Abstract
Aim:
In a search for finding novel therapeutic agents, extracts from an endemic Lebanese plant, Astragalus angulosus, were evaluated for their potential in-vitro antibacterial and antibiofilm activities against three Gram-positive bacterial strains; Staphylococcus epidermidis (CIP444), Staphylococcus aureus (ATCC25923), and Enterococcus faecalis (ATCC29212); in addition to two Gram-negative strains, Escherichia coli (ATCC35218) and Pseudomonas aeruginosa (ATCC27853).
Materials and Methods:
The plant was collected in April of 2013 and divided into several different portions, then its extracts were obtained by maceration using two different solvents. Extract analysis followed directly where microtiter broth dilution method was employed to assess antibacterial activity, while antibiofilm potential was tested using colorimetric method.
Results:
Whole plant ethanolic extract showed the highest bacteriostatic effect at a concentration of 12.78 mg/ml and also was the most versatile exerting its effect against 3 different strains. Other extracts also exhibited an effect but at higher concentrations and each against a single strain. Regarding antibiofilm activity, the majority of the extracts were able to eradicate >50% of S. epidermidis preformed biofilm, where the highest activity was obtained with flower fraction extracted in water, achieving 67.7% biofilm eradication at 0.2 mg/ml.
Conclusions:
This plant possesses a promising potential in regard to eradicating bacteria and their biofilms and it is the first contributing step of establishing a library for the endemic Lebanese plants in this domain.
KEY WORDS: Antibacterial, antibiofilm, Astragalus angulosus, phytochemical screening, minimal inhibitory concentration, minimal bactericidal concentration
INTRODUCTION
Ever since ancient times, people relied on drugs from natural sources to cure their diseases [1], that is true since medicinal plants are considered to be the richest biological resource of drugs in traditional and modern medicine, food supplements, pharmaceuticals and intermediates for synthetic drugs, where it is estimated that 14-28% of higher plants are used medicinally [2]. The importance of plants as a source of medicine is depicted in a study by the World Health Organization, estimating that a substantial percentage of the population in developing countries relies on medicinal plants, where also people in developed countries are increasingly gaining interest in this domain [3].
Despite the fact that the pharmacological industries have produced a relatively limited number of new antibiotics in the past few decades, microbial resistance to these drugs have increased [4]. This problem was further accentuated by the over prescription and misuse of traditional antibiotics [5]. Furthermore, modern synthetic medication poses a major risk to patients’ health where such adverse drug reactions (ADRs) occur almost daily in medium sized hospitals and outpatient panels; this results in substantial morbidity and mortality in addition to the decreasing efficacy of these products [1,6]. In addition to the previous implications, drug resistance severely increases the cost of medical care where the estimated cost of treating a case of tuberculosis (including drugs, procedures, and hospitalization) increases from $12,000 for a drug-susceptible strain to $180,000 for a multidrug-resistant strain [7]. Therefore, the decreasing efficacy of synthetic drugs and the increasing contraindications of their usage make the usage of natural drugs an encouraging need again [1].
Another concern of the work at hand focuses on bacterial biofilms which can be defined as a community of microorganisms attached to a surface and embedded in their self-produced matrix. Moreover, organisms in this state of existence will exhibit new phenotypic characteristics rather than that of the planktonic state [8]. More specifically in the current study, we will be dealing with Staphylococcus epidermidis biofilms, in which the strain used here (CIP444) was isolated from an infected implanted device [9]. We are interested specifically in the S. epidermidis biofilm since although it is usually described as a commensal species and a permanent colonizer of human skin, it is among the most common sources of infections on indwelling medical devices [10].
Biofilms can be very problematic in various aspects of our lives ranging from medical to industrial areas. In addition to their increased resistance to antimicrobial agents, biofilms can form on many medical implants such as catheters, artificial hips, and contact lenses. The most worrisome fact is that cells existing in a biofilm can become 10-1000 times more resistant to antimicrobial agents, mainly through the production of extracellular polymeric substance matrix that hinders the access of antibiotics to the bacterial cells. These infections can often only be treated by removal of the implant, thus increasing the trauma to the patient and the cost of the treatment. It has been estimated that biofilms are associated with 65% of nosocomial infections and that treatment of these biofilm-based infections costs >$1 billion annually [11].
Therefore, it is of utmost importance to seek a novel therapeutic agent that has a minimum impact on patient’s health and is cost-effective. The concern to unravel better and safer ways to treat microbial infections and their consequences encouraged us to hunt for natural products that might provide such solution. To achieve our goal, we have relied on plant secondary metabolites such as phenols, saponins, flavonoids and many others that possess antimicrobial potential [2]. Our chosen plant in this work is a species endemic to Lebanon, Astragalus angulosus, whose extracts were used in antibacterial and antibiofilm testing in an aim to unravel a new therapeutic agent.
MATERIALS AND METHODS
Plant Material
A. angulosus was collected from Yammouneh in Bekaa (34.116046, 36.037830) during its flowering period in April (2013). It was identified in accordance with the two well-known guides of Lebanon’s flora [12,13].
Preparation of the Extracts
The plant was rinsed and divided into several fractions; whole plant, flowers, leaves, green stems, and brown stems. The phytochemicals were extracted by maceration and performed over multiple stages where after finely chopping the plant portions, each fraction (50-100 g according to the abundance of each part) was fully submerged in a suitable volume of distilled water and ethanol (100%) in separate light blocking beakers and agitated at ambient temperature for 8-12 h then for the same duration at 37°C. They were then filtered and the process repeated for the obtained marks. Finally, the resulting fractions were concentrated using a rotary evaporator under reduced pressure at 60°C for aqueous and 40°C for ethanolic fractions, and finally, they were frozen at −80°C and freeze-dried using a lyophilizer (Christ Alpha 1-4 LDplus, Martin Christ, Germany) for 2-3 days at −20°C to obtain the powders to study. To assess the obtained powders, they were dissolved in sterile distilled water, and aliquots with a defined concentration were prepared for further analysis.
Phytochemical Screening
For the purpose of this test, aliquots from the ethanolic and water extract of each fraction of the plant were prepared and evaluated by phytochemical qualitative reactions for common plant secondary metabolites; these include tannins, resins, coumarins, saponins, alkaloids, phenols, terpenoids, volatile oils, and flavonoids [14-16]. The results were evaluated according to the response of the extract to these tests, mainly color change and/or precipitate formation.
Antibacterial Testing
Bacterial strains, media, and reagents
Five referenced strains belonging either to the American Type Culture Collection or to the Collection of “Institut Pasteur” were used in this study. Three Gram-positive strains, S. epidermidis (CIP444), Staphylococcus aureus (ATCC25923), and Enterococcus faecalis (ATCC29212); two Gram-negative strains, Escherichia coli (ATCC35218) and Pseudomonas aeruginosa (ATCC27853). CIP444 is a strain that was isolated from a patient with an infected implanted device in the Mignot hospital of Versailles, France [9].
This strain was identified and characterized for many features by Pr. Ali Chokr and deposited to be enclosed within the micro-organisms of the collection of “Institut Pasteur” in 2007 [9,17-19]. All strains were stored at −80°C in glycerol stocks and used as required. Brain heart infusion broth/agar (BHI/BHA), tryptone soya broth (TSB) and Mueller-Hinton broth (MHB) were purchased from HIMEDIA (Mumbai, India) and then prepared as per indicated by the manufacturer.
Minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) assays
MICs and MBCs were determined using the microtiter broth dilution method as recommended by the Clinical and Laboratory Standards Institute [20]. The different bacterial strains were grown in BHI overnight and then bacterial suspensions of each were prepared using MHB at 5 × 105 CFU/ml (confirmed by viable count) to be used for inoculation. 100 µl of each extract was used to perform serial two-fold dilutions in MHB using a 96-well flat-bottom polystyrene tissue culture-treated microtiter plate (Corning®Costar® 3598; Corning, NY 14831, USA) then 100 µl of the previously prepared suspensions were inoculated into each well. Positive growth control lacking any plant extract and negative growth control lacking a bacterial inoculum were taken into account. The plates were then incubated at 37°C for 24 h, the MIC of each extract was the lowest concentration with no visible growth in its corresponding well. Then, all wells with no visible growth were plated on BHA to determine the MBC which is the lowest concentration able to reduce the initial bacterial inoculum by >99.9%. For comparison, these strains were tested using the same procedure against standard common antibiotics including tetracycline, nalidixic acid, polymyxin, rifampicin, and ampicillin (results not shown).
Antibiofilm Susceptibilty Testing against a Preformed Biofilm
Biofilm formation
Assay of biofilm formation in polystyrene plates was performed essentially according to a standard procedure [21], where S. epidermidis (CIP444) was grown in TSB medium overnight at 37°C, then a bacterial suspension of concentration 4.16 × 105 CFU/ml was prepared in TSB supplemented with 0.25% glucose. 120 µl of this bacterial suspension were inoculated into each well of a sterile 96-well flat bottom plate except for column 12 that was used as a control and filled only with sterile TSB medium; then, the plates were incubated for 24 h at 37°C. The biomass and any non-adherent bacteria were then discarded by gently washing the plates with 0.9% NaCl physiologic water and the remaining biofilm was fixed by incubating the plates for 50 min at 50°C [9].
Antibiofilm activity assay
After the fixation of the formed biofilm as previously described, each well of the microtiter plate was filled with 100 µl of sterile physiologic water for use as a diluent of our plant extracts. The serial 1:2 dilution was then performed with 100 µl of each plant extract, and the plates were then incubated at 37°C during 18 h. Tests were performed in quadruple. The wells were then washed 2 times with saline water, filled with 100 µl of crystal violet 0.1% and incubated at room temperature for 10 min. The stain is then discarded, and the wells are washed 3 times with saline water. Finally, they were filled with 100 µl of physiologic water and the OD490 nm is measured. One microtiter plate was skipped of treatment with the plant extracts and used as an untreated positive control.
Statistical Analysis
Antibiofilm tests were performed in quadruple, and the results were expressed as mean values ± standard errors of the means. Differences in the biofilm eradication potentials were evaluated by an unpaired non-parametric analysis of variance (Kruskal-Wallis test) followed by Dunn’s multiple comparisons test of each mean versus the mean of the untreated (positive) control. Approximate eradication efficiency (percentage) was calculated according to the following equation: (Average [control wells]−Average (treated wells)/Average (control wells)]×100. GraphPad Prism® Software (Version 6.05; GraphPad Software, Inc.) was used for statistical analysis, considering a P < 0.05 to be statistically significant.
RESULTS
Phytochemical Screening
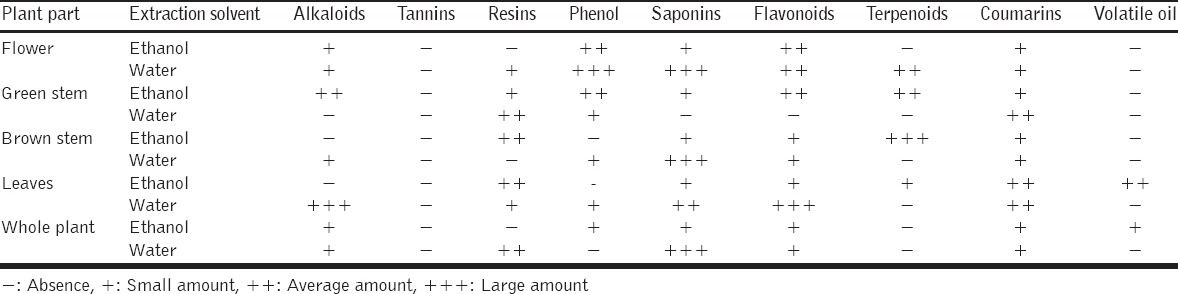
Saponins, coumarins, and flavonoids were present in all fractions and portions of this plant as represented in Table 1. Other secondary metabolites were also present but in variable amounts with respect to the different fractions, and noticeably low abundance of volatile oils and complete absence of the tannins.
Table 1.
Results of phytochemical screening of A. angulosus
MIC and MBC Results
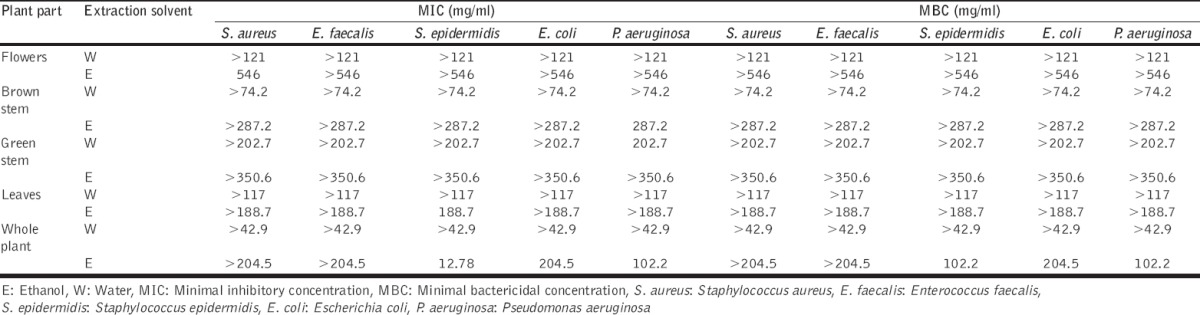
After testing the extracts according to the method specified earlier, the entire raw findings are displayed in Table 2. For some extracts, no MIC and/or MBC was obtained at the used concentration and hence the symbol (>) was used to indicate that a higher concentration might be needed to achieve an effect.
Table 2.
MIC and MBC results of A. angulosus against 5 bacterial strains
Among the 10 extracts, only 5 exhibited a bacteriostatic effect. The most significant being whole plant ethanolic fraction which exerted its effects on three bacterial strains (S. epidermidis, E. coli, and P. aeruginosa), with the lowest being 12.78 mg/ml for S. epidermidis as shown in Figure 1 and it is also notable that P. aeruginosa was the most sensitive organism where 3 fractions were able to suppress its growth (brown stem ethanolic, aqueous green stem and whole plant ethanolic fractions) with the lowest being that of whole plant ethanolic at 102.2 mg/ml. The other fractions’ MICs ranged between 188.7 mg/ml for leaves ethanolic on S. epidermidis to 546 mg/ml of flowers ethanolic fraction on S. aureus.
Figure 1.
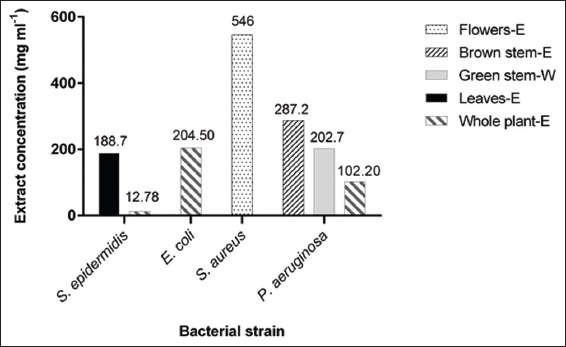
Significant minimal inhibitory concentration results of the tested fractions of Astragalus angulosus (E: Ethanol, W: Water)
Regarding the MBC, only the whole plant ethanolic fraction was active among the 10 extracts as seen in Figure 2, and against 3 of the tested strains, ranging from 102.2 mg/ml for S. epidermidis to 204.5 mg/ml for E. coli and P. aeruginosa.
Figure 2.
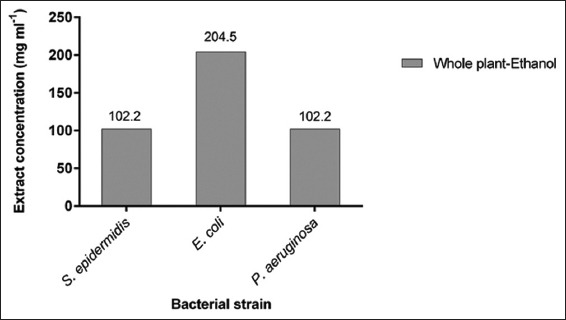
Minimal bactericidal concentration results of the active bactericidal extract of Astragalus angulosus
Antibiofilm Activity Results
As demonstrated in Figures 3 and 4, in general, all our extracts were able to eradicate the S. epidermidis preformed biofilm but with variable patterns. For example, flower, brown stem and whole plant (water and ethanol fraction) also green stem and leaves (water fraction) extracts performed better under lower concentrations, and this was confirmed also by the statistical analyses performed. While green stem and leaves (ethanolic fraction) extracts had better eradication capacities at higher concentrations.
Figure 3.
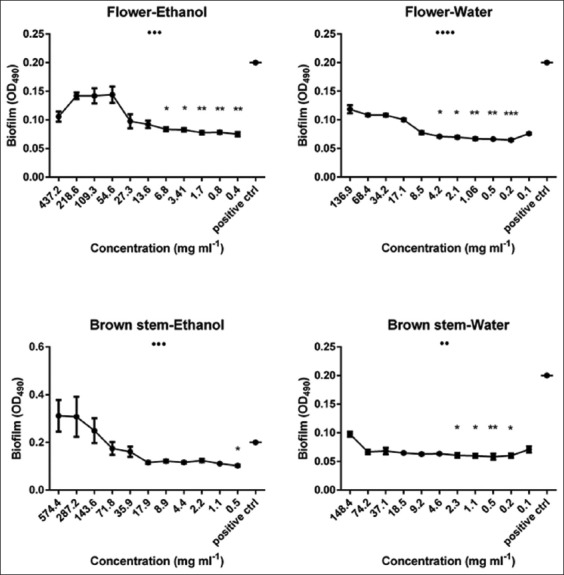
Biofilm eradication potential of 4 Astragalus angulosus extracts against a preformed Staphylococcus epidermidis biofilm assessed by spectrophotometric method. Results are displayed as mean biofilms plus standard error of the mean. •P<0.05, ••P<0.01, •••P<0.001, ••••P<0.0001 (analysis of variance by Kruskal-Wallis test). *P<0.05, **P<0.01, ***P<0.001 (Dunn’s post-test comparing the mean biofilm for each category (extract concentration) with that of the untreated control, OD490: Optical density at 490 nm
Figure 4.
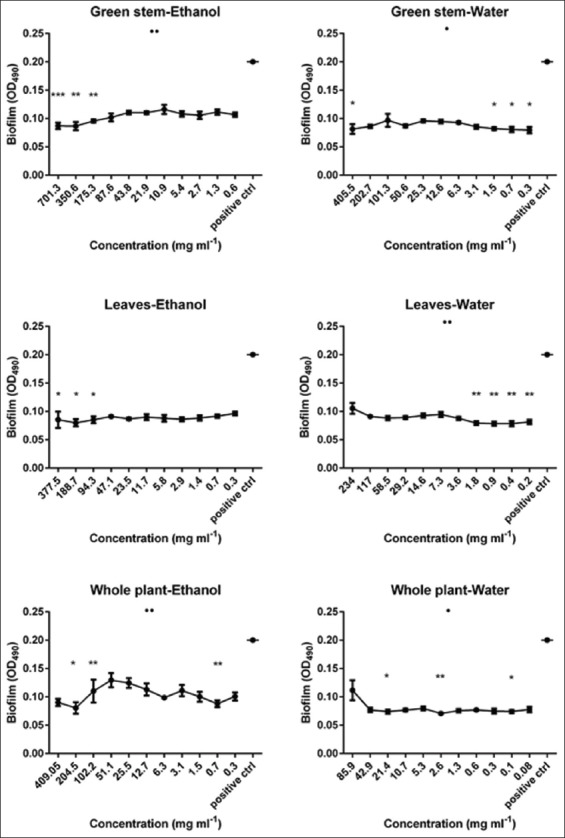
Biofilm eradication potential of 4 Astragalus angulosus extracts against a preformed Staphylococcus epidermidis biofilm assessed by spectrophotometric method. Results are displayed as mean biofilms plus standard error of the mean. •P<0.05, ••P<0.01, •••P<0.001, •••P<0.0001 (analysis of variance by Kruskal-Wallis test). *P<0.05, **P<0.01, ***P<0.001 (Dunn’s post-test comparing the mean biofilm for each category (extract concentration) with that of the untreated control, OD490: Optical density at 490 nm
As shown in Figure 3, the highest overall significance belongs to the flower water extract which also achieved the highest eradication activity at a very low concentration (~67.7% at 0.2 mg/ml, P < 0.0001). Other extracts followed the same pattern such as flower ethanol (~62.5% at 0.4 mg/ml, P < 0.01), brown stem, and whole plant (both fractions) in addition to green stem and leaves (water fraction) but with a lesser impact and significance. By contrast, we noticed that green stem (ethanolic fraction) exerted best eradication efficiency at higher concentrations (~56.5% at 701.3 mg/ml, P < 0.001) and the same goes for leaves ethanolic fraction, but the overall test was insignificant according to the Kruskal-Wallis evaluation regarding the latter.
DISCUSSION
Increasing drug resistance to traditional treatments observed in several bacterial strains, in addition to harmful ADRs and substantial cost of treatment shifted the pace toward finding novel therapeutic agents, natural products such as plants and plant-derived compounds were the primary target due to their efficacy, safety, and lower cost.
Native to China, Astragalus has been used for centuries in traditional Chinese medicine. There are actually over 2,000 species of Astragalus; however, the two related species - Astragalus membranaceus and Astragalus mongholicus - are the ones primarily used for health purposes [22].
Historically, Astragalus has been used in traditional medicine. Usually in combination with other herbs, as antioxidant, to support and enhance the immune system, for chronic hepatitis, other viral infections and as an adjunctive therapy for cancer. It is also used as a folk or traditional remedy for colds and upper respiratory infections, and for heart disease [23-26].
The biological activities of the Lebanese plants are not well explored. Only a few studies on some biological activities of certain plant genera such as Allium and Berberis are available [27-29].
To the best of our knowledge, the activities of the endemic Lebanese plants are not yet studied.
In this study, we chose a Lebanese endemic species of Astragalus which is A. angulosus in an effort to find novel agents that could aid in fighting bacterial infections and their biofilms. In the present work, we assessed and tested for the first time the antibacterial and antibiofilm activities of water and ethanolic extracts from the flower, leaves, brown stem, green stem, and whole plant portions of A. angulosus against five bacterial strains.
MIC and MBC values indicate that not all extracts exhibited an activity. Regarding the MIC assay, only 5 out of 10 were able to inhibit bacterial growth, among which the whole plant ethanolic fraction was most significant where it achieved the lowest MIC and had an effect against multiple strains. We also noticed that one of our tested strains, E. faecalis was insensitive to all treatments used, that is, independently of the strain being a Gram-positive one but rather probably related to the strain itself or the nature of our extracts and their targets. It is worthy to note that the most successful extracts contained fair amounts of several secondary metabolites mainly flavonoids, coumarins, and volatile oils. Furthermore, it was evident that most of the active extracts were ethanolic fraction extracts, which are characterized by containing flavonoids and phenols that are known antimicrobial agents [30,31].
It is not surprising that bactericidal activity was also detected with our leading extract which is the whole plant ethanolic fraction, which was the only extract with bactericidal activity and that was against 3 strains as well. This could be due to a potential synergistic effect between the different phytochemical constituents found in the whole plant. The synergism effect in plant extracts was also highlighted in literature as in the study of Ncube et al., in 2012, on the antimicrobial synergism within plant extract combinations from three South African medicinal bulbs [32].
The other problem at hand is the infections caused by biofilm-related formation on indwelling medical devices, where the introduction of synthetic and artificial devices into body systems have provided the microorganisms with a way for evading host defenses and invading the host [33,34], where S. epidermidis is a major cause of these infections. This issue is not to be taken lightly where research has shown that an important cause of nosocomial infections is the catheter-associated bloodstream infections, with an estimated occurrence of 50,000-100,000 cases a year in the United States with the skin being the most common source of organisms causing catheter-related infections [35].
Our tests evaluated the ability of the extracts to disrupt and eradicate preformed biofilms, and the results were quite promising. We noticed a significant effect of some extracts, especially the flower water fraction in biofilm eradication and at low concentrations. Therefore, exposure to subinhibitory concentrations of some of our extracts could substantially affect the preformed S. epidermidis biofilms. We also observed that while some extracts showed better performance at higher concentrations, others were to some extent active irrespective of the used concentration.
By contrast to the antibacterial activity tests, the most successful extracts in the antibiofilm activity assays where the water extracts that contained higher saponin content, which prompt us to hypothesize that it may have been acting as a kind of detergent against the preformed biofilms without excluding the effects of the other metabolites detected here such as alkaloids, resins, and phenols which are slightly more frequent in water extracts than in ethanolic ones. This is corroborated by the fact that our test involved preformed biofilm and as such the mode of action of the extracts in this test would be different, and that is, due to the absence of any bacteria in our biofilms where we were trying to combat the structure of the biofilm left by the bacteria and not the bacteria themselves.
This is supported by a study of Ye et al., in 2015, on the plant Camellia oleifera. They found that the extracts of this plant were rich in saponin identified as camelliagenin which shows significant inhibition on the biofilm of E. coli and S. aureus and it is related to the decrease of a component of the bacterial biofilm, the extracellular DNA. On the other hand, in 2015, Santiago et al. showed that Acalypha wilkesiana fraction extracts rich in saponins prevent the biofilm formation by methicillin-resistant S. aureus (MRSA). Bioactive fraction rich in saponins inhibited the production of MRSA biofilm by preventing the initial cell-surface attachment [36,37].
After observing the “disjunction” between the active extracts in antibacterial tests and antibiofilm tests, it was clear that some extracts could decrease the viability of preformed biofilms through mechanism(s) that are different from mere growth inhibition of bacterial cells in the planktonic form. Therefore, in our view, there are different compounds responsible for the results seen in our performed tests and further analysis should be done to try to pinpoint the exact effectors.
In end, we can say that the results obtained in this study, taken together, provide promising evidence on the antibacterial and antibiofilm potential and effectiveness of the Lebanese endemic plant A. angulosus. Further biochemical testing and the separation of the secondary metabolites by chromatography methods are required to identify the exact compounds responsible for the observed effects. The ability of the extracts to prevent biofilm formation rather than just its eradication, then antioxidant, anticoagulant, and other biological activities, in addition to their toxicity on human cells remain to be further elucidated.
Our results showed that at relatively low concentrations some of our extracts displayed promising antibacterial and antibiofilm capabilities making them attractive for additional studies where we can hopefully label them as “novel therapeutic agents.”
ACKNOWLEDGMENTS
The authors are grateful to the Lebanese University for its financial support. We thank Eng. Dominique Choueiter for his help in the identification of the plant. We also thank the English Language Center at Rafic Hariri campus of the Lebanese University for critical reading of the manuscript.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Petrovska BB. Historical review of medicinal plants’ usage. Pharmacogn Rev. 2012;6:1–5. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and Extraction: A Review. Int Pharm Sci. 2011;1:98–106. [Google Scholar]
- 3.World Health Organization. Monographs on Selected Medicinal Plant. Vol. 1. Geneva: WHO Library Cataloguing in Publication Data; 1999. [Google Scholar]
- 4.Nascimento GG, Locatelli J, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz J Microbiol. 2000;31:247–56. [Google Scholar]
- 5.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: A clinician’s guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140:795–801. doi: 10.7326/0003-4819-140-10-200405180-00009. [DOI] [PubMed] [Google Scholar]
- 7.Cohen ML. Epidemiology of drug resistance: Implications for a post-antimicrobial era. Science. 1992;257:1050–5. doi: 10.1126/science.257.5073.1050. [DOI] [PubMed] [Google Scholar]
- 8.O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 9.Chokr A, Watier D, Eleaume H, Pangon B, Ghnassia JC, Mack D, et al. Correlation between biofilm formation and production of polysaccharide intercellular adhesin in clinical isolates of coagulase-negative staphylococci. Int J Med Microbiol. 2006;296:381–8. doi: 10.1016/j.ijmm.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Otto M. Staphylococcus epidermidis – The ‘accidental’ pathogen. Nat Rev Microbiol. 2009;7:555–67. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–9. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 12.Tohmé G, Tohmé H. Illustrated Flora of Lebanon. Beirut, Lebanon: National Council for Scientific Research; 2007. [Google Scholar]
- 13.Mouterde P. New Flora of Lebanon and Syria. Beirut: Editions of Cath. Impr; 1966. [Google Scholar]
- 14.Farhan H, Rammal H, Hijazi A, Hamad H, Badran B. Phytochemical screening and extraction of polyphenol from stems and leaves of a Lebanese Euphorbia macrolada schyzoceras Boiss. Ann Biol Res. 2012;3:149–56. [Google Scholar]
- 15.Mohsen H. Beirut, Lebanon: Lebanese University; 2012. Evaluation of anticancerous and antioxidant effects of two Lebanese plants Euphorbia macroclada and Eryngium creticum. [Google Scholar]
- 16.Rawat V, Upadhyaya K. Evaluation of antimicrobial activity and preliminary phytochemical screening of Mesua ferrea seeds extract. J Nat Prod. 2012;6:17–26. [Google Scholar]
- 17.Chokr A, Leterme D, Watier D, Jabbouri S. Neither the presence of ica locus, nor in vitro-biofilm formation ability is a crucial parameter for some Staphylococcus epidermidis strains to maintain an infection in a guinea pig tissue cage model. Microb Pathog. 2007;42:94–7. doi: 10.1016/j.micpath.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Kogan G, Sadovskaya I, Chaignon P, Chokr A, Jabbouri S. Biofilms of clinical strains of Staphylococcus that do not contain polysaccharide intercellular adhesin. FEMS Microbiol Lett. 2006;255:11–6. doi: 10.1111/j.1574-6968.2005.00043.x. [DOI] [PubMed] [Google Scholar]
- 19.Sadovskaya I, Chaignon P, Kogan G, Chokr A, Vinogradov E, Jabbouri S. Carbohydrate-containing components of biofilms produced in vitro by some staphylococcal strains related to orthopaedic prosthesis infections. FEMS Immunol Med Microbiol. 2006;47:75–82. doi: 10.1111/j.1574-695X.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 20.Cinical and Laboratory Standards Institute. Broth dilution procedures. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard. 9th ed. Wayne, PA, USA: Cinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 21.Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Center for Complementary and Integrative Health. Herbs at a Glance-Astragalus. Rockville Pike, Maryland, USA: Bethesda, National Center for Complementary and Integrative Health; 2012. [Google Scholar]
- 23.Labed A, Ferhat M, Labed-Zouad I, Kaplaner E, Zerizer S, Voutquenne-Nazabadioko L, et al. Compounds from the pods of Astragalus armatus with antioxidant, anticholinesterase, antibacterial and phagocytic activities. Pharm Biol 2016. :1–7. doi: 10.1080/13880209.2016.1200632. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Yu Y, Brad K, Xie W, Zhang XY. The screening and evaluation of herbs and identification of herbal combinations with anti-viral effects on Newcastle disease virus. Br Poult Sci. 2016;57:34–43. doi: 10.1080/00071668.2015.1119245. [DOI] [PubMed] [Google Scholar]
- 25.Xue H, Gan F, Zhang Z, Hu J, Chen X, Huang K. Astragalus polysaccharides inhibits PCV2 replication by inhibiting oxidative stress and blocking NF-κB pathway. Int J Biol Macromol. 2015;81:22–30. doi: 10.1016/j.ijbiomac.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 26.Tang J, Cai J, Liu R, Wang J, Lu Y, Wu Z, et al. Immunostimulatory effects of artificial feed supplemented with a Chinese herbal mixture on Oreochromis niloticus against Aeromonas hydrophila. Fish Shellfish Immunol. 2014;39:401–6. doi: 10.1016/j.fsi.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Sharifi-Rad J, Mnayer D, Tabanelli G, Stojanovic-Radic ZZ, Sharifi-Rad M, Yousaf Z, et al. Plants of the genus Allium as antibacterial agents: From tradition to pharmacy. Cell Mol Biol (Noisy-le-grand) 2016;62:57–68. [PubMed] [Google Scholar]
- 28.Mnayer D, Fabiano-Tixier AS, Petitcolas E, Hamieh T, Nehme N, Ferrant C, et al. Chemical composition, antibacterial and antioxidant activities of six essentials oils from the Alliaceae family. Molecules. 2014;19:20034–53. doi: 10.3390/molecules191220034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Merahbi R, Liu YN, Eid A, Daoud G, Hosry L, Monzer A, et al. Berberis libanotica Ehrenb extract shows anti-neoplastic effects on prostate cancer stem/progenitor cells. PLoS One. 2014;9:e112453. doi: 10.1371/journal.pone.0112453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maddox CE, Laur LM, Tian L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr Microbiol. 2010;60:53–8. doi: 10.1007/s00284-009-9501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cazarolli LH, Zanatta L, Alberton EH, Figueiredo MS, Folador P, Damazio RG, et al. Flavonoids: Prospective drug candidates. Mini Rev Med Chem. 2008;8:1429–40. doi: 10.2174/138955708786369564. [DOI] [PubMed] [Google Scholar]
- 32.Ncube B, Finnie JF, Van Staden J. In vitro antimicrobial synergism within plant extract combinations from three South African medicinal bulbs. J Ethnopharmacol. 2012;139:81–9. doi: 10.1016/j.jep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 33.Dickinson GM, Bisno AL. Infections associated with prosthetic devices: Clinical considerations. Int J Artif Organs. 1993;16:749–54. [PubMed] [Google Scholar]
- 34.Khardori N, Yassien M. Biofilms in device-related infections. J Ind Microbiol. 1995;15:141–7. doi: 10.1007/BF01569817. [DOI] [PubMed] [Google Scholar]
- 35.Adal KA, Farr BM. Central venous catheter-related infections: A review. Nutrition. 1996;13:208–21. doi: 10.1016/s0899-9007(96)91126-0. [DOI] [PubMed] [Google Scholar]
- 36.Santiago C, Lim KH, Loh HS, Ting KN. Prevention of cell-surface attachment and reduction of penicillin-binding protein 2a (PBP2a) level in methicillin-resistant Staphylococcus aureus biofilms by Acalypha wilkesiana. BMC Complement Altern Med. 2015;15:79–86. doi: 10.1186/s12906-015-0615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye Y, Yang Q, Fang F, Li Y. The camelliagenin from defatted seeds of Camellia oleifera as antibiotic substitute to treat chicken against infection of Escherichia coli and Staphylococcus aureus. BMC Vet Res. 2015;11:214–224. doi: 10.1186/s12917-015-0529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


