Abstract
Background:
The medicinally important phytochemicals present in Syzygium cumini seeds probably accounts for its wide use in traditional systems of medicines in India, like Ayurveda, Unani, and Siddha.
Aim:
The aim of the study was to determine the antioxidant potential of three different geographical variants of S. cumini seeds and to compare the phenolic profiling to know the effect of geographical variation in phenolic composition.
Materials and Methods:
Total phenolic and flavonoid content of S. cumini seeds were analyzed. Antioxidant activities in terms of 2,2-diphenyl-1-picrylhydrazyl, 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), nitric oxide and superoxide radical scavenging assays were performed. The most active fractions were subjected to high-performance liquid chromatography (HPLC) profiling to identify the phenolic composition.
Results:
Among all the fractions, 70% methanol fraction of S. cumini seed showed significant antioxidant potential. There existed a linear correlation between phenolic content and antioxidant activity. HPLC profiling of 70% methanol (ME) fractions of all the variants revealed the presence of phenolic compounds with high concentrations of ellagic acid and gallic acid. The differences in phenolic concentration due to geographical changes might be the reason for higher antioxidant potential showed by 70% ME of Trivandrum variant.
Conclusion:
70% methanolic fraction of S. cumini can act as a novel source of natural antioxidant.
KEY WORDS: Antioxidant activity, high-performance liquid chromatography, total flavonoid content, total phenolic content, Syzygium cumini
INTRODUCTION
The recent trend in the knowledge of free radicals and biology is producing a medical revolution that ensures a new age of health and disease management. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are the predominant by-products of cellular redox processes. These free radicals possess both toxic and beneficial effects. ROS and RNS exert beneficial effects on cellular responses and immune function at low or moderate level. They generate oxidative stress at high concentrations, which is a deleterious process that can damage cell structure and function [1]. Oxidative stress is an important factor in the progression of chronic degenerative diseases including coronary heart disease, cancer, and arthritis [2]. The human body can counteract this oxidative stress with the support of exogenous antioxidants and by producing various endogenous antioxidants. For the past few decades, the secondary metabolites from plants have been well known for their antioxidant potential.
Medicinal plants are an important source of antioxidants. Phenolic compounds from natural products are gaining importance because of their relatively safe and wide acceptance by consumers. The increasing interest in the search for natural replacements for synthetic antioxidants has led to the antioxidant evaluation of a number of plant species. Antioxidants have the ability to counteract the damaging effects of free radicals inside our body. If free radicals formed are left unchallenged, they would eventually lead to the etiology of a wide range of diseases.
Plant phenolics are the most fascinating antioxidants which include predominantly phenolic acids, flavonoids, and tannins. Researchers and food manufacturers have become more engrossed in polyphenols due to their potent antioxidant properties, their richness in the diet, and their ability to prevent various oxidative stress associated diseases [3]. The inverse relationship between the dietary intake of fruits and vegetables and the chance of oxidative stress associated diseases has been partially accredited to phenolics [4]. The phenolic compounds in plants are reported to have antidiabetic, anticancer, anti-inflammatory, antimutagenic, antimicrobial, and other activities.
Syzygium cumini (L.) Skeels, belonging to the family Myrtaceae, is one of the best-known species and is often distributed in Asia (East India, Malaysia, and China). S. cumini is widely used in traditional systems of medicines in India, such as Ayurveda, Unani, and Siddha. Different parts of S. cumini are reported to have several medicinal properties like antidiabetic [5], antimicrobial [6], anti-inflammatory [7] and free radical scavenging potential [8,9]. The seeds have been reported to possess compounds such as jambosine, gallic acid (GA), ellagic acid, corilagin, 3,6-hexahydroxy diphenoylglucose, 1-galloylglucose, 3-galloylglucose, quercetin, β-sitoterol and 4,6 hexahydroxydiphenoylglucose [10,11].
The phytochemical content of active fraction is subject to large variations due to variety, age, maturity of the plants used, season, geo-agro-climatic conditions, agronomical practices, post-harvest handling, storage, processing, etc. The active principle thus can vary tremendously and that, in turn, would affect the biopotency. It is in this context that chemical profiling of the plants is important to produce products with consistent quality. Therefore, the aim of this present study was to compare the antioxidant activity and profiling of S. cumini seeds collected from three different geographical locations.
MATERIALS AND METHODS
Chemicals
GA, chlorogenic acid (ClA), caffeic acid, syringic acid, coumaric acid, ferulic acid (FA), ellagic acid, cinnamic acid, catechol, myricetin, quercetin, kaempferol, apigenin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), sodium carbonate, aluminum chloride, potassium acetate and all other chemicals and biochemicals unless otherwise noted were from Sigma (St. Louis, MO, USA). All the positive controls used were of high-performance liquid chromatography (HPLC) grade. Folin Ciocalteau reagent, Methanol and acetic acid of HPLC grade were supplied by Merck, Germany. All other chemicals used were of the standard analytical grade.
Plant Material
The fully mature S. cumini fruits were collected from Trivandrum - TVM (8° 29’ N, 76° 59’ E), Trichy - TCH (10° 48’ N, 78° 41’ E) and from Malampuzha - MPA (10.7° N, 76.6° E). The samples were authenticated by Dr. E. S. Santhosh Kumar, Technical Officer, Jawaharlal Nehru Tropical Botanic Garden and Research Institute (JNTBGRI), Palode, Trivandrum, Kerala and voucher specimens (Collection No: SC-APNP-CSIR-100, 101 and 102) were deposited in the herbarium of JNTBGRI, Palode, Trivandrum, Kerala, India. Samples were collected during the month of April and stored at −80°C until processed.
Preparation of Plant Fractions
S. cumini seeds were separated from fruits and washed well using distilled water. The seeds thus obtained were dried in the oven at 40°C for 24 h, seed coats were removed, and seeds were coarsely powdered using a motor and pestle. 2 kg dry powder was fractioned sequentially with hexane (HE), ethyl acetate (EA), methanol (ME), 70% ME, and water (WE) at room temperature (27 ± 1°C). The extraction process was repeated till each solvent became colorless. These fractions were filtered through Whatman No. 1 filter paper. The fractions were evaporated in rotavapor and stored at 4°C, protected from light and humidity for further analysis.
Yield of Extracts
The extract (1 mL) was pipetted out to a pre-weighed petri dish and kept in the oven for 4 h at 100°C. The weight of the petri dish was then measured. The petri dish was kept in the oven till the weight become constant. The difference in weight of the petri dish gave the yield of extract in 1 mL.
Determination of Total Phenolic Content (TPC)
The TPC was determined by Folin–Ciocalteau method [12] with slight modifications. Briefly, different concentrations of fractions (20-100 µL) were taken, and 80 µL of Folin–Ciocalteau reagent and 200 µL sodium carbonate (20%) were added, made up to 700 µL using distilled water and incubated at ambient temperature (25-27°C) for 90 min. The color developed was measured at 760 nm using a multimode reader (Biotek, USA). The phenolic contents were calculated using a standard curve for GA, and the result was expressed as mg GA equivalents (GAE) per gram dry weight of fraction (mg GAE/g). All measurements were performed in triplicates.
Determination of Total Flavonoid Content (TFC)
The TFC was estimated using standard procedures described by Chang et al. [13] with slight modifications. Different concentrations of fractions were diluted with 150 µL of ethanol. Further, 10 µL of 10% aluminum chloride solution and 1 M potassium acetate (10 µL) was added and made up to 280 µL using distilled water. The final solution was mixed well and incubated at room temperature for 40 min. The absorbance was measured at 415 nm using a multimode reader (Biotek, USA). Quercetin was used as a standard, and results were expressed as mg quercetin equivalents (QE) per gram dry weight of fraction (mg QE/g).
Based on the antioxidant assays, TPC and TFC, the active fraction of all the variants was selected for further studies.
Antioxidant Assays
DPPH scavenging activity
The DPPH scavenging activity of different fractions was evaluated according to the method of Brand-Williams et al. [14]. 1 mL of 0.1 mM DPPH solution in ME was mixed with 1 mL of each fraction at varying concentrations. The corresponding blank sample was prepared, and GA was used as reference standard. Mixture of 1 mL ME and 1 mL DPPH solution was used as control. The mixture was shaken well and incubated for 30 min in the dark. The reaction was carried out in triplicate, and the decrease in absorbance was measured at 517 nm after incubation using a multiplate reader (Synergy 4 Biotek. USA). The scavenging activity was expressed as IC50 (µg/mL). The % inhibition was calculated using the formula:

Nitric oxide (NO) scavenging activity
NO scavenging activity was estimated according to the method of Marcocci et al. [15] with slight modification. The reaction mixture contained 1 mL of 10 mM sodium nitroprusside (SNP), phosphate buffered saline (pH 7.4) and various concentration of fractions in a final volume of 1.1 mL. After incubation for 150 min at room temperature, pipetted out 100 µL into wells plate and 100 µL of Griess reagent was added. The mixture was incubated for 10 min at 25°C. The pink chromophore generated was measured spectrophotometrically at 540 nm against a blank sample. All tests were performed in triplicates. Ascorbic acid was used as the standard. The percentage inhibition of NO radical generation was calculated using the following formula:

Superoxide radical scavenging activity
Superoxide radical scavenging activity of different fractions was measured by the reduction of NBT according to a previously reported method [16]. The non-enzymatic phenazine methosulfate-nicotinamide adenine dinucleotide (PMS/NADH) system generates superoxide radicals, which reduce nitro blue tetrazolium (NBT) to a purple formazan. The 1 mL reaction mixture contained phosphate buffer (20 mM, pH 7.4), NADH (73 µM), NBT (50 µM), PMS (15 µM) and various concentrations of the sample solution. After incubation for 5 min at room temperature, the absorbance at 562 nm was measured against an appropriate blank to determine the quantity of formazan generated. Quercetin was used as the standard, and the percentage radical scavenging capacity was determined using the formula:

ABTS scavenging activity
ABTS scavenging potential of each fraction were analyzed by the method of Arnao et al. [17] with some modifications. The working solution was prepared by mixing the stock solutions - 7 mM ABTS and 2.45 mM potassium persulfate solution in equal quantities, and allowing them to react for 14 h at room temperature in the dark. The solution was then diluted with ethanol to make the absorbance in the range 0.70 ± 0.01 units at 734 nm. Different concentrations of fractions were allowed to react with 1 mL of the ABTS solution for 7 min, and the absorbance was taken at 734 nm. The ABTS scavenging capacity of each fraction was compared with that of ascorbic acid, and percentage inhibition was calculated as:

HPLC - Diode Array Detector (DAD) Analysis of Phenolic Compounds in 70% ME Fractions
The identification and quantification of phenolic compounds present in most active fractions of three variants of S. cumini were performed with a Shimadzu HPLC system containing two LC-8A preparative liquid chromatography pump units, a C18 reverse phase column (Phenomenex, 5 µm, 250×4.6 mm2 dia.), and a (DAD; SPD-M10A VP) with a wavelength range of 200-450 nm. The fractions and 13 reference standards, namely, GA, ClA, caffeic acid, syringic acid, coumaric acid, FA, ellagic acid, cinnamic acid, catechol, myricetin, quercetin, kaempferol, and apigenin were prepared in HPLC grade ME at a concentration of 1 mg/mL and filtered through a 0.45 µm filter. Each sample (20 µL) were injected, and the HPLC analysis was done according to the standard method [18] with slight modifications. The mobile phase used was water: acetic acid (98:2, v/v) as solvent A and methanol:acetic acid (98:2, v/v) as solvent B with a time program of 0-15 min 15% B, 16-20 min 50% B, 21-35 min 70% B, 36-50 min 100% B. The flow rate was 1 mL/min and the column temperature was set at 30°C. Identification and quantification of the phenolic compounds were done by comparing the retention time and characteristic absorption spectra from the DAD with those of the authentic standards. To minimize variation in quantification, samples were taken in triplicates. Data acquisition and analysis were carried out using Shimadzu Class-VP version 6.14 SP1 software.
RESULTS
Yield of Extracts
Plants are rich in medicinally active and economically important compounds. Solvent extraction helps in segregating and concentrating the active compounds. Initial extraction with hexane defatted the extract. EA, ME, 70% ME and water were sequentially used for the extraction. The yield of more than 70% was found for the methanolic fraction followed by 10-20% by 70% methanolic fraction of all the variants [Figure 1]. EA, hexane, and water fraction together constitute for only 6-8% yield in all the variants.
Figure 1.

Graph showing percentage yield in different fractions of three different variants of Syzygium cumini seeds. TVM: Trivandrum variant, TCH: Trichy variant, MPA: Malampuzha variant
TPC
All the variants were initially analyzed for their total phenolic and flavonoid contents. The TPC of all fractions of S. cumini was expressed as milligram of GAE/g dry weight of fractions as represented in Figure 2a. All the fractions of S. cumini contained a significant amount of phenolic compounds. The highest phenolic content was exhibited by 70% methanolic fraction for all the three variants (TVM - 906 ± 7.2, TCH - 808.5 ± 3.9, MPA - 864.4 ± 5.6 mg GAE/g dry weight of fractions) and ME fraction of TVM (757.3 ± 6.2 mg GAE/g dry weight of each fraction). TPC increased in the following order for all the three variants: Hexane fraction <aqueous fraction <EA fraction <ME fraction <70% ME fraction [Figure 2a].
Figure 2.

(a) Total phenolic content (mg GA equivalents/g dry wt.) (b) total flavonoid content (mg QE/g dry wt.) in different fractions of three different variants of Syzygium cumini seeds. Values are the means ± standard deviation of three replicated samples. Duncan’s multiple range test was conducted and data in the same column with different letters/symbols indicate statistically significant differences among groups at P < 0.05. TVM: Trivandrum variant, TCH: Trichy variant, MPA: Malampuzha variant
Total Flavonoid Content
Total flavonoid content for all the three variants of S. cumini was evaluated and had been represented in Figure 2b. Results revealed that 70% methanolic fraction of TVM variant possessed highest flavonoid content (233.8 ± 5.5 mg QE/g DW) compared to other variants. The total flavonoid content increased in the following order for all the three variants: Hexane fraction <aqueous fraction <EA fraction <ME fraction <70% ME fraction.
DPPH Radical Scavenging Activity
DPPH radical scavenging activity is one of the best methods to evaluate the antioxidant properties of natural products. DPPH• (1,1-Diphenyl-2-picrylhydrazyl radical) can accept an electron to become a stable diamagnetic molecule. The radical scavenging power of the sample was measured by the decrease in absorbance due to DPPH• at 517 nm, showing the formation of its reduced form, DPPH, which was yellow in color. The purple colored methanolic solution shows a strong absorption band at 517 nm due to the presence of odd electron.
In the present study, the DPPH radical scavenging activity increased in the following order- aqueous fraction <EA fraction <ME fraction <70% ME fraction. 70% ME fraction exhibited highest DPPH scavenging activity [Figure 3a]. The IC50 value of 70% ME fraction was found to be 5.1 µg/mL, 5.5 µg/mL, 6.2 µg/mL respectively for TVM, TCH, and MPA variants. However, the activity of each fraction was found to be less when compared to the standard, GA (1.8 ± 0.77 µg/mL). Methanol fraction also exhibited potential DPPH scavenging activity. The IC50 values for DPPH scavenging activity of EA, ME and water fractions of S. cumini seeds are represented in Figure 3a.
Figure 3.

(a) 2,2-diphenyl-1-picrylhydrazyl (b) nitric oxide (c) super oxide (d) 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging activities of EA, ME, 70% ME and WEs of different variants of Syzygium cumini. EA: EA fraction, ME: ME fraction, 70% ME: 70% methanol fraction, WE: Water fraction, TVM; Trivandrum variant, TCH: Trichy variant, MPA: Malampuzha variant. Each value represents mean ± standard deviation (SD) from triplicate measurements. Values are means ± SD; n = 3; *Represents groups differ significantly from control group (P ≤ 0.05)
NO Scavenging Activity
Due to the presence of unpaired electron, NO is classified as a free radical and is a potent pleiotropic inhibitor of biological processes such as relaxation of smooth muscle, neuronal signaling and regulation of cell-mediated toxicity [19]. In addition to ROS, NO is also involved in inflammation, cancer, and other pathological conditions [20,21] and this free radical reacts with superoxide anion and form a potentially cytotoxic molecule, the peroxynitrite. Peroxynitrite causes nitration or hydroxylation of aromatic compounds especially tyrosine and also triggers adduct formation with dissolved carbon dioxide in body fluids and damages various proteins [22]. SNP generates NO at physiological pH which reacts with oxygen to produce nitrite ions. A pink chromosphere was formed when nitrite ions react with Griess reagent, whose absorbance was measured at 540 nm.
The results from the assay illustrated that the 70% ME fraction of all variants demonstrated higher NO scavenging potential. The IC50 value of 70% ME fractions were 4.23 ± 0.34 µg/mL, 5.23 ± 0.24 µg/mL, 6.24 ± 0.31 µg/mL respectively for TVM, TCH, and MPA variants [Figure 3b]. The standard, curcumin, demonstrated NO radical scavenging potential with an IC50 value of 17.35 ± 2.3 µg/mL which was comparable to that of 70% ME fractions and ME fraction of TVM variant (IC50 value of 16.92 ± 2.3 µg/mL). The IC50 values for NO scavenging activity of EA, ME, 70% ME and aqueous fractions are represented in Figure 3b.
Superoxide Radical Scavenging Activity
Superoxide anion radical is biologically quite toxic and is one of the strongest ROS among the free radicals and get converted to other harmful ROS such as hydrogen peroxide and hydroxyl radical, damaging biomolecules which results in chronic diseases [23]. The biological toxicity of superoxide is due to its capacity to inactivate iron-sulfur cluster containing enzymes, generate the highly reactive hydroxyl radical PMS-NADH systems by oxidation of NADH and assayed by the reduction of NBT [24]. The consumption of superoxide anion in the reaction mixture is indicated by the decrease in absorbance at 560 nm.
The results of the assay showed that 70% ME and ME fractions of all variants exhibited significant superoxide radical scavenging activity [Figure 3c] which was highly comparable with the standard, catechin, with an IC50 value 83.99 ± 2.34 µg/mL. The IC50 value of 70% ME fractions were 28.83 ± 2.14 µg/mL, 34.72 ± 1.24 µg/mL, 39.46 ± 2.64 µg/mL, respectively, for TVM, TCH and MPA variants. The IC50 value for superoxide radical scavenging activity of all fractions of all variants is represented in Figure 3c.
ABTS Scavenging Potential
ABTS is a protonated radical which has a characteristic maximum at 734 nm and the interaction with the fraction, or standard Trolox suppresses the absorbance of ABTS radical, and the results were expressed as Trolox equivalent antioxidant capacity value [Figure 3d].
The results from the study indicated that both ME and 70% ME fractions of all variants effectively scavenge ABTS radicals and the scavenging potential increased in a dose- dependent manner. The standard, trolox, exhibited an IC50 - 2.96 ± 0.87 µg/mL. Among the fractions 70% ME fraction of TVM variant exhibited highest ABTS scavenging ability (IC50 - 1.43 ± 0.05 µg/mL).
HPLC-DAD Analysis of Phenolic Compounds
The phenolic compounds of 70% ME fractions of three different variants were identified [Figure 4]. All fractions were individually spiked with each standard and recorded an increased peak height at almost same retention time, indicating the presence of those compounds. The results showed that 70% ME fraction possessed the major quantity of phenolic acids and flavonoid compounds. Among the fractions, 70% ME fraction of TVM and MPA variants showed the highest concentration of ellagic acid (222.2 mg/g DW, 350.2 mg/g DW) and GA (272.2 mg/g DW, 341.24 mg/g DW, respectively). TCH variant (70% ME fraction) showed the presence of GA (21.6 mg/g DW), quercetin (13.2 mg/g DW) and other phenolic acids such as cinnamic acid, ClA, ellagic acid, and FA. The major phenolic compounds quantified from the active fraction of all the variants have been depicted in Figure 5.
Figure 4.
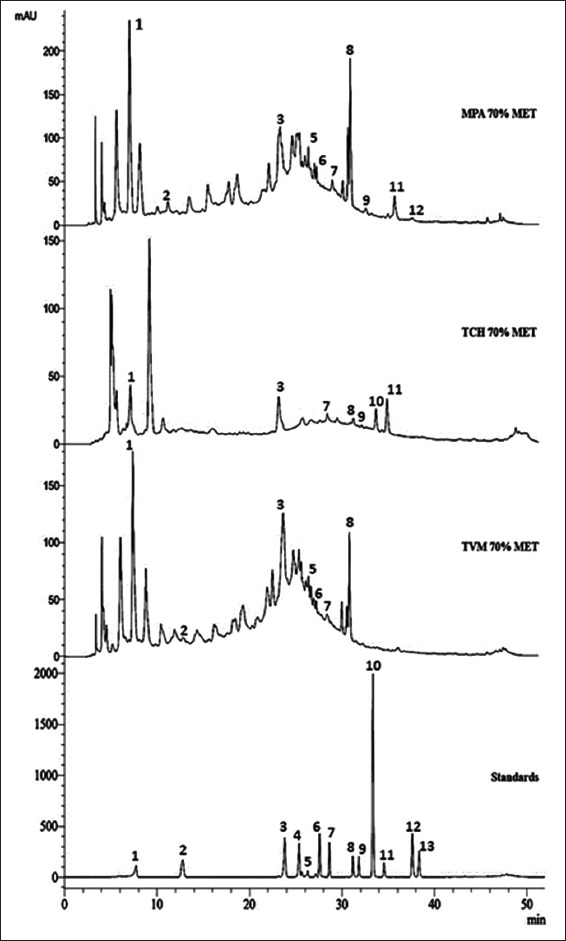
Representative high-performance liquid chromatography-diode array detector chromatograms of mixed standards and 70% methanol fractions of Trivandrum variant, Trichy variant and Malampuzha variant recorded at 280 nm. Standards are (1) gallic acid, (2) catechol, (3) chlorogenic acid, (4) caffeic acid, (5) syringic acid, (6) coumaric acid, (7) ferulic acid, (8) ellagic acid, (9) myricetin, (10) cinnamic acid, (11) quercetin, (12) kaempferol, (13) apigenin
Figure 5.

Prominent phenolic compounds (mg/g dry wt.) 70% methanol fractions of Syzygium cumini seeds quantified by high-performance liquid chromatography-diode array detector. Values are the means ± standard deviation of three replicated samples. GA: Gallic acid, ClA: Chlorogenic acid, FA: Ferulic acid, EA: Elagic acid. TVM: Trivandrum variant, TCH: Trichy variant, MPA: Malampuzha variant
DISCUSSION
In the present study, the antioxidant potential of S. cumini fractions of three geographical variants was assessed in terms of their phenolic and flavonoid content in addition to their free radical scavenging efficacies. Bajpai et al., [25] reported that 50% methanolic extract of seeds possessed 108.7 mg/g of TPC. An independent study using methanolic fraction of leaves had reported the presence of 610.32 mg/g of TPC and 451.5 mg/g of total flavonoid content [26]. In contrast, our results suggested higher TPC in 70% methanolic fraction of S. cumini seeds that were reported to contain in leaves, fruit, and pulp. This may be because sequential extraction of seeds using solvents would have fractionated phenolic and flavonoid compounds in 70% ME.
The radical scavenging activities such as DPPH, ABTS, NO, superoxide radical scavenging potential of the fractions were analyzed. The electron donating ability of S. cumini seed fractions were measured by the bleaching action of 1,1-Diphenyl-2-picrylhydrazyl radical purple colored solution. A study by Banerjee et al. [27] reported that ethanolic and methanolic fractions of S. cumini seeds showed a DPPH radical scavenging activity of 140 mg/GAE/g dry weight and 19.1 mg/g dry weight, respectively. From the results of the present study, the exhibition of higher DPPH radical scavenging activity demonstrated by 70% ME and ME fractions may be due to the presence of phenolic constituents that are more capable of donating hydrogen to a free radical and scavenge the radicals. In addition, 70% ME and ME fractions of all variants showed significant ABTS and NO radical scavenging potential which was significantly higher than the results reported by Lekha et al. (2013) [28] using EA fraction of S. cumini seeds. Although superoxide is a weak oxidant, powerful and dangerous hydroxyl radicals that can contribute to oxidative stress are generated from superoxides. Earlier reports on S. cumini fruit skin demonstrated IC50 value of 260 µg/mL for scavenging superoxide radicals [29]. The results from the present study demonstrated significant superoxide radical protection by 70% ME and ME fractions of all variants. The demonstration of significant radical scavenging activities (DPPH, ABTS, NO, and superoxide radicals) especially by the 70% methanolic fractions of all the variants of S. cumini seeds may be due to the presence of phenolic compounds in the fraction.
The phenolic profiling of active fractions of all the variants indicated the presence of prominent phenolic compounds which were known for their antioxidant potential. GA, ellagic acid, ClA, and FA were found predominantly in 70% ME fraction of TVM variant. These phenolic acids have been very well known for the antioxidant activity [30-32], and these compounds were found in 70% methanolic fraction of all variants of S. cumini seeds. This difference in the composition of phenolics in 70% methanolic fractions of TVM variant may be responsible for its higher antioxidant efficacy when compared with the other variants. A positive correlation was reportedly observed between TPC and antioxidant activity [33,34]. A similar correlation between TPC and antioxidant activity was also reflected among the fractions in the present study.
In summary, 70% ME fraction of three variants of S. cumini seeds demonstrated significant radical scavenging activities which may be attributed to the higher levels of total phenolic compounds. TVM variant showed the best activity which can be attributed to the presence of highest phenolic content and relative composition of phenolics and flavonoids among all the variants.
CONCLUSION
The present study made an attempt to evaluate and compare the antioxidant potential of S. cumini fractions from different geographical locations of India and to correlate the activity with their phenolic content. The study demonstrated the fractionation of higher levels of phenolic compounds in 70% methanolic fractions of all variants resulting in increased radical scavenging potential. The active principle or individual polyphenols that may vary in their levels among the variants due to differences in geographical locations imparts significant contribution to their efficacy.
ACKNOWLEDGMENTS
The authors are thankful to Department of Science and Technology, India, and Council for Scientific and Industrial Research, India for the financial support. The authors are also grateful to Dr. E. S. Santhosh Kumar, Technical Officer, Jawaharlal Nehru Tropical Botanic Garden and Research Institute (JNTBGRI), Palode, Trivandrum, Kerala for accepting voucher specimens and identifying the plant species.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Oyagbemi AA, Azeez OI, Saba AB. Interactions between reactive oxygen species and cancer: The roles of natural dietary antioxidants and their molecular mechanisms of action. Asian Pac J Cancer Prev. 2009;10:535–44. [PubMed] [Google Scholar]
- 2.Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20:21138–56. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanda BL, Sultana G, Radhakrishnan TT. Determination of phytochemicals and antioxidant activity of Acorus calamus rhizome. J Drug Deliv Ther. 2014;4:117–21. [Google Scholar]
- 4.Grosso G, Stepaniak U, Micek A, Stefler D, Bobak M, Pajak A. Dietary polyphenols are inversely associated with metabolic syndrome in Polish adults of the HAPIEE study. Eur J Nutr 2016. doi: 10.1007/s00394-016-1187-z. doi: 10.1007/s00394-0161187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bose SN, Sepaha GC. Clinical observations on the antidiabetic properties of Pterocarpus marsupium and Eugenia jambolana. J Indian Med Assoc. 1956;27:388–91. [PubMed] [Google Scholar]
- 6.Chandrasekaran M, Venkatesalu V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J Ethnopharmacol. 2004;91:105–8. doi: 10.1016/j.jep.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Chauduri AK, Pal S, Gomes A, Bhattacharya S. Antiinflammatory and related actions of Syzygium cumini seed. Phytother Res. 1990;4:5–10. [Google Scholar]
- 8.Silva DH, Plaza CV, Bolzani VS, Cavalheiro AJ, Gamboa IC. Antioxidants from fruits and leaves of Eugenia jambolana, an myrtaceae species from Atlantic Forest. Plant Med. 2006;72:187. [Google Scholar]
- 9.Banerjee A, Dasgupta N, De B. In vitro antioxidant activity of Syzygium cumini fruit. Food Chem. 2005;90:727–733. [Google Scholar]
- 10.Rastogi RP, Mehrotra BN. Compendium of Indian Medicinal Plants. Vol. 1. Lucknow: Central Drug Research Institute; 1990. pp. 388–9. [Google Scholar]
- 11.Sagrawat H, Mann A, Kharya M. Pharmacological potential of Eugenia Jambolana: A review. Pharmacogenesis Mag. 2006;1:96–104. [Google Scholar]
- 12.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58. [Google Scholar]
- 13.Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–82. [Google Scholar]
- 14.Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 15.Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun. 1994;201:748–55. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- 16.Fontana M, Mosca L, Rosei MA. Interaction of enkephalins with oxyradicals. Biochem Pharmacol. 2001;61(10):1253–7. doi: 10.1016/s0006-2952(01)00565-2. [DOI] [PubMed] [Google Scholar]
- 17.Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73:239–44. [Google Scholar]
- 18.Chen QC, Zhang WY, Jin W, Lee IS, Min BS, Jung HJ, et al. Flavonoids and isoflavonoids from Sophorae Flos improve glucose uptake in vitro. Planta Med. 2010;76:79–81. doi: 10.1055/s-0029-1185944. [DOI] [PubMed] [Google Scholar]
- 19.Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–98. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- 20.Nabavi SM, Ebrahimzadeh MA, Nabavi SF, Hamidinia A, Bekhradnia AR. Determination of antioxidant activity, phenol and flavonoids content of Parrotia persica Mey. Pharmacologyonline. 2008a;2:560–7. [Google Scholar]
- 21.Nabavi SM, Ebrahimzadeh MA, Nabavi SF, Jafari M. Free radical scavenging activity and antioxidant capacity of Eryngium caucasicum Trautv and Froripia subpinnata. Pharmacologyonline. 2008b;3:19–25. [Google Scholar]
- 22.Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–80. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 23.Al-Mamun M, Yamaki K, Masumizu T, Nakai Y, Saito K, Sano H, et al. Superoxide anion radical scavenging activities of herbs and pastures in northern Japan determined using electron spin resonance spectrometry. Int J Biol Sci. 2007;3:349–55. doi: 10.7150/ijbs.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulcin I, Huyut Z, Elmastas M, Aboul-Enein H. Radical scavenging and antioxidant activity of tannic acid. Arabian J Chem. 2010;3:43–53. [Google Scholar]
- 25.Bajpai M, Pande A, Tewari SK, Prakash D. phenolic content and antioxidant activity of some food and medicinal plants. Int J Food Sci Nutr. 2005;56:287–91. doi: 10.1080/09637480500146606. [DOI] [PubMed] [Google Scholar]
- 26.Ruan ZP, Zhang LL, Lin YM. Evaluation of the antioxidant activity of Syzygium cumini leaves. Molecules. 2008;13:2545–56. doi: 10.3390/molecules13102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee J, Narendhirakannan RT. Biosynthesis of silver nanoparticles from Syzygium cumini (L.). seed extract and evaluation of their in vitro antioxidant activities. Dig J Nanomater Biostruct. 2011;6:961–8. [Google Scholar]
- 28.Lekha KN, Maleeka B, Geetha S. In vitro-Antioxidant activity of the seed and leaf extracts of Syzygium cumini. IOSR JESTFT. 2013;7:54–62. [Google Scholar]
- 29.Archana B, Nabasree D, Bratati D. In vitro study of Syzygium cumini fruit. Food Chem. 2005;90:727–33. [Google Scholar]
- 30.Kähkönen MP, Hopia AI, Heinonen M. Berry phenolics and their antioxidant activity. J Agric Food Chem. 2001;49:4076–82. doi: 10.1021/jf010152t. [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan M, Sudheer AR, Menon VP. Ferulic acid: Therapeutic potential through its antioxidant property. J Clin Biochem Nutr. 2007;40:92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kono Y, Kobayashi K, Tagawa S, Adachi K, Ueda A, Sawa Y, et al. Antioxidant activity of polyphenolics in diets. Rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochim Biophys Acta. 1997;1335:335–42. doi: 10.1016/s0304-4165(96)00151-1. [DOI] [PubMed] [Google Scholar]
- 33.Piluzza G, Bullitta S. Correlation between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm Biol. 2011;49:240–7. doi: 10.3109/13880209.2010.501083. [DOI] [PubMed] [Google Scholar]
- 34.Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12:221. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]


