Highlights
-
•
We compare gyrification and surface area in prefrontal and parietal cortex in young cannabis users and controls.
-
•
Frequent cannabis use was associated with reduced gyrification in prefrontal subregions.
-
•
Reduced gyrification in cannabis users was associated with poorer performance.
-
•
Sensitive periods during neurodevelopment may be affected by frequent cannabis use.
Keywords: Cannabis, Marijuana, Gyrification, Prefrontal and parietal cortex, Emerging adults, Cognition
Abstract
Background
Regions undergoing maturation with CB1 receptors may be at increased risk for cannabis-induced alterations. Here, we examine the relationships between cannabis use and prefrontal (PFC) and inferior parietal gyrification and surface area (SA) in youth.
Methods
Participants included 33 cannabis users and 35 controls (ages 18–25). Exclusions included co-morbid psychiatric/neurologic disorders and heavy other drug use. Multiple regressions and Pearson r correlations examined the effects of cannabis use on gyrification, SA and cognition.
Results
Cannabis use was associated with decreased gyrification in: ventral-medial PFC (RH: [FDR corrected p = .02], LH: [FDR corrected p = .02]); medial PFC (RH: [FDR corrected p = .02], LH: [FDR corrected p = .02]); and frontal poles (RH: [FDR corrected p = .02], LH: [FDR corrected p = .02]). No differences were observed in bilateral hemispheres, PFC, dorsolateral, ventrolateral, or inferior parietal ROIs. Cannabis use was associated with marginally decreased SA in left: medial PFC [FDR corrected p = .09], and ventral lateral PFC: [FDR corrected p = .09]. In cannabis users, increased gyrification was associated with improved working-memory performance in right medial (p = .003), ventral-medial (p = .03), and frontal pole ROIs (p = .007).
Conclusions
Cannabis use was associated with reduced gyrification in PFC regions implicated in self-referential thought and social cognition. Results suggest that these gyrification characteristics may have cognitive implications.
1. Introduction
Cannabis is the second most used drug after alcohol, with 22.9% of high school seniors and 20% of college students using in the past month, and perhaps most alarmingly, one in every 15 seniors reporting daily use (Johnston et al., 2014). Cannabis legislation changes are sweeping across the United States. Policy experts predict that increased access and reduced price will lead to increased usage, especially in young adults who are the heaviest users (Caulkins et al., 2012). Late adolescence and emerging adulthood is a period of ongoing neurodevelopment, with pruning of inefficient gray matter connections (Gogtay et al., 2004, Gogtay and Thompson, 2010). Healthy adult rats demonstrate enhanced binding of cannabinoid (CB1) receptors within areas such as the prefrontal cortex (PFC) (Verdurand et al., 2011) in comparison to juveniles, suggesting increased reliance upon the cannabinoid system with age. Indeed, converging lines of animal and human evidence have suggested that this is a sensitive period that may be particularly vulnerable to cannabis-induced neurocognitive effects (Jager and Ramsey, 2008, Meier et al., 2012; see Lisdahl et al., 2013 for review).
Preclinical animal models suggest that endogenous endocannabinoid signaling in the PFC influences executive functioning (EF) performance (for review see Egerton et al., 2006). In humans, significant CB1 receptor density has been measured in the PFC, a region associated with mood regulation and EF, and throughout the cortex (Goldberg, 2009, Terry et al., 2009; see Yurgelun-Todd, 2007). Therefore, disruption of the endogenous cannabinoid system during adolescence may particularly impact the integrity later developing regions, such as the PFC and parietal lobes (Gogtay et al., 2004, Gogtay and Thompson, 2010). Indeed, daily cannabis users demonstrate significant, though reversible, downregulation of the CB1 density in PFC and other cortical regions including the parietal lobes (Hirvonen et al., 2012). Further, cannabis-using youth demonstrate impairments in executive functioning, including complex attention, inhibitory control, and working memory (Harvey et al., 2007, Hanson et al., 2010, Medina et al., 2007, Lisdahl and Price, 2012).
Previous structural magnetic resonance imaging (MRI) research has demonstrated that regular (weekly or more) cannabis using adolescents demonstrate larger PFC (including orbitofrontal cortex) volume in female cannabis users (Medina et al., 2009) and reduced medial orbitofrontal volumes in a primarily male sample (Churchwell et al., 2010). Our group has found reduced medial orbitofrontal (mOFC) and inferior parietal volumes in this same sample of young adults (Price et al., 2015) compared to controls, and other groups have found that earlier age of onset significantly predicted decreased right superior PFC thickness (Lopez-Larson et al., 2011). Recent MRI advances have yielded new measurements of cortical architecture that may be more sensitive to drug effects than volume or cortical thickness. One such candidate is local gyrification index, or a 3-dimensional ratio representing the degree of folding on the outer surface relative to buried cortex within neighboring sulci, which may also be calculated for regions of interest (Schaer et al., 2008, Schaer et al., 2012). Several candidate theories attempt to explain the primary driving mechanisms of gyrification development, including cortico-cortical mechanical tension, morphogenetic, and differential cortical expansion rate influences (Richman et al., 1975; see Van Essen, 1997, Toro and Burnod, 2005, Ronan et al., 2013, Tallinen et al., 2014; see Kriegstein et al., 2006, Hilgetag and Barbas, 2006, White et al., 2010 for reviews). Another measure is cortical surface area (SA), which is a reflection of the amount of area on the cortical surface represented in mm2 (Dale et al., 1999).
Age-related changes in cortical surface area (SA) and other surface characteristics, including gyral and sulcal shape, have been noted in several preliminary studies. Schnack et al. (2015) measured SA changes between MRI scans in 504 subjects. Results from the study found age-related changes in SA such that adolescence is a period in which the cortex is greatly expanding and reaches the maximum individual peak in SA during this time. Further, the same study found that those with the highest IQ had the greatest rate of cortical SA change during this period. Magnotta et al. (1999) found a significant relationship between age with gyral and sulcal shape in a sample of 148 participants aged 18–82. A more recent two-year longitudinal study with 52 participants found overall decreases in gyrification index in youth who were between the ages of 11 and 17 at baseline, with significant widening of sulci and loss of SA within the frontal cortex (Alemán-Gómez et al., 2013). Other samples have found reduced PFC surface complexity in teens compared to children (Su et al., 2013), and reduced PFC gyrification in young adults compared to early teens (Klein et al., 2014). Further, increased gyrification has been associated with enhanced vocabulary knowledge in typically developing youth (Wallace et al., 2013). In a large cohort of 322 healthy adults spanning ages 20–85, SA decreases were most robust within the dorsomedial frontal, and PFC gyrification decreases were observed with older age (Hogstrom et al., 2012). Sex differences in folding have also been noted with females demonstrating greater gyrification in PFC compared to males (Luders et al., 2004, Mutlu et al., 2013). Lastly, a large longitudinal study in 647 participants found an inverted-U shaped trajectory of SA maturation between the ages of 3 and 30 (Raznahan et al., 2011). Changes in SA appeared to peak later than cortical thickness in the large cohort. The same study found that gyrification index (note: this index differs from the Schaer et al., 2008 LGI measure) and convex hull area influence SA changes during early to late adolescents; however, late adolescent changes in SA may be most attributed by reductions in gyrification in comparison to reduced convex hull area. Further, SA may peak at later developmental periods compared to other cortical measures such as volume (Wierenga et al., 2014). Preliminary evidence suggests that later developing regions, such as the PFC (Gogtay et al., 2004), continue to undergo gyrification, cortical surface shape, and SA changes during adolescents and young adulthood.
While several studies have demonstrated a great degree of genetic influences on cortical thickness, gray, and white matter volume (see Douet et al., 2014), studies of gyrification or surface characteristics among small samples of monozygotic (MZ) twins demonstrate observable differences (Bartley et al., 1997, Biondi et al., 1998, Mohr et al., 2004, White et al., 2002), suggesting that environmental factors may influence the shape of the cortical surface (see White et al., 2010) especially in secondary and tertiary sulci (Lohmann et al., 1999). For example, Hasan et al. (2011) found that PFC gyrification was no more similar in MZ twins compared to dizygotic twins. Therefore, compared to other brain characteristics, such as gray and white matter volume, surface morphometry values (including gyrification) appear to be significantly influenced by environmental factors compared to genetics, although this needs to be confirmed in larger sample sizes.
Therefore, gyrification may reflect changes sensitive to repeated behavioral or environmental influences, such as substance use, although additional research in emerging adults is needed. To our knowledge, only one study has examined surface morphology in a sample of young cannabis users (Mata et al., 2010). Mata et al. (2010) examined sulcal concavity, a measure similar yet distinct from a 3-dimensional gyrification value. The study noted decreased sulcal concavity in the left PFC and bilateral temporal lobes of young adult cannabis users compared to controls (Mata et al., 2010). The study also failed to find any significant differences in global SA after controlling for potential confounds, suggesting a unique characteristic of sulcal curvature differences in regions undergoing neuromaturation in young cannabis users (Mata et al., 2010). The same study did not examine sub-regional differences in SA or how sulcal differences between cannabis users and non-users relate to downstream behavioral phenotypes, such as neuropsychological function.
Because cannabis use has an age of onset (SAMHSA, 2014) that overlaps with continued PFC gyrification development (Su et al., 2013, Klein et al., 2014), examining the impact of cannabis use on gyrification remains an important area to investigate. The current study examined whether cannabis use status predicted PFC or parietal gyrification in a sample of adolescents and emerging adults. Surface morphology may be related to cortical thickness and volume (Alemán-Gómez et al., 2013). Given that both reductions in cortical thickness and volume (Lopez-Larson et al., 2011, Price et al., 2015) and reductions in PFC sulcal concavity (Mata et al., 2010) were previously found in young cannabis users, we predicted that cannabis users would demonstrate reduced gyrification and SA in PFC and parietal regions. Reduced SA and gyrification may be most pronounced in both inferior frontal and parietal regions that show reductions in volume (Churchwell et al., 2010, Price et al., 2015) Within regions that differed between cannabis users and controls, follow-up analyses examined brain–behavior relationships in both groups.
2. Materials and methods
2.1. Participants
Participants included 68 (33 cannabis-users, 35 controls) right-handed adolescents and emerging adults between the ages of 18–25 (21 male and 12 female cannabis-users; 15 male and 20 female controls) from a larger imaging genetics study (PI: Lisdahl, NIH R03 DA027457). Exclusion criteria included MRI contraindications; history of chronic medical or neurologic illness or injury (meningitis, HIV, epilepsy, brain tumor, traumatic brain injury, >2 min of unconsciousness and concussion symptoms, stroke, cerebral palsy, Parkinson's disease, Huntington's disease, high blood pressure, diabetes, chronic migraines); history of a learning disability; complications during birth/premature birth; prenatal exposure to alcohol (>4 drinks/day or >7 drinks/week) or illicit drugs (>10 uses); current use of psychoactive medication; preexisting DSM-IV Axis I disorders independent of substance use; current pregnancy; >20 lifetime use occasions of any of the following drug categories (stimulants, ecstasy, inhalants, hallucinogens, sedatives, or opiates); and refusal to remain abstinent from all drugs and alcohol for at least seven days. Individuals that classified as very heavy alcohol drinkers (>8 standard drinks per week on average) were also excluded. Eligible participants consisted of two groups, cannabis users (>25 past year and >50 lifetime joints) and controls (≤5 past year and <15 lifetime joints). Groups were matched as closely as possible on age, education, ethnicity, gender, and verbal IQ.
2.2. Procedures
The Institutional Review Board at the University of Cincinnati approved all aspects of this study. Participants were recruited through advertisements in a local free newspaper and fliers. Those interested were screened by phone for exclusionary criteria, which have been described in further detail elsewhere (see Price et al., 2015; Lisdahl and Price, 2012). Briefly, a semi-structured interview based on DSM-IV-TR criteria for Axis I psychotic, anxiety, and mood disorders was administered (see First et al., 2001). Next, eligible participants completed either one or two sessions. Those with moderate or greater substance use completed the questionnaires, drug use interview, neuropsychological battery, and MRI scan in two sessions (typically 2–3 days apart; see biological samples below). Participants were paid $160 for two sessions (5.5 h) ($110 for one, 3.5 h) and received parking reimbursement, local substance treatment resources and images of their brain.
2.3. Screening inventories and questionnaires
2.3.1. Demographic information
Participants completed a Background Questionnaire outlining demographic variables (see Table 1).
Table 1.
Demographic, substance use information according to group.
| Cannabis users (n = 33) M (SD) [range] |
Controls (n = 35) M (SD) [range] |
|
|---|---|---|
| Age | 21.21 (2.34) [18–25] |
21.14 (2.33) [18–25] |
| % Female | 36.4% | 57.1% |
| % Caucasian | 66.7% | 65.7% |
| WRAT-4 Reading Standard Score |
103.36 (15.86) [73–134] |
101.37 (10.13) [81–120] |
| Education | 13.12 (1.75) [9–17] |
13.91 (1.72) [11–18] |
| Beck Depression Inventory Total-2* |
5.12 (3.84) [0–17] |
3.31 (3.39)* [0–14] |
| Past year nicotine use** | 1788.67 (2532.57) [0–7350] |
455.86 (1093.0) [0–3680] |
| Cotinine levels** | 3.87 (2.39) [0–6] |
1.29 (2.08) [0– 6] |
| Past year alcohol use** | 282.91 (382.10) [0–1724] |
101.8 (166.38) [0–878] |
| Past year marijuana use** | 548.36 (771.07) [26–3895] |
.40 (1.22) [0–5] |
| Past year other drug use** | 9.82 (30.31) [0–171] |
.11 (.53) [0–3] |
Notes: ANOVAs and Chi-squares tests assessed group differences; *p < .05; **p < .01. On average, cannabis users reported increased depressive symptoms, higher cotinine levels, and increased past year drug and alcohol use. Drug use categories were as follows: nicotine (including cigarettes, chewing tobacco/snuff/pipe, cigars/hookah), alcohol, MJ, and ‘other’ drug use, which was a total in standardized units (hits or pills) including all of the following categories: stimulants, ecstasy, inhalants, hallucinogens, sedatives, and opioids.
2.3.2. Biological samples
Participants were administered a urine toxicology screen using the One Step Drug Screen Test, a breathalyzer test, and female participants were administered a pregnancy test. Those who tested positive for drugs and/or alcohol except cannabis and nicotine were excluded. Metabolite levels were further examined for participants that tested positive for cannabis via mass spectrometry testing. Session 2 total THC metabolite ratios, controlling for creatinine, were subtracted from session 1 total ratios to ensure there were no increases or current use while in the study (see Goodwin et al., 2008). Thus, if the difference in ratios reached >50 ng/mL, the participant was excluded. Cotinine levels measured recent nicotine use or exposure.
2.3.3. Drug use
Past year drug use was measured using a modified version of the Time-Line Follow-Back (Sobell et al., 1979) interview (see Table 1 for categories). Drug use was measured by the number of standard units (cigarettes or cigars for nicotine; standard drinks for alcohol; joints for cannabis; tablets for ecstasy; grams for stimulants; number of hits or pills for inhalants, hallucinogens, and opioids; and pills or hits for sedatives). The Customary Drinking and Drug Use Record (CDDR), measured lifetime and past 3-month substance use, withdrawal symptoms, DSM-IV abuse and dependence criteria, and substance-related difficulties (Brown et al., 1998, Stewart and Brown, 1995).
2.3.4. Self-reported mood
The Beck Depression Inventory-II (Beck et al., 1996) assessed current depressive symptomology.
2.4. Neuropsychological assessments
2.4.1. Premorbid verbal intelligence/quality of education
The Wide Range Achievement Test-4th edition (WRAT-4) Reading subtest (Wilkinson, 2006) measured estimates of verbal intelligence and quality of education for group comparison purposes (see Manly et al., 2002).
2.4.2. Complex attention
Complex attention was assessed using the total correct responses in the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) Letter Number Sequencing (LNS) and the Paced Auditory Serial Attention Test (PASAT). The LNS is a subscale of the WAIS-III Working Memory Index and measures the ability to retain and manipulate bits of information over several separate trials (Wechsler, 1997). The PASAT is a working memory task in which participants must retain two serially presented numbers and perform a summation roughly every 2 s (Gronwall, 1977). LNS and PASAT total scores were used for the current study.
2.4.3. Cognitive inhibition
The D-KEFS Color Word Interference Test Inhibition condition total completion time assessed inhibitory ability (Delis and Kaplan, 2000). For this task, participants were required to read the color of ink a color word is printed in (inhibition condition).
2.5. MRI data acquisition
2.5.1. Parameters
T1-weighted, 3-D SPGR anatomical brain scans were obtained on a 4T Varian Unity MRI scanner using a modified driven equilibrium Fourier transform (MDEFT) sequence (FOV = 25.6 cm, 256 × 256 × 192 matrix, slice thickness = 1 mm, in-plane resolution = 1 × 1 mm, TR = 13 ms, TE = 5.3 ms, flip angle = 22°). A neuroradiologist at the Center for Imaging Research reviewed anatomical scans, and participants with noted abnormalities were excluded from this sample.
2.6. MRI processing
2.6.1. PFC local gyrification analysis
Images were preprocessed in FreeSurfer version 5.3 (Dale et al., 1999). Average local gyrification indices (LGI) were created using a radius set to 20 mm for each region listed below, in order to maximize sensitivity (Schaer et al., 2008, Schaer et al., 2012), and cortical surface-based anatomical atlas (Destrieux et al., 2010). Regions of interest (ROIs) included bilateral: dorsal lateral PFC (DLPFC); medial PFC (mPFC); frontal pole; ventral medial PFC (vmPFC); ventral lateral PFC (vlPFC); and inferior parietal (infPariet). Control regions reflecting the average LGI and SA for each of the left and right hemispheres was included to test whether results were diffuse or specific to a priori defined ROIs.
2.6.2. Surface area analysis
As part of the FreeSurfer processing stream (Dale et al., 1999) SA was computed for each participant. Corresponding with the ROIs listed above in the LGI analysis (see Section 2.6.1), SA was calculated for all ROIs and the hemisphere control regions.
2.6.3. Operating system
Mac Pro with: OS X version 10.6.8, 12GB of memory, and 2×2.26 GHz Quad-Core Intel Xeon.
2.7. Statistical analyses
All analyses were conducted using SPSS. ANOVAs, Mann–Whitney U-test (drug variables), and Chi-square tests were run to examine potential demographic differences as well as differences in past year drug use histories between drug groups. Variables that either significantly differed between groups or may impact neural architecture were entered as covariates (Medina et al., 2007, Medina et al., 2008). Covariates included WRAT-4 Reading scaled score, age, gender, past year alcohol use, cotinine levels, and current depressive symptoms.
General linear modeling (GLM) in SPSS was used to examine whether cannabis group status was significantly associated with a priori defined LGI or SA ROIs. Standard least squares multiple regression was used; block one included covariates, and block two included cannabis group status. All dependent variables were normally distributed and there was no evidence of multicollinearity. Significance was determined if p < .05, and correction for multiple comparisons was calculated for each hemisphere's results utilizing Benjamini and Hochberg's False Discovery Rate correction (FDR; Benjamini and Hochberg, 1995). All FDR corrections were computed for the left and right hemispheres separately.
In the cannabis users, Pearson r correlations were run between cognitive performance (complex attention and cognitive inhibition; see Price et al., 2015) and gyrification or SA ROIs that significantly differed between groups. Significance was determined if p < .05 (after FDR correction).
3. Results
3.1. Demographic and mood information
3.1.1. Demographic and self-report variables
ANOVAs and Chi-squares were run to test differences between cannabis users and controls. There were significant differences in self-reported BDI-II depressive symptoms, with cannabis users reporting on average 2 more symptoms than controls, but still within the minimal range of symptoms [F(1,66) = 4.24, p = .04]. Groups did not differ in ethnicity [22 Caucasian cannabis users and 23 Caucasian controls [X2(4) = 3.86, p = .43], gender [X2(1) = 2.9, p = .09], past year Cahalan alcohol drinking patterns criteria [X2(5) = 4.3, p = .51], age [F(1,66) = .02, p = .90], WRAT-4 Reading standard score [F(1,66) = .39, p = .54], education [F(1,66) = 3.6, p = .06], annual income [F(1,66) = .17, p = .68], or body mass index [F(1,65) = .46, p = .50].
3.1.2. Drug variables
Mann–Whitney U tests revealed significant differences between cannabis users and controls in past year nicotine (U = 244.5, p < .01), recent nicotine use (U = 199.5, p ≤ .01), past year alcohol use (U = 334.5, p = .003), past year cannabis use (U = 0.00, p ≤ .01), and past year other drug use (measured as standardized hits or pills of stimulants, ecstasy, inhalants, hallucinogens, sedatives and opiates; U = 236, p ≤ .01). The cannabis group used more of these substances in comparison to controls, although the other drug use category was relatively low for the vast majority of the cannabis users and our exclusion criteria consistent with ≤ 20 lifetime uses of any drug category.
3.2. Gyrification results
3.2.1. Cannabis group
After controlling for WRAT-4 Reading scaled score, age, gender, past year alcohol use, cotinine levels, and current depressive symptoms, cannabis users demonstrated significantly reduced gyrification in bilateral medial PFC (Right: [t(59) = −2.9, beta = −.41, p = .005; FDR corrected p = .02] and Left: [t(59) = −3.1, beta = −.45, p = .003; FDR corrected p = .02]); bilateral frontal poles (Right: [t(59) = −2.7, beta = −.38, p = .009; FDR corrected p = .02] and Left: [t(59) = −3.1, beta = −.46, p = .003; FDR corrected p = .02]); and bilateral ventral-medial PFC (Right: [t(59) = −2.8, beta = −.40, p = .006; FDR corrected p = .02] and Left: [t(59) = −3.0, beta = −.44, p = .004; FDR corrected p = .02]) (see Fig. 1).
Fig. 1.
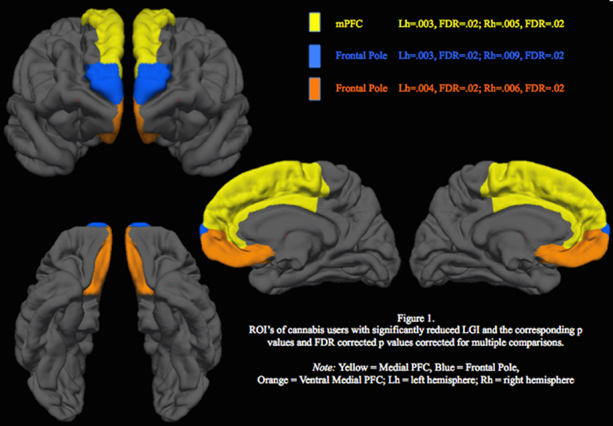
ROI's of cannabis users with significantly reduced LGI and the corresponding p values and FDR corrected p values corrected for multiple comparisons. Note: yellow = medical PFC; blue = frontal pole; orange = ventral medial PFC; Lh = left hemisphere; Rh = right hemisphere; LGI = local gyrification index.
No significant group differences were observed in LGI for total hemisphere (control region) (Right: [t(58) = −1.6, beta = −.25, p = .11] and Left: [t(58) = −.62, beta = −.09, p = .54]); dorsolateral PFC (Right: [t(59) = .05, beta = .007, p = .96] and Left: [t(59) = 1.5, beta = .2, p = .15]); ventral lateral PFC (Right: [t(59) = −1.5, beta = −.23, p = .13] and Left: [t(59) = −.7, beta = −.11, p = .49]); or bilateral inferior parietal cortex (Right: [t(60) = −1.9, beta = −.28, p = .06] and Left [t(60) = −.41, beta = −.06, p = .69]). There were no regions where cannabis users showed significant increases compared to controls.
3.3. Surface area results
3.3.1. Cannabis group
After controlling for WRAT-4 Reading scaled score, age, gender, past year alcohol use, cotinine levels, and current depressive symptoms, cannabis users demonstrated significantly reduced SA in the left ventral medial [t(60) = −2.5, beta = −.35, p = .02; FDR corrected p = .09], and left ventral lateral PFC [t(60) = −2.7, beta = −.32, p = .008; FDR corrected p = .09], although these findings were only marginally significant after correcting for multiple comparisons. No significant group differences were observed in total hemisphere (control region) SA (Right: [t(59) = −1.1, beta = −.12, p = .26] and Left: [t(59) = −1.9, beta = −.2, p = .07]); bilateral medial PFC (Right: [t(60) = −1.0, beta = −.12, p = .30] and Left: [t(60) = −1.8, beta = −.22, p = .08]); bilateral dorsolateral PFC (Right: [t(60) = −.65, beta = −.07, p = .52] and Left: [t(60) = −1.7, beta = −.19, p = .10]); right ventral medial PFC [t(60) = −1.5, beta = −.21, p = .14]; right ventral lateral PFC [t(60) = −1.4, beta = −.16, p = .18]; or bilateral inferior parietal (Right: [t(60) = −1.3, beta = −.15, p = .20] and Left: [t(60) = −.53, beta = −.07, p = .60]).
3.4. Brain–behavior results
3.4.1. Cannabis group
Positive correlations were found between increased gyrification and improved LNS performance in the right medial PFC [r = .50, n = 33, p = .003], right ventral medial PFC [r = .38, n = 33, p = .03] and right frontal pole [r = .46, n = 33, p = .007].
3.4.2. Controls
No significant correlations were observed between brain regions that significantly differed between groups and neuropsychological performance in controls.
4. Discussion
This study examined whether cannabis use status predicted prefrontal or parietal local gyrification index (LGI) and surface area (SA) in a sample of otherwise healthy adolescents and emerging adults. Consistent with the predicted hypotheses, after controlling for reading ability, age, gender, past year alcohol use, cotinine levels, and current depressive symptoms, cannabis users had reduced LGI in bilateral medial frontal, ventral medial, and frontal poles. No significant differences were found in hemispheric or inferior parietal LGI, suggesting that aberrant gyrification may be localized to particular PFC regions in emerging adults. Further, group differences in SA in orbitofrontal areas were consistent with LGI findings, but did not pass correction for multiple comparisons.
Decreased gyrification in right medial, ventral medial, and frontal pole regions, were associated with poorer performance on complex attention in cannabis users, suggesting that reduced gyrification confers a functional deficit. This is consistent with previous studies suggesting increased gyrification is associated with better cognitive functioning (Wallace et al., 2013) and may reflect improved cognitive control (Luders et al., 2012).
Present findings are consistent with prior research demonstrating unique PFC surface morphology characteristics in cannabis using youth (Mata et al., 2010). Specifically, Mata et al. (2010) found reduced sulcal concavity in the PFC of cannabis users in comparison to non-users and failed to find global hemispheric differences in SA. In the current study we found significantly reduced LGI in medial, ventral medial, and frontal poles in cannabis users compared to controls. We found no significant differences in inferior parietal LGI and marginal differences in SA, while in an overlapping sample we previously reported subtle volume abnormalities in this region (Price et al., 2015). Though we did not examine the relationship between either LGI or SA and other cortical measures in this study, surface area, gyrification, and cortical thickness appear have distinct patterns in neurodevelopment from ages 6 to 22 (Raznahan et al., 2011). We also found unique patterns in cannabis effects between two cortical morphometry measures; after controlling for covariates including age and gender, results from the current study suggest that frequent cannabis use may influence LGI in a more diffuse PFC distribution compared to SA since we found only marginal reductions of SA in two PFC regions (left: ventral lateral and ventral medial PFC) among cannabis users compared to controls. Therefore, while gyrification may be partially related to gray matter volume and SA, it likely reflects a novel measure of brain maturation (Klein et al., 2014). Though Mata et al. (2010) did not find global hemispheric group differences in SA, perhaps the influence of frequent cannabis use on SA is restricted to regions with later SA development. Changes in global SA during late adolescents may be primarily driven by reduced global gyrification index (Raznahan et al., 2011) and may differ from influences driving cortical thickness maturation (Wierenga et al., 2014). Future studies may want to examine how cannabis use impacts neurodevelopment utilizing multiple measures of cortical morphometry (LGI, cortical thickness, volume, and SA).
Frequent cannabis-using youth report using cannabis to cope with stressors or relax (Boys et al., 2001, Mitchell et al., 2007, Bonn-Miller et al., 2007, Johnson et al., 2010, Benschop et al., 2015), although continued use may negatively impact regions underlying healthy affective processing (Etkin et al., 2011). For example, the medial portions of the PFC are implicated in self-referential thought, regulation of stress response, autonomic regulation, emotional processing, and social cognition (Urry et al., 2009, Somerville et al., 2013, Bado et al., 2014; for reviews see Uddin et al., 2007, Hänsel and von Känel, 2008). Ventral medial portions of the PFC play a role in regulating amygdala activity, contextual decision-making, fear response and extinction, anticipatory responses, and social processing (Aoki et al., 2014, Lonsdorf et al., 2014, Rudorf and Hare, 2014, Spoormaker et al., 2014, Motzkin et al., 2014, Motzkin et al., 2015). Animal studies suggest that the inferior frontal regions also play a vital role in insight or one's ability to imagine consequences of behavior in new situations (Lucantonio et al., 2012). The frontal pole underlies detecting contextual change, and reward-related decision-making (Pollmann and Manginelli, 2009, Kovach et al., 2012). Therefore, additional studies examining functional consequences of cannabis use in youth may focus on affective processing, reward processing, and mood symptomatology. In addition, given the potential impact of endocannabinoid signaling on PFC activation (Filbey et al., 2010), future studies may want to examine whether genotypes related to endocannabinoid signaling interact with cannabis exposure to predict frontolimbic structural integrity in youth.
Further, there is also evidence of functional abnormalities as evidenced on fMRI studies of inhibitory control and complex working memory (see Tapert et al., 2007, Schweinsburg et al., 2008, Jacobsen et al., 2007). In an overlapping sample, our lab previously reported smaller inferior parietal volumes with small effect size among cannabis using emerging adults, although this finding did not survive multiple corrections (see Price et al., 2015). Taken together, later to develop PFC regions may be more consistently susceptible to morphological abnormalities associated with cannabis use during youth.
The current study was cross-sectional; therefore, it is not possible to determine whether the LGI and SA results reflect premorbid characteristics that co-occur with the onset of cannabis use. For example, there is evidence that smaller OFC volumes and increased impulsivity predict earlier age of cannabis use onset during adolescence (Cheetham et al., 2012). However, there are no studies to date that have examined whether other cortical morphometric measures (SA or gyrification) predict the onset of cannabis use, so it is not known whether these measures are sensitive premorbid markers for addiction risk, or cannabis use potential. On the other hand, it is not known whether these morphological features are more sensitive to environmental influence later in the course of development compared to volume or cortical thickness. Therefore, larger prospective longitudinal studies are needed to examine the influence of cannabis use on multiple morphometric measures (volume, LGI, SA, cortical thickness).
There are other limitations that need to be considered. Alcohol and cannabis use among youth are highly comorbid (Johnston et al., 2011). Although the current study excluded “very heavy” drinkers, statistically controlled for past year alcohol use, and did not find any relationship between past year alcohol use and gyrification or SA, it is possible that some of the findings are associated with combined or simultaneous cannabis and alcohol use. Lastly, due to the potential impact of various workstations on results (Gronenschild et al., 2012) results of the current study may be specific to the particular operating system, FreeSurfer version, imaging acquisition and preprocessing. Thus, replication using different imaging parameters or processing techniques is warranted.
5. Conclusion
In conclusion, this study found that regular cannabis users had less complex PFC gyrification, especially in medial and ventral medial regions. Cannabis users also demonstrated marginal reductions in orbitofrontal surface area. Reduced gyrification was significantly correlated with poorer working memory. These findings may reflect alterations in synaptic connections, resulting in reduced prefrontal complexity and poorer cognitive functioning in adolescent onset cannabis users. This adds to converging lines of evidence that suggest that adolescence and emerging adulthood is a sensitive period for drug-induced neurocognitive effects. Understanding the impact of regular cannabis use on neurodevelopment during adolescence and emerging adulthood remains a significant public health priority and additional prospective longitudinal studies are warranted.
Conflict of interest
None declared.
Acknowledgements
This research was funded by the National Institute on Drug Abuse (NIDA; R03 DA027457) and the University of Cincinnati Center for Environmental Genetics Pilot Program (P30 ES06096). Dr. Lisdahl was also funded by NIDA (R01 DA030354) during manuscript preparation.
Contributor Information
Skyler G. Shollenbarger, Email: Skyler.Shollenbarger@gmail.com.
Jenessa Price, Email: JenessaPrice@gmail.com.
Jon Wieser, Email: JonWieser@netzero.com.
Krista Lisdahl, Email: Krista.Medina@gmail.com.
References
- Alemán-Gómez Y., Janssen J., Schnack H., Balaban E., Pina-Camacho L., Alfaro-Almagro F., Castro-Fornieles J., Otero S., Baeza I., Moreno D., Bargalló N., Parellada M., Arango C., Desco M. The human cerebral cortex flattens during adolescence. J. Neurosci. 2013;33(38):15004–15010. doi: 10.1523/JNEUROSCI.1459-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki R., Matsumoto M., Yomogida Y., Izuma K., Murayama K., Sugiura A., Camerer C.F., Adolphs R., Matsumoto K. Social equality in the number of choice options is represented in the ventromedial prefrontal cortex. J. Neurosci. 2014;34(18):6413–6421. doi: 10.1523/JNEUROSCI.4427-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bado P., Engel A., Oliveira-Souza R., Bramati I.E., Paiva F.F., Basilio R., Sato J.R., Tovar-Moll F., Moll J. Functional dissociation of ventral frontal and dorsomedial default mode network components during resting state and emotional autobiographical recall. Hum. Brain Mapp. 2014;35(7):3302–3313. doi: 10.1002/hbm.22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley A.J., Jones D.W., Weinberger D.R. Genetic variability of human brain size and cortical gyral patterns. Brain. 1997;120(2):257–269. doi: 10.1093/brain/120.2.257. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; San Antonio, TX: 1996. Beck Depression Inventory-II. [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Soc. Ser. B: Method. 1995;57(1):289–300. [Google Scholar]
- Benschop A., Liebregts N., van der Pol P., Schaap R., Buisman R., van Laar M., van den Brink W., de Graaf R., Korf D.J. Reliability and validity of the Marijuana Motives Measure among young adult frequent cannabis users and associations with cannabis dependence. Addict. Behav. 2015:4091–4095. doi: 10.1016/j.addbeh.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Biondi A., Nogueira H., Dormont D., Duyme M., Hasboun D., Zouaoui A., Chantôme M., Marsault C. Are the brains of monozygotic twins similar? A three-dimensional MR study. Am. J. Neuroradiol. 1998;36(2):1361–1367. [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller M.O., Zvolensky M.J., Bernstein A. Marijuana use motives: concurrent relations to frequency of past 30-day use and anxiety sensitivity among young adult marijuana smokers. Addict. Behav. 2007;32(1):49–62. doi: 10.1016/j.addbeh.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Boys A., Marsden J., Strang J. Understanding reasons for drug use amongst young people: a functional perspective. Health Educ. Res. 2001;16(4):457–469. doi: 10.1093/her/16.4.457. [DOI] [PubMed] [Google Scholar]
- Brown S.A., Meyer M.G., Lippke L., Tapert S.F., Stewart D.G., Vik P.W. Psychometric evaluation of the customary drinking and drug use record (CDDR): a measure of adolescent alcohol and drug involvement. J. Stud. Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Caulkins J.P., Kilmer B., MacCoun R.J., Pacula R., Reuter P. Design considerations for legalizing cannabis: lessons inspired by analysis of California's Proposition 19. Addiction. 2012;107(5):865–871. doi: 10.1111/j.1360-0443.2011.03561.x. [DOI] [PubMed] [Google Scholar]
- Cheetham A., Allen N.B., Whittle S., Simmons J.G., Yücel M., Lubman D.I. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol. Psychiatry. 2012;71(8):684–692. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Churchwell J.C., Lopez-Larson M., Yurgelun-Todd D.A. Altered frontal cortical volume and decision making in adolescent cannabis users. Front. Psychol. 2010;1:225. doi: 10.3389/fpsyg.2010.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Delis D.C., Kaplan E. Psychological Corporation; San Antonio, Texas: 2000. Delis–Kaplan Executive Functioning Scale Manual. [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douet V., Chang L., Cloak C., Ernst T. Genetic influences on brain developmental trajectories on neuroimaging studies: from infancy to young adulthood. Brain Imaging Behav. 2014;8(2):234–250. doi: 10.1007/s11682-013-9260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A., Allison C., Brett R.R., Pratt J.A. Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neurosci. Biobehav. Rev. 2006;30(5):680–695. doi: 10.1016/j.neubiorev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F.M., Schacht J.P., Myers U.S., Chavez R.S., Hutchison K.E. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. 2010;35:967–975. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. New York Psychiatric Institute Biometrics Research Department; New York: 2001. Clinical Interview for DSM-IVTR (SCID-I): User's Guide and Interview – Research Version. [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., 3rd, Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through earlyadulthood. Proc. Natl. Acad. Sci. U.S.A. 2004;17:17. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Thompson P.M. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn. 2010;72(1):6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg E. Oxford University Press; New York, NY: 2009. The New Executive Brain: Frontal Lobes in a Complex World. [Google Scholar]
- Goodwin R.S., Darwin W.D., Chiang C.N., Shih M., Li S.I., Huestis M.A. Urinary elimination of 11-nor-9-carboxy-Δ9-tetrahydrocannabinal in cannabis users during continuously monitored abstinence. J. Anal. Toxicol. 2008;32:562–569. doi: 10.1093/jat/32.8.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronenschild E.H.B.M., Habets P., Jacobs H.I.L., Mengelers R., Rozendaal N., van Os J., Marcelis M. The effects of freeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS ONE. 2012;7(6):e38234. doi: 10.1371/journal.pone.0038234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwall D.M. Paced auditory serial-addition task: a measure of recovery from concussion. Percept. Mot. Skills. 1977;44(April (2)):367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Hänsel A., von Känel R. The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? Biopsychosoc. Med. 2008;2 doi: 10.1186/1751-0759-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K.L., Winward J.L., Schweinsburg A.D., Medina K., Brown S.A., Tapert S.F. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict. Behav. 2010;35(11):970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey M.A., Sellman J.D., Porter R.J., Frampton C.M. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007;26(3):309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Hasan A., McIntosh A., Droese U., Schneider-Axmann T., Lawrie S., Moorhead T., Tepest R., Maier W., Falkai P., Wobrock T. Prefrontal cortex gyrification index in twins: an MRI study. Eur. Arch. Psychiatry Clin. Neurosci. 2011;261(7):459–465. doi: 10.1007/s00406-011-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgetag C.C., Barbas H. Role of mechanical factors in the morphology of the primate cerebral cortex. PLoS Comput. Biol. 2006;2(3):e22. doi: 10.1371/journal.pcbi.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J., Goodwin R., Li C., Terry G., Zoghbi S., Morse C., Pike V.W., Volkow N.D., Huestis M.A., Innis R.B. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry. 2012;17(6):642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogstrom L.J., Westlye L.T., Walhovd K.B., Fjell A.M. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cereb. Cortex. 2012 doi: 10.1093/cercor/bhs231. [DOI] [PubMed] [Google Scholar]
- Jacobsen L.K., Pugh K.R., Constable R.T., Westerveld M., Mencl W.E. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent marijuana users. Biol. Psychiatry. 2007;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jager G., Ramsey N.F. Long-term consequences of adolescent cannabis exposure on the development of cognition brain structure and function: an overview of animal and human research. Curr. Drug Abuse Rev. 2008;1(2):114–123. doi: 10.2174/1874473710801020114. [DOI] [PubMed] [Google Scholar]
- Johnson K., Mullin J.L., Marshall E.C., Bonn-Miller M.O., Zvolensky M. Exploring the mediational role of coping motives for marijuana use in terms of the relation between anxiety sensitivity and marijuana dependence. Am. J. Addict. 2010;19(3):277–282. doi: 10.1111/j.1521-0391.2010.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L.D., O’Malley P.M., Bachman J.G., Schulenberg J.E. Institute for Social Research, The University of Michigan; Ann Arbor: 2011. Monitoring the Future National Survey Results on Drug Use 1975–2010. Volume II: College Students and Adults Ages 19–50. 312 pp. [Google Scholar]
- Johnston L.D., O’Malley P.M., Bachman J.G., Schulenberg J.E., Miech R.A. Institute for Social Research, The University of Michigan; Ann Arbor: 2014. Monitoring the Future national Survey Results on Drug Use 1975–2013: Volume II, College Students and Adults Ages 19–55. 424 pp. [Google Scholar]
- Klein D., Rotarska-Jagiela A., Genc E., Sritharan S., Mohr H., Roux F., Han C.E., Kaiser M., Singer W., Uhlhaas P.J. Adolescent brain maturation and cortical folding: evidence for reductions in gyrification. PLoS ONE. 2014;9(1):1–10. doi: 10.1371/journal.pone.0084914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach C.K., Daw N.D., Rudrauf D., Tranel D., O’Doherty J.P., Adolphs R. Anterior prefrontal cortex contributes to action selection through tracking of recent reward trends. J. Neurosci. 2012;32(25):8434–8442. doi: 10.1523/JNEUROSCI.5468-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A., Noctor S., Martínez-Cerdeño V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat. Rev. Neurosci. 2006;7(11):883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Lisdahl K.M., Gilbart E.R., Wright N.E., Shollenbarger S.G. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front. Psychiatry. 2013;4:1–19. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl K.M., Price J.S. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J. Int. Neuropsychol. Soc. 2012;18(4):678–688. doi: 10.1017/S1355617712000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G., von Cramon D.Y., Steinmetz H. Sulcal variability of twins. Cereb. Cortex. 1999;9(7):754–763. doi: 10.1093/cercor/9.7.754. [DOI] [PubMed] [Google Scholar]
- Lonsdorf T.B., Haaker J., Kalisch R. Long-term expression of human contextual fear and extinction memories involves amygdala, hippocampus and ventromedial prefrontal cortex: a reinstatement study in two independent samples. Soc. Congn. Affect. Neurosci. 2014;9(12):1973–1983. doi: 10.1093/scan/nsu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson M.P., Bogorodzki P., Rogowska J., McGlade E., King J.B., Terry J., Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav. Brain Res. 2011;220(1):164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E., Kurth F., Mayer E.A., Toga A.W., Narr K.L., Gaser C. The unique brain anatomy of meditation practitioners: alterations in cortical gyrification. Front. Hum. Neurosci. 2012;6(34) doi: 10.3389/fnhum.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E., Narr K.L., Thompson P.M., Rex D.E., Jancke L., Steinmetz H., Toga A.W. Gender differences in cortical complexity. Nat. Neurosci. 2004;7(8):799–800. doi: 10.1038/nn1277. [DOI] [PubMed] [Google Scholar]
- Lucantonio F., Takahashi Y.K., Hoffman A.F., Chang C.Y., Bali-Chaudhary S., Shaham Y., Lupica C.R., Schoenbaum G. Orbitofrontal activation restores insight lost after cocaine use. Nat. Neurosci. 2012;17:1092–1099. doi: 10.1038/nn.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnotta V.A., Andreasen N.C., Schultz S.K., Harris G., Cizadlo T., Heckel D., Nopoulos P., Flaum M. Quantitative in vivo measurement of gyrification in the human brain: changes associated with aging. Cereb. Cortex. 1999;9:151–160. doi: 10.1093/cercor/9.2.151. [DOI] [PubMed] [Google Scholar]
- Manly J.J., Jacobs D.M., Touradji P. Reading level attenuates differences in neuropsychological test performance between African American and White elders. J. Int. Neuropsychol. Soc. 2002;8:341–348. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- Mata I., Perez-Iglesias R., Roiz-Santiañez R., Tordesillas-Gutierrez D., Pazos A., Gutierrez A., Vazquez-Barquero J.L., Crespo-Facorro B. Gyrification brain abnormalities associated with adolescence and early-adulthood cannabis use. Brain Res. 2010;1317:297–304. doi: 10.1016/j.brainres.2009.12.069. [DOI] [PubMed] [Google Scholar]
- Medina K., Hanson K.H., Schweinsburg A.D., Cohen-Zion M., Nagel B.J., Tapert S.F. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. J. Int. Neuropsychol. Soc. 2007;13(5):807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K., McQueeny T., Nagel B.J., Hanson K.L., Schweinsburg A.D., Tapert S.F. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol.: Clin. Exp. Res. 2008;32(3):386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K., McQueeny T., Nagel B.J., Hanson K.L., Yang T.T., Tapert S.F. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict. Biol. 2009;14(4):457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M.H., Caspi A., Ambler A., Harrington H., Houts R., Keefe R.E., McDonald K., Ward A., Poulton R., Moffitt T.E. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. U.S.A. 2012;109(40):15980. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell H., Zvolensky M.J., Marshall E.C., Bonn-Miller M.O., Vujanovic A.A. Incremental validity of coping-oriented marijuana use motives in the prediction of affect-based psychological vulnerability. J. Psychopathol. Behav. Assess. 2007;29(4):277–288. [Google Scholar]
- Mohr A., Weisbrod M., Schellinger P., Knauth M. The similarity of brain morphology in healthy monozygotic twins. Brain Res. Cogn. Brain Res. 2004;20(1):106–110. doi: 10.1016/j.cogbrainres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Motzkin J.C., Philippi C.L., Wolf R.C., Baskaya M.K., Koenigs M. Ventromedial prefrontal cortex lesions alter neural and physiological correlates of anticipation. J. Neurosci. 2014;34(31):10430–10437. doi: 10.1523/JNEUROSCI.1446-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin J.C., Philippi C.L., Wolf R.C., Baskaya M.K., Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol. Psychiatry. 2015;77(3):276–284. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu A., Schneider M., Debbané M., Badoud D., Eliez S., Schaer M. Sex differences in thickness, and folding developments throughout the cortex. NeuroImage. 2013;82:200–207. doi: 10.1016/j.neuroimage.2013.05.076. [DOI] [PubMed] [Google Scholar]
- Pollmann S., Manginelli A.A. Anterior prefrontal involvement in implicit contextual change detection. Front. Hum. Neurosci. 2009;3 doi: 10.3389/neuro.09.028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.S., McQueeny T., Shollenbarger S.G., Browning E.L., Wieser J., Lisdahl K.M. Effects of marijuana use on prefrontal and parietal volumes and cognition in emerging adults. Psychopharmacology. 2015 doi: 10.1007/s00213-015-3931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., Giedd J.N. How does your cortex grow? J. Neurosci. 2011;31(19):7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D.P., Stewart R.M., Hutchinson J.W., Caviness V.S., Jr. Mechanical model of brain convolutional development. Science. 1975;189(4196):18–21. doi: 10.1126/science.1135626. [DOI] [PubMed] [Google Scholar]
- Ronan L., Voets N., Catarina R., Alexander-Bloch A., Hough M., Mackay C., Crow T.J., James A., Giedd J.N., Fletcher P.C. Differential tangential expansion as a mechanism for cortical gyrification. Cereb. Cortex. 2013;(March) doi: 10.1093/cercor/bht082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudorf S., Hare T.A. Interactions between dorsolateral and ventromedial prefrontal cortex underlie context-dependent stimulus valuation in goal-directed choice. J. Neurosci. 2014;34(48):15988–15996. doi: 10.1523/JNEUROSCI.3192-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M., Cuadra M.B., Schmansky N., Fischl B., Thiran J.P., Eliez S. How to measure cortical folding from MR images: a step-by-step tutorial to compute local gyrification index. J. Vis. Exp. 2012;(59):e3417. doi: 10.3791/3417. DOI 10.37913417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M., Cuadra M.B., Tamarit L., Lazeyras F., Eliez S., Thiran J.-P. A surface-based approach to quantify local cortical gyrification. IEEE Trans. Med. Imaging. 2008;27(2):161–170. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- Schnack H.G., van Haren N.M., Brouwer R.M., Evans A., Durston S., Boomsma D.I., Kahn R.S., Hulshoff Pol H.E. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb. Cortex. 2015;25(6):1608–1617. doi: 10.1093/cercor/bht357. [DOI] [PubMed] [Google Scholar]
- Schweinsburg A.D., Brown S.A., Tapert S.F. The influence of marijuana use on neurocognitive functioning in adolescents. Curr. Drug Abuse Rev. 2008;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L.C., Maisto S.A., Sobell M.B., Cooper A.M. Reliability of alcohol abusers’ self-reports of drinking behavior. Behav. Res. Ther. 1979;17(2):157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Ruberry E.J., Dyke J.P., Glover G., Casey B.J. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol. Sci. 2013;24(8):1554–1562. doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoormaker V.I., Gvozdanovic G.A., Sämann P.G., Czisch M. Ventromedial prefrontal cortex activity and rapid eye movement sleep are associated with subsequent fear expression in human subjects. Exp. Brain Res. 2014;232(5):1547–1554. doi: 10.1007/s00221-014-3831-2. [DOI] [PubMed] [Google Scholar]
- Stewart D.G., Brown S.A. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- Su S., White T., Schmidt M., Kao C.Y., Sapiro G. Geometric computation of human gyrification indexes from magnetic resonance images. Hum. Brain Mapp. 2013;34:1230–1244. doi: 10.1002/hbm.21510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallinen T., Chung J.Y., Biggins J.S., Mahadevan L. Gyrificaiton from constrained cortical expansion. Proc. Natl. Acad. Sci. U.S.A. 2014;111(35):12667–12672. doi: 10.1073/pnas.1406015111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert S.F., Schweinsburg A.D., Drummond S.A., Paulus M.P., Brown S.A., Yang T.T., Frank L.R. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194(2):173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry G.E., Liow J., Zoghbi S.S., Hirvonen J., Farris A.G., Lerner A., Tauscher J.T., Schaus J.M., Phebus L., Felder C.C., Morse C.L., Hong J.S., Pike V.W., Halldin C., Innis R.B. Quantitation of cannabinoid CB1 receptors in healthy human brain using positron emission tomography and an inverse agonist radioligand. NeuroImage. 2009;48(2):362–370. doi: 10.1016/j.neuroimage.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R., Burnod Y. A morphogenetic model for the development of cortical convolutions. Cereb. Cortex. 2005;15(12):1900–1913. doi: 10.1093/cercor/bhi068. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Iacoboni M., Lange C., Keenan J.P. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn. Sci. 2007;11(4):153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Urry H.L., van Reekum C.M., Johnstone T., Davidson R.J. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage. 2009;47(3):852–863. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385(6614):313. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Verdurand M., Nguyen V., Stark D., Zahara D., Gregoire M.C., Greguric I., Zavitsanou K. Comparison of cannabinoid CB1 receptor binding in adolescent and adult rats: a positron emission tomography study using [18F]MK-9470. Int. J. Mol. Imaging. 2011 doi: 10.1155/2011/548123. Article ID 548123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace G.L., Robustelli B., Dankner N., Kenworthy L., Giedd J.N., Martin A. Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders. Brain. 2013;136(6):1956–1967. doi: 10.1093/brain/awt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Third edition. The Psychological Corporation; San Antonio: 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- White T., Andreasen N.C., Nopoulos P. Brain volumes and surface morphology in monozygotic twins. Cereb. Cortex. 2002;12:486–493. doi: 10.1093/cercor/12.5.486. [DOI] [PubMed] [Google Scholar]
- White T., Su S., Schmidt M., Kao C., Sapiro G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010;72(1):36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga L.M., Langen M., Oranje B., Durston S. Unique developmental trajectories of cortical thickness and surface area. NeuroImage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. Wide Range, Inc.; Wilmington, DE: 2006. Wide Range Achievement Test, 4th Edition (WRAT-4) Manual. [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr. Opin. Neurobiol. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]


