Abstract
Background/Aim:
Some of the antifungal drugs used in the current treatments regime are responding to antimicrobial resistance. In rural areas of Southern Tanzania, indigenous people use antifungal drugs alone or together with medicinal plants to curb the effects of antibiotic resistance. This study documented ethnobotanical information of medicinal plants used for managing fungal infections in the Southern Highlands of Tanzania and further assess their safety.
Materials and Methods:
Ethnobotanical survey was conducted in Makete and Mufindi districts between July 2014 and December 2015 using semi-structured questionnaires followed by two focus group discussions to verify respondents’ information. Cytotoxicity study was conducted on extracts of collected plants using brine shrimp lethality test and analyzed by MS Excel 2013 program.
Results:
During this survey about 46 plant species belonging to 28 families of angiosperms were reported to be traditionally useful in managing fungal and other health conditions. Among these, Terminalia sericea, Aloe nutii, Aloe lateritia, Zanthoxylum chalybeum, Zanthoxylum deremense, and Kigelia africana were frequently mentioned to be used for managing fungal infections. The preparation of these herbals was mostly by boiling plant parts especially the leaves and roots. Cytotoxicity study revealed that most of the plants tested were nontoxic with LC50 > 100 which implies that most compounds from these plants are safe for therapeutic use. The dichloromethane extract of Croton macrostachyus recorded the highest with LC50 value 12.94 µg/ml. The ethnobotanical survey correlated well with documented literature from elsewhere about the bioactivity of most plants.
Conclusions:
The ethnobotanical survey has revealed that traditional healers are rich of knowledge to build on for therapeutic studies. Most of the plants are safe for use; and thus can be considered for further studies on drug discovery.
KEY WORDS: Ethnobotanical, fungal, brine shrimp test, medicinal plants, traditional medicine
INTRODUCTION
The history of mankind has continuously remained interlocked to the surrounding environment. The first civilizations realized that there were plants with healing potential. The value of plants has a long history in saving human beings cutting across different cultures in the world [1]. Utilization of medicinal plants by individuals lies on the knowledge accumulated through the interaction of people with the environment and the diffusion of information, traditionally transmitted orally through subsequent generations [2]. In the contemporary world of conventional medicine, the practice of herbal medicine has attracted more attention and is becoming accepted globally [3]. Traditional medicine is not well documented in most African societies [4]. However, the practices and resources have been orally transferred from one generation to another thus limiting its reliability.
Documenting the indigenous knowledge through ethnobotanical studies is important for sustainable utilization of medicinal plants in drug discovery. Several active compounds have been discovered from plants based on ethnobotanical information, some used directly as therapeutic drugs [3]. Therefore, the focus of the study was to collect and document information on the use of antifungal medicinal plants and their therapeutic practices among the Hehe and Kinga tribe in Southern Highland of Tanzania. The information could further help scientific research in drug development.
MATERIALS AND METHODS
Study Area
The study was conducted in Mufindi District found in Iringa Region and Makete District based in Njombe Region. Makete District is one of the six districts of Iringa Region and is located in the Southern Highlands of Tanzania about 115 km from the regional headquarters (Figure 1). It is situated within 9°15’0” S 34°10’0” E [5]. Mufindi district on the other hand lies between 08°35’40’’S 035°17’20’’E. Both districts are dominated by Hehe, Kinga and Bena ethnic tribes. Furthermore, these districts experience high levels of migration and mobility (61.4%) caused by seasonal workers to numerous plantations in the areas and being a logistical hub for transport infrastructural facilities by road and railway (Tanzania-Zambia route) [6]. These unique dynamics increase the risk for HIV transmission in the communities. Most of the livelihoods are from agriculture which is the major source of subsistence, occupying about 80 % of the households in the districts [5]. Other activities include livestock keeping, timber production, and petty businesses at small scale. Most household members are thus compelled to engage in multiple jobs and activities to make a living [5].
Figure 1.
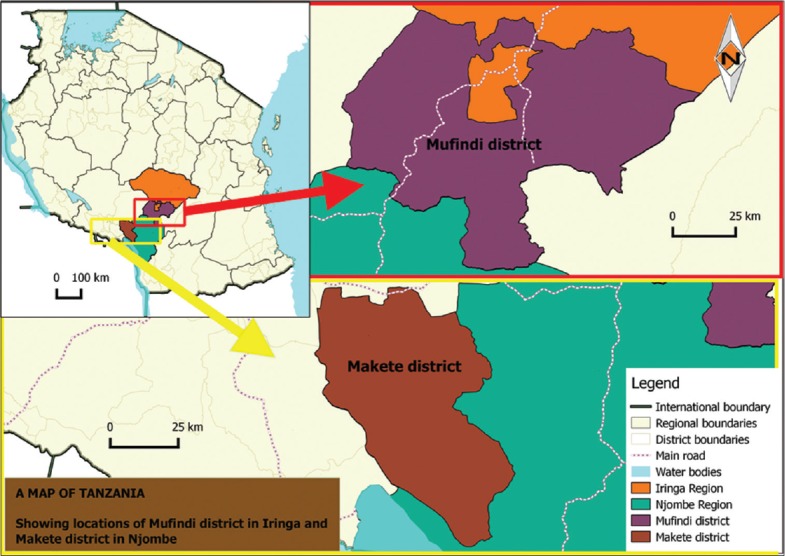
Map of Tanzania showing the study areas (Mufindi and Makete Districts) Ethnobotanical survey
During the ethnobotanical survey that was done between July 2014 and September 2015 semi-structured questionnaire was used as data collection tools to interview traditional health practitioners, elders and selected villagers who have knowledge on medicinal plants. This study employed a purposive sampling, in which selection of respondents do only focus to people who are considered by the community as having exceptional knowledge about the use of plants such as traditional healers, herbalists and elders. The questionnaire aimed to collect and document ethnobotanical information of plants that are used to treat various infections including fungal infections. Documentation of plants, parts used and their preparations whenever possible was done. Focus group discussion was employed to validate information collected using questionnaire method.
Collection of Plant Materials
Identification of plant species was done by the botanist from the Department of Botany, University of Dar es Salaam, Tanzania, and all voucher specimens were deposited at the Institute of Traditional Medicine, Muhimbili University of Health and Allied Sciences. Collection of the identified plants was aided by the traditional health practitioners and elders. Decision on which plant and/or part of plant to be collected for further studies was mainly influenced by the information given by respondents in the field validated first by focus group discussion and by literature.
Reagents
Absolute ethanol, dichloromethane, and petroleum ether were purchased from Fluka Chemie GmbH (Sigma-Aldrich®, Zwijndrecht, Netherlands), dimethyl sulfoxide (DMSO) was purchased from Sigma® (Poole, Dorset, UK) while sea salt was prepared locally by evaporating water collected from the Indian Ocean, along the Dar es Salaam Coast.
Extraction and Concentration
Plant materials from the field were cut into small pieces, air-dried and ground using a machine grinder consequently soaked, sequentially using petroleum ether, dichloromethane, and ethanol for 48 h for each solvent. The method of percolation was employed during extraction process. The crude extracts were obtained by concentrating the filtrate in vacuo using a rotary evaporator with the bath temperature maintained at 40°C. The crude extract obtained was placed in the refrigerator for few hours and then subjected to freeze drier to remove solvent that could have remained.
Brine Shrimp Lethality Test
The brine shrimp lethality assay was used as an indication for bioactivity of different tested plant extracts as well as investigation for toxicity [7,8]. Artificial seawater was prepared by dissolving 3.8 g of sea salt in 1 L of distilled water. Brine shrimp eggs (2 g) were added and left for 24 h to hatch in light condition. Stock solutions (40 mg/mL) of all extracts were dissolved in DMSO. Different levels of concentrations (240, 120, 80, 40, 24, 8, 4.5, 3, 1.5 and 1 µg/ml) were prepared by drawing different volumes from the stock solutions and then added into vials, each containing ten brine shrimps larvae. The volume was adjusted with the prepared artificial seawater. Each level of concentration was tested in duplicate. The negative control contained brine shrimp, artificial seawater and DMSO (0.6%) only. The vials were incubated under light for 24 h. The dead larvae were counted and mean percentage mortality calculated.
Data Analysis
The mean percentage mortality was plotted against the logarithm of concentrations and the concentration killing 50% of the larvae (LC50) were determined from the graph using Microsoft Excel 2013 computer software. Regression equation obtained enabled calculation of lethal concentrations, i.e., LC50, LC16, and LC84. The 95% confidence interval was then calculated using method reported by Litchfield and Wilcoxon [9]. The results were used to document safety and cytotoxicity activity of plant extracts.
RESULTS
Ethnobotanical Survey
During the ethnobotanical survey, a total of 40 respondents (traditional healers, herbalists, and elders) were interviewed from the selected regions. 5 different villages in Njombe and Iringa regions were visited for the survey including three villages; Tambalang’ombe, Mayale, Kingege, and Ifwagi from Mufindi, Iringa region as well as Lupalilo and Maliwa villages of Makete district in Njombe region. These villages were chosen based on the information of registered or known traditional health practitioners obtained from the District Medical offices.
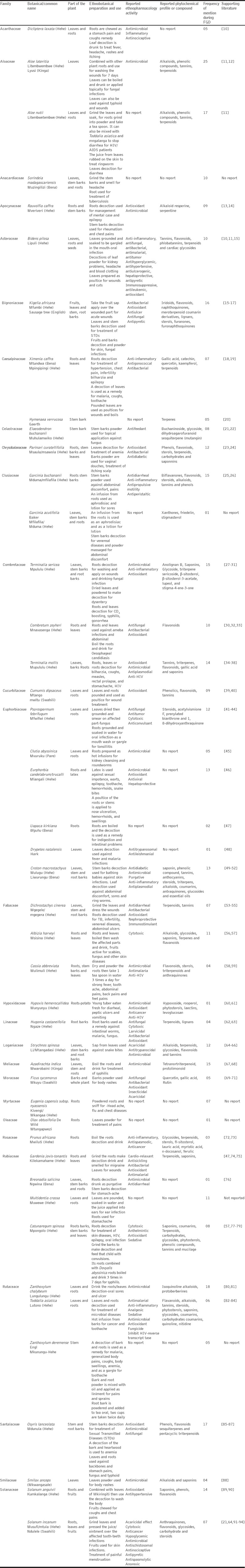
A total of 46 plant species used by the Hehe, Bena and Kinga tribe for the treatment of various microbial related ailments were documented [Table 1]. The plants represent about 28 families with the most prominent families being Euphorbiaceae (6 species), Combretaceae, and Rubiaceae (4 species each) and followed by Rutaceae, and Fabaceae (each with 3 species). Most of the ethnobotanical information were related to fungal infections since the study focused on documenting plants that were used in managing fungal infections among these ethnic groups. Out of 46 reported plant species, 14 (32%) had similar cited antifungal activity while 8 (18%) of plant species traditionally used for managing other nonfungal infections in Mufindi and Makete districts were reported by the literature to have antifungal activity [Table 1].
Table 1.
List of medicinal plants reported for managing various diseases in Iringa and Njombe regions
Brine Shrimp Lethality Assay
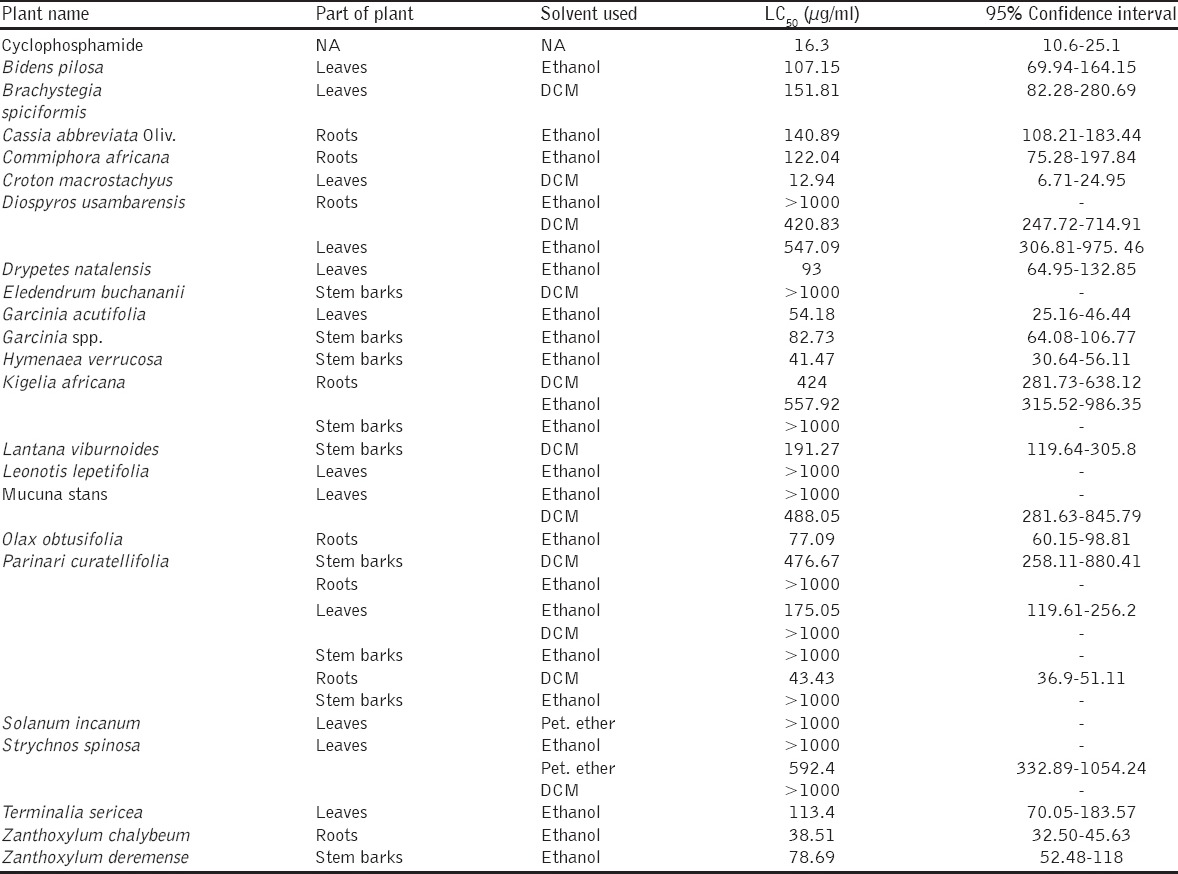
The brine shrimp test is used as a preliminary test for testing toxicity of a plant and anticancer activity after a single dose administration. In this study, the LC50 values were clustered per Moshi et al., [95]. The LC50 of <1.0 µg/ml is considered highly toxic; LC50 1.0-10.0 µg/ml is toxic; LC50 10.0-30.0 µg/ml - moderately toxic; LC50 > 30 < 100 µg/ml - mildly toxic and LC50 > 100 µg/ml as nontoxic. Studies done by Moshi et al., [96,97] provided the evidence that plant extract with the LC50 <20 µg/ml could be a source for anticancer compounds. The results from this study revealed that most (77.1%) of the plants tested were nontoxic with LC50 value <100 [Table 2]. The present findings imply that most compounds from these plants were safe for therapeutic use. Among the tested plant extracts dichloromethane extract of Croton macrostachyus had moderate toxicity with LC50 value 12.94 µg/ml.
Table 2.
Brine shrimp toxicity results of medicinal plants used in Southern Highland regions
DISCUSSION
Ethnobotanical Survey
Plant-based traditional medicine system continues to play an essential role in primary health care for the wider communities irrespective of the locality. This work has revealed the potential herbal medicines used in managing fungal infection in Njombe and Iringa Regions which are leading in spread of HIV infection in Tanzania with about 14.8% and 9.1% HIV prevalence, respectively [6,94]. Association of opportunistic fungal infections and HIV have been reported from the early days of the HIV/AIDS pandemic in Tanzania and worldwide [98]. The majority of the people living with HIV/AIDS are susceptible to fungal and bacterial opportunistic infections due to immunity suppression [37]. Availability of fungal herbal medicines may subsidize the effect of antifungal drugs resistance and availability to patients due to recurring fungal infections. The findings showed that remedies used in these communities consisted of one or a combination of two or more plant species. According to the traditional health practitioners, combinations of different plant species increases the efficiency of medicine and improves the cure’s power which could be due synergistic effects in treatment of various diseases. Most of plant species collected have been documented to be used in different African communities for the treatment of skin diseases [12]. Furthermore, the study noted that there was a wide use of the leaf part which could be considered as a good sign for the conservation of the environment and ensures sustainable utilization of plants.
Among the frequently mentioned plants, included Terminalia sericea, Aloe nutii, Aloe lateritia, Zanthoxylum chalybeum, Zanthoxylum deremense, and Kigelia africana. The claims on these plants have a special merit as they are also recorded in the literature to be useful in managing various microbial infections. Pharmacological studies by several authors have demonstrated the potency of the mentioned plants in terms of antifungal activity [12,16,21,27,30,81,99,100]. However, the proportion of claims made by traditional health practitioners in Makete and Mufindi districts concerning some of the plants documented in this study and which are supported by literature evidence of proven biological activity or similar ethnobotanical uses elsewhere is remarkable. The results also confirmed the supportive role of traditional health practitioners in offering health-care services to local communities in addition to available conventional medical cares.
Brine Shrimp Lethality Assay
Apart from efficacy, safety of herbal medicines is of paramount importance as little is documented about many plants that are used in traditional medicine. Findings from various studies have recommended brine shrimp assay as one of the methods for preliminary investigations of toxicity. This assay is also used in screening bioactive compounds from medicinal plants popularly used for several purposes and for monitoring the isolation of such biologically active compounds [101-103]. This work present few results from plant extracts that were tested for toxicity against brine shrimps. However, not all collected plant samples were screened for toxicity since during extraction yield was very little or none for some samples to be used for the testing. Findings obtained in this study showed that 77.1% of plant tested to be nontoxic supporting the popular use of medicinal plants by communities since they are regarded as safe therapeutic agents. Unlike other plants, C. macrostachyus exhibited high toxicity level that suggests its potential for anticancer agents. The LC50 of C. macrostachyus (12.94 µg/ml) is not statistically different to the standard anticancer drug cyclophosphamide (16.3 µg/ml). Other similar study undertaken on stem barks of this plant to evaluate cytotoxicity and acute toxicity in mice demonstrated the toxicity of the plant resulting in mortality of tested organisms [104]. The genus Croton has been reported to demonstrate moderate to high toxicities with proven the anticancer activity [51]. This knowledge triggers the use of plant products as complementary and alternative therapies both as direct and adjuvant remedy. A growing body of literature suggests the cancer preventive and therapeutic potential of phytochemicals and a lot of research has focused on the cellular mechanisms by which these phytochemicals interfere with the carcinogenic process. With the ability to target a variety of signaling pathways, phytochemicals are considered to be promising therapeutic agents against tumors with limited toxicity to normal cells.
CONCLUSION
The ethnobotanical survey has revealed that traditional health practitioners are rich in knowledge of fungal medicinal plants in these areas. These plants though have received little attention from modern biomedical research could be a promising source of knowledge for the discovery of useful remedies if this wealth is preserved through proper documentation and research. Most of the plants collected were ascertained to be safe for use and hence could be considered for further scientific studies. The reported species may be used for the development of new, affordable, and effective herbal formulations for antifungal health-care management or used in drug discovery.
ACKNOWLEDGMENTS
Authors are grateful to all traditional health practitioners in the study area for their support on data collection and sharing their knowledge on folk medicinal plants. Much appreciation goes to the Late Dr Joseph Magadula who participated in the initial planning of the work. Furthermore, Mr. Haji Selemani a botanist from the Department of Botany, University of Dar es salaam for identification of plant species studied. The study received the financial support from the Swedish Research Council.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Hosseinzadeh S, Jafarikukhdan A, Hosseini A, Armand R. The application of medicinal plants in traditional and modern medicine: A review of Thymus vulgaris. Int J Clin Med. 2015;6:635–42. [Google Scholar]
- 2.Singh KR, Dwivedi BS, Singh R. Traditional wisdom of farmers: An experience towards the sustainable development of livestock. Indian J Tradit Knowl Inaugural Issue. 2002;1:70–4. [Google Scholar]
- 3.Khan MH, Yadava PS. Antidiabetic plants in Thoubal district of Manipur, Northeast India. Indian J Tradit Knowl. 2010;9:510–4. [Google Scholar]
- 4.Chhabra SC, Uiso FC, Mshiu EN. Phytochemical screening of Tanzanian medicinal plants. I. J Ethnopharmacol. 1984;11:157–79. doi: 10.1016/0378-8741(84)90037-0. [DOI] [PubMed] [Google Scholar]
- 5.Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC), National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), and Macro International Inc. Tanzania HIV/AIDS and Malaria Indicator Survey 2007-08. Dares, Salaam, Tanzania. 2008. [Last accessed on 2015 Nov 10]. pp. 109–25. Available from: https://www.dhsprogram.com/pubs/pdf/AIS6/AIS6_05_14_09.pdf .
- 6.Mwaipopo R. Evaluation of TAHEA Supported “Mama Mkubwa” Initiative in Makete District, Iringa Region. Tanzania: UNICEF; 2005. [Last accessed on 2015 Nov 10]. p. 110. Available from: https://www.unicef.org/infobycountry/files/Tanzania_Mama_Mkubwa.pdf . [Google Scholar]
- 7.Sam TW. Toxicity testing using the brine shrimp Artemia salina. In: Colegate SM, Molyneux RJ, editors. Bioactive Natural Products Detection, Isolation, and Structural Determination. Boca Raton, FL: CRC Press; 1993. pp. 442–56. [Google Scholar]
- 8.Sharma N, Gupta PC, Singh A, Rao CV. Brine shrimp bioassay of Pentapetes phoenicea Linn. and Ipomoea carnea Jacq. Leaves. Der Pharm Lett. 2013;5:162–7. [Google Scholar]
- 9.Litchfield JT, Jr, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 10.Rojas JJ, Ochoa VJ, Ocampo SA, Muñoz JF. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complement Altern Med. 2006;6:2. doi: 10.1186/1472-6882-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiguba R, Ononge S, Karamagi C, Bird SM. Herbal medicine use and linked suspected adverse drug reactions in a prospective cohort of Ugandan inpatients. BMC Complement Altern Med. 2016;16:145. doi: 10.1186/s12906-016-1125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moshi MJ, van den Beukel CJ, Hamza OJ, Mbwambo ZH, Nondo RO, Masimba PJ, et al. Brine shrimp toxicity evaluation of some Tanzanian plants used traditionally for the treatment of fungal infections. Afr J Tradit Complement Altern Med. 2006;4:219–25. doi: 10.4314/ajtcam.v4i2.31211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Njau EA, Alcorn J, Ndakidemi P, Chirino-Trejo M, Buza J. Antimicrobial and antioxidant activity of crude extracts of Rauvolfia caffra var. Caffra (Apocynaceae) from Tanzania. Int J Biol. 2014;6:156–67. [Google Scholar]
- 14.Erasto P, Mbwambo ZH, Nondo RS, Namrita Lall N, Lubschagne A. Antimycobacterial, antioxidant activity and toxicity of extracts from the roots of Rauvolfia vomitoria and R. caffra. Spatula DD. 2011;1:73–80. [Google Scholar]
- 15.Ezeonwumelu JO, Julius AK, Muhoho CN, Ajayi AM, Oyewale AA, Tanayen JK, et al. Biochemical and histological studies of aqueous extract of Bidens pilosa Leaves from Ugandan rift valley in rats. Br J Pharmacol Toxicol. 2011;2:302–9. [Google Scholar]
- 16.Saini S, Kaur H, Verma B, Ripudaman Singh SK. Kigelia africana (Lam). Benth. An overview. Nat Prod Radiance. 2009;8:190–7. [Google Scholar]
- 17.Dos Santos MM, Olaleye MT, Ineu RP, Boligon AA, Athayde ML, Barbosa NB, et al. Antioxidant and antiulcer potential of aqueous leaf extract of Kigelia africana against ethanol-induced ulcer in rats. EXCLI J. 2014;13:323–30. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhen J, Guo Y, Villani T, Carr S, Brendler T, Mumbengegwi DR, et al. Phytochemical Analysis and Anti-Inflammatory Activity of the Extracts of the African Medicinal Plant Ximenia caffra. J Anal Methods Chem 2015. 2015:948262. doi: 10.1155/2015/948262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair JJ, Mulaudi RB, Chukwujekwu JC, Van Heerden FR. Antigonococcal activity of Ximenia caffra Sond. (Olacaceae) and identification of the active principle. [Last Accessed on 2015 Nov 28];South Afr J Bot. 2013 86:111–5. Available from: http://www.dx.doi.org/10.1016/j.sajb.2013.02.170 . [Google Scholar]
- 20.Cunningham A, Martin SS, Langenheim JH. Resin acids from two amazonian species of Hymenaea. Phytochemistry. 1973;12:633–5. [Google Scholar]
- 21.Hamza OJ, van den Bout-van den Beukel CJ, Matee MI, Moshi MJ, Mikx FH, Selemani HO, et al. Antifungal activity of some Tanzanian plants used traditionally for the treatment of fungal infections. J Ethnopharmacol. 2006;108:124–32. doi: 10.1016/j.jep.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Tsujino Y, Ogoche YIJ, Tazaki H, Fujimori T, Mori K. Buchaninoside, a steroidal glycoside from Elaeodendron buchananii. Phytochemistry. 1995;40:753–6. [Google Scholar]
- 23.Boora F, Chirisa E, Mukanganyama S. Evaluation of Nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. J Food Proc 2014. 2014. p. 9. Available from: http: //www.dx.doi.org/10.1155/2014/918018 .
- 24.Sylvanus U, Olakunle F, Amos J, Olutayo O. Antibacterial activity and phytochemical evaluation of the leaf root and stem bark extracts of Parinari curatellifolia (planch. ex benth) Int J Adv Chem. 2014;2:178–81. [Google Scholar]
- 25.Balemba OB, Bhattarai YY, Stenkamp-Strahm CC, Mellau LS, Mawe GM. The traditional anti-diarrheal remedy Garcinia buchananii stem bark extract, inhibits propulsive motility and fast synaptic potentials in the guinea pig distal colon. Neurogastroenterol Motil. 2010;22:1332–9. doi: 10.1111/j.1365-2982.2010.01583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boakye PA, Brierley SM, Pasilis SP, Balemba OB. Garcinia buchananii bark extract is an effective anti-diarrheal remedy for lactose-induced diarrhea. J Ethnopharmacol. 2012;142:539–47. doi: 10.1016/j.jep.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 27.Moshi MJ, Mbwambo ZH. Some pharmacological properties of extracts of Terminalia sericea roots. J Ethnopharmacol. 2005;97:43–7. doi: 10.1016/j.jep.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 28.Eldeen IM, Elgorashi EE, Mulholland DA, van Staden J. Anolignan B: A bioactive compound from the roots of Terminalia sericea. J Ethnopharmacol. 2006;103:135–8. doi: 10.1016/j.jep.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Mochizuki M, Hasegawa N. Anti-inflammatory effect of extract of Terminalia sericea roots in an experimental model of colitis. J Health Sci. 2007;53:329–31. [Google Scholar]
- 30.Fyhrquist P, Mwasumbi L, Haeggström CA, Vuorela H, Hiltunen R, Vuorela P. Ethnobotanical and antimicrobial investigation on some species of Terminalia and Combretum (Combretaceae) growing in Tanzania. J Ethnopharmacol. 2002;79:169–77. doi: 10.1016/s0378-8741(01)00375-0. [DOI] [PubMed] [Google Scholar]
- 31.Nkobole N, Houghton PJ, Hussein A, Lall N. Antidiabetic activity of Terminalia sericea constituents. Nat Prod Commun. 2011;6:1585–8. [PubMed] [Google Scholar]
- 32.Mutasa T, Mangoyi R, Mukanganyama S. The effects of Combretum zeyheri leaf extract on ergosterol synthesis in Candida albicans. JHerbs Spices Med Plants. 2015;21:211–7. [Google Scholar]
- 33.Masengu C, Zimba F, Mangoyi R, Mukanganyama S. Inhibitory activity of Combretum zeyheri and its S9 metabolites against Escherichia coli, Bacillus subtilis and Candida albicans. J Microb Biochem Technol. 2014;6:228–35. [Google Scholar]
- 34.Liu M, Katerere DR, Gray AI, Seidel V. Phytochemical and antifungal studies on Terminalia mollis and Terminalia brachystemma. Fitoterapia. 2009;80:369–73. doi: 10.1016/j.fitote.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Baba-Moussa F, Akpagana K, Bouchet P. Antifungal activities of seven West African Combretaceae used in traditional medicine. J Ethnopharmacol. 1999;66:335–8. doi: 10.1016/s0378-8741(98)00184-6. [DOI] [PubMed] [Google Scholar]
- 36.Moshi MJ, Mbwambo ZH, Kapingu MC, Mhozya VH, Marwa C. Antimicrobial and brine shrimp lethality of extracts of Terminalia mollis laws. Afr J Tradit Complement Altern Med. 2006;3:59–69. [Google Scholar]
- 37.Kisangau DP, Lyaruu HV, Hosea KM, Joseph CC. Use of traditional medicines in the management of HIV/AIDS opportunistic infections in Tanzania: A case in the Bukoba rural district. J Ethnobiol Ethnomed. 2007;3:29. doi: 10.1186/1746-4269-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masoko P, Eloff JN. The diversity of antifungal compounds of six South African Terminalia species (Combretaceae) determined by bioautography. Afr J Biotechnol. 2005;4:1425–31. [Google Scholar]
- 39.Nivedhini V, Chandran R, Parimelazhagan T. Chemical composition and antioxidant activity of Cucumis dipsaceus Ehrenb. Ex Spach fruit. Int Food Res J. 2014;21:1465–72. [Google Scholar]
- 40.Urs SK, Kumar HN, Chandana E, Chauhan JB. Evaluation of the antioxidant activity of Cucumis dipsaceus. J Microbiol Biotechnol Res. 2013;3:32–40. [Google Scholar]
- 41.Zubair MF, Oladosu IA, Olawore NO, Usman LA, Fakunle CO, Hamid AA, et al. Bioactive steroid from the root bark of Psorospermum corymbiferum. Chin J Nat Med. 2011;9:0264–6. [Google Scholar]
- 42.Abou-Shoer M, Boettner FE, Chang C, Cassady JM. Antitumour and cytotoxic xanthones of Psorospermum febrifugum. Phytochemistry. 1988;27:2795–800. [Google Scholar]
- 43.Kupchan SM, Streelman DR, Sneden AT. Psorospermin, a new antileukemic xanthone from Psorospermum febrifugum. JNat Prod. 1980;43:296–301. doi: 10.1021/np50008a010. [DOI] [PubMed] [Google Scholar]
- 44.Bum EN, Naami YF, Soudi S, Rakotonirina SV, Rakotonirina A. Psorospermum febrifugum spach (Hypericaceae) decoction antagonized chemically-induced convulsions in mice. Int J Pharmacol. 2006;1:118–21. [Google Scholar]
- 45.de Boer HJ, Kool A, Broberg A, Mziray WR, Hedberg I, Levenfors JJ. Anti-fungal and anti-bacterial activity of some herbal remedies from Tanzania. J Ethnopharmacol. 2005;96:461–9. doi: 10.1016/j.jep.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 46.Gupta N, V\ishnoi G, Wal A, Wal P. Medicinal value of Eurphorbia Tirucalli. Syst Rev Pharm. 2013;4:40–6. [Google Scholar]
- 47.Ngbolua KN, Tshibangu DS, Mpiana PT, Mihigo SO, Mavakala BK, Ashande MC, et al. Anti-sickling and antibacterial activities of some extracts from Gardenia ternifolia subsp. Jovis-tonantis (Welw.) Verdc. (Rubiaceae) and Uapaca heudelotii Baill. (Phyllanthaceae) J Adv Med Pharm Sci. 2015;2:10–9. [Google Scholar]
- 48.Malebo HM, Tanja W, Cal M, Swaleh SA, Omolo MO, Hassanali A, et al. Antiplasmodial, anti-trypanosomal, anti-leishmanial and cytotoxicity activity of selected Tanzanian medicinal plants. Tanzan J Health Res. 2009;11:226–34. doi: 10.4314/thrb.v11i4.50194. [DOI] [PubMed] [Google Scholar]
- 49.Teugwa MC, Sonfack DC, Fokom R, Penlap BV, Amvam ZP. Antifungal and antioxidant activity of crude extracts of three medicinal plants from Cameroon pharmacopeia. J Med Plant Res. 2013;7:1537–42. [Google Scholar]
- 50.Bantie L, Assefa S, Teklehaimanot T, Engidawork E. In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus Hocsht. (Euphorbiaceae) against Plasmodium berghei in mice. BMC Complement Altern Med. 2014;14:79. doi: 10.1186/1472-6882-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meireles DR, Fernandes HM, Rolim TL, Batista TM, Mangueira VM, de Sousa TK, et al. Toxicity and antitumor efficacy of Croton polyandrus oil against Ehrlich ascites carcinoma cells. Rev Bras Farmacogn. 2016;26:751–8. [Google Scholar]
- 52.Salatino A, Salatino ML, Negri G. Traditional uses, chemistry and pharmacology of croton species (Euphorbiaceae) J Braz Chem Soc. 2007;18:11–33. [Google Scholar]
- 53.Eisa MM, Almagboul AZ, Omer ME, Elegami AA. Antibacterial activity of Dichrostachys cinerea. Fitoterapia. 2000;71:324–7. doi: 10.1016/s0367-326x(99)00171-9. [DOI] [PubMed] [Google Scholar]
- 54.Adikay S, Konganti B, Prasad KV. Effects of alcoholic extract of roots of Dichrostacchys cinerea Wight & Arn. Against cisplatin-induced nephrotoxicity in rats. Nat Prod Radiance. 2009;8:12–8. [Google Scholar]
- 55.Jayakumari S, Rao GH, Anbu J, Ravichandiran V. Antidiarrhoeal activity of Dichrostachys cinerea (L.). WIGHT & ARN. Int J Pharm Pharm Sci. 2011;3:61–3. [Google Scholar]
- 56.Kokila K, Priyadharshini SD, Sujatha V. Phytopharmacological properties of Albizia species: A review. Int J Pharm Pharm Sci. 2013;5:70–3. [Google Scholar]
- 57.Moshi MJ, Kamuhabwa A, Mbwambo ZH, De Witte P. Cytotoxic screening of some tanzania medicinal plants. [Last accessed on 2014 Apr 26];East Centre Afr Pharm J. 2003 6:50–6. Available from: http://www.ajol.info/index.php/ecajps/article/viewFile/9700/14051 . [Google Scholar]
- 58.Leteane MM, Ngwenya BN, Muzila M, Namushe A, Mwinga J, Musonda R, et al. Old plants newly discovered Cassia sieberiana D.C. and Cassia abbreviata. Oliv. Root extracts inhibit in vitro HIV-1c replication in peripheral blood mononuclear cells (PBMCs) by different modes of action. J Ethnopharmacol. 2012;141:48–56. doi: 10.1016/j.jep.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 59.Erasto P, Majinda RR. Bioactive proanthocyanidins from the root bark of Cassia abbreviate. Int J Biol Chem Sci. 2011;5:2170–9. [Google Scholar]
- 60.Katerere DR, Eloff JN. Anti-bacterial and anti-oxidant activity of Hypoxis hemerocallidea (Hypoxidaceae): Can leaves be substituted for corms as a conservation strategy? South Afr J Bot. 2008;74:613–6. [Google Scholar]
- 61.Sikhakhane X. Evaluating the anticancer and antimicrobial properties of extracts from Hypoxis hemerocallidea (African potato) A Dissertation Submitted in Fulfillment of the Requirement for the Degree Magistrae Scientiae in Biochemistry at the University of Johannesburg. 2014. [Last accessed on 2015 Jun 04]. p. 262. Available from: http://www.hdl.handle.net/10210/11393 .
- 62.Baraza LD, Joseph CC, Munissi JJ, Nkunya MH, Arnold N, Porzel A, et al. Antifungal rosane diterpenes and other constituents of Hugonia castaneifolia. Phytochemistry. 2008;69:200–5. doi: 10.1016/j.phytochem.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 63.Lyantagaye LS. Medicinal potential of Commiphora sponthulata, Hugonia casteneifolia and Raphidiocystis chrysocoma indigenous to Tanzania. J Chem Biol Phys Sci. 2013;4:287–92. [Google Scholar]
- 64.Madzimure J, Nyahangare ET, Hamudikuwanda H, Hove T, Belmain SR, Stevenson PC, et al. Efficacy of Strychnos spinosa (Lam.). and Solanum incanum L. aqueous fruit extracts against cattle ticks. Trop Anim Health Prod. 2013;45:1341–7. doi: 10.1007/s11250-013-0367-6. [DOI] [PubMed] [Google Scholar]
- 65.Chukwudi US, Stephen BO. Phytochemical screening and antimicrobial properties of the leaf and stem bark extracts of Strychnos spinosa. Nat Sci. 2013;11:123–8. [Google Scholar]
- 66.Isa AI, Awouafack MD, Dzoyem JP, Aliyu M, Magaji RA, Ayo JO, et al. Some Strychnos spinosa (Loganiaceae) leaf extracts and fractions have good antimicrobial activities and low cytotoxicities. BMC Complement Altern Med. 2014;14:456. doi: 10.1186/1472-6882-14-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siddiqui S, Faizi S, Siddiqui BS. Constituents of Azadirachta indica Isolation and structure elucidation of a new antibacterial tetranortriterpenoid, mahmoodin, and a new protolimonoid, Naheedin. J Nat Prod. 1992;55:303–10. doi: 10.1021/np50081a005. [DOI] [PubMed] [Google Scholar]
- 68.Khalid SA, Duddeck H, Gonzalez-Sierra M. Isolation and characterization of an antimalarial agent of the neem tree Azadirachta indica. JNat Prod. 1989;52:922–6. doi: 10.1021/np50065a002. [DOI] [PubMed] [Google Scholar]
- 69.Salem MZ, Salem AZ, Camacho LM, Ali HM. Antimicrobial activities and phytochemical composition of extracts of Ficus species: An over view. Afr J Microbiol Res. 2013;7:4207–19. [Google Scholar]
- 70.Saleh B, Hammoud R, Al-Mariri A. Antimicrobial activity of Ficus sycomorus L. (Moraceae) leaf and stem-bark extracts against multidrug resistant human pathogens. J Inst Nat Fibres Med Plants. 2015;61:39–49. [Google Scholar]
- 71.Romeh AA. Phytochemicals from Ficus sycomorus L. leaves act as insecticides and acaricides. Afr J Agric Res. 2013;8:3571–9. [Google Scholar]
- 72.Bii C, Korir KR, Rugutt J, Mutai C. The potential use of Prunus africana for the control, treatment and management of common fungal and bacterial infections. J Med Plants Res. 2010;4:995–8. [Google Scholar]
- 73.Kadu CA, Parich A, Schueler S, Konrad H, Muluvi GM, Eyog-Matig O, et al. Bioactive constituents in Prunus africana : Geographical variation throughout Africa and associations with environmental and genetic parameters. Phytochemistry. 2012;83:70–8. doi: 10.1016/j.phytochem.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 74.Chhabra SC, Mahunnah RL, Mshiu EN. Plants used in traditional medicine in eastern Tanzania. V. Angiosperms (Passifloraceae to Sapindaceae) J Ethnopharmacol. 1991;33:143–57. doi: 10.1016/0378-8741(91)90173-b. [DOI] [PubMed] [Google Scholar]
- 75.Gakunju DM, Mberu EK, Dossaji SF, Gray AI, Waigh RD, Waterman PG, et al. Potent antimalarial activity of the alkaloid nitidine, isolated from a Kenyan herbal remedy. Antimicrob Agents Chemother. 1995;39:2606–9. doi: 10.1128/aac.39.12.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sibandze GF, van Zyl RL, van Vuuren SF. The anti-diarrhoeal properties of Breonadia salicina. Syzygium cordatum and Ozoroa sphaerocarpa when used in combination in Swazi traditional medicine. J Ethnopharmacol. 2010;132:506–11. doi: 10.1016/j.jep.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 77.Surabhi S, Leelavathi S. Anti-oxidant property of ethanolic extract of Catunaregam spinosa Thunb. Int J Drug Dev Res. 2010;2:399–403. [Google Scholar]
- 78.Madhavan V, Vedavathi B, Raju A, Murali A, Yoganarasimhan S. Sedative activity studies on the aqueous and alcohol extracts of the stem bark of Madanaphala - An ayurvedic drug (Catunaregam spinosa (Thunberg) Tiruvengadam) Asian J Tradit Med. 2011;6:203–10. [Google Scholar]
- 79.Varadharajan M, Sunkam Y, Magadi G, Rajamanickam D, Reddy D, Bankapura V. Pharmacognostical studies on the root bark and stem bark of Catunaregam spinosa (Thunb.) Tiruv. (Madanaphala) - An Ayurvedic drug. Spatula DD. 2014;4:89–99. [Google Scholar]
- 80.Kato A, Moriyasu M, Ichimaru M, Nishiyama Y, Juma FD, Nganga JN, et al. Isolation of Alkaloidal constituents of Zanthoxylum usambarense and Zanthoxylum chalybeum using ion-pair HPLC. J Nat Prod. 1996;59:316–8. [Google Scholar]
- 81.Olila D, Olwa-Odyek, Opuda-Asibo J. Antibacterial and antifungal activities of extracts of Zanthoxylum chalybeum and Warburgia ugandensis Ugandan medicinal plants. Afr Health Sci. 2001;1:66–72. [PMC free article] [PubMed] [Google Scholar]
- 82.Praveena A, Suriyavathana M. Phytochemical characterization of Toddalia asiatica. L Var. Floribunda stem. Asian J Pharm Clin Res. 2013;6:148–51. [Google Scholar]
- 83.Kariuki HN, Kanui TI, Yenesew A, Patel N, Mbugua PM. Antinocieptive and anti-inflammatory effects of Toddalia asiatica (L) Lam. (Rutaceae) root extract in Swiss albino mice. Pan Afr Med J. 2013;14:133. doi: 10.11604/pamj.2013.14.133.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tan GT, Pezzuto JM, Kinghorn AD, Hughes SH. Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J Nat Prod. 1991;54:143–54. doi: 10.1021/np50073a012. [DOI] [PubMed] [Google Scholar]
- 85.Yeboah EM, Majinda RR. Radical scavenging activity and total phenolic content of extracts of the root bark of Osyris lanceolata. Nat Prod Commun. 2009;4:89–94. [PubMed] [Google Scholar]
- 86.Yeboah EM, Majinda RR, Kadziola A, Muller A. Dihydro-beta-agarofuran sesquiterpenes and pentacyclic triterpenoids from the root bark of Osyris lanceolata. J Nat Prod. 2010;73:1151–5. doi: 10.1021/np900597w. [DOI] [PubMed] [Google Scholar]
- 87.Ooko EA. Evaluation of Anti-microbial activity of Osyris lanceolata (East African Sandalwood) JKUAT Abstracts of Post Graduate Thesis. 2009. [Last accessed on 2015 May 04]. Available from: http://www.journals.jkuat.ac.ke/index.php/pgthesis_abs/article/view/573 .
- 88.Adebayo-Tayo BC, Adegoke AA. Phytochemical and microbial screening of herbal remedies in Akwa Ibom State, South Southern Nigeria. J Med Plants Res. 2008;2:306–10. [Google Scholar]
- 89.Elekofehinti OO, Kade IJ. Aqueous extract of Solanum anguivi Lam. fruits (African Egg Plant) inhibit Fe2+ and SNP induced lipid peroxidation in Rat’s brain – In vitro. Der Pharm Lett. 2012;4:1352–9. [Google Scholar]
- 90.Elekofehinti OO, Kamdem JP, Bolingon AA, Athayde ML, Lopes SR, Waczuk EP, et al. African eggplant (Solanum anguivi Lam.). fruit with bioactive polyphenolic compounds exerts in vitro antioxidant properties and inhibits Ca2+- induced mitochondrial swelling. Asian Pac J Trop Biomed. 2013;3:757–66. doi: 10.1016/S2221-1691(13)60152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beaman-Mbaya V, Muhammed SI. Antibiotic action of Solanum incanum linnaeus. Antimicrob Agents Chemother. 1976;9:920–4. doi: 10.1128/aac.9.6.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sambo HS, Pam CS, Dahiru D. Effect of aqueous extract of Solanum incanum fruit on some serum biochemical parameters. Agric Bus Technol J. 2013;10:82–6. [Google Scholar]
- 93.Regassa A. The use of herbal preparations for tick control in western Ethiopia. J S Afr Vet Assoc. 2000;71:240–3. doi: 10.4102/jsava.v71i4.722. [DOI] [PubMed] [Google Scholar]
- 94.Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC), National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), and ICF International. Tanzania HIV/AIDS and Malaria Indicator Survey 2011-12: Key Findings. TACAIDS, ZAC, NBS, OCGS, and ICF International. 2013. [Last accessed on 2015 Jun 04]. pp. 103–19. Available from: http://www.measuredhs.com/pubs/pdf/AIS11/AIS11.pdf .
- 95.Moshi MJ, Innocent E, Magadula JJ, Otieno DF, Weisheit A, Mbabazi PK, et al. Brine shrimp toxicity of some plants used as traditional medicines in Kagera Region, north western Tanzania. Tanzan J Health Res. 2010;12:63–7. doi: 10.4314/thrb.v12i1.56287. [DOI] [PubMed] [Google Scholar]
- 96.Moshi MJ, Cosam JC, Mbwambo ZH, Kapingu M, Nkunya MH. Testing beyond ethnomedical claims: Brine shrimp lethality of some Tanzanian plants. Pharm Biol. 2004;42:547–51. [Google Scholar]
- 97.Moshi MJ, Mbwambo ZH, Nondo RS, Masimba PJ, Kamuhabwa A, Kapingu MC, et al. Evaluation of ethnomedical claims and brine shrimp toxicity of some plants used in Tanzania as traditional medicines. Afr J Tradit Complement Altern Med. 2006;3:48–58. [Google Scholar]
- 98.National Institute for Medical Research (NIMR), TB/HIV/Malaria: Challenges to the health systems in Africa in the era of globalization. Proceedings of the 19th Annual joint Scientific Conference of the National Institute for Medical Research, Arusha International Conference Centre, Arusha, Tanzania. March, 15-17. 2004:102. [Google Scholar]
- 99.Fahmy NM, Al-Sayed E, Singab AN. Genus Terminalia : A phytochemical and biological review. (Montin.) species. Med Aromat Plants. 2015;4:218. [Google Scholar]
- 100.Amri E, Kisangau D. P. Ethnomedicinal study of plants used in villages around Kimboza forest reserve in Morogoro, Tanzania. [Last accessed on 2015 Jun 16];J Ethnobiol Ethnomed. 2012 8:1. doi: 10.1186/1746-4269-8-1. Available from: http://www.ethnobiomed.com/content/8/1/1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quignard EL, Pohlit AM, Nunomura SM, Pinto AC, Santos EV, Morais SK, et al. Screening of plants found in Amazonas state for lethality towards brine shrimp. Acta Amazon. 2003;33:93–104. [Google Scholar]
- 102.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–4. [PubMed] [Google Scholar]
- 103.Neuwinger HD. African Ethnobotany: Poisons and Drugs: Chemistry, Pharmacology and Toxicology. Weinheim: Chapman and Hall, GmbH; 1996. pp. 4–5. [Google Scholar]
- 104.Mbiantcha M, Nguelefack TB, Ndontsa BL, Tane P, Kamanyi A. Preliminary Assessment of toxicity of Croton macrostachyus stem bark (Euphorbiaceae) extracts. Int J Pharm Chem Biol Sci. 2013;3:113–2. [Google Scholar]


