Abstract
Background:
An unceasing threat of resistance of malarial parasites to available antimalarial drugs makes the development of new drugs imperative. Natural plant-based products are an alternative source for discovering new antimalarial drugs.
Aim:
To determine the prophylactic efficacy of a traditionally used plant-based drug on prevention of malaria in endemic villages of Odisha, India.
Methods:
A total of 267 healthy human volunteers of both sexes, aged 18-60 years were enrolled in Odisha, India, to receive either minimum 20 doses of aqueous extract of Traditional Plant-based Malaria Prophylactic drug 74, twice a week (experimental group), or no drug (control group) for 14 weeks. The primary criterion was the occurrence of malaria positive cases confirmed through expert microscopy during the study period. Analyses were by per-protocol (PP) and modified intention-to-treat (mITT).
Results:
A significant (P < 0.01) reduction (64%) of malaria incidence was observed in the experimental group compared to control group, 12.3% and 26.6%, respectively, as PP analysis. However, the reduction was nonsignificant as per mITT analysis (P = 0.22). The experimental group showed a relative risk of 0.36 compared to control group.
Conclusion:
This preliminary study constitutes a potential “proof of concept” for the development of malaria prophylactic drug and provide a scientific basis for the use of traditional remedy as a malaria preventive by tribal populations in India.
KEY WORDS: Herbal prophylaxis, India, malaria, malaria-endemic population, traditional medicine
INTRODUCTION
Malaria, a disease caused by the parasite of the genus Plasmodium, remains major public health concerns in tropical and subtropical countries. 214 million cases of malaria and over 438,000 deaths occurred globally in 2015 [1]. 58% of the malaria deaths occurred in the poorest 20% of the world’s population, and these patients receive only poor quality care and have catastrophic economic consequences from their illness [2].
In India, malaria is one of the most common infectious diseases among tribal population with 22.6% of clinical episodes due to Plasmodium falciparum and 42% of episodes due to Plasmodium vivax globally [3,4]. In Odisha, eastern state of India, malaria is the leading cause of mortality and morbidity in the tribal population [5]. In many other states of India, malaria transmission intensity varies with season with high transmission after the monsoon rains in autumn and winter. In such conditions, a suitable control strategy implemented during these seasons may have a good impact on the reduction of malaria burden.
Malaria infection can be prevented using (anti-liver stage) chemoprophylaxis. However, most of the available drugs are confronted with the evolution of drug resistance [6-8]. On the other hand, the safety is a major concern in chemoprophylaxis. For instance, primaquine, atovaquone, and doxycycline are contraindicated in pregnant women and children. Moreover, many of the drugs are not affordable and inaccessible to the needy populations.
As in almost all tropical endemic countries, malaria in India affects particularly people living in rural, remote areas, where most often affordable modern drugs are not available and where poor health-care infrastructure cannot assure prompt and appropriate treatment. The use of herbal medicinal plants especially those used in traditional medicine for the prevention of malaria is common in population of malaria endemic areas, among these, the use of Traditional Plant-based Malaria Prophylactic 74 (TPMP74) [9].
TPMP74 is a coded polyherbal traditional remedy, widely used in the form of aqueous extract (decoction), and all the plants are well recognized for its medicinal properties for a long time in Ayurveda. Each plant and aqueous extract of TPMP74 have been standardized through organoleptic, physicochemical, phytochemical, microbial, heavy metal, pesticide residue, and high-performance thin layer chromatography analysis [10].
Furthermore, it has also been demonstrated that TPMP74 possesses an inhibitory effect on the Plasmodium yoelii hepatic stages in vitro (50% inhibitory concentration = 0.74 mg/mL) with a therapeutic index of 9.54 and in mice treated with 2000 mg/kg/day, peak parasitemia reduction by 81% in the experimental 2.35% ± 0.14% as compared to controls 12.62% ± 0.52% (P < 0.001) [11].
Thus, TPMP74 which is considered as an important malaria prophylactic remedy needs to be further studied for its efficacy in human volunteers. The aim of this prospective comparative field study was to evaluate the efficacy of TPMP74 aqueous extract on lowering the occurrence of malaria in study population of malaria-endemic villages of Odisha, India.
MATERIALS AND METHODS
Ethical Issues
The protocol was approved by the Institutional Ethics Committee of the Foundation for Revitalization of Local Health Traditions (FRLHT), Bengaluru (currently ITD-HST) (Protocol number: 05 TPPM-K). Written informed consent was taken from all the study participants. They were free to withdraw from the study at any time. Volunteers were followed closely and were given conventional treatment if necessary. The study was registered in Clinical Trial Registry - India (CTRI) and trial registration number is CTRI/2014/05/004610.
Settings
A prospective comparative field study was conducted in two rural villages (Tunpar and Kellar) of Koraput district (17° 50’N and 20° 30’N, 81° 27’ and 84° 10’ E) of Odisha state, India, during the period of high malaria transmission (June to December 2009). The two villages were situated within a radius of 3 km having similar climate, rainfall, and socioeconomic status [12] and had higher malaria incidences in the year 2007 and 2008 (unpublished data - Laxmipur PHC, Koraput district). The annual parasite incidence (API; cases/1000 persons/year) of Koraput district is 20.3 with 97% of P. falciparum cases [13,14]. The entomological and epidemiological parameters in these two villages were similar in intensity for malaria transmission (unpublished data-malaria baseline report, FRLHT, 2009).
Design
The study was designed as a prospective comparative study of volunteers recruited from Tunpar village who served as the experimental group to receive traditional prophylactic herbal remedy with those similarly recruited from Kellar village to serve as the control group given no specific malaria preventive medication. Owing to the very different nature of the intervention, it was not possible to blind the volunteers to the intervention being taken or to randomize the volunteers in the same village into two groups.
Participants
Before the start of the study, meetings were held in both the study villages to explain the purpose, methods of the study, and to answer their questions. People who were interested to participate in the study as volunteer were screened by medical examination and blood smears. Inclusion criteria for the study were (1) age 18-60 years, (2) having no chronic illness or symptomatic malaria, (3) having no parasites in the blood smear, and (4) healthy, without any co-morbid conditions. Noninclusion criteria were pregnancy, lactating mothers, those taking medication for any other complaints, and those unable or unwilling to provide written informed consent.
Intervention
TPMP74 aqueous extract (decoction) was freshly prepared on the day of administration by harvesting plant parts from the preidentified sources in the study area. The trained village health workers (VHWs) prepared the decoction in a central place (Government primary school, Tunpar) of the village. 45 mL of lukewarm decoction was administered twice a week (Tuesday and Friday) during the early evening (on an empty stomach) for 14 weeks to the participants of the experimental group and compliance on consumption of decoction was observed by the VHWs. The whole procedure was monitored by the study investigator. Samples of freshly prepared decoctions were analyzed to ensure the total dissolved solids of 16.32 ± 0.03% w/v, which confirmed the quality of decoction administered.
A data documentation system for the administration of decoction was devised and used by trained VHWs. This consisted of health card held by each volunteer and an attendance register held by the VHWs to record compliance and adherence to the study protocol. This health card was labeled with the volunteers’ number, name, and a table to record clinical findings on the day of administration of decoction.
Follow-up
Volunteers of both groups were actively and passively followed during the study period. The active follow-up was done biweekly and consisted of questioning the volunteers for the occurrence of symptoms consistent with malaria including fever, headache, body ache, and malaise. The passive follow-up was done through continuous availability of VHWs and auxiliary nurse midwife in the village, to evaluate any medical complaint at any time during the study period. Any volunteer, who complained of symptom potentially related to malaria, was given a complete medical check-up and laboratory assessment through blood smear examination. The positively diagnosed malaria cases were treated as per the standard guidelines of National Institute of Malaria Research, Government of India [15]. Apart from this clinical follow-up, all the volunteers were screened for malaria infection by blood smear examination at the end of the study. As per the study protocol, experimental group participants, who have received a minimum of 20 doses of TPMP74 out of the total of 28 doses in the study, were considered for statistical analysis. Volunteers were also monitored for intake of any antimalarial drugs as well as other drugs during the study period.
Laboratory Methods
Blood films were stained with Giemsa for 30 min, and parasite counts were done using standard WHO criteria [16].
Outcome Measures
The outcome of the study was based on the incidence of malaria as measured by laboratory diagnosis (blood smear) in both the experimental and control groups.
Statistical Analysis
Research pro forma was developed to enter baseline details, laboratory findings and follow-up details including regular consumption of the decoction. Every detail was entered by the VHWs and checked by investigators. All data were entered into an Excel sheet using MS Excel. Descriptive statistics, proportions, rates, and protective efficacies along with the required standard deviation and confidence intervals, Chi-square tests, per-protocol (PP) analysis and modified intention-to-treat (mITT) analysis were done using SPSS 18.0 (SPSS Inc., Chicago, USA). Since these were prospective cohort groups, relative risk was computed taking the incidence rate and the 95% corresponding confidence interval of malaria in experimental and control groups.
RESULTS
Participants
A total of 578 volunteers were enrolled in this study, 292 (male 142, female 150) in the experimental group and 286 (male 143, female 143) in the control group. Of the 292 volunteers enrolled in the experimental group, 149 (51%) did not complete the minimum 20 doses and 30 volunteers (10.2%) did not complete follow-up. Of the 286 volunteers enrolled in the control group, 132 (46.1%) did not complete follow-up. The main reason for loss to follow-up during the study period was migration of volunteers to another location as these are tribal people depend on daily wages. Thus, there were a total of 113 volunteers in the experimental group and 154 volunteers in the control group who completed the follow-up [Figure 1].
Figure 1.
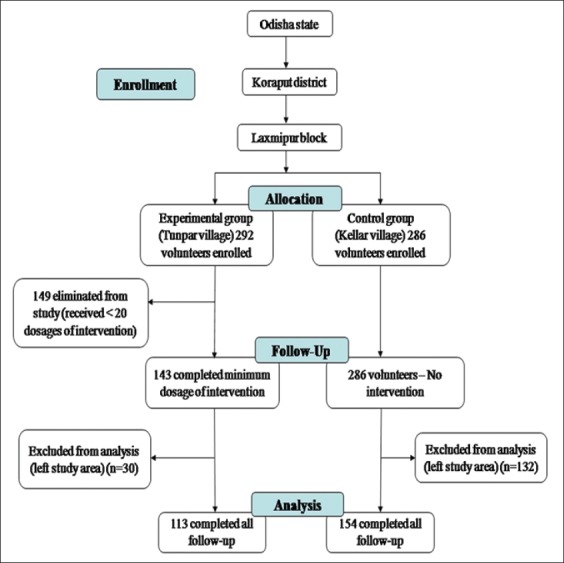
Village trial flow chart
Baseline Characteristics
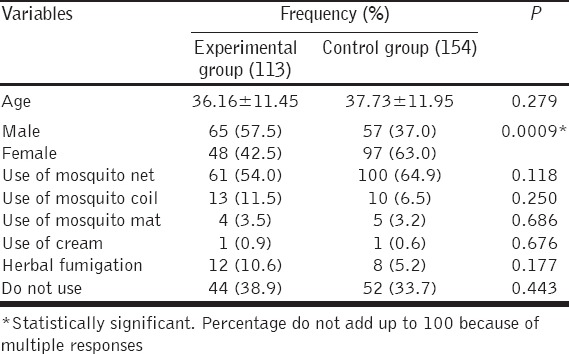
Baseline demographic and characteristics of participants in experimental and control groups are shown in Table 1. There was no statistically significant difference in age and use of malaria preventive measures between the two groups except for the gender. This shows that the volunteers of study and control groups have similar characteristics.
Table 1.
Baseline demographic and characteristics of participants in experimental and control groups
Impact of the Study on Malaria Incidence
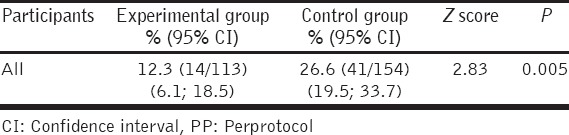
The incidence of microscopically confirmed malaria in the two groups is presented in Table 2. PP analysis demonstrated that overall the incidence of malaria during the entire malaria endemic season was 14 among the 113 of the experimental group (incidence rate = 12.3%) and 41 among the 154 of the control group (incidence rate = 26.6%). The difference is statistically significant (P = 0.005). The experimental group showed a relative risk of 0.36, compared to the control group meaning that the risk to get malaria in the control group was 2.8 times higher than in the experimental one. However, mITT analysis was not significant (P = 0.22), where specific minimum standard criteria were minimum of 20 dosages during the 14 weeks of administration of TPMP74. However, due to the limited sample sizes, the power of the test was too low to be conclusive (28.9%). Hence, a higher number of volunteers could demonstrate a significant difference with a mITT analysis. One volunteer in the experimental group and two volunteers in the control group had P. vivax infection, whereas the remainder had P. falciparum malaria. However, there was no statistically significant difference in age, gender and use of malaria preventive measures among the volunteers who were positively diagnosed for malaria.
Table 2.
Incidence rate of malaria in experimental and control group (in PP analysis)
DISCUSSION
In this study, we wished to ascertain whether a plant-based preparation used by traditional healers in Odisha, India, as malaria preventive remedy has a demonstrable prophylactic potential. The selection of one over others traditional medicines was based on its perceived value to prevent, rather than to treat the malaria infection because drugs capable of providing prophylactic cover by inhibiting the liver stages have some advantages over other chemotherapeutic drugs [6].
We opted first to attempt to gather field evidence showing that the remedy as traditionally administered does indeed have an effect on the acquisition of malaria. A similar approach had been adopted in other studies [17,18]. In this field study, healthy volunteers were selected from two villages. For practical reasons (i.e.: No placebo remedy), it was difficult to recruit volunteers in the same village to serve as an experimental and a control group. Thus, volunteers from one village were the experimental group to whom the remedy was administered and those from the other served as the control group. The two villages were carefully selected to have similar malaria epidemiology. We are aware that such a study has limitations, principally a high attrition rate due to the migration of volunteers out of the village for economical reasons, and because of difficulties of some to adhere to the 28 (minimum 20) doses that needed to be administered during the study period. Nonetheless, we obtained clear indication that the remedy reduced the number of recorded microscopically confirmed malaria episodes in residents of malaria-endemic villages with constrained resources (a risk reduction of 64% in the PP analysis).
Intermittent preventive treatment (IPT) is a common approach followed in malaria transmission season to protect against malaria in endemic areas. Dicko et al. in Mali found a reduction of 42.5% in annual incidence of clinical malaria when IPT with sulfadoxine-pyrimethamine is given at 8-week intervals in children targeting the transmission season [19]. Several studies have shown that IPT given to pregnant women, children and infants has reduced the incidence of clinical episodes of malaria by 20-60% depending on the transmission duration and seasonality [20-22].
Unlike the use of other malaria control strategies such as use of insecticide-impregnated material and IPT that requires daily implementation and external agency, whereas this traditional herbal usage strategy requires only training on the use of locally available herbs with standard preparation procedures. This simple strategy can be delivered through VHWs, local nongovernmental and governmental organizations. These herbal remedies may be affordable and accessible to the endemic population and can contribute to reducing the malaria burden in the communities, particularly in rural settings where health services are insufficient and inaccessible. It is also essential to achieve the cultural and social acceptance by the communities for a successful implementation of any malaria control strategy. As TPMP74 is a local health traditional practice by healers of their own community, it is quite easy for their acceptance.
Usually, the search for lead compounds from ethnopharmacological preparation is initiated on individual fractions or compounds isolated from each plant component from which it is prepared. However, traditional healers often use polyherbal preparations in the belief that not only each herb has a specific beneficiary role but also that the combination of these effects is important. The underlying principle is that plants combination may be synergistic or may potentiate the action of the individual plants [23]. To capture this, we opted to assess the first whether the traditional remedy, as administered to subjects, has an impact on malarial infections. Indications of a positive effect from such a study would then justify testing the same preparation under in vivo and in vitro experimental settings.
Earlier clinical observational studies on TPMP74 have shown that the 45 mL of lukewarm decoction is safe and tolerable in healthy human participants [24]. The dose, dosage (twice a week), form of medicine, and duration of intervention were followed as per the recommendations of traditional healers [9]. In this study, the participants of experimental group were closely monitored for safety and any adverse events. None of the participants showed any serious adverse event except 10 volunteers who developed on the 3rd dosage mild but self-limiting gastric irritation and nausea.
The prophylactic activity observed in villagers was mirrored in that observed when the same formulation of the remedy was assayed in vivo in mice challenged by P. yoelii yoelii sporozoites inoculation. In addition to a 3-fold reduction in peak parasitemia in treated as compared to control mice, a 1-day delay was observed for the 1st day of patency in the treated mice (6 days vs. 5 days in the controls-after sporozoites inoculation). Because of the exponential increase of the asexual parasites every 24 h, such a delay could reflect a dramatic reduction and/or delay in merozoites emerging after liver stage development indicating potential prophylactic activity [11].
CONCLUSION
The simple strategy of using plant-based remedy is likely to be one of the effective strategies in reducing malaria burden in areas like Koraput district where local people still depend on the local plants to combat malaria. This approach of malaria control needs further research in other areas where malaria transmission remains high and where traditional herbal remedies have a major impact. For the benefit of the large population who adheres to the traditional medicine practices, there is a need for further pre-clinical and multicentric trials to evaluate efficacy and safety on long-term ingestion of commonly used traditional herbal malaria prophylaxis.
ACKNOWLEDGMENTS
We express sincere gratitude to the constant encouragement given by Prof. Darshan Shankar, Vice-chancellor, ITD-HST, Bengaluru, Dr. Unnikrishnan P.M., UNU-IAS, Japan and Mr. John G., THREAD, Odisha, during the entire study period. We are also grateful to the traditional healers for their unreserved support in sharing their knowledge; volunteers who participated in the study and local NGO, THREAD - Team for Human Resource Education and Action for Development, Odisha for continuous support. Dr. PSS Sundar Rao, Bengaluru and staff of National Institute of Malaria Research, Bengaluru field station. The authors also acknowledge the support of Manipal University, Manipal, India.
Footnotes
Source of Support: We also acknowledge the funding support provided by ETC COMPAS (Grant number: 075012/India/001) and ETC CAPTURED programme, The Netherlands (Grant number: DGIS/D),
Conflict of Interest: None declared.
REFERENCES
- 1.WHO. World Malaria Report 2015. 2015. [Last accessed on 2016 Jul 29]. Available from: http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/
- 2.Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: What’s new, what’s needed: A summary. Am J Trop Med Hyg. 2004;71(2 Suppl):1–15. [PubMed] [Google Scholar]
- 3.Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA, et al. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 2010;7:e1000290. doi: 10.1371/journal.pmed.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, et al. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhingra N, Jha P, Sharma VP, Cohen AA, Jotkar RM, Rodriguez PS, et al. Adult and child malaria mortality in India: A nationally representative mortality survey. Lancet. 2010;376:1768–74. doi: 10.1016/S0140-6736(10)60831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazier D, Rénia L, Snounou G. A pre-emptive strike against malaria’s stealthy hepatic forms. Nat Rev Drug Discov. 2009;8:854–64. doi: 10.1038/nrd2960. [DOI] [PubMed] [Google Scholar]
- 7.Kain KC, Shanks GD, Keystone JS. Malaria chemoprophylaxis in the age of drug resistance. I. Currently recommended drug regimens. Clin Infect Dis. 2001;33:226–34. doi: 10.1086/321817. [DOI] [PubMed] [Google Scholar]
- 8.Collins WE, Jeffery GM. Primaquine resistance in Plasmodium vivax. Am J Trop Med Hyg. 1996;55:243–9. doi: 10.4269/ajtmh.1996.55.243. [DOI] [PubMed] [Google Scholar]
- 9.Nagendrappa PB, Naik MP, Payyappallimana U. Ethnobotanical survey of malaria prophylactic remedies in Odisha, India. J Ethnopharmacol. 2013;146:768–72. doi: 10.1016/j.jep.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 10.API. The Ayurvedic Pharmacopeia of India-Part I. 1st ed. Vol. 2. New Delhi: Department of AYUSH; 1990. pp. 124–5. [Google Scholar]
- 11.Nagendrappa PB, Franetich JF, Gay F, Lorthiois A, Venkatasubramanian P, Mazier D. Antiplasmodial activity of traditional polyherbal remedy from Odisha, India: Their potential for prophylactic use. Asian Pac J Trop Biomed. 2015;5:982–6. [Google Scholar]
- 12.District portal Koraput. Government of Odisha. [Last accessed on 2016 Jul 29]. Available from: http://www.ordistricts.nic.in/district_profile/aboutus.php .
- 13.Das LK, Pani SP. Clinical manifestations of severe forms of P. falciparum malaria in Koraput district of Orissa state, India. J Vector Borne Dis. 2006;43:140–3. [PubMed] [Google Scholar]
- 14.Daash A, Srivastava A, Nagpal BN, Saxena R, Gupta SK. Geographical information system (GIS) in decision support to control malaria-a case study of Koraput district in Orissa, India. J Vector Borne Dis. 2009;46:72–4. [PubMed] [Google Scholar]
- 15.NIMR. Guidelines for Diagnosis and Treatment of Malaria in India-2011. New Delhi: National Institute of Malaria Research; 2011. [Google Scholar]
- 16.WHO. Basic Malaria Microscopy - Part I: Learner’s Guide. 2010. [Last accessed on 2016 Jul 29]. Available from: http://www.who.int/malaria/publications/atoz/9241547820/en/
- 17.Graz B, Willcox ML, Diakite C, Falquet J, Dackuo F, Sidibe O, et al. Argemone mexicana decoction versus artesunate-amodiaquine for the management of malaria in Mali: Policy and public-health implications. Trans R Soc Trop Med Hyg. 2010;104:33–41. doi: 10.1016/j.trstmh.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Willcox ML, Graz B, Falquet J, Diakite C, Giani S, Diallo D. A “reverse pharmacology” approach for developing an anti-malarial phytomedicine. Malar J. 2011;10(Suppl 1):S8. doi: 10.1186/1475-2875-10-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dicko A, Sagara I, Sissoko MS, Guindo O, Diallo AI, Kone M, et al. Impact of intermittent preventive treatment with sulphadoxine-pyrimethamine targeting the transmission season on the incidence of clinical malaria in children in Mali. Malar J. 2008;7:123. doi: 10.1186/1475-2875-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schellenberg D, Menendez C, Kahigwa E, Aponte J, Vidal J, Tanner M, et al. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: A randomised, placebo-controlled trial. Lancet. 2001;357:1471–7. doi: 10.1016/S0140-6736(00)04643-2. [DOI] [PubMed] [Google Scholar]
- 21.Massaga JJ, Kitua AY, Lemnge MM, Akida JA, Malle LN, Rønn AM, et al. Effect of intermittent treatment with amodiaquine on anaemia and malarial fevers in infants in Tanzania: A randomised placebo-controlled trial. Lancet. 2003;361:1853–60. doi: 10.1016/s0140-6736(03)13504-0. [DOI] [PubMed] [Google Scholar]
- 22.Chandramohan D, Owusu-Agyei S, Carneiro I, Awine T, Amponsa-Achiano K, Mensah N, et al. Cluster randomised trial of intermittent preventive treatment for malaria in infants in area of high, seasonal transmission in Ghana. BMJ. 2005;331:727–33. doi: 10.1136/bmj.331.7519.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasoanaivo P, Wright CW, Willcox ML, Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar J. 2011;10(Suppl 1):S4. doi: 10.1186/1475-2875-10-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FRLHT. Foundation for Revitalization of Local Health Traditions - Annual Report. 2007. [Last accessed on 2016 Jul 29]. Available from: http://www.researchgate.net/profile/Prakash_BN2/publication/233780529_Traditional_Herbal_Medicine_for_Malaria_Prevention/links/0fcfd50b6eb46189d9000000.pdf .


