Abstract
Background:
Hydrogen peroxide (H2O2) is an oxidant agent and this molecule naturally occurs in the body as a product of aerobic metabolism. Geraniol is a plant-derived natural antioxidant. The aim of this study was to determine the role of geraniol on hepatic fatty acids alterations following H2O2-induced oxidative stress in male rats.
Methods:
After randomization, male Wistar rats were divided into four groups (n = 7 each group). Geraniol (50 mg/kg, dissolved in corn oil) and H2O2 (16 mg/kg, dissolved in distilled water) were administered by an intraperitoneal injection. Administrations were performed during 30 days with 1-day interval.
Results:
Administration of H2O2 resulted with a significant increase in malondialdehyde (MDA) and a significant decrease in glutathione (GSH) peroxidase glutathione level; geraniol restored its effects on liver. However, hepatic catalase (CAT) activities were significantly higher in H2O2, geraniol, and geraniol+H2O2 groups than control group. The ratio of hepatic total saturated fatty acids increased in H2O2-treated animals compared with control. In addition, hepatic total unsaturated fatty acids reduced in H2O2 group compared with control. The percentages of both hepatic total saturated and unsaturated fatty acids were not different between geraniol+H2O2 and control groups.
Conclusions:
H2O2-induced oxidative stress may affect fatty acid composition in liver and body. Geraniol can partly restore oxidative hepatic damage because it cannot completely reverse the H2O2-induced increase in hepatic CAT activities. Moreover, this natural compound can regulate hepatic total saturated and unsaturated fatty acids percentages against H2O2-induced alterations.
KEY WORDS: Fatty acids, geraniol, hydrogen peroxide, liver, oxidative stress
INTRODUCTION
Reactive oxygen species (ROS) in particular hydroxyl and superoxide radicals, and non-radical oxidants such as hydrogen peroxide (H2O2), hypochlorous acid, and peroxynitrite are generated in organism, mainly as an outcome of aerobic metabolism [1,2]. However, the excessive productions of ROS can impair essential cellular components such as nucleic acids, proteins, and polyunsaturated fatty acids [3]. Even some other molecules such as peroxynitrite (ONOO–) and H2O2 are not free radicals; they are accepted to generate free radicals through many biochemical reactions in various cases [4]. In general, all organisms are well protected against free radical damage by endogenous oxidative enzymes such as catalase (CAT) and glutathione (GSH) peroxidase (GSH-Px). Whenever the balance between ROS generation and antioxidant defense is lost, “oxidative stress” results through a series of stages dysregulates the cellular functions leading to several pathophysiological conditions [5,6]. Several nonenzymatic antioxidant compounds such as phenolics, ascorbic acid, tocopherol, and other dietary compounds play an essential role in defending the body against free radical damage by scavenging or neutralizing oxidizing molecules and maintaining redox balance [7].
Recent studies have reported that the plant kingdom offers a wide range of natural antioxidant molecules including phenolic acids, flavonoids, and other secondary metabolites, and they can be useful for the treatment of various disorders [8,9]. Geraniol, a natural acyclic monoterpene, is the primary component of oils of rose and palmarosa [10] and several essential oils such as lemon, ginger, and orange [11]. This natural molecule possesses diverse biological effects, being an antioxidant [12], antibacterial [13], anti-inflammatory [14], and antiangiogenic [15] agent.
The mitochondrial respiratory chain is responsible for the primary source of ROS production in cells because this mechanism consumes about 80-90% of oxygen that a person utilizes and produces most of the ROS generated in the body. Another essential formation of ROS especially occurs in the liver [16]. This study aims to assess a possible protective role of geraniol on liver fatty acids composition in H2O2-induced oxidative stress. For this purpose, in our study biochemical analyses such as liver tissue and serum MDA, hepatic GSH-Px, GSH, and CAT concentrations besides liver and serum fatty acids percentages were evaluated.
MATERIAL AND METHODS
Animals and Experimental Procedure
A total of 28 adult Wistar albino male rats (230 ± 10 g body weight) were obtained from Experimental Research Unit of Firat University (Elazig, Turkey). The animals were housed under standard light/darkness cycle (lights on from 0700 to 1900 h), at a regular temperature (21 ± 1°C) and humidity (55 ± 5%) with free access to fresh water and food. The experimental applications were confirmed by Ethical Committee of Firat University (Document No: 146/2011-11), and the rats were treated in strict compliance with the international laws on the use and care of experimental animals.
Groups of animals were randomly divided into four groups as control, naringenin, lead acetate, and naringenin+lead acetate (n = 7 each group). Control group animals received vehicle solutions only. Geraniol (50 mg/kg, dissolved in corn oil) and H2O2 (16 mg/kg, dissolved in distilled water) were administered by intraperitoneal injection. Administrations were performed during 30 days with 1-day interval. The animals were sacrificed at the end of 30 days. Blood and hepatic tissue were obtained from animals. Serum and liver samples were stored at −20°C until the assays were performed.
H2O2, geraniol and other chemicals were obtained from Sigma (Dorset, UK) unless otherwise indicated.
Determination of Liver and Serum Oxidative Stress-related Parameters
Protein concentration was analyzed using Lowry method [17]. MDA level was measured at 532 nm and expressed as nmol g protein−1 [18]. CAT was measured by determining the decomposition of H2O2 at 240 nm and was expressed as kg protein−1 [19]. GSH-Px was analyzed according to Lawrence and Burk method [20] and was expressed as IU g protein−1. GSH concentrations were determined using the method of Sedlak and Lindsay [21]. All analyzes were performed using a UV-visible spectrophotometer (Shimadzu-2R, Tokyo, Japan).
Lipid Extraction and Measurement of Fatty Acid Percentages
The liver tissue samples (3 g) were homogenized for analyzes. Nonlipid contaminants in the lipid solution were purified through the addition of 0.88% KCl solution. The total lipids were extracted by a mixture of hexane/isopropanol (3:2 v/v) according to the previous method [22]. Fatty acids were converted into methyl esters via adding of 2% sulfuric acid (v/v) in methanol [23]. These methyl esters were separated by a gas chromatography and measured via flame/ionization detection system (Shimadzu GC-17 Ver3, Japan). The chromatography process was performed via capillary column (Machery-Nagel, Germany) using nitrogen as a vehicle gas (flow rate 800 µl/min).
Statistical Analysis
Results were expressed as a mean±standard error of mean. Data were analyzed using one-way analysis of variance followed by post-hoc Tukey’s honestly significant difference (HSD) test (SPSS 12.0 for Windows as a software program). For all analyzes, P < 0.05 was considered statistically significant.
RESULTS
Effects of Geraniol on Hepatic MDA, GSH, GSH-Px, and CAT Activity against H2O2-Treatment
Figure 1 represents the hepatic MDA concentration (nmol g protein−1) of the groups. Administration of H2O2 caused significant increases (P < 0.001) MDA concentrations (23.5 ± 1.3) compared with control (16.3 ± 0.7), whereas its levels were lower in the liver of the geraniol+H2O2 (17.8 ± 1.4) group animals compared with H2O2-treated group. In H2O2-treated animals, liver GSH (Figure 2, nmol g protein−1) and GSH-Px (Figure 3, IU g protein−1) levels (2.9 ± 0.2 and 50.8 ± 6.5, respectively) were significantly lower (P < 0.001 and P < 0.01, respectively) than control group (3.5 ± 0.1 and 63.4 ± 7.7, respectively). These values were significantly increased following coadministration of geraniol with H2O2 in geraniol+H2O2 group (3.7 ± 0.2 and 77.9 ± 8.6, respectively) rats compared with H2O2 alone-treated animals (P < 0.001). Liver CAT activity (Figure 4, kg protein−1) increased in the geraniol (113.9 ± 9.2), H2O2 (120.9 ± 9.4) and geraniol plus H2O2 (115.2 ± 10.4) groups compared with control (59.9 ± 5.5) group (P < 0.001). In geraniol+H2O2 group animals, CAT levels were generally lower than the H2O2 but this effect did not differ between groups.
Figure 1.
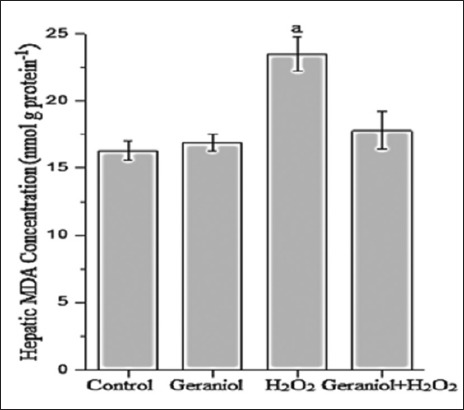
Hepatic malondialdehyde concentration of the groups. aP<0.001 vs. control group, (one-way analysis of variance followed by post-hoc Tukey’s honestly significant difference test), n = 7 for each group
Figure 2.
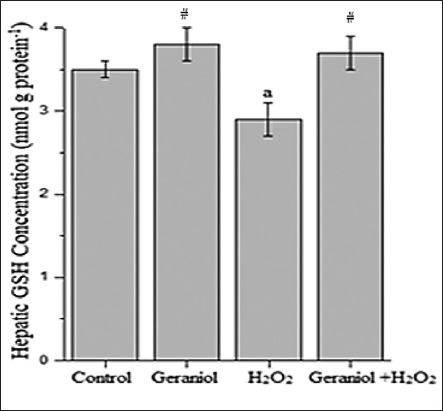
Hepatic glutathione concentration of the groups. aP<0.001 vs. control and #P<0.001 vs. H2O2-treated group, (one-way analysis of variance followed by post-hoc Tukey’s honestly significant difference test), n = 7 for each group
Figure 3.
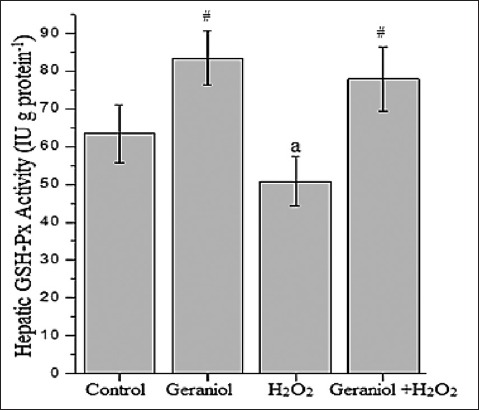
Hepatic glutathione-Px activity of the groups. aP<0.01 vs. control #P<0.001 vs. H2O2-treated group, (one-way analysis of variance followed by post-hoc Tukey’s honestly significant difference test), n=7 for each group
Figure 4.
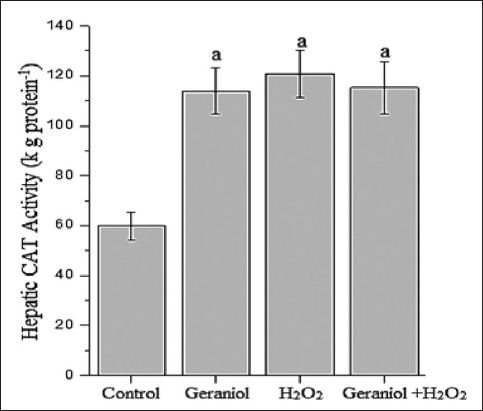
Hepatic catalase activity of the groups. aP<0.001 vs. control group, (one-way analysis of variance followed by post-hoc Tukey’s honestly significant difference test), n=7 for each group
Effect of Geraniol on Serum MDA Concentration against H2O2-Treatment
We examined that effect of geraniol on the liver as well as body oxidant status in rats’ serum after H2O2 treatment (Figure 5). There was no statistical difference between geraniol plus H2O2 group (8.5 ± 0.4) and control (7.2 ± 0.3) in MDA levels (nmol g protein−1). We found that there was a significant increase in serum MDA level of H2O2-treated group (12.8 ± 0.5) when compared with control animals (P < 0.001). However, MDA concentrations were found to be lower in geraniol plus H2O2-treated animals compared with H2O2 group.
Figure 5.
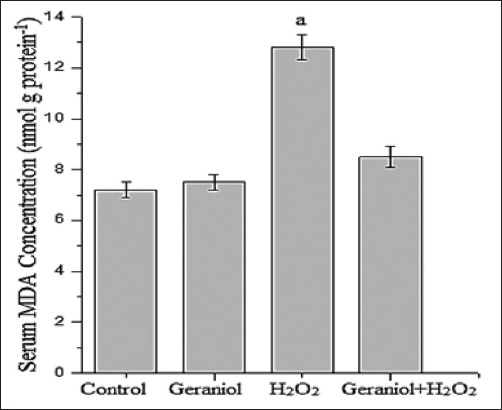
Serum malondialdehyde concentration of the groups. aP<0.001 vs. control group, (one-way analysis of variance followed by post-hoc Tukey’s honestly significant difference test), n=7 for each group
Effect of Geraniol on Hepatic Fatty Acid Percentages against H2O2-Treatment
The values regarding effects of geraniol and H2O2 on the total and individual fatty acids were summarized in Table 1. The differences in mean total saturated fatty acids (∑SFA) were detected between H2O2 and control groups (P < 0.05). There was no a significant alteration in ∑SFA percentage of control group compared to geraniol alone and geraniol plus H2O2 groups. The ratio of total unsaturated FA (∑USFA) was significantly lower in H2O2-treated animals compared to control group (P < 0.05). Although it was not statistically significant, the monounsaturated fatty acids (MUFA) levels of H2O2 and control groups, the liver MUFA concentration was found to be lower in the geraniol and geraniol plus H2O2 groups compared to control (P < 0.001 and P < 0.01, respectively).
Table 1.
The percentages of fatty acids in liver
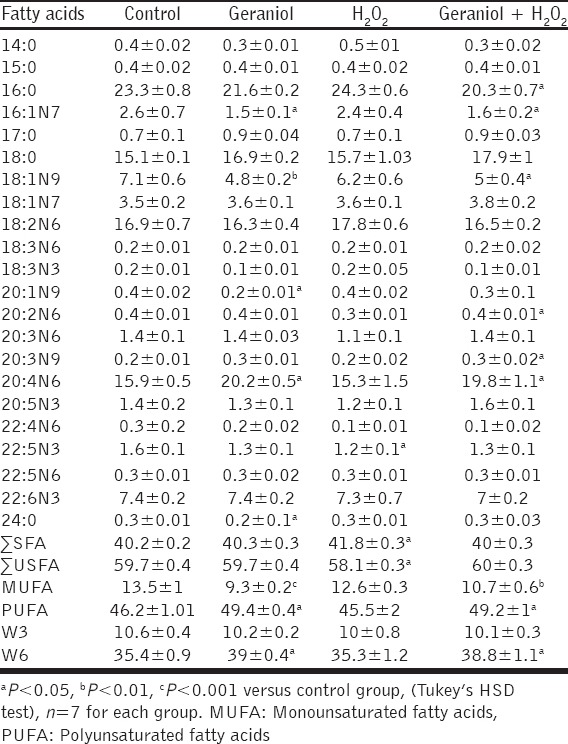
The significant difference in the mean polyunsaturated fatty acids (PUFA) was detected in geraniol and geraniol+H2O2 groups compared with control group (P < 0.05). Although not statistically significant, the PUFA levels of H2O2 and control groups, its liver ratio was found to be higher in the geraniol and geraniol plus H2O2 groups compared with control group animals (P < 0.05). No overt differences in mean W3 percentages were observed among groups. There was no statistical difference between H2O2 and control group in the W6 levels. However, this FA percentage was found to be higher in geraniol and geraniol+H2O2 groups compared to control (P < 0.05).
Effect of Geraniol on Serum Fatty Acid Percentages against H2O2-Treatment
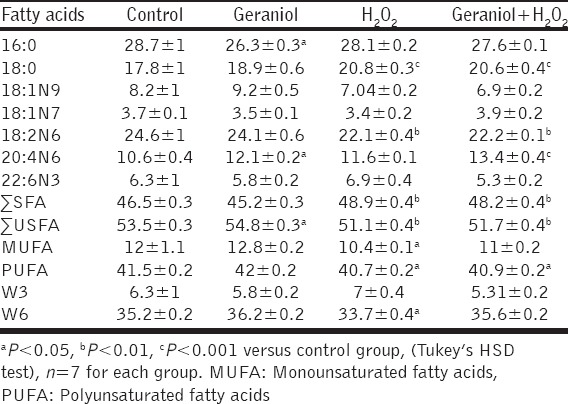
The differences in mean 18:0 percentages were detected in alone H2O2-treated and geraniol plus H2O2 groups compared to control rats (P < 0.001). No overt differences in this FA percentage were observed between control and geraniol-treated groups. There was a significant difference in H2O2 and geraniol+H2O2 groups compared to control animals in the mean 18:2n6 ratio (P < 0.01). The differences of these FA levels were not detected between geraniol and control groups.
In the rats administered geraniol, ∑SFA level was similar to control rats. However, the ∑SFA concentration was significantly affected by H2O2 and geraniol plus H2O2 treatment compared to control animals (P < 0.01). Serum ∑USFA level was significantly higher in the geraniol-treated animals compared to control rats (P < 0.05). ∑USFA concentration was found to be lower in the H2O2 and geraniol+H2O2 groups compared to control animals (P < 0.01). Although not statistically significant, MUFA level was determined to be higher in the geraniol-treated animals compared to control animals. However, the concentrations of MUFA were significantly lower in H2O2 and geraniol+H2O2 groups compared to control animals (P < 0.05). Serum PUFA level was significantly lower in H2O2 and geraniol+H2O2-treated groups compared with control rats (P < 0.05). Although not statistically significant, PUFA percentage was determined to be higher in the geraniol-treated animals compared to control group. Administration of H2O2 decreased serum w6 level compared to control rats (P < 0.05). However, the W6 percentages of the geraniol+H2O2 group were similar to the control group. The values regarding effects of geraniol and H2O2 on the total and individual fatty acids in the serum were summarized in Table 2.
Table 2.
The percentages of fatty acids in serum
DISCUSSION
The study provides an argument for the protective role of geraniol on changes of liver and serum oxidative stress related molecules, enzymes, and fatty acids in rats with H2O2-induced hepatic damage. Intraperitoneal administrations of both geraniol and H2O2 affected antioxidant status in the liver and serum of rats. While H2O2 increased MDA levels, geraniol restored its effects on the liver and serum lipid peroxidation in male rats. Treatment of geraniol to animals inhibited H2O2-induced decrease of GSH and GSH-Px concentrations in the liver. However, geraniol did not reverse the H2O2-induced increase in CAT activity completely.
Lipid peroxidation is an important toxic pathway because it involves the removal of hydrogen from fatty acid chains mediated by ROS [24,25] this way can lead to cell death in the body. The endogenous antioxidant enzyme includes GSH-Px that catalyzes the reduction of H2O2 to water through the oxidation of reduced GSH. CAT also participated in this conversion [26]. It was reported that oral administration of geraniol to 7,12-dimethylbenz(a)anthracene (DMBA)-treated mice significantly increased the activities of enzymatic antioxidants (CAT and GSH-Px) and non-enzymatic antioxidant (GSH) level in red blood cells, and skin tissues. Moreover, these parameters did not differ between geraniol alone treated and control animals [27]. Ibrahim et al. noticed [28] that 30 days administration of geraniol restores effects of the fructose-induced metabolic syndrome on hepatic and serum lipid peroxides related parameters in rats.
Hepatic nitric oxide (NO) content elevates in the fructose-induced model, possibly because of the increased synthesis of inducible NO-synthase activated by NF-κB [29,30]. Moreover, this mechanism is related with the increased lipid peroxidation and the decreased non-protein thiols (NPSH). Geraniol can suppress liver NO and lipid peroxides and enriches NPSH [28] via the activation of both GSH-Px and other reductase enzymes [31]. These reports that are related to hepatic lipid peroxidation and GSH-Px are consistent with our data. However, in this study, we observed that geraniol was not completely able to restore H2O2-induced increase in the hepatic CAT activity. Andrade et al. reported [32] that the inhalation of geraniol was increased serum alanine aminotransferase activity and hepatic lipid hydroperoxide in rats. Moreover, rats exposed to geraniol had higher CAT, SOD, and GSH-Px activities. The authors suggested that the lipoperoxide generation could be a result of ineffective antioxidant enzyme activities because these reactions were not sufficient to inhibit the ROS action and the lipoperoxide production in the liver of these animals. Koek et al. reported [33] that the activity of antioxidants enzymes increases early stage in the nonalcoholic steatohepatitis, but these alterations tend to decrease with progression of hepatic pathogenesis. The high concentration of H2O2 can be related with high activity of GSH-Px and CAT because these enzymes play an essential role in the elimination of H2O2. CAT is the most efficient enzyme in this interaction; also, it is so efficient that it cannot be saturated with H2O2 at any concentration [34]. Therefore, we can suggest that geraniol has an antioxidant role, but this effect is not full capacity on the H2O2-induced oxidative pathway in the liver of male rats.
Geraniol has anticancer efficacy that may be related to the inhibition of HMG-CoA reductase [35]. The latter role also confirms geraniol antiatherogenic effect, recently Jayachandran et al. suggested [14] that this natural monoterpene can ameliorate fructose-induced obesity and dyslipidemia in hamsters [28]. In 2011, it was reported [36] that geraniol activates in vitro several peroxisome proliferator-activated receptor (PPAR) subspecies. The interaction with geraniol and PPAR nuclear receptors that regulate the expression of target genes involved in lipid metabolism [37] is an important result because this data may provide a new treatment option for metabolic disorders such as hyperlipidemia, obesity, and diabetes. During the recent years, it was suggested that the antiatherogenic effect of geraniol is related to the activation of lipoprotein lipase to inhibit serum triglycerides, as well as lecithin cholesterol acyl transferase to elevate high-density lipoprotein cholesterol [14,28]. In this study, we first observed that the use of geraniol decreased high liver ∑SFA level caused by H2O2-treatment. Moreover, this monoterpene can protect the reduction of hepatic ∑USFA level induced by H2O2. Similar results were partly obtained for serum ∑SFA and ∑USFA levels.
As regards the individual fatty acids, the hepatic levels of palmitoleic acid, oleic acid, and eicosenoic acid, which are the member of MUFA, were affected by geraniol treatment. The percentages of hepatic oleic acid, palmitoleic acid, and eicosenoic acid decreased in the geraniol group and partly geraniol plus H2O2 groups. Therefore, the percentage of total MUFA decreased these groups. Interestingly, serum MUFA percentage did not differ between geraniol and control, while it decreased in H2O2 and geraniol plus H2O2 groups. Although not statistically significant, the percentage of hepatic arachidonic acid (20:4n6), which is a member of PUFA and is a partly essential fatty acid, was determined to be lower in the H2O2-treated rats, its level significantly increased in H2O2 and geraniol+H2O2 groups. In humans, levels of MUFA, such as oleic acid, are increased to the age of 18 years [38]. Several other PUFA particularly arachidonic acids are also decreased with age in the older persons [38]. Therefore, it can be said that these fatty acids change with metabolic and environmental factors over the years. Alterations in fatty acid composition of cells and its membrane are known to influence the activity of G proteins and protein kinase C (PKC) [39]. PKC and G proteins play a modulatory role in the regulating blood pressure [40]. Recently, it was reported that this natural compound can ameliorate the elevated systolic blood pressure against the fructose-induced metabolic syndrome [28]. Oleic acid is converted into nitrated-oleic acid in the presence of NO. The molecular mechanisms associated with physiologic roles of nitrated oleic acid remain unknown. It was suggested [41] that nitrated oleic exists in the blood and several organs, where it promotes vessel relaxation and modulation of immune system cells. However, we did not determine whether geraniol and H2O2 affected blood pressure or NO-related metabolic parameters. Interestingly, although it was not statistically significant, oleic acid serum percentage was found to be higher in the geraniol-injected rats and lower in H2O2 and geraniol plus H2O2 groups. Otherwise, this fatty acid decreased in the liver of geraniol-treated group. Therefore, it can be said that the oleic acid and related fatty acids may have tissue dependent specific roles.
In this study, we observed that H2O2 causes oxidative stress in the liver and body; this effect can be restored partly through geraniol administration because it cannot reverse the H2O2-induced increase in CAT enzyme completely. In addition, this natural compound can be regulated in the liver several fatty acids percentage such as ∑SFA and ∑USFA levels against H2O2-induced alteration. However, geraniol has a distinct effect on other fatty acids like individual and total levels; this situation can be related to its other metabolic effects and interactions.
ACKNOWLEDGMENTS
We thank Dr. Z. Isik Solak Gormus for technical assistance and reviewing the use of English in the manuscript. This research was supported by the Scientific Research Projects Unit of Adiyaman University (ADYUBAP) project, number FEFBAP/2012-0001.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Halliwell B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet. 1994;344:721–4. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 2.Poulsen HE, Prieme H, Loft S. Role of oxidative DNA damage in cancer initiation and promotion. Eur J Cancer Prev. 1998;7:9–16. [PubMed] [Google Scholar]
- 3.Bandyopadhyay U, Das D, Banerjee RK. Reactive oxygen species: Oxidative damage and pathogenesis. Curr Sci. 1999;77:658–66. [Google Scholar]
- 4.Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40:959–75. doi: 10.1016/s0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- 5.Pillai CK, Pillai KS. Antioxidants in health. Indian J Physiol Pharmacol. 2002;46:1–5. [PubMed] [Google Scholar]
- 6.Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984;222:1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutteridge JM, Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci. 2000;899:136–47. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 8.Dai J, Mumper RJ. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–52. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira DM, Valentão P, Pereira JA, Andrade PB. Phenolics: From chemistry to biology. Molecules. 2009;14:2202–11. [Google Scholar]
- 10.Khan AQ, Khan R, Qamar W, Lateef A, Rehman MU, Tahir M, et al. Geraniol attenuates 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced oxidative stress and inflammation in mouse skin: Possible role of p38 MAP Kinase and NF-?B. Exp Mol Pathol. 2013;94:419–29. doi: 10.1016/j.yexmp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Vieira A, Heidor R, Cardozo MT, Scolastici C, Purgatto E, Shiga TM, et al. Efficacy of geraniol but not of ß-ionone or their combination for the chemoprevention of rat colon carcinogenesis. Braz J Med Biol Res. 2011;44:538–45. doi: 10.1590/s0100-879x2011007500037. [DOI] [PubMed] [Google Scholar]
- 12.Tiwari M, Kakkar P. Plant derived antioxidants - Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol In Vitro. 2009;23:295–301. doi: 10.1016/j.tiv.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Bard M, Albrecht MR, Gupta N, Guynn CJ, Stillwell W. Geraniol interferes with membrane functions in strains of Candida and Saccharomyces. Lipids. 1988;23:534–8. doi: 10.1007/BF02535593. [DOI] [PubMed] [Google Scholar]
- 14.Jayachandran M, Chandrasekaran B, Namasivayam N. Effect of geraniol, a plant derived monoterpene on lipids and lipid metabolizing enzymes in experimental hyperlipidemic hamsters. Mol Cell Biochem. 2015;398:39–53. doi: 10.1007/s11010-014-2203-3. [DOI] [PubMed] [Google Scholar]
- 15.Wittig C, Scheuer C, Parakenings J, Menger MD, Laschke MW. Geraniol suppresses angiogenesis by downregulating vascular endothelial growth factor (VEGF)/VEGFR-2 signaling. PLoS One. 2015;10:e0131946. doi: 10.1371/journal.pone.0131946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proulx M, du Souich P. Inflammation-induced decrease in hepatic cytochrome P450 in conscious rabbits is accompanied by an increase in hepatic oxidative stress. Res Commun Mol Pathol Pharmacol. 1995;87:221–36. [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 18.Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966;16:359–64. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- 19.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71:952–8. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 21.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 22.Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem. 1978;90:420–6. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- 23.Chistie WW. Gas Chromatography and Lipids (Reprinted) Glasgow: The Oily Press; 1990. pp. 1–184. [Google Scholar]
- 24.Abuja PM, Albertini R. Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin Chim Acta. 2001;306:1–17. doi: 10.1016/s0009-8981(01)00393-x. [DOI] [PubMed] [Google Scholar]
- 25.Faine LA, Rodrigues HG, Galhardi CM, Ebaid GM, Diniz YS, Padovani CR, et al. Effects of olive oil and its minor constituents on serum lipids, oxidative stress, and energy metabolism in cardiac muscle. Can J Physiol Pharmacol. 2006;84:239–45. doi: 10.1139/y05-124. [DOI] [PubMed] [Google Scholar]
- 26.Preiser JC. Oxidative stress. JPEN J Parenter Enteral Nutr. 2012;36:147–54. doi: 10.1177/0148607111434963. [DOI] [PubMed] [Google Scholar]
- 27.Manoharan S, Selvan MV. Chemopreventive potential of geraniol in 7, 12-dimethylbenz (a) anthracene (DMBA) induced skin carcinogenesis in Swiss albino mice. J Environ Biol. 2012;33:255–60. [PubMed] [Google Scholar]
- 28.Ibrahim SM, El-Denshary ES, Abdallah DM. Geraniol, alone and in combination with pioglitazone, ameliorates fructose-induced metabolic syndrome in rats via the modulation of both inflammatory and oxidative stress status. PLoS One. 2015;10:e0117516. doi: 10.1371/journal.pone.0117516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spruss A, Kanuri G, Uebel K, Bischoff SC, Bergheim I. Role of the inducible nitric oxide synthase in the onset of fructose-induced steatosis in mice. Antioxid Redox Signal. 2011;14:2121–35. doi: 10.1089/ars.2010.3263. [DOI] [PubMed] [Google Scholar]
- 30.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–8. [PubMed] [Google Scholar]
- 31.Singh BK, Tripathi M, Chaudhari BP, Pandey PK, Kakkar P. Natural terpenes prevent mitochondrial dysfunction, oxidative stress and release of apoptotic proteins during nimesulide-hepatotoxicity in rats. PLoS One. 2012;7:e34200. doi: 10.1371/journal.pone.0034200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrade BF, Braga CP, Dos Santos KC, Barbosa LN, Rall VL, Sforcin JM, et al. Effect of inhaling Cymbopogon martinii essential oil and geraniol on serum biochemistry parameters and oxidative stress in rats. Biochem Res Int 2014. 2014:493183. doi: 10.1155/2014/493183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koek GH, Liedorp PR, Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin Chim Acta. 2011;412:1297–305. doi: 10.1016/j.cca.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Spolarics Z, Wu JX. Role of glutathione and catalase in H2O2 detoxification in LPS-activated hepatic endothelial and Kupffer cells. Am J Physiol. 1997;273:G1304–11. doi: 10.1152/ajpgi.1997.273.6.G1304. [DOI] [PubMed] [Google Scholar]
- 35.Carnesecchi S, Bradaia A, Fischer B, Coelho D, Schöller-Guinard M, Gosse F, et al. Perturbation by geraniol of cell membrane permeability and signal transduction pathways in human colon cancer cells. J Pharmacol Exp Ther. 2002;303:711–5. doi: 10.1124/jpet.102.039263. [DOI] [PubMed] [Google Scholar]
- 36.Katsukawa M, Nakata R, Koeji S, Hori K, Takahashi S, Inoue H. Citronellol and geraniol, components of rose oil, activate peroxisome proliferator-activated receptor a and ? And suppress cyclooxygenase-2 expression. Biosci Biotechnol Biochem. 2011;75:1010–2. doi: 10.1271/bbb.110039. [DOI] [PubMed] [Google Scholar]
- 37.Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 38.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 39.EscribáPV Ozaita A, Ribas C, Miralles A, Fodor E, Farkas T, et al. Role of lipid polymorphism in G protein-membrane interactions: Nonlamellar-prone phospholipids and peripheral protein binding to membranes. Proc Natl Acad Sci U S A. 1997;94:11375–80. doi: 10.1073/pnas.94.21.11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Escribá PV, Sánchez-Dominguez JM, Alemany R, Perona JS, Ruiz-Gutiérrez V. Alteration of lipids, G proteins, and PKC in cell membranes of elderly hypertensives. Hypertension. 2003;41:176–82. doi: 10.1161/01.hyp.0000047647.72162.a8. [DOI] [PubMed] [Google Scholar]
- 41.Trostchansky A, Rubbo H. Nitrated fatty acids: Mechanisms of formation, chemical characterization, and biological properties. Free Radic Biol Med. 2008;44:1887–96. doi: 10.1016/j.freeradbiomed.2008.03.006. [DOI] [PubMed] [Google Scholar]


