Abstract
Aim:
To evaluate the protective effect of Nigella sativa (NS) against nephrotoxicity of methotrexate (MTX) in mice.
Materials and Methods:
Four groups of Swiss albino male mice, eight in each group were used. The study was carried on between October 2014 and April 2015. Group 1 (control) were administered 0.3 ml distilled water orally daily for 21 days and injected with normal saline (0.25 ml) IP weekly. Group 2 (MTX group) were treated with MTX, 10 mg/kg IP weekly, while Group 3 were treated with 0.125 ml of NS oil by mouth daily and injected with normal saline (0.25 ml) IP weekly. Group 4 received 0.125 ml of NS oil by mouth daily and injected with 10 mg/kg MTX IP weekly. Oral treatments were administered using a special curved smooth tip nontraumatic metal needle and IP injections were given for 3 weeks at days 7, 14 and 21. Animals were sacrificed at day 23. Malondialdehyde (MDA) and glutathione (GSH) measurements were performed on kidney homogenate. Histopathology of the kidneys were prepared and examined.
Results:
MTX has resulted in a small elevation in MDA and reduction in GSH levels in kidney homogenate which was returned back to control values when NS and MTX were administered in combination. Statistical significance was achieved with elevation of GSH by MTX and NS compared to MTX alone. MTX caused histopathological changes suggesting nephrotoxicity in 6 animals out of 8, while no changes were found in all animals treated with MTX and NS.
Conclusion:
NS is protective against MTX-induced nephrotoxicity.
KEY WORDS: Antioxidant, methotrexate, Nigella sativa, kidney toxicity
INTRODUCTION
Methotrexate (MTX) was introduced in 1948 and remains one of the most commonly used antimetabolite agents in cancer therapy. It was approved for the management of various types of malignancies such as leukemia, breast cancer, and lymphoma [1]. It has been used also for nonmalignant diseases such as rheumatoid arthritis [2], psoriasis [3] and for the treatment of ectopic pregnancy [4]. MTX has serious side effects including hepatotoxicity, myelosuppression, pulmonary disorder, gastrointestinal toxicity [5,6] and nephrotoxicity [7]. There are various ways to minimize MTX nephrotoxicity such as intravenous hydration, leucoverin rescue, alkalinization of urine and the use of glucarpidase [8]. There is an increasing evidence from animal studies supporting the protective effect of a medicinal plant Nigella sativa (NS, black cumin) against nephrotoxicity produced by gentamicin [9], paracetamol [10], cadmium [11], cyclosporine A [12], doxorubicin [13], and cisplatin [14]. The protective effect of NS is mainly attributed to its antioxidant potential which has been inferred from the ability of NS in reversing drug induced changes in parameters of oxidative stress, malondialdehyde (MDA) and glutathione (GSH) levels, toward placebo levels [15,16]. This study, therefore, was designed to investigate the effect of NS against MTX-induced nephrotoxicity in mice.
MATERIALS AND METHODS
Animal Handling
Thirty-two adult Swiss albino male mice were obtained from the animal house at Basrah College of Medicine. Their weights ranged between 20 and 30 g and their ages between 4 and 6 weeks. The study was conducted between October 2014 and April 2015. The animals were kept for acclimatization in separate cages in the animal house 2 weeks before the study, with a 12:12 h light/dark cycle and at a room temperature around 25°C. A standard food was prepared in the form of pellets containing carbohydrate, starch, moisture, and a crude protein not <20% and a crude fat not <6% of the total weight of the pellet. Food and ordinary drinking water were provided ad-libitum. The animals were carefully handled in accordance with the internationally accepted guidelines for handling laboratory animals (National Institutes for Health USA Publication, 1985), and measures were undertaken to minimize pain and discomfort during experimentation. The study protocol was approved by a local Institutional Ethical Committee.
Preparation of NS
Seeds of NS were purchased from a local market in Basrah, and authenticated by an expert herbalist. A voucher specimen was kept in the Department of Pharmacology for future reference. Viability of the seeds was tested by cultivating 100 seeds in a small garden, implanted numbers of plants were counted and their growth was followed up for at least 4 weeks.
Extraction of the NS essential oil was performed in the Marine Science Center at Basrah University. The seeds were crushed by electric grinder into a fine powder then the essential oil was extracted with petroleum ether at 40-60°C via a soxholet apparatus (25% yield of essential oil) and the solvent was removed by rotatory evaporator under vacuum, then the oil was stored in dark sealed containers.
Study Design
The animals were divided into 4 groups (8 mice in each group). The first group (control) received (0.3 ml) of distilled water orally every day for 21 days and at the same time injected with (0.25 ml) normal saline IP weekly at days 7, 14 and 21 of the experiment. The second group (MTX group) were given distilled water (0.3 ml) orally each day and injected with MTX IP weekly (MTX ampule, Ebewe-Pharma, Austria) at a dose of (10 mg/kg) at days 7, 14 and 21, while animals in the third group received 0.125 ml of NS oil by mouth daily and injected with normal saline (0.25 ml) IP at the same weekly schedule. Animals in the fourth group received the same treatment of Group 3 but instead of normal saline, the animals were injected with MTX at a dose 10 mg/kg IP. Oral treatment was administered using a special curved smooth tip nontraumatic metal needle. Animals in all groups were sacrificed at day 23 after an overnight fasting. The following parameters were obtained; MDA and GSH in tissue homogenate of the kidney, specimens of kidney for histopathological examination, body weight and kidneys weight of the animals were measured and a ratio kidneys weight to animal body weight was calculated.
Measurement of MDA in Kidney Homogenates
MDA levels in kidney homogenates were measured by thiobarbituric acid (TBA) test, based on the reaction of MDA with TBA which produces a pink pigment that can be measured by a spectrophotometer at 532 nm [17].
Measurement of GSH in Kidney Homogenates
Enzyme-linked immunosorbent assay (ELISA) kit specific for mice (Cusabio reagents, Cusabio Laboratories, Wuhan, China) was used for measurement of GSH levels in kidney homogenates. This kit employs a technique known as “double antibody sandwich technique.” ELISA (HumaReader HS, Germany) is the equipment used for the measurement.
Histopathological Examination
Slides were prepared in duplicates from kidneys specimens, stained with hematoxylin and eosin. The slides were coded, blindly examined by a senior histopathologist using light microscopy (Olympus CX-series). Histopathological changes were graded as previously described by Morsy et al., [18]: 0 (Normal), 1 (mild), 2 (moderate), and 3 (severe), for 0%, 25%, 50%, and 75% histopathological changes of fields examined, respectively.
Statistical Analysis
SPSS version 19 (IBM, NY, USA) was used for statistical analysis. A nonparametric Mann–Whitney U-test for independent samples was used for comparing variables. The data were presented as mean ± SD, P < 0.05 is considered significant.
RESULTS
Effect of NS, MTX and their Combination on MDA in Kidney Homogenate
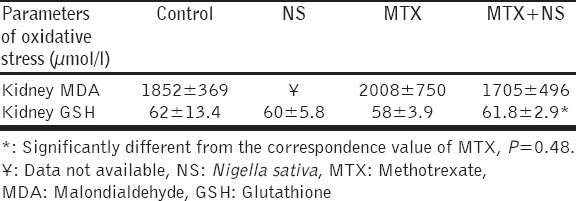
There was an increase in MDA level in the homogenate of the kidney in the group treated with MTX compared to the control group, however, the change was small and did not achieve statistical significance (1852 ± 369 to 2008 ± 750 µmol/L). MTX with NS had resulted in a small and insignificant reduction in MDA levels compared to the MTX treated group (1705 ± 496 vs. 2008 ± 750 µmol/L). The effect of NS on MDA levels was discarded from the analysis because of unexplained variability in the data possibly due to technical errors in measurement [Table 1].
Table 1.
Effect of NS, MTX and their combination on MDA and GSH levels in tissue homogenate of the kidney
Effect of NS, MTX and their Combination on GSH in Kidney Homogenate
There was a small and insignificant reduction in GSH level in the group of mice treated with MTX compared to the control. Treatment with NS has also resulted in a small and insignificant reduction in GSH compared to the control. While treatment with the combination MTX and NS resulted in elevation in GSH level which is statistically higher than the value of GSH of the MTX treatment (P = 0.048) but still lower than the level of the control (P = 0.065) [Table 1].
Effect of NS, MTX and their Combination on Animal Body Weight
Changes in animal body weight from day 1 to day 23 were recorded and a mean change from the corresponding pretreatment value was calculated for each treatment. The body weight of animals treated with normal saline (control group) for 23 days was increased compared to pretreatment value with a mean change of 1.02 ± 3.27 g, however, this increase did not achieve statistical significance, while a reduction in animal body weights were observed in the animals treated with MTX (−2.53 ± 2.41 g) or NS (−2.03 ± 2.57 g); these changes were significantly different from the control value (P = 0.01). Animal body weights increased again toward the control values in the group treated with the combination NS and MTX (1.01 ± 0.25 g) [Figure 1a].
Figure 1.
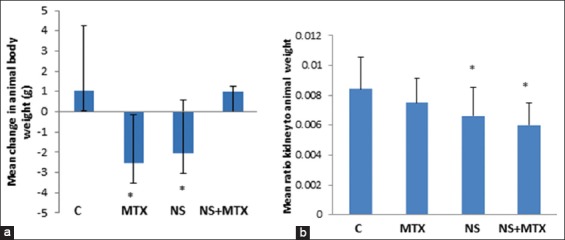
(a) Changes in animal body weights between day 1 (before treatment) and after 23 days of treatment with MTX, NS and the combination, (b) the ratio of kidney weights to animal weights. C: Control, MTX: Methotrexate, NS: Nigella sativa, NS+MTX: Combination of NS and MTX. *: Significantly different from the control (P=0.05)
Effect of NS, MTX and their Combination on Animal Kidney Weight Body Weight Ratio
A baseline kidney weight was not possible to obtain for comparison with kidney weight at day of sacrificing the animals, therefore, for this analysis it was assumed that kidney weight is proportionally related to animal’s body weight, such proportion is approximately valid as described by Gad, 2008 [19].
The ratios of kidney to animal weight for NS and the combination NS + MTX were significantly lower than the value of the control, P = 0.05 [Figure 1b]. The ratio for MTX was lower than that for the control but it did not reach statistical significance.
Histopathological Examination
Control and NS treated groups
There were no histopathological changes found in the control group or in the group treated with NS (Score 0). Glomeruli and renal tubules maintain normal morphological features [Table 2, Figure 2a and b].
Table 2.
Scoring of histopathological changes of kidney damage induced by treatments with NS, MTX and their combination
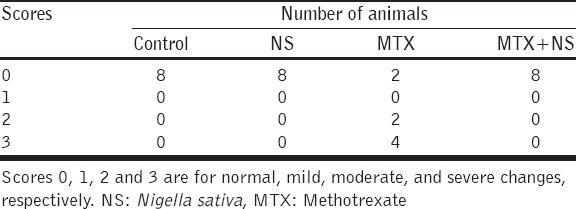
Figure 2.
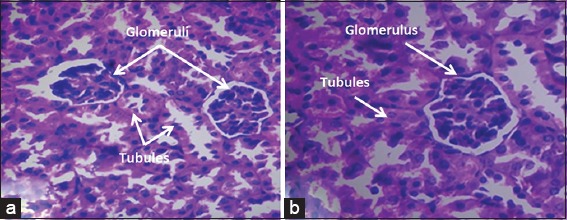
Renal tissue from a mouse treated with normal saline, (a) Nigella sativa, (b) histopathological features revealed normal glomeruli and tubules (×40 hematoxylin and eosin)
MTX treated group
Treatment with MTX produced no changes in two animals (Score 0), moderate changes in two (Score 2) and in four animals severe changes were seen (Score 3). The changes involved renal tubules and seemed not involving the glomeruli; these changes were focal degeneration of tubular epithelial cells, interstitial chronic inflammatory cell infiltration of lymphocytes and plasma cells and perinephric fat necrosis [Figure 3a].
Figure 3.
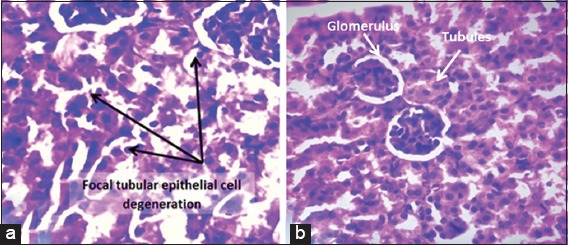
Renal tissue of a mouse treated with methotrexate (MTX), (a) showed severe focal degeneration of tubular epithelial cells (×40, hematoxylin and eosin [H&E]), (b) it is a renal tissue from a mouse treated with the combination Nigella sativa and MTX. Normal glomeruli and tubules are seen (×40, H&E)
NS and MTX groups
There were no histopathological changes (Score 0) detected in all animals (n = 8) treated with the combination NS + MTX [Figure 3b].
DISCUSSION
MTX is a drug basically used for its cytotoxic, anti-inflammatory, and antiproliferative effects [20], but organ toxicities are frequently reported, and may result in discontinuation of treatment. Thus attempts to minimize these toxicities are well appreciated. NS is a medicinal plant with a wide range of properties and effects on various body systems including hepatoprotective and nephroprotective properties [10,21].
This study was then designed to investigate the protective effect of NS against nephrotoxicity induced by MTX. Mice were selected as a convenient model for MTX nephrotoxicity.
Two parameters of oxidative stress (MDA and GSH) were assessed. Treatment with MTX has resulted in a slight increase in the level of MDA in kidney homogenate which is then slightly decreased in the group treated with NS and MTX combination; the changes were small and did not achieve statistical significance.
The rise in MDA and reduction in GSH may reflect oxidative effect of MTX, and the changes of these parameters toward placebo values following the combination NS with MTX may be attributed to the antioxidant effect of NS [22].
Histopathological examination of the kidneys was performed and used as a tool for confirmation of toxicity induced by MTX and to evaluate the nephroprotective effect of NS. There were no changes detected in the control and NS groups, while in the MTX treated group; tubular epithelial cell degeneration ranging in severity was observed in 6 of 8 treated animals.
This study revealed that MTX is toxic to the tubules and seems not affecting the glomeruli. This result, in part, is in agreement with the study of Morsy et al., 2013 [18] who reported nephrotoxicity in rats following IP administration of MTX at a dose of 7 mg/kg/day for 3 consecutive days. These authors observed severe histopathological changes including degeneration of the renal tubules as a result of direct toxicity of MTX with disruption of in-between tubules basement membranes with cystic luminal dilatation and flattening of lining cells, but in contrast to the findings of this study, these authors had reported degeneration of glomeruli induced by MTX treatment. There were many possibilities for the lack of effect of MTX on glomeruli in this study; first, it could be attributed to spacing of MTX dosing since in this study a dose of 10 mg/kg was given once weekly for 21 days rather than daily for 3 consecutive days; second, species differences may also contribute to differences in MTX effect on the glomeruli; finally, changes in glomeruli may exist but may not be seen by ordinary microscope.
NS treatment had resulted in preventing histopathological changes in the kidneys in all animals treated with NS and MTX.
MTX causes renal toxicity by different mechanisms including direct tubular toxicity [7] possibly due to oxidative stress [23] or due to accumulation and precipitation of MTX or its metabolites in distal tubules causing intrarenal obstruction leading to tubular damages. On the other hand, this may further decreases renal elimination of MTX leading to increased plasma levels and more toxicity.
It is not known for NS to have an effect on accumulation of MTX in renal tubules; therefore, it is possible for NS to minimize tubular damage caused by direct toxicity of MTX through its antioxidant potential [22].
Animal body weights were measured at the beginning and at the end of the experiment. There was a reduction in body weight in the group treated with MTX and in the group treated with NS. Reduction in body weight by MTX was reported by de Araújo et al. [24] which may be attributed to intestinal mucositis a finding noticed in this study and resulting in reduction in food consumption in the MTX and NS treated animals, in addition, diarrhea was noticed in animals treated with MTX, both these findings may contribute to weight loss in the animals. Reduction in body weight by NS and the mechanism behind this effect is not clearly understood.
Changes in animal’s body weight were paralleled with changes in kidneys weight. For the sake of comparison, changes in kidney weight were presented as a ratio of kidney to animal body weight. Small kidneys were observed in the group treated with NS or even smaller in the MTX treated group.
The significance of these changes and their effect on kidney function needs further investigations.
In medical practice absence of persistent kidney protective agents against drugs which produce kidney toxicity, herbs may offer an important alternative treatment in preventing kidney toxicity. It can be concluded that the medicinal plant NS (black cumin) is protective to the kidney against toxic effects of MTX. As NS demonstrates a good safety profile [25] it is worth trying NS orally along with MTX treatment to those patients who need this type of treatment.
Study Limitations
The mouse as a small animal has limited amount of blood sufficient to perform additional test such as renal function test or parameters of oxidative stress in the serum, therefore, the study depended on parameters obtained from homogenates of kidney tissue with further confirmation by histopathological examination.
ACKNOWLEDGMENTS
The authors would like to express appreciation and thanks to Dr. Sawsan S. Al-Haroon, assistant Professor, Department of Pathology, Basrah College of Medicine, for histopathological examination.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Weidmann A, Foulkes AC, Kirkham N, Reynolds NJ. Methotrexate toxicity during treatment of chronic plaque psoriasis: A case report and review of the literature. Dermatol Ther (Heidelb) 2014;4:145–56. doi: 10.1007/s13555-014-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazlewood GS, Barnabe C, Tomlinson G, Marshall D, Devoe D, Bombardier C. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: Abridged Cochrane systematic review and network meta-analysis. BMJ. 2016;353:i1777. doi: 10.1136/bmj.i1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrascosa JM, de la Cueva P, Ara M, Puig L, Bordas X, Carretero G, et al. Methotrexate in moderate to severe psoriasis: Review of the literature and expert recommendations. Actas Dermosifiliogr. 2016;107:194–206. doi: 10.1016/j.ad.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Cecchino GN, Araujo Júnior E, Elito Júnior J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417–23. doi: 10.1007/s00404-014-3266-9. [DOI] [PubMed] [Google Scholar]
- 5.Jakubovic BD, Donovan A, Webster PM, Shear NH. Methotrexate-induced pulmonary toxicity. Can Respir J. 2013;20:153–5. doi: 10.1155/2013/527912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neves C, Jorge R, Barcelos A. The network of methotrexate toxicity. Acta Reumatol Port. 2009;34:11–34. [PubMed] [Google Scholar]
- 7.Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 8.Tuffaha HW, Al Omar S. Glucarpidase for the treatment of life-threatening methotrexate overdose. Drugs Today (Barc) 2012;48:705–11. doi: 10.1358/dot.2012.48.11.1871575. [DOI] [PubMed] [Google Scholar]
- 9.Yaman I, Balikci E. Protective effects of nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp Toxicol Pathol. 2010;2:183–90. doi: 10.1016/j.etp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Aycan IÖ, Tokgöz O, Tüfek A, Alabalik U, Evliyaoglu O, Turgut H, et al. The use of thymoquinone in nephrotoxicity related to acetaminophen. Int J Surg. 2015;13:33–7. doi: 10.1016/j.ijsu.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Erboga M, Kanter M, Aktas C, Sener U, Fidanol Erboga Z, Bozdemir Donmez Y, et al. Thymoquinone ameliorates cadmium-induced nephrotoxicity, apoptosis, and oxidative stress in rats is based on its anti-apoptotic and anti-oxidant properties. Biol Trace Elem Res. 2016;170:165–72. doi: 10.1007/s12011-015-0453-x. [DOI] [PubMed] [Google Scholar]
- 12.Uz E, Bayrak O, Uz E, Kaya A, Bayrak R, Uz B, et al. Nigella sativa oil for prevention of chronic cyclosporine nephrotoxicity: An experimental model. Am J Nephrol. 2008;28:517–22. doi: 10.1159/000114004. [DOI] [PubMed] [Google Scholar]
- 13.Elsherbiny NM, El-Sherbiny M. Thymoquinone attenuates Doxorubicin-induced nephrotoxicity in rats: Role of Nrf2 and NOX4. Chem Biol Interact. 2014;223:102–8. doi: 10.1016/j.cbi.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Hosseinian S, Khajavi Rad A, Hadjzadeh MA, Mohamadian Roshan N, Havakhah S, Shafiee S. The protective effect of Nigella sativa against cisplatin-induced nephrotoxicity in rats. Avicenna J Phytomed. 2016;6:44–54. [PMC free article] [PubMed] [Google Scholar]
- 15.Bayrak O, Bavbek N, Karatas OF, Bayrak R, Catal F, Cimentepe E, et al. Nigella sativa protects against ischaemia/reperfusion injury in rat kidneys. Nephrol Dial Transplant. 2008;23:2206–12. doi: 10.1093/ndt/gfm953. [DOI] [PubMed] [Google Scholar]
- 16.Tayman C, Cekmez F, Kafa IM, Canpolat FE, Cetinkaya M, Tonbul A, et al. Protective effects of Nigella sativa oil in hyperoxia-induced lung injury. Arch Bronconeumol. 2013;49:15–21. doi: 10.1016/j.arbres.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Morsy MA, Ibrahim SA, Amin EF, Kamel MY, Rifaai RA, Hassan MK. Curcumin ameliorates methotrexate-induced nephrotoxicity in rats. Adv Pharmacol Sci 2013. 2013:3–1. doi: 10.1155/2013/387071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gad SC. Animal Models in Toxicology. 2nd ed. New York, NY: CRC/Taylor & Francis; 2008. pp. 814–5. [Google Scholar]
- 20.Shen S, O’Brien T, Yap LM, Prince HM, McCormack CJ. The use of methotrexate in dermatology: A review. Australas J Dermatol. 2012;53:1–18. doi: 10.1111/j.1440-0960.2011.00839.x. [DOI] [PubMed] [Google Scholar]
- 21.Kushwah DS, Salman MT, Singh P, Verma VK, Ahmad A. Protective effects of ethanolic extract of Nigella sativa seed in paracetamol induced acute hepatotoxicity in vivo. Pak J Biol Sci. 2014;17:517–22. doi: 10.3923/pjbs.2014.517.522. [DOI] [PubMed] [Google Scholar]
- 22.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–8. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 23.Devrim E, Cetin R, Kiliçoglu B, Ergüder BI, Avci A, Durak I. Methotrexate causes oxidative stress in rat kidney tissues. Ren Fail. 2005;27:771–3. doi: 10.1080/08860220500244823. [DOI] [PubMed] [Google Scholar]
- 24.de Araújo AA, Borba PB, de Souza FH, Nogueira AC, Saldanha TS, Araújo TE, et al. In a methotrexate-induced model of intestinal mucositis, olmesartan reduced inflammation and induced enteropathy characterized by severe diarrhea, weight loss, and reduced sucrose activity. Biol Pharm Bull. 2015;38:746–52. doi: 10.1248/bpb.b14-00847. [DOI] [PubMed] [Google Scholar]
- 25.Qidwai W, Hamza HB, Qureshi R, Gilani A. Effectiveness, safety, and tolerability of powdered Nigella sativa (kalonji) seed in capsules on serum lipid levels, blood sugar, blood pressure, and body weight in adults: Results of a randomized, double-blind controlled trial. J Altern Complement Med. 2009;15:639–44. doi: 10.1089/acm.2008.0367. [DOI] [PubMed] [Google Scholar]


