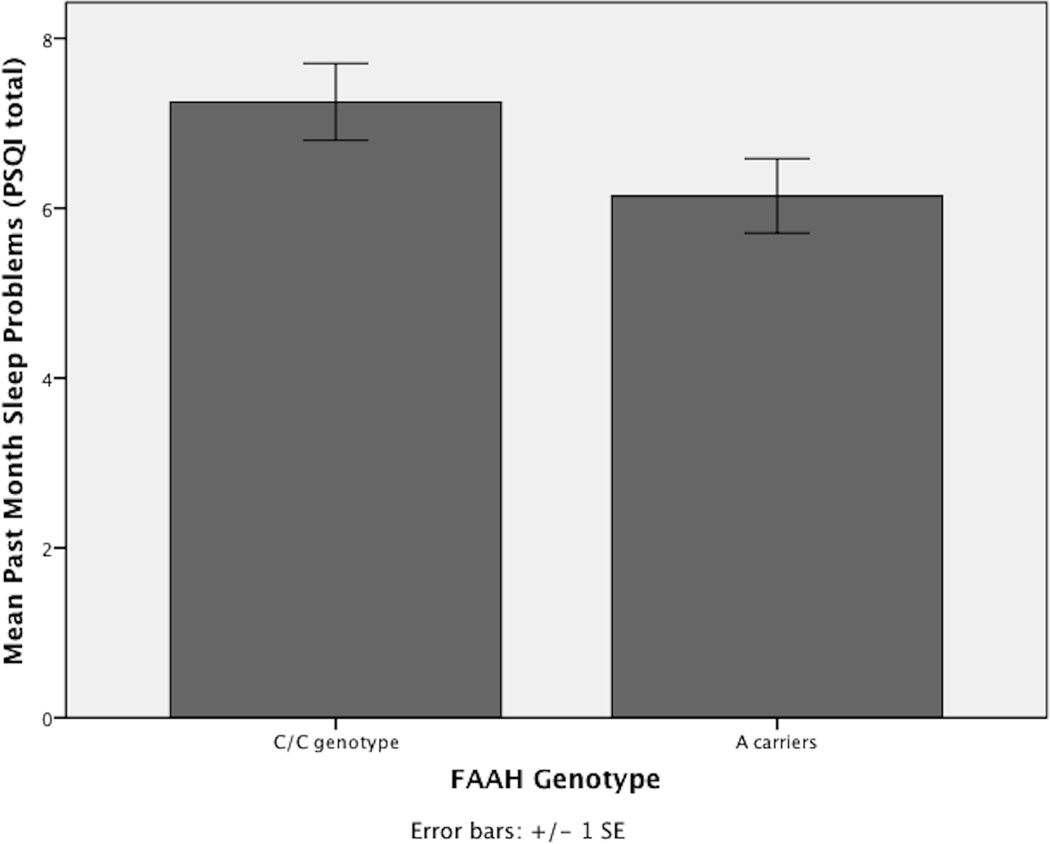
Cannabis Use: Past year cannabis use was significantly associated with greater sleep problems [t (31) = 2.83, beta=.46, p< .01]. FAAH Genotype: FAAH genotype significantly predicted sleep problems [t (31) = −2.09, beta=−.36, p< .05], with C/C genotypes demonstrating increased sleep problems compared to A carriers (see Figure 2). CNR1 Genotype: CNR1 genotype did not significantly predict sleep problems [t (31) = 1.08, beta=.74, p=.29]. All other covariates were not significantly associated with sleep problems.
Figure 2.
After controlling for age, gender, ethnicity, length of cannabis abstinence, and past year alcohol, nicotine, and cannabis use, participants with the FAAH C/C genotype reported significantly more sleep problems than FAAH A Carriers.
Regression Three: Block Three (Depressive Symptoms Added to Test Mediation)
Cannabis Use: With the addition of depressive symptoms, greater past year cannabis still significantly predicted more sleep problems [t (30) = 3.98, beta=.43, p< .001]. FAAH Genotype: FAAH genotype was no longer a significant predictor of sleep problems [t (30) = −.37, beta=−.05, p= .72] when depressive symptoms were added to the model. CNR1 Genotype: CNR1 genotype was not significantly related to sleep quality [t (30) = .37, beta=.04, p= .71]. Depressive Symptoms: Depressive symptoms positively predicted more sleep problems [t (30) = 6.39, beta=.74, p< .001]. All other covariates were not significantly associated with sleep quality.
Regression Three: Block Four (Moderators)
Depressive Symptoms*Cannabis Use: Depressive symptoms did not significantly interact with past year cannabis use in predicting sleep problems, although a trend level association was observed [BDI-II without sleep question: t (27) = 1.85, beta=.34, p= .08; BDI-II with sleep question: t (27) = 1.97, beta=.35, p= .06] in that those with both greater depressive symptoms and greater past year cannabis use demonstrated the most sleep problems. FAAH Genotype*Cannabis Use: FAAH genotype did not significantly interact with cannabis use in predicting sleep problems [t (27) = −1.25, beta=−.25, p = .22]. CNR1 Genotype*Cannabis Use: CNR1 genotype did not significantly interact with cannabis use in predicting sleep problems [t (27) = .17, beta=.02, p = .87].
Outliers
In order to determine if any of the participants in the sample disproportionately influenced the regression models, DFBETA analyses for past year cannabis use and BDI-II were conducted. No participants were above the DFBETA cutoff for either past year cannabis use or BDI-II scores, indicating no outliers in the sample.
Discussion
Although previous studies have established a connection between cannabinoids and impaired sleep, to our knowledge, this is the first study to analyze the relationship between cannabis use, FAAH genotype, CNR1 genotype, depressive symptoms, and sleep. As predicted, after controlling for length of cannabis abstinence, we found a dose-dependent relationship between increased past year cannabis use and greater past month self-reported sleep problems. This relationship was not significantly moderated by depressive symptoms, but a trend level relationship was found, such that those with more cannabis use and greater depressive symptoms had the most impaired sleep. Also consistent with our hypothesis, FAAH C/C genotypes reported significantly reduced sleep quality compared to A carriers, and this relationship was mediated by depressive symptoms (Figure 3). CNR1 genotype did not predict sleep quality.
Figure 3.
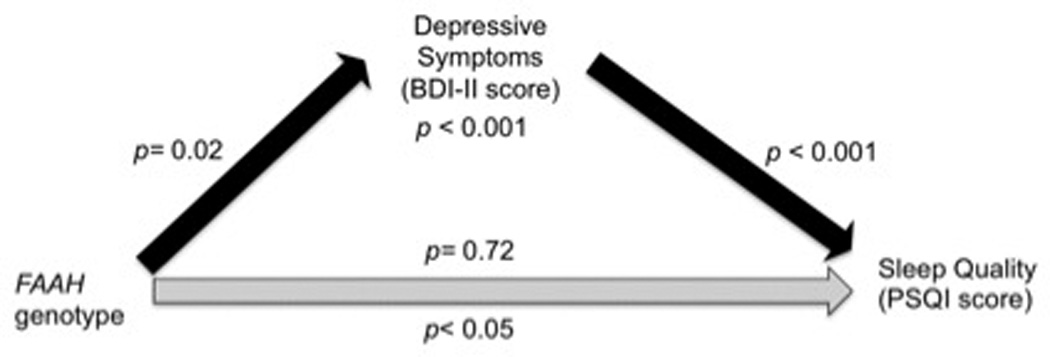
Depressive symptoms (BDI-II score) fully mediated the relationship between FAAH genotype and sleep quality (PSQI score) in the current sample of cannabis users. Prior to including depressive symptoms in the regression model, FAAH genotype significantly predicted sleep quality (p< 0.05). After including depressive symptoms in the model, sleep quality was no longer associated with FAAH genotype (p= 0.72), but was predicted by depressive symptoms (p< 0.001). In separate regression models, FAAH genotype significantly predicted depressive symptoms (p= 0.02), which predicted sleep quality (p< 0.001).
These results build upon previous studies finding that cannabis and its components may alter sleep architecture (18, 21–23). In other studies, THC reduces time to sleep onset (16,–17), and indeed, individuals often report using cannabis to promote sleep (2). Our findings suggest that this increased somnolence may be offset by sleep disruptions in that the heaviest cannabis users report the poorest sleep quality.
While increased recreational cannabis use has been associated with sleep problems, medicinal cannabinoid preparations, such as Sativex® and dronabinol, are used to treat insomnia in those with chronic pain (63, 64). These formulations typically contain either a lower dose of THC (e.g., 5 mg of dronabinol) or both a reduced dose of THC and a 1:1 combination of THC and CBD, in the case of Sativex® (63, 65, 66). Such cannabinoid preparations are not widely available for a variety of reasons, including regulations on research of drugs similar to prohibited substances (66). Many individuals instead use recreational cannabis for purported medical reasons, including to improve sleep (67). Most recreational cannabis is much higher in THC than in CBD, with published mean THC concentrations at 8.8% and CBD at 0.4% in the U.S. in 2008 (68) and a more recent analysis found 14.88% THC in cannabis seized in Australia (69). This difference between recreationally available cannabis and medicinal preparations is noteworthy, as THC and CBD impact sleep differently, and their effects depend upon dose (18). Thus, individuals using Sativex® or dronabinol may experience different sleep quality and architecture compared to those using other forms of cannabis. These differences in content aside, it is additionally possible that the hypnotic properties of cannabinoids may improve sleep in those with insomnia while disrupting sleep in those without severe sleep problems. Thus, various factors may determine the impact of cannabinoids on sleep.
Sleep problems are most commonly associated with cannabis withdrawal (70–71). Sleep difficulty due to cannabis withdrawal typically lasts about six days (72). Strange dreams take longer to subside, with researchers reporting their occurrence 45 days into withdrawal (72). However, as the PSQI inquires about “bad dreams” rather than “strange dreams,” this particular symptom is likely not influencing PSQI scores in the current sample. Our participants were abstinent 6–53 days, and 95% of them were abstinent for longer than six days (Table 2). Thus, most participants were likely beyond the most severe withdrawal symptoms at the time of questionnaire completion. However, 90% of our subjects started their abstinence within the past month; therefore they may have been experiencing some extent of withdrawal during the month for which the PSQI was completed. Still, length of abstinence was not statistically related to sleep quality within our sample. Further, as only 24.4% of participants reported ever experiencing sleep problems upon abstinence from any drug (including cannabis), these findings likely represent a persistent relationship with cannabis use, independent of withdrawal symptoms. To our knowledge, no other studies have demonstrated an association between chronic cannabis use and persistent sleep problems after controlling for length of abstinence. Yet, these results need to be confirmed in longitudinal studies as well as studies experimentally controlling for abstinence.
Table 2.
Cannabis Abstinence Characteristics
| Length of cannabis abstinence | N | % |
|---|---|---|
| 6–7 days (≤ 1 week) | 7 | 17.1 |
| 8–14 days (1–2 weeks) | 24 | 58.5 |
| 15–21 days (2–3 weeks) | 4 | 9.8 |
| 22–28 days (3–4 weeks) | 2 | 4.9 |
| 29–53 days (4–7 weeks) | 4 | 9.8 |
| Mean= 14.59 days (SD= 11.06) | ||
Since our data are cross-sectional and dose-dependent, the direction of causality between cannabis use and sleep problems cannot be determined. Heavy cannabis use is associated with CB1 receptor downregulation, which decreases endocannabinoid signaling (73). Reduced endogenous cannabinoid activity is related to poorer sleep (4). Therefore, the observed sleep problems could be due to greater CB1 downregulation with increased cannabis use. Alternatively, it is possible that sleep problems preceded cannabis use, as many people report using cannabis to alleviate sleep problems (2). Even so, sleep problems still persist in chronic users. It is possible that cannabis use serves as a negative reinforcer for sleep problems by worsening already poor sleep and leading to greater sleep disruption once use is terminated, thereby perpetuating a cycle. Of note, one research group recently found that daily cannabis users have increased circadian entrainment to the 24-hour day compared to controls, with no differences in measures of total sleep time (74). Thus, cannabis may serve as a cue to regulate the sleep-wake cycle. It is unclear whether cannabis users may simultaneously experience increased entrainment and disrupted sleep. Further research should explore the impact of chronic cannabis use on the sleep-wake cycle, taking into account THC and CBD content.
Our findings that FAAH A Carriers reported better sleep quality aligns with animal literature showing that increased endocannabinoid activity promotes sleep (6, 10–13). Although we are not aware of other human studies investigating the relationship between endocannabinoid genes and sleep, one study reported a significantly greater decrease in fatigue for FAAH C/C genotypes after 10mg amphetamine administration compared to A carriers (75). This may be related to decreased somnolence with lower endocannabinoid activity. These researchers also examined three FAAH SNPs together as a haplotype block, finding that haplotype CCC was significantly associated with lower fatigue after 10mg administration, while haplotype TAT was associated with higher fatigue (75). Given these findings, future studies may consider more closely investigating the individual and group impact of other FAAH SNPs.
We found that the relationship between FAAH genotype and sleep was driven by depressive symptoms. This finding held even after removing the sleep quality question from the BDI-II. The one other human study that investigated FAAH and depression found a marginally higher frequency of rs324420 A carrying adults in those with major depressive disorder and bipolar disorder compared to controls (49). In contrast, in our sample, C/C genotype significantly predicted more depressive symptoms. A notable difference between these two studies is that only those with minimal to moderate depressive symptoms were included in the current study. Therefore, more work should be done to further investigate the relationship between FAAH and a full range of depressive symptoms and in those with independent major depressive disorder.
Contrary to our predictions, CNR1 genotype was not associated with depressive symptoms or sleep quality. It is possible that within our cannabis using sample, hereditary variation in CB1 receptor density may not be related to depression and sleep, as heavy cannabis use downregulates CB1 signaling (73). Thus, the impact of CNR1 may be masked in the presence of significant CB1 downregulation. Previous studies investigating CNR1's impact on depressive symptoms examined different polymorphisms (rs1049353; rs806368, rs1049353, rs806371, rs806377 and rs1535255; AAT(n)) from the current study (rs2180619), making it difficult to compare findings (47–49). However, one study examining CNR1 rs2180619 found higher anxiety in those with both the G/G genotype and the S/S genotype of the serotonin transporter gene (5–HTTLPR) (52). Thus, this literature remains mixed and limited.
There are additional limitations to the current study. Firstly, this study relied on measures of self-report, which are less ideal than objective measures. Additionally, the present study has a relatively small sample size for investigating relationships between genetic variants and behavior. Indeed, this pilot candidate gene association study may not be sufficiently powered to detect CNR1's effects, as several genetic variants of small effect typically converge to influence a given behavioral phenotype (76). Therefore, multiple environmental and genetic influences likely contribute to sleep. As such, our observation that depressive symptoms mediated the relationship between FAAH and sleep quality is a finding that should be replicated in future studies with larger sample sizes. Additionally, the present study has somewhat low power (55% power) for investigating the impact of multiple interaction terms. Thus, studies with larger sample sizes should further examine these relationships.
Overall, this study provides further insight into the relationship between cannabis, cannabinoid genetics, and sleep. Increased past year cannabis use was related to poorer past month sleep quality in a sample of emerging adults, even after controlling for length of abstinence. Furthermore, we found novel, yet preliminary, evidence that FAAH genotype (C/C) predicts sleep problems, and this relationship was mediated by depressive symptoms. These findings suggest that sleep problems may be an important target for intervention in cannabis-dependent individuals. Additionally, cannabinoid genetics may play a role in sleep problems and should be further investigated in human samples.
Acknowledgments
This research was funded by the National Institute on Drug Abuse (NIDA; R03 DA027457) and the University of Cincinnati Center for Environmental Genetics Pilot Program (P30 ES06096). Dr. Lisdahl was also funded by NIDA (R01 DA030354) during manuscript preparation.
Footnotes
Financial Disclosures
The authors report no relevant financial conflicts.
Contributor Information
Kristin E. Maple, Department of Psychology, University of Wisconsin—Milwaukee
Kymberly A. McDaniel, Department of Psychology, University of Wisconsin—Milwaukee
Skyler G. Shollenbarger, Department of Psychology, University of Wisconsin—Milwaukee
Krista M. Lisdahl, Department of Psychology, University of Wisconsin—Milwaukee
References
- 1.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975–2012. Volume II: College Students and Adults 19–50. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2013. [Google Scholar]
- 2.Boys A, Marsden J, Strang J. Understanding reasons for drug use amongst young people: A functional perspective. Health Educ Res. 2001;16(4):457–469. doi: 10.1093/her/16.4.457. [DOI] [PubMed] [Google Scholar]
- 3.Gates PJ, Albertella L, Copeland J. The effects of cannabinoid administration on sleep: A systematic review of human studies. Sleep Med Rev. 2014;18(6):477–487. doi: 10.1016/j.smrv.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Murillo-Rodriguez E, Poot-Ake A, Arias-Carrion O, Pacheco-Pantoja E, Fuente-Ortegon A, Arankowsky-Sandoval G. The emerging role of the endocannabinoid system in the sleep-wake cycle modulation. Cent Nerv Syst Agents Med Chem. 2011;11(3):189–196. doi: 10.2174/187152411798047780. [DOI] [PubMed] [Google Scholar]
- 5.Goonawardena A, Plano A, Robinson L, Ross R, Greig I, Pertwee R, Hampson RE, Platt B, Riedel G. Modulation of food consumption and sleep-wake cycle in mice by the neutral CB1 antagonist ABD459. Behav Pharmacol. 2015;26(3):289–303. doi: 10.1097/FBP.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murillo-Rodriguez E, Blanco-Centurion C, Sanchez C, Piomelli D, Shiromani P. Anandamide enhances extracellular levels of adenosine and induces sleep: An in vivo microdialysis study. Sleep. 2003;26(8):943–947. doi: 10.1093/sleep/26.8.943. [DOI] [PubMed] [Google Scholar]
- 7.Murillo-Rodriguez E, Cabeza R, Mendez-Diaz M, Navarro L, Prospero-Garcia O. Anandamide-induced sleep is blocked by SR141716A, a CB1 receptor antagonist and by U73122, a phospholipase C inhibitor. Neuroreport. 2001;12(10):2131–2136. doi: 10.1097/00001756-200107200-00018. [DOI] [PubMed] [Google Scholar]
- 8.Santucci V, Storme J, Soubrié P, Fur G. Arousal-enhancing properties of the CB1 cannabinoid receptor antagonist SR 141716A in rats as assessed by electroencephalographic spectral and sleep-waking cycle analysis. Life Sci. 1996;58(6):PL103–PL110. doi: 10.1016/0024-3205(95)02319-4. [DOI] [PubMed] [Google Scholar]
- 9.Pava MJ, den Hartog CR, Blanco-Centurion C, Shiromani PJ, Woodward JJ. Endocannabinoid modulation of cortical up-states and NREM Sleep. PLoS One. 2014;9(2):e88672. doi: 10.1371/journal.pone.0088672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrera-Solís A, Vásquez K, Prospéro-García O. Acute and subchronic administration of anandamide or oleamide increases REM sleep in rats. Pharmacol Biochem Behav. 2010;95(1):106–112. doi: 10.1016/j.pbb.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Méndez-Díaz M, Caynas-Rojas S, Santacruz V, Ruiz-Contreras A, Aguilar-Roblero R, Prospéro-García O. Entopeduncular nucleus endocannabinoid system modulates sleep–waking cycle and mood in rats. Pharmacol Biochem Behav. 2013;107:29–35. doi: 10.1016/j.pbb.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Murillo-Rodriguez E, Sánchez-Alavez M, Navarro L, Martinez-González D, Drucker-Colín R, Prospéro-Garcia O. Anandamide modulates sleep and memory in rats. Brain Res. 1998;812:270–274. doi: 10.1016/s0006-8993(98)00969-x. [DOI] [PubMed] [Google Scholar]
- 13.Rueda-Orozco P, Soria-Gómez E, Montes-Rodríguez C, Pérez-Morales M, Prospéro-García O. Intrahippocampal administration of anandamide increases REM sleep. Neurosci Lett. 2010;473:158–162. doi: 10.1016/j.neulet.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Huitron-Resendiz S, Sanchez-Alavez M, Wills DN, Cravatt BF, Henriksen SJ. Characterization of the sleep-wake patterns in mice lacking fatty acid amide hydrolase. Sleep. 2004;27(5):857–865. doi: 10.1093/sleep/27.5.857. [DOI] [PubMed] [Google Scholar]
- 15.Chait L. Subjective and behavioral effects of marijuana the morning after smoking. Psychopharmacology. 1990;100:328–333. doi: 10.1007/BF02244601. [DOI] [PubMed] [Google Scholar]
- 16.Cousens K, DiMascio A. (−) Delta 9 THC as an hypnotic: an experimental study of three dose levels. Psychopharmacologia. 1973;33:355–364. doi: 10.1007/BF00437513. [DOI] [PubMed] [Google Scholar]
- 17.Gorelick DA, Goodwin RS, Schwilke E, Schroeder JR, Schwope DM, Kelly DL, Ortemann-Renon C, Bonnet D, Huestis MA. Around-the-clock oral THC effects on sleep in male chronic daily cannabis smokers. Am J Addict. 2013;22:510–514. doi: 10.1111/j.1521-0391.2013.12003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholson A, Turner C, Stone B, Robson P. Effect of delta 9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J Clin Psychopharmacol. 2004;24(3):305–313. doi: 10.1097/01.jcp.0000125688.05091.8f. [DOI] [PubMed] [Google Scholar]
- 19.Karacan I, Fernandez Salas A, Coggins WJ. Sleep electroencephalographic electrooculographic characteristics of chronic marijuana users. I. Ann N Y Acad Sci. 1976;282:348e74. doi: 10.1111/j.1749-6632.1976.tb49909.x. [DOI] [PubMed] [Google Scholar]
- 20.Colten HR, Altevogt BM. Sleep physiology. In: Colten HR, Altevogt BM, editors. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: The National Academies Press; 2006. pp. 33–53. [PubMed] [Google Scholar]
- 21.Feinberg I, Jones R, Walker JM, Cavness C, March J. Effects of high dosage delta-9-tetrahydrocannabinol on sleep patterns in man. Clin Pharmacol Ther. 1975;17(4):458–466. doi: 10.1002/cpt1975174458. [DOI] [PubMed] [Google Scholar]
- 22.Freemon FR. The effect of Delta 9-tetrahydrocannabinol on sleep. Psychopharmacology. 1974;35:39–44. [Google Scholar]
- 23.Tassinari CA, Ambrosetto G, Peraita-Adrado MR, Gastaut H. The neuropsychiatric syndrome of Δ9-tetrahydrocannabinol and cannabis intoxication in naïve subjects: a clinical and polygraphic study during wakefulness and sleep. In: Nahas GG, et al., editors. Marihuana and Medicine. New York, NY: Humana Press; 1999. pp. 649–664. [Google Scholar]
- 24.Chagas MHN, Crippa JAS, Zuardi AW, Hallak JEC, Machado-de-Sousa JP, Hirotsu C, Maia L, Tufik S, Andersen M. Effects of acute systemic administration of cannabidiol on sleep-wake cycle in rats. J Psychopharmacol. 2013;27(3):312–316. doi: 10.1177/0269881112474524. [DOI] [PubMed] [Google Scholar]
- 25.Monti JM. Hypnoticlike effects of cannabidiol in the rat. Psychopharmacology. 1977;55(3):263–265. doi: 10.1007/BF00497858. [DOI] [PubMed] [Google Scholar]
- 26.Murillo-Rodriguez E, Sarro-Ramirez A, Sanchez D, Mijangos-Moreno S, Tejeda-Padron A, Poot-Ake A, Guzman K, Pacheco-Pantoja E, Arias-Carrion O. Potential effects of cannabidiol as a wake-promoting agent. Curr Neuropharmacol. 2014;12(3):269–272. doi: 10.2174/1570159X11666131204235805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen-Zion M, Drummond SP, Padula CB, Winward J, Kanady J, Medina KL, Tapert SF. Sleep architecture in adolescent marijuana and alcohol users during acute and extended abstinence. Addict Behav. 2009;34(11):976–979. doi: 10.1016/j.addbeh.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dijk D. Regulation and functional correlates of slow wave sleep. J Clin Sleep Med. 2009;5:S6–S15. [PMC free article] [PubMed] [Google Scholar]
- 29.Gillin JC, Drummond SPA. Medication and substance use. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia, PA: W.B. Saunders Company; 2000. pp. 1176–1195. [Google Scholar]
- 30.Fairman BJ, Anthony JC. Are early-onset cannabis smokers at an increased risk of depression spells? J Affect Disord. 2012;138:54–62. doi: 10.1016/j.jad.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: A cohort study. BMJ. 2002;325:1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasic D, Weerasinghe S, Asbridge M, Langille DB. Longitudinal associations of cannabis and illicit drug use with depression, suicidal ideation and suicidal attempts among Nova Scotia high school students. Drug Alcohol Depend. 2013;129:49–53. doi: 10.1016/j.drugalcdep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 33.van Laar M, van Dorsselaer S, Monshouwer K, de Graaf R. Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction. 2007;102:1251–1260. doi: 10.1111/j.1360-0443.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- 34.Rey JM, Tennant CC. Cannabis and mental health. BMJ. 2002;325:1183–1184. doi: 10.1136/bmj.325.7374.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayatbakhsh MR, Najman JM, Jamrozik K, Mamun AA, Alati R, Bor W. Cannabis and anxiety and depression in young adults: A large prospective study. J Am Acad Child Adolesc Psychiatry. 2007;46(3):408–417. doi: 10.1097/chi.0b013e31802dc54d. [DOI] [PubMed] [Google Scholar]
- 36.Lev-Ran S, Le Foll B, McKenzie K, George TP, Rehm J. Cannabis use and cannabis use disorders among individuals with mental illness. Compr Psychiatry. 2013;54:589–598. doi: 10.1016/j.comppsych.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Wittchen HU, Frohlich C, Behrendt S, Gunther A, Rehm J, Zimmermann P, Lieb R, Perkonigg A. Cannabis use and cannabis use disorders and their relationship to mental disorders: A 10-year prospective-longitudinal community study in adolescents. Drug Alcohol Depend. 2007;88(Suppl 1):S60–S70. doi: 10.1016/j.drugalcdep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: Associations with white matter volume and marijuana use. J Child Psychol Psychiatry. 2007;48(6):592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medina KL, Shear PK. Anxiety, depression, & behavioral symptoms of executive dysfunction in ecstasy users: Contributions of polydrug use. Drug Alcohol Depend. 2007;87(2–3):303–311. doi: 10.1016/j.drugalcdep.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McQueeny T, Padula CB, Price JS, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behav Brain Res. 2011;224(1):128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hillard C, Weinlander K, Stuhr K. Contributions of endocannabinoid signaling to psychiatric disorders in humans: Genetic and biochemical evidence. Neuroscience. 2012;204:207–229. doi: 10.1016/j.neuroscience.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witkin J, Tzavara E, Nomikos G. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol. 2005;16(5–6):315–331. doi: 10.1097/00008877-200509000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Ashton CH, Moore PB, Gallagher P, Young AH. Cannabinoids in bipolar affective disorder: a review and discussion of their therapeutic potential. J Psychopharmacol. 2005;19(3):293–300. doi: 10.1177/0269881105051541. [DOI] [PubMed] [Google Scholar]
- 44.Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology. 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- 45.Hill M, Gorzalka B. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol. 2005;15:593–599. doi: 10.1016/j.euroneuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Adamczyk P, Golda A, McCreary A, Filip M, Przegalinski E. Activation of endocannabinoid transmission induces antidepressant-like effects in rats. J Physiol Pharmacol. 2008;59(2):217–228. [PubMed] [Google Scholar]
- 47.Barrero F, Ampuero I, Morales B, Vives F, De Dios Luna Del Castillo J, Hoenicka J, Yébene-Garcia J. Depression in Parkinson's disease is related to a genetic polymorphism of the cannabinoid receptor gene (CNR1) The Pharmacogenomics J. 2005;5:135–141. doi: 10.1038/sj.tpj.6500301. [DOI] [PubMed] [Google Scholar]
- 48.Mitjans M, Serretti A, Fabbri C, Gastó C, Catalán R, Fañanás L, Arias B. Screening genetic variability at the CNR1 gene in both major depression etiology and clinical response to citalopram treatment. Psychopharmacology. 2013;227:509–519. doi: 10.1007/s00213-013-2995-y. [DOI] [PubMed] [Google Scholar]
- 49.Monteleone P, Bifulco M, Maina G, Tortorella A, Gazzerro P, Proto M, Di Filippo C, Monteleone F, Canestrelli B, Buonerba G, Bogetto F, Maj M. Investigation of CNR1 and FAAH endocannabinoid gene polymorphisms in bipolar disorder and major depression. Pharmacol Res. 2010;61:400–404. doi: 10.1016/j.phrs.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: A focus on insomnia. Sleep Med Rev. 2010;14(4):227–238. doi: 10.1016/j.smrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Agrawal A, Lynskey MT. Candidate genes for cannabis use disorders: findings, challenges and directions. Addiction. 2009;104(4):518–532. doi: 10.1111/j.1360-0443.2009.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazary J, Lazary A, Gonda X, Benko A, Molnar E, Hunyady L, Juhasz G, Bagdy G. Promoter variants of the cannabinoid receptor 1 gene (CNR1) in interaction with 5-HTTLPR affect the anxious phenotype. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1118–1127. doi: 10.1002/ajmg.b.31024. [DOI] [PubMed] [Google Scholar]
- 53.Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J Int Neuropsychol Soc. 2012;18(4):678–688. doi: 10.1017/S1355617712000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 55.Goodwin RS, Darwin WD, Chiang CN, Shih M, Li S-H, Huestis MA. Urinary elimination of 11-nor-9-carboxy-9-tetrahydrocannabinol in cannabis users during continuously monitored abstinence. J Anal Toxicol. 2008;32(8):562–569. doi: 10.1093/jat/32.8.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behav Res Ther. 1979;17(2):157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- 57.Brown SA, Meyer MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the customary drinking and drug use record (CDDR): A measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 58.Stewart DG, Brown SA. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- 59.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 60.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index (PSQI): A new instrument for psychiatric research and practice. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 61.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 62.Flanagan J, Gerber A, Cadet J, Beutler E, Sipe J. The fatty acid amide hydrolase 385 A/A (P129T) variant: haplotype analysis of an ancient missense mutation and validation of risk for drug addiction. Hum Genet. 2006;120:581–588. doi: 10.1007/s00439-006-0250-x. [DOI] [PubMed] [Google Scholar]
- 63.Russo EB, Guy GW, Robson PJ. Cannabis, pain, and sleep: Lessons from therapeutic clinical trials of Sativex®, a cannabis-based medicine. Chem Biodivers. 2007;4:1729–1743. doi: 10.1002/cbdv.200790150. [DOI] [PubMed] [Google Scholar]
- 64.Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, Jamison RN. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J Pain. 2008;9(3):254–264. doi: 10.1016/j.jpain.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 65.Neff GW, O’Brien CB, Rajender Reddy K, Bergasa ND, Regev A, Molina E, Amaro R, Rodriguez MJ, Chase V, Jeffers L, Schiff E. Preliminary observation with dronabinol in patients with intractable pruritus secondary to cholestatic liver disease. Am J Gastroenterol. 2002;97(8):2117–2119. doi: 10.1111/j.1572-0241.2002.05852.x. [DOI] [PubMed] [Google Scholar]
- 66.Hall W, Weier M. Assessing the public health impacts of legalizing recreational cannabis use in the USA. Clin Pharmacol Ther. 2015;97(6):607–615. doi: 10.1002/cpt.110. [DOI] [PubMed] [Google Scholar]
- 67.Belendiuk KA, Babson KA, Vandrey R, Bonn-Miller MO. Cannabis species and cannabinoid concentration preference among sleep-disturbed medicinal cannabis users. Addict Behav. 2015;50:178–181. doi: 10.1016/j.addbeh.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 68.Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, Ross SA, Khan IA, ElSohly MA. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55(5):1209–1217. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 69.Swift W, Wong A, Li KM, Arnold JC, McGregor IS. Analysis of cannabis seizures in NSW, Australia: Cannabis potency and cannabinoid profile. PLoS One. 2013;8(7):e70052. doi: 10.1371/journal.pone.0070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Budney A, Hughes J, Moore B, Novy P. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58:917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- 71.Haney M, Ward A, Comer S, Foltin R, Fischman M. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- 72.Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112(3):393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- 73.Hirvonen J, Goodwin RS, Li C-T, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17(6):642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whitehurst LN, Fogler K, Hall K, Hartmann M, Dyche J. The effects of chronic marijuana use on circadian entrainment. Chronobiol Int. 2015;32(4):561–567. doi: 10.3109/07420528.2015.1004078. [DOI] [PubMed] [Google Scholar]
- 75.Dlugos A, Hamidovic A, Hodgkinson C, Goldman D, Palmer A, de Wit H. More aroused, less fatigued: fatty acid amide hydrolase gene polymorphisms influence acute response to amphetamine. Neuropsychopharmacology. 2010;35(3):613–622. doi: 10.1038/npp.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winham SJ, Biernacka JM. Gene-environment interactions in genome-wide association studies: Current approaches and new directions. J Child Psychol Psychiatry. 2013;54(10):1–24. doi: 10.1111/jcpp.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]