Abstract
Aim:
Chenopodium opulifolium is a specie of the Chenopodiaceae commonly used as vegetables in local diet and for treating different ailment in Uganda. This study was conducted to evaluate the antioxidant, antinociceptive and anti-inflammatory effects of the aqueous extract of C. opulifolium leaves (AECO).
Materials and Methods:
The dried leaf of the plant was extracted by maceration in water. Qualitative and quantitative phytochemical analysis, antioxidants, and membrane stabilizing effects were determined in the extract. The extract was then investigated for acute toxicity, antinociceptive (writhing, hot plate and open field test), and anti-inflammatory (egg albumin-induced paw edema) effects in rodents.
Results:
Phytochemical analysis revealed the presence of alkaloids, tannins, phlobatannins, flavonoids, and saponins in AECO. Total caffeic acid derivatives and total flavonoids content were 91.7 mgCAE/g sample and 94.7 mgRE/g sample, respectively. AECO demonstrated antioxidant effects in both 1,1-diphenyl-2-picryl-hydrazyl and NO assays. Significant membrane stabilizing activity was observed in both the heat and hypotonic solution-induced lysis of erythrocytes. The acute toxicity test showed that AECO (5000 mg/kg) did not cause any significant change in behavior or death in rats. AECO (100-400 mg/kg) produced a significant antinociceptive effect in both the writhing and hot plate tests, but no significant reduction in the locomotory activity in mice. Furthermore, the extract significantly (P < 0.05) reduced egg albumin-induced rat paw edema by 44.2%, 44.5%, and 51.2%, respectively, after 120 min.
Conclusion:
The results showed that C. opulifolium extract possesses significant antioxidant, antinociceptive and anti-inflammatory effects, and these affirm the reasons for its folkloric use.
KEY WORDS: Anti-inflammatory, caffeic acid, Chenopodium opulifolium, erythrocytes, writhing
INTRODUCTION
Medicinal plants represent unique biodiversity resources on the African continent contributing immensely to the health and wellness of its population over the centuries. The contributions of the variety of medicinal plants to local economies, cultural integrity, and health care and ultimately the well-being are increasingly being discussed [1]. In Sub-Saharan Africa for example, households in rural areas rely heavily on plant resources for food, fodder, and herbal medicine. The World Health Organization decade old statistics estimated that about 80% of the African population depends on herbal medicines for some aspect of primary health care needs [2]. Inaccessible and unaffordable modern medicines are major factors that still promote the use of medicinal plants among rural Ugandans for their daily health care needs [3]. Medicinal plants being a dominant component of the traditional medicine practice in Uganda, is used for the prevention, diagnosis, and treatment of social, mental and physical illness [4]. One of such plants used is Chenopodium opulifolium Schrad. ex Koch & Ziz, with a synonym Chenopodium ugandae (Aellen) Aellen, a member of the Chenopodiaceae family that grows in Uganda [4].
The Chenopodiaceae is a large family comprising about 102 genera and 1400 species. The genus chenopodium includes a variety of weedy herbs (more than 200 species) that are native to Europe, Africa, Asia, North and South America [5]. The importance of the Chenopodium species was due to their variety of medicinal properties, commercially used as spices or drugs because of the presence of useful secondary metabolites. The most characteristic constituents are flavonoids, essential oils, and terpenes [6]. The reported biological activities of the chenopods include the antiviral, antimicrobial, antifungal, anti-inflammatory, analgesic, and immunomodulatory effects [7].
C. opulifolium known locally as “Kavumbavumba” in central or “Omwentago” in Western Uganda has been reported to have several ethnobotanical uses [8,9]. The leaf is used as vegetable in local diets in East Africa [10]. The leaves or root decoction is drunk to induce menstruation and hasten childbirth and to relieve irregular painful menstruation [11]. The leaves can be used as eye ointment or macerated leaf used for female asthenia, and abdominal colic for newborns [12]; and for managing pediatrics cases of febrile illnesses [13]. C. opulifolium leaf is reported to be used in the management of Herpes simplex viral infection in HIV/AIDS [14]. The water decoction or infusion of its leaves has been reported to be used for the treatment of malaria and cough, either alone or in combination with Azadirachta indica, Momordica foetida, Ocimum gratissimum, or Vernonia amygdalina [15].
The ethnobotanical information on this plant did not match the pharmacological or toxicological information. C. opulifolium medicinal properties have not been extensively studied in biological systems, and no published data are available in scientific literatures. This study was, therefore, carried out in an attempt to screen for the antioxidant, antinociceptive, and anti-inflammatory effects of the aqueous extract of C. opulifolium (AECO) leaves.
MATERIALS AND METHODS
Materials
Chemicals and drugs
Glacial acetic acid, indomethacin, sodium nitrite (NaNO2), aluminum chloride (AlCl3), sodium nitroprusside, and quercetin were all product of Sigma-Aldrich, USA.
Animals
Adult Wister rats (120-180 g) or Swiss mice (18-25 g) of either sex were used throughout this study. The animals were kept under environmentally controlled laboratory room conditions at 21-27°C. The animals were housed in cages lined with wood shavings and were fed with standard pelletized feed (Nuvita® Animal Feeds Ltd., Jinja Uganda) and water ad-libitum. The animal experiments were conducted according to the National Institute of Health Guide for the care and use of laboratory animals [16] and ethical guidelines for investigation of experimental pain in conscious animals [17].
Plant Material
The leaves of C. opulifolium locally known as “omwentago” were collected in Ishaka, Bushenyi District, in Western Uganda. The plant was authenticated by Assoc Prof Dominic Byarugaba, a botanist at Kampala International University; a voucher specimen was prepared as herbarium sample and deposited at the school of pharmacy herbarium (KIU-WC/10/001). Fresh leaves of the plant were dried by placing them under shade, and then, reduced to fine powder by grinding using a metallic mortar and pestle in Kampala International University pharmacy laboratory.
Extraction
Extraction of C. opulifolium powdered leaf was done by maceration method. Briefly, powdered leaf (100 g) was soaked in 1 L of distilled water and shaken for 48 h on a laboratory rotator (Nuve SL 350 Quality System, Digisystem Laboratory Inc., Taiwan). The extract was filtered and evaporated over water bath at 50°C and was finally dried in the oven set at 40°C. The extract obtained was darkish brown in color denoted as AECO and was stored in the refrigerator at 4°C.
Qualitative Phytochemical Screening
The aqueous extract used for pharmacological screening was subjected to preliminary qualitative phytochemical analysis using standard protocols [18,19]. The presence of the compounds to be tested was rated as positive (+) or negative (−). These compounds included tannins, phlobotannins, saponins, terpenoids, flavonoids, alkaloids, and reducing sugars.
Determination of Total Caffeic Acid Derivatives Content (TCAD)
TCAD in the aqueous extract was determined using the spectrophotometric method with Arnow’s reagent [20]. 0.2 mL of AECO (1 mg/mL), 1 mL HCl (0.5 N), and 1 mL Arnow’s reagent and 1 mL NaOH (1 M) were mixed in test tubes and allowed to stand for 5 min. The volume was made up to 5 mL and after 30 min, the absorbance was read at 500 nm with a spectrophotometer (Spectronic 21D Milton Roy, USA). Caffeic acid content was determined from caffeic acid calibration curve with a linear regression curve (R2 = 0.999).
Determination of Total Flavonoids Content in AECO Leaves
The total flavonoid content (TFC) in the extract was determined by the AlCl3 spectrophotometric method as described by Sultana et al. [21]. 1 mL of AECO (1 mg/mL) was dispensed into three separate test tubes. After that, 0.3 mL of 10% (w/v) NaNO2 was added to the test tubes, and left to react for 5 min, 0.3 mL of 10% (w/v) AlCl3 was added and left for 1 min to react. Thereafter, 2 mL of 1M NaOH was added and the mixtures shaken. Aliquots of the mixtures were transferred to a cuvette, and the absorbance values measured with a spectrophotometer (Spectronic 21D Milton Roy) at 510 nm. A mixture of 1 mL of 80% (v/v) methanol, 4 mL of deionized water, 0.3 mL of 10% (w/v) NaNO2, 0.3 mL of 10% (w/v) AlCl3, and 2 mL of 1 M NaOH was prepared and used as the blank. Rutin was used as a standard for the calibration curve (R2 = 0.985).
Evaluation of Free Radical Scavenging Effects of AECO
1,1-Diphenyl-2-picryl-hydrazyl (DPPH) assay
The free radical scavenging properties of AECO were assessed using DPPH spectrophotometric assay by Aderogba et al. [22] with slight modifications. To 2 mL of AECO (12.5, 25, 50, 100, 200 and 400 µg/mL in methanol), quercetin (25 µg/mL) or methanol (2 mL) were added 3 mL of freshly prepared DPPH solution (0.1 mM) in methanol. The mixture was incubated in the dark cupboard for 30 min at room temperature, and the absorbance was measured at 514 nm on an ultraviolet (UV)/vis spectrophotometer (INESA). The percentage free radical scavenging activity was calculated accordingly:
% inhibition=[(absorbance of control–absorbance of test sample)/absorbance of control] ×100%
Nitric oxide free radical scavenging assay
Nitric oxide radical scavenging activity assay according to the method described by Kumaran and Karunakaran [23] was used. Briefly, sodium nitroprusside (10 mM in 0.1 M sodium phosphate buffer, pH 7.4) was mixed with AECO (12.5, 25, 50, 100, 200 and 400 µg/mL) or quercetin (25 µg/mL) and incubated at room temperature for 150 min. The control contained the same reaction mixture except the extract. After incubation period, 0.5 mL of Griess reagent (equal volume of 1% sulfanilamide in 5% phosphoric acid and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride) was added. The absorbance was read at 546 nm in a UV/vis Spectrophotometer (INESA).
Evaluation of Membrane Stabilizing Properties
Preparation of 10% erythrocytes suspension
Blood was collected by cardiac puncture from healthy male Wistar rats lightly anesthetized with ether. The collected blood was mixed with equal volume of sterilized Alsever solution; packed red cells were obtained by repeated centrifugation (3000 rpm for 10 min), in isotonic phosphate buffer solution (154 mM NaCl in 10 mM sodium phosphate buffer, pH 7.4). The packed cell was re-suspended to 10% (v/v) in the isotonic buffer.
Effect of AECO on heat-induced rat erythrocytes hemolysis
The effects of AECO on hemolysis of rat red blood cell (RBC) induced by heat was evaluated according to the modified method by Anosike et al. [24]. Briefly, AECO and indomethacin were dissolved in isotonic phosphate buffer solution (154 mM NaCl in 10 mM sodium phosphate buffer, pH 7.4). The assay mixture consisted of 5 mL graded doses of extract (100, 200, 400, 800 and 1000 µg/mL); and 0.5 mL of 10% RBC suspension. The control was prepared as above without the extract, while RBC was omitted from the extract control tubes. Each sample was prepared and arranged in quadruplicate sets (4 sets per dose). A pair of the tubes was incubated at 54°C for 20 min in a regulated water bath. The another pair was maintained at −10°C in a freezer for 20 min. Afterward, the tubes were centrifuged at 4000 rpm for 3 min, and the hemoglobin contents of the supernatant were estimated at 540 nm using Spectronic 21 D (Milton Roy) Spectrophotometer.
Effect of AECO on hypotonic solution induced rat erythrocytes hemolysis
The effects of AECO on hemolysis of rat RBC induced by hypotonic solution was evaluated according to the method described by Shinde et al. [25] with some modifications. The extract was dissolved in hypotonic sodium phosphate buffer solution (50 mM NaCl in 10 mM sodium phosphate buffer, pH 7.4). The hypotonicity-induced hemolysis assay mixture consisted of 5 mL graded concentration (100, 200, 400, 800 and 1000 µg/mL), in hypotonic solution, and 0.5 mL of 10% RBC suspension.
The control was prepared as above except that the extract was omitted, while the extract controls lacked erythrocyte suspension. Each sample was prepared and arranged in quadruplicate sets (4 sets per dose). The mixture was incubated for 1 h at room temperature (23 ± 2°C), and afterward, centrifuged for 3 min at 4000 rpm. The absorbance of the hemoglobin content of the supernatant was estimated at 540 nm using Spectronic 21D (Milton Roy) spectrophotometer.
Acute Toxicity Study
Acute toxicity refers to adverse effects occurring within a short time of administration of a single dose of a substance or multiple doses given within 24 h. The safety of the leaf extract was tested as described by Adzu and Haruna [26]. Rats were grouped into five groups of three rats each. The extract was administered orally to four groups in increasing doses of 250, 500, 1000 and 5000 mg/kg body weight, and the fifth group (control group) received distilled water at 10 mL/kg body weight. All the rats were kept in the same conditions. They were observed for pharmacotoxic signs continuously for 4 h and at the 24 h post administration to score for mortality. Thereafter, they were observed for up to 7 days post administration. Pharmacotoxic signs include salivation, diarrhea, piloerection, sedation, restlessness, sensitivity to sound and touch, gasping, cyanosis, blood in urine and edema.
Evaluation of Antinociceptive Properties of AECO
Acetic acid-induced writhing in mice
The method as described by Koster et al. [27] was used. Five groups of mice (n = 5) were used in this study comprising the vehicle (0.1 mL/10 g distilled water), indomethacin (10 mg/kg), or AECO (100, 200, 200 mg/kg). Each mice were placed singly inside the plexiglass observation chamber immediately after oral treatment for 1 h before injection of 0.6% acetic acid (0.1 mL/10 g body weight). The frequency of writhing occurring between 5 and 20 min was counted. Writhing movement was accepted as a contraction of the abdominal muscles accompanied by stretching of hind limbs.
Hot Plate Test
Thermal noxious stimulus was produced in mice by placing them on the hot plate (UgoBasile hot/cold plate 35100, Italy) according to a method described by Turner [28]. Five groups of mice (n = 5) were used, in this study, comprising the vehicle (0.1 mL/10 g distilled water), pentazocine (5 mg/kg), or AECO (100, 200, 200 mg/kg). The mice were placed singly on the hot plate which was maintained at 55 ± 1°C. Reaction time was recorded when the animals licked their fore and hind paws or jumped; at before (0) and 30, 60, 90 and 120 min after administration of test drugs. The mice which reacted within 20 s were selected for the study. The mean percentage maximum possible effect (% MPE) was calculated as:

Assessment of Effect of AECO on Locomotory Activity in Open-field
To assess the possible nonspecific muscle relaxants or the sedative effects of AECO, the motor performance of the mice was evaluated on the open-field apparatus (Archer, 1973). Groups of mice (n = 5) were treated with vehicle (0.1 mL/10g, p.o.), AECO (100, 200, 400 mg/kg, p.o.) 1 h before the performance of the test. The mice were placed in the center of the UgoBasile activity cage apparatus and allowed to have free ambulation for 5 min of observation of the locomotion frequency (horizontal activity and vertical activity).
Evaluation of In Vivo Anti-inflammatory Activity in Egg Albumin-induced Edema in Rat Paw
The phlogistic agent employed in this study to induce acute inflammation was fresh egg albumin according to the method described by Akah and Nwambie [29]. The rats were fasted overnight prior to the experiment and were divided into five groups of five rats each. Group 1 (negative control) received distilled water (10 mL/kg body weight), Groups 2-4 received AECO (100 -400 mg/kg body weight), while Group 5 (positive control) received indomethacin (10 mg/kg). All treatments were orally administered using oral canula.
Edema was induced 30 min later in all the rats by a single subplantar injection of 0.1 mL of raw egg albumin to the left hind paw. Edema formation was taken as increase in paw circumference measured by wrapping a white cotton thread around the injected paw and measuring the circumference on a meter rule [30]. Paw circumference was taken at 30, 60, 90 and 120 min after egg albumin injection. The percentage inhibition of edema was calculated using the formula:
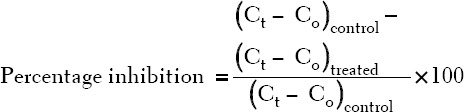
Where Ct - paw (edema) circumference at time (t) after albumin injection and Co - paw (edema) circumference before egg albumin injection.
Statistical Analysis of Data
Results were expressed as mean ± standard error of the mean and statistical comparison of means was performed using analysis of variance, all levels of significance were set as P < 0.05. Significant main effects were further analyzed by post-hoc test using Bonferroni’s multiple comparison test. Graphs and statistical analysis were performed using Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) and GraphPad Prism® software version 5.01 (GraphPad Software, Inc. La Jolla, CA 92037 USA).
RESULTS
Result of Phytochemical Analysis of AECO
The screening for the secondary metabolites revealed the presence of alkaloids, tannins, phlobotannins, flavonoids, and saponins (data not shown). Steroids, terpenoids, and reducing sugars were not detected in AECO. The TCAD determined by the Arnow’s reagent method showed that AECO contained an amount equivalent to 91.7 ± 0.01 mgCAE/g of sample. The amount of TFC in the extract estimated from the rutin calibration curve was 94.7 ± 0.03 mgRE/g of the sample (Figure 1).
Figure 1.
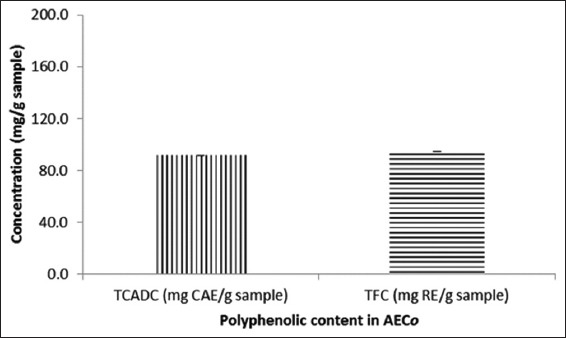
Total caffeic acid derivatives content and total flavonoid content in aqueous extract of Chenopodium opulifolium
In Vitro Antioxidant Effects of AECO
The results as shown in Figure 2 below revealed that AECO possesses in vitro antioxidant activity by mopping free radicals generated in both the DPPH and NO free radical scavenging assays. There was a corresponding increase in antiradical activity with an increase in concentration. The antiradical activity was higher in the NO than in the DPPH scavenging assay. Quercetin (25 µg/mL) activity was better than AECO (400 µg/mL) in the DPPH assay but lower in the NO assay.
Figure 2.
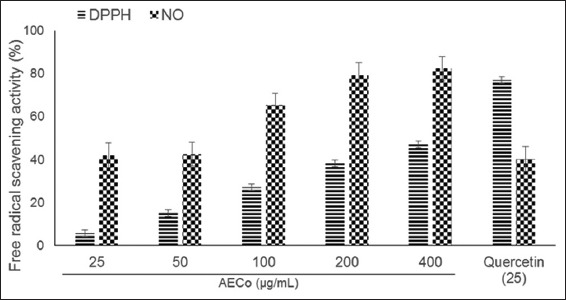
In vitro antioxidant activity of aqueous extract of Chenopodium opulifolium
Membrane Stabilizing Activity
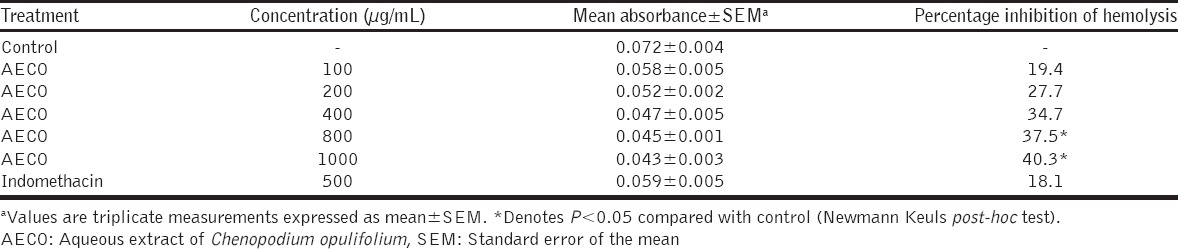
AECO (100 -1000 µg/mL) demonstrated in vitro anti-inflammatory activity by its ability to inhibit heat-induced rat erythrocytes hemolysis (Table 1). The high percentage inhibition of hemolysis (36.5%, 44.0% and 49.6%) obtained for doses 400, 800 and 1000 µg/mL, respectively, was statistically significant (P < 0.05). Similarly, results, as shown in Table 2, revealed that AECO (100-1000 µg/mL) inhibited hypotonic solution-induced rat erythrocytes hemolysis in a concentration-dependent manner. The high percentage inhibition of hemolysis (37.5% and 40.3%) obtained for concentrations 800 and 1000 µg/mL, respectively, was statistically significant (P < 0.05).
Table 1.
Membrane stabilizing effect of AECO on heat-induced hemolysis of red blood cell
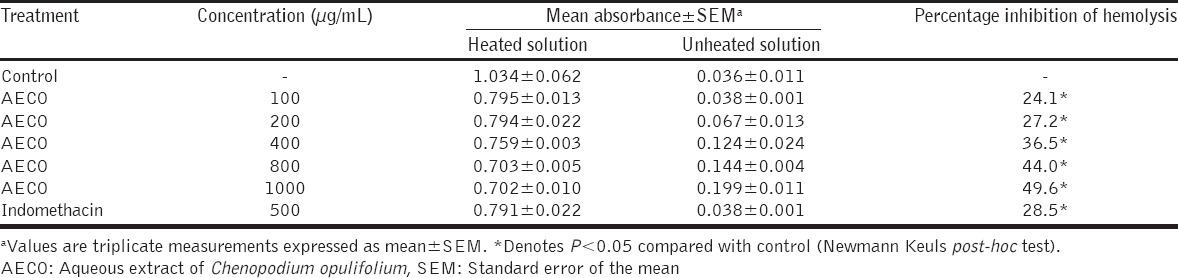
Table 2.
Membrane stabilizing effect of AECO on hypotonicity - induced hemolysis of red blood cell
Acute Toxicity
In the acute toxicity test of the AECO, there was no mortality or any signs of behavioral changes or pharmacotoxicity observed after oral administration of extract of the different doses even to the highest dose administered (5000 mg/kg body weight) in rats. No noticeable sign of toxicity was observed in all test doses until the end of the study period.
Anti-nociceptive Effect of AECO in Writhing Test
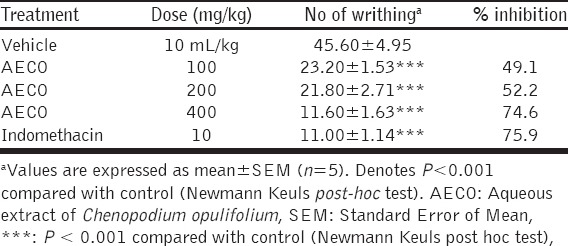
The pretreatment of mice with AECO 100, 200 and 400 mg/kg caused a significant (P < 0.001) and dose-dependent decrease in number of abdominal constriction in mice compared to control (Table 3). The percentage of inhibition of constriction was calculated as 49.1%, 52.2%, and 74.6% by AECO at 100, 200, and 400 mg/kg, respectively. Indomethacin (10 mg/kg) showed significant (P < 0.001) inhibition of abdominal constriction (75.9%).
Table 3.
Antinociceptive activity of AECO in acetic acid-induced abdominal constriction test in mice
Antinociceptive Effect of AECO in Hot Plate Test
In this model of thermally-induced nociception, pretreatment of mice with AECO (100-200 mg/kg) significantly prolong reaction time significantly (P < 0.05) compared to control as shown in the percent maximal possible effect curve (Figure 3). Dose-dependent maximal effect was observed at 60 min following administration. Pentazocine (5 mg/kg) showed a significant (P < 0.001) maximal possible effects when compared to control.
Figure 3.
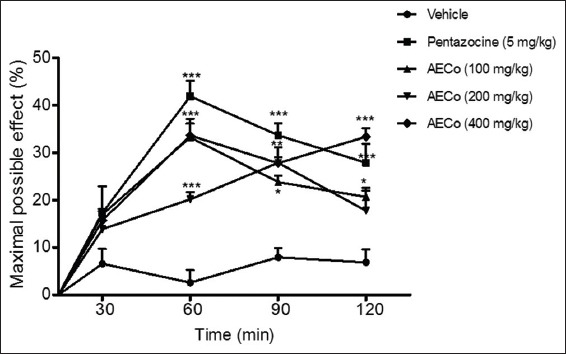
The effect of orally administered aqueous extract of C. opulifolium on the hot plate test. Result expressed as mean±standard error of the mean (n=5). The statistical significance was calculated by two-way analysis of variance followed by Bonferroni’s test. *P<0.05, **P<0.01, ***P<0.001 when compared to vehicle
Effects of AECO on Mice in the Open Field Test
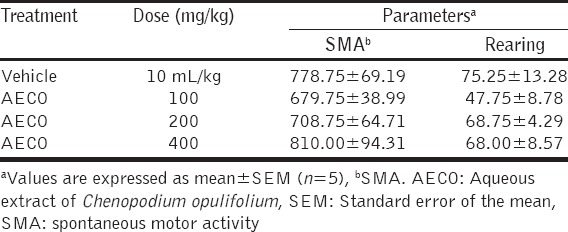
Evaluation of the effect of oral administration of AECO on the spontaneous motor activity and rearing in mice did not show any significant alteration (P > 0.05) in those activities when compared with control (Table 4).
Table 4.
The effects of orally administered AECO in the open field test in mice
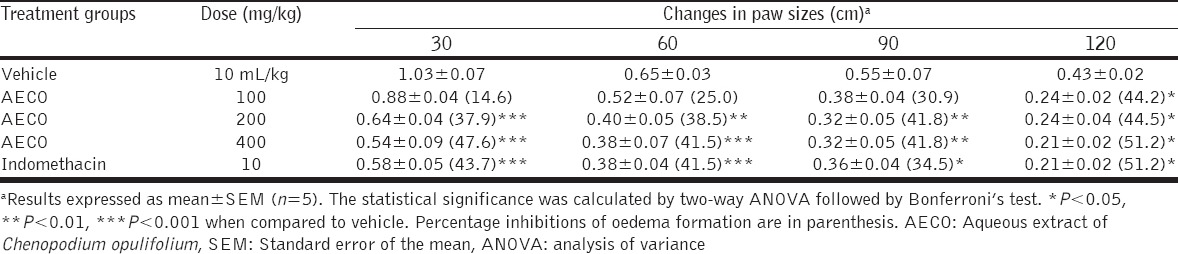
Anti-inflammatory Effect of AECO in Egg Albumin - Induced Edema in Rat Paw
The results obtained from this experiment are shown in Table 5. Egg albumin induced edema in all experimental animals at different time points, and maximum edema formation was observed at 30 min post albumin injection. Pre-treatment with AECO (100, 200 and 400 mg/kg) produced statistically significant (P < 0.001) and time-dependent inhibition of the edematous response during the 120 min duration measurement. The percentage inhibition of edema formation was 44.2%, 44.5%, and 51.2% by AECO 100, 200, and 400 mg/kg, respectively, while indomethacin administered at 10 mg/kg inhibited edema by 51.2%.
Table 5.
Anti-inflammatory effects of AECO on egg albumin-induced rat paw oedema
DISCUSSION
The findings of this study showed the antioxidants, antinociceptive and anti-inflammatory activities of AECO leaves. The phytochemical screening of AECO used in the pharmacological tests revealed the presence of alkaloids, tannins, phlobotannins, flavonoids, and saponins. Quantification of caffeic acid derivatives and TFC in the extract showed an appreciable amount of polyphenolics. The species of the family Chenopodiaceae are widely distributed weedy herbs often used commercially as spices or drugs because of the presence of useful secondary metabolites [31]. Phenolics, flavonoids, saponins, and triterpenoids were reported to be the major phytoconstituents of the Chenopodium genus, and about 300 and 79 compounds have been reportedly isolated [7]. AECO showed weak antiradical activity in the DPPH and potent activity in NO free radical scavenging assays. DPPH free radical scavenging assay is a primary assay for investigating the antioxidant properties of extracts. Antioxidant potentials usually correlate well with the phenolic composition of extract.
In a concentration-dependent manner, the extract shows membrane stability activity in both the heat and hypotonic solution-induced hemolysis of rat erythrocytes. The ability to protect the erythrocytes from stress-induced lytic effect of heat and hypotonic solution demonstrate the in vitro anti-inflammatory properties of the extract. During inflammation, there are lyses of lysosomes which release their component enzymes that produce a variety of disorders. The lysosomal enzymes are implicated in the pathogenesis of articular tissue degradation in several rheumatic diseases. Drugs by stabilizing the membrane can prevent the rupture of the lysosomes and inhibit the release of lysosomal enzymes [32]. The RBC membrane stabilization test serves as a model for lysosomal membrane stabilization since several agents capable of releasing hydrolytic enzymes from lysosomes also injure erythrocytes [24]. The presence of polyphenols such as flavonoids and tannins in AECO may contribute to the anti-inflammatory effects observed. Flavonoids are known to exert profound stabilizing effects on lysosomes membrane which is often attributed to their free radical scavenging properties [33].
Results of the acute toxicity test of the AECO showed that there was no mortality or any significant change in the behavior of the rats recorded up to the dose of 5000 mg/kg body weight. Acute toxicity test provides preliminary information on the toxic nature of a material for which no other toxicological information is available [34]. Based on the results of the preliminary toxicity testing on AECO, the highest dose was found to be non-lethal, hence we can safely conclude that median lethal dose (LD50) is >5000 mg/kg body weight. The high safety margin of AECO may partly explain the historical use of C. opulifolium decoction or infusion in managing fever associated with malaria parasite infection in Uganda [13]. The information from acute toxicity studies is often used in dose setting for other studies, thus the set dose for anti-inflammatory screening of AECO was lower than 1/10th fraction of the highest tolerated dose [35,36].
C. opulifolium extract showed similar antinociceptive effect to Indomethacin in the acetic acid-induced abdominal writhing test. This model characterized by stereotypical behavior of abdominal constriction involves peripheral nociceptive mechanisms. Most importantly is the release of biogenic amines (e.g., histamine and serotonin), bradykinin, prostaglandin E2 and PGF2α which activates visceral nociceptors [37].
Thermally-induced nociception in the hot plate is considered to be selective for centrally acting analgesic compounds (morphine-like drugs). AECO showed positive activity in this test. In order to rule out the chances of false positive effect of AECO, we evaluated its effect on spontaneous locomotor function in the activity meter cage. AECO did not alter the ambulatory and rearing activities. The results revealed that the observed antinociceptive effect of AECO was not as a result of sedation or impairment of motor activity in mice. Taken together, effect in acetic acid-induced writhing and hot plate test revealed that AECO antinociceptive activity might involve peripheral, spinal and supraspinal inhibition of pain.
The AECO leaves exhibited an anti-inflammatory effect by inhibiting the edema induced by egg albumin in rat paw. This model is used as an in vivo model of acute inflammation that can be used to screen agents for anti-inflammatory effect [29,30]. The results showed that AECO suppressed inflammation at the early phase, an indication that it may be blocking the release of the early phase mediators. Histamine, bradykinin, serotonin, and prostaglandins are inflammatory factors released from damaged cells, and they are mediators of inflammation and pain. Prostaglandins are products of arachidonic acid metabolism via the cyclooxygenase pathway [29].
CONCLUSION
This study clearly revealed that the AECO leaf contains polyphenolics (flavonoids, caffeic acid, and tannins) that showed antioxidant, antinociceptive, and anti-inflammatory activities. This result provides a scientific credit for the use of C. opulifolium in traditional medicine as a remedy against inflammatory conditions and thus a candidate for further studies.
ACKNOWLEDGMENTS
We acknowledge the kind assistance of Associate Professor Dominic Byarugaba for plant authentification and Mr. Pascal Mwembembezi and Ronald, in the school of Pharmacy, Kampala International University, Western Campus for technical assistance.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Okigbo RN, Eme UE, Ogbogu S. Biodiversity and conservation of medicinal and aromatic plants in Africa. Biotechnol Mol Bio Rev. 2008;3:27–134. [Google Scholar]
- 2.WHO. Traditional Medicine Strategy 2002-5. 2002, WHO/EDM/TRM. Geneva: World Health Organization; 2002. [Google Scholar]
- 3.Kamatenesi-Mugisha M, Oryem-Origa H. Medicinal plants used to induce labour during childbirth in western Uganda. J Ethnopharmacol. 2007;109:1–9. doi: 10.1016/j.jep.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Tabuti JR, Kukunda CB, Kaweesi D, Kasilo OM. Herbal medicine use in the districts of Nakapiripirit, Pallisa, Kanungu, and Mukono in Uganda. J Ethnobiol Ethnomed. 2012;8:35. doi: 10.1186/1746-4269-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith BD. Eastern North America as an independent center of plant domestication. Proc Natl Acad Sci U S A. 2006;103:12223–8. doi: 10.1073/pnas.0604335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bylka W, Kowalewski Z. Flavonoids in Chenopodium album L and Chenopodium opulifolium L. Herba Pol. 1997;43:208–13. [Google Scholar]
- 7.Kokanova-Nediakova Z, Nedialkov PT, Nikolov SD. The genus Chenopodium: Phytochemistry, ethnopharmacology and pharmacology. Pharmacogn Rev. 2009;3:280–306. [Google Scholar]
- 8.Mugisha-Kamatenesi M, Deng AL, Ogendo JO, Omolo EO, Mihale MJ, Otim M, et al. Indigenous knowledge of field insect pests and their management around Lake Victoria basin in Uganda. Afr J Environ Sci Technol. 2008;2:342–8. [Google Scholar]
- 9.Esezah K, Godwin A, Fredrick A, Ogwal-Okeng J. Antifungal medicinal plants used by communities adjacent to Bwindi impenetrable National Park, South Western Uganda. Eur J Med Plants. 2015;7:184–92. [Google Scholar]
- 10.Ruffo CK, Birnie A, Tengnas B. RELMA Technical Handbook Series. Nairobi, Kenya: RELMA; 2002. Edible Wild Plants of Tanzania; p. 27. [Google Scholar]
- 11.Mugisha-Kamatenesi M, Oryem-Origa H, Odyek O. Medicinal plants used in some gynaecological morbidity ailments in Western Uganda. Afr J Ecol. 2007;45:34–40. [Google Scholar]
- 12.Ssegawa P, Kasenene JM. Medicinal plant diversity and uses in the Sango bay area, Southern Uganda. J Ethnopharmacol. 2007;113:521–40. doi: 10.1016/j.jep.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Nalumansi P, Kamatenesi-Mugisha M, Godwin A. Medicinal plants used in Paediatric ealth care in Namungalwe Sub County, Iganga District, Uganda. Nova J Med and Biol Sci. 2014;2:1–14. [Google Scholar]
- 14.Kisangau DP, Lyaruu HV, Hosea KM, Joseph CC. Use of traditional medicines in the management of HIV/AIDS opportunistic infections in Tanzania: A case in the Bukoba rural district. J Ethnobiol Ethnomed. 2007;3:29. doi: 10.1186/1746-4269-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeap SK, Ho WY, Beh BK, Liang WS, Ky H, Yousr AH, et al. Vernonia amygdalina, an ethnoveterinary and ethnomedically used green vegetable with multiple bioactivities. J Med Plants Res. 2010;4:2787–812. [Google Scholar]
- 16.National Institute of Health. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academic Press 1996; 1996. [Google Scholar]
- 17.Zimmermann M. Ethical guidelines for investigations of experimental pain in animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 18.Sofowara A. Ibadan, Nigeria: Spectrum Books Ltd; 1993. Medicine Plants and Traditional Medicine in Africa. [Google Scholar]
- 19.Evans WC. 15th ed. Edinburgh: Habid, W.B Saunders; 2002. Trease and Evans Pharmacognosy. [Google Scholar]
- 20.Benedec D, Vlase L, Hanganu D, Oniga I. Antioxidant potential and polyphenolic content of Romanian Ocimum basilicum. Digest J Nanomat Biostruct. 2012;7:1263–70. [Google Scholar]
- 21.Sultana B, Farooq A, Muhammad A. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–80. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aderogba MA, Okoh EK, Adelanwa TA, Obuotor EM. Antioxidant properties of the Nigerian piliostigma species. J Bio Sci. 2004;4:501–3. [Google Scholar]
- 23.Kumaran A, Karunakaran RJ. Antioxidant and free radical scavenging activity of an aqueous extract of coleus aromaticus. Food Chem. 2006;97:109–14. [Google Scholar]
- 24.Anosike CA, Obidoa O, Ezeanyika LU. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum) DARU J Pharm Sci. 2012;20:76. doi: 10.1186/2008-2231-20-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinde UA, Phadke AS, Nair AM, Mungantiwar AA, Dikshit VJ, Sarsf MN. Membrane stabilization activity - A possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia. 1989;70:251–7. [Google Scholar]
- 26.Adzu B, Haruna AK. Studies on the use of Zizyphus spina-christi against pain in rats and mice. Afr J Biotechnol. 2007;6:1317–24. [Google Scholar]
- 27.Koster R, Anderson M, DeBeer EJ. Acetic acid for analgesic screening. Fed Proc. 1959;18:412. [Google Scholar]
- 28.Turner RA. In: Analgesics: Screening Methods in Pharmacology. Turner RA, Hebban P, editors. New York: Academic Press; 1965. p. 100. [Google Scholar]
- 29.Akah PA, Nwambie IA. Evaluation of Nigerian traditional medicinal plants used for rheumatoid disorders. J Ethnopharamacol. 1994;42:179–82. doi: 10.1016/0378-8741(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 30.Ajayi AM, Tanayen JK, Balogun SO, Ibrahim A, Ezeonwumelu JO, Kiplagat D, et al. Anti-inflammatory and analgesic properties of ethanolic stem bark extract of Ficus trichopoda in rats. Pharmacogn J. 2011;2:43–7. [Google Scholar]
- 31.Dembitsky V, Shkrob I, Hanus LO. Ascaridole and related peroxides from the genus Chenopodium. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152:209–15. doi: 10.5507/bp.2008.032. [DOI] [PubMed] [Google Scholar]
- 32.Ignarro LJ. Effects of anti-inflammatory drugs on the stability of rat liver lysosomes in vitro. Biochem Pharmacol. 1971;20:2847–60. doi: 10.1016/0006-2952(71)90196-1. [DOI] [PubMed] [Google Scholar]
- 33.Oyedapo OO, Akinpelu BA, Akinwunmi KF, Adeyinka MO, Sipeolu FO. Red blood cell membrane stabilizing potentials of extracts of Lantana camara and its fractions. Int J Plant Physiol Biochem. 2010;2:46–51. [Google Scholar]
- 34.Asare GA, Addo P, Bugyei K, Gyan B, Adjei S, Otu-Nyarko LS, et al. Acute toxicity studies of aqueous leaf extract of Phyllanthus niruri. Interdiscip Toxicol. 2011;4:206–10. doi: 10.2478/v10102-011-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NC3RS. National Centre for Replacement, Refinement, and Reduction of Animals in Resaerch. 2007 [Google Scholar]
- 36.Bala A, Kar B, Haldar PK, Mazumder UK, Bera S. Evaluation of anticancer activity of Cleome gynandra on Ehrlich’s Ascites Carcinoma treated mice. J Ethnopharmacol. 2010;129:131–4. doi: 10.1016/j.jep.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Duarte ID, Nakamura M, Ferreira SH. Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz J Med Biol Res. 1988;21:341–3. [PubMed] [Google Scholar]


