Abstract
Background
Both Attention Deficit Hyperactivity Disorder (ADHD) and chronic cannabis (CAN) use have been associated with brain structural abnormalities, although little is known about the effects of both in young adults.
Methods
Participants included: those with a childhood diagnosis of ADHD who were CAN users (ADHD_CAN; n=37) and non-users (NU) (ADHD_NU; n=44) and a local normative comparison group (LNCG) who did (LNCG_CAN; n=18) and did not (LNCG_NU; n=21) use CAN regularly. Multiple regressions and MANCOVAs were used to examine the independent and interactive effects of a childhood ADHD diagnosis and CAN group status and age of onset (CUO) on subcortical volumes and cortical thickness.
Results
After controlling for age, gender, total brain volume, nicotine use, and past-year binge drinking, childhood ADHD diagnosis did not predict brain structure; however, persistence of ADHD was associated with smaller left precentral/postcentral cortical thickness. Compared to all non-users, CAN users had decreased cortical thickness in right hemisphere superior frontal sulcus, anterior cingulate, and isthmus of cingulate gyrus regions and left hemisphere superior frontal sulcus and precentral gyrus regions. Early cannabis use age of onset (CUO) in those with ADHD predicted greater right hemisphere superior frontal and postcentral cortical thickness.
Discussion
Young adults with persistent ADHD demonstrated brain structure abnormalities in regions underlying motor control, working memory and inhibitory control. Further, CAN use was linked with abnormal brain structure in regions with high concentrations of cannabinoid receptors. Additional large-scale longitudinal studies are needed to clarify how substance use impacts neurodevelopment in youth with and without ADHD.
Keywords: ADHD, ADHD persistence, cannabis, marijuana, early onset, young adults, MRI, cortical thickness
1. INTRODUCTION
Attention-Deficit/Hyperactivity Disorder (ADHD) is the most common neurodevelopmental disorder in childhood, with worldwide prevalence estimated at 5.3% (Polanczyk et al., 2007). ADHD is characterized by developmentally inappropriate inattention, impulsiveness, and hyperactivity (DSM-5). Meta-analyses have found several cognitive deficits associated with ADHD, especially in sustained attention and executive functions such as working memory, response inhibition, risky decision-making, and planning and shifting (Hervey et al., 2004; Lijffijt et al., 2005; Oosterlaan et al., 1998; Willcutt et al., 2005, 2012). Consistent with these deficits in executive functioning, individuals with childhood diagnosis of ADHD demonstrate comorbidity with substance use disorders (SUD), including increased risk for earlier onset of substance use (e.g., Charach et al., 2011; Lee et al., 2011; Molina et al., 2007; Sullivan and Rudnik-Levin, 2001), including cannabis (CAN) use (Lee et al., 2011; Molina et al., 2013; Pingault et al., 2013). Of concern, CAN use is on the rise in the United States, with 23% of high school seniors and approximately 20% of college students reporting past month use (Johnston et al., 2015). Cannabis is independently associated with neurocognitive deficits, especially in prefrontal regions (Lisdahl et al., 2014); therefore, CAN exposure may be particularly concerning in youth with ADHD.
ADHD in childhood and early adolescence appears to affect several neuronal regions, with abnormalities seen in the prefrontal cortex (PFC), anterior (ACC) and posterior cingulate cortex, basal ganglia, insula, cerebellum and parietal, temporal and occipital cortices (Castellanos and Proal, 2012; Castellanos et al., 2003; Cherkasova and Hechtman, 2009; Frodl and Skokauskas, 2012; Pastura et al., 2011; Peng et al., 2013). Adolescence is marked by ongoing neurodevelopment, including pruning of the cortical gray matter and increases in white matter (Barnea-Goraly et al., 2005; Bava et al., 2010; Giedd et al., 1999; Giorgio et al., 2010; Gogtay and Thompson, 2010; Jernigan et al., 1991; Simmonds et al., 2014; Sowell et al., 2002; Toga et al., 2006). It has been proposed that brain structural abnormalities in childhood ADHD represent a delay in this neuromaturation, as one large prospective longitudinal study demonstrated that children with ADHD had a marked delay in cortical, especially in PFC regions, compared to controls (Shaw et al., 2007). Some have suggested that as children with ADHD age through adolescence, brain differences normalize. Indeed, some cross-sectional and longitudinal studies have noted childhood ADHD structural abnormalities in striatal regions improve as they transition to adolescence (Castellanos et al., 2002; McAlonan et al., 2009). Therefore, studying the neurocognitive correlates of ADHD in children may not generalize to adolescents and young adults.
Around half of those with childhood ADHD will demonstrate persistent ADHD symptoms into adulthood (Barkley et al., 2002), chiefly related to inattention (Faraone et al., 2006). Persistent adult ADHD is linked with poorer academic achievement and underemployment (Pingault et al., 2011; Polderman et al., 2010). In a prospective longitudinal study following 152 children with ADHD and 139 matched controls, Castellanos and colleagues (2002) noted that abnormalities observed in childhood ADHD in the PFC, temporal, and cerebellar regions continued to be abnormal as the cohort aged into adolescence. Consistent with these findings in another longitudinal study, Shaw and colleagues (2014) found that individuals with ADHD demonstrated abnormal striatal development from childhood into adolescence compared to controls (Shaw et al., 2014).
Studies conducted in adults with ADHD (which have been primarily cross-sectional, disproportionately male, and the majority above age 25) suggest that enduring symptoms result from permanently reduced cortical thickness or volumes in several brain regions. These include the frontal cortex: superior frontal gyrus (Almeida et al., 2010; Biederman et al., 2008; Makris et al., 2007; Proal et al., 2011; Seidman et al., 2006), dorsolateral prefrontal cortex (DLPFC; Makris et al., 2007), precentral gyrus (Proal et al., 2011; Almeida Montes et al., 2013; Makris et al., 2007), ACC (Amico et al., 2011; Biederman et al., 2008; Makris et al., 2007; Proal et al., 2011; Seidman et al., 2006), inferior frontal gyrus (IFG; Depue et al., 2010), middle frontal gyrus (Proal et al., 2011) and orbitofrontal cortex (OFC; Hesslinger et al., 2002; Almeida Montes et al., 2013). Reduced volume and thickness have been noted in other cortical regions, including the occipital cortex (Ahrendts et al., 2011; Proal et al., 2011), parietal cortex [postcentral gyrus (Almeida Montes et al., 2013), inferior parietal (Makris et al., 2007; Proal et al., 2011), precuneus (Proal et al., 2011), superior parietal (Almeida Montes et al., 2013), and temporal pole (Proal et al., 2011). Subcortical regions that are abnormal in adults with ADHD include the caudate (Almeida et al., 2010; Almeida Montes et al., 2010; Onnink et al., 2014; Proal et al., 2011; Seidman et al., 2011), amygdala (Frodl et al., 2010), hippocampus in medicated individuals (Onnink et al., 2014), nucleus accumbens (Seidman et al., 2006), and cerebellum (Biederman et al., 2008). Therefore, studies in adults with persistent ADHD have found brain structural abnormalities that are also seen in childhood (Castellanos et al., 2003; Castellanos and Proal, 2012; Cherkasova and Hechtman, 2009; Frodl and Skokaukas, 2012; Pastura et al., 2011; Peng et al., 2013). However, it is notable that most of these studies included samples that were, on average, older than twenty-five years of age (Ahrendts et al., 2011; Almeida et al., 2010; Almeida Montes et al., 2010; Amico et al., 2011; Biederman et al., 2008; Clerkin et al., 2013; Frodl et al., 2010; Hesslinger et al., 2002; Makris et al., 2007; Mattfeld et al., 2014; Onnink et al., 2014; Perlov et al., 2008; Seidman et al., 2011, 2006; Almeida Montes et al., 2013) when most gray matter neuromaturation is complete (e.g., Giedd et al., 1999) and in older samples, gray matter may be reducing due to aging. For example, older adults may actually be demonstrating more rapid reductions in gray matter and white matter compared to younger samples. Further, middle-aged or older adults with ADHD may have different comorbidity profiles (e.g., more severe SUD or metabolic disorders), or more severe trajectories than those who demonstrate remission of symptoms during adolescence or young adulthood. Therefore, studies in middle-aged adults may not necessarily generalize to adolescents and young adults who were diagnosed with ADHD in childhood.
Two longitudinal studies, to date, have focused on brain structural differences in those who had persistent versus remitted ADHD as they age into late adolescence and young adulthood (Proal et al., 2011; Shaw et al., 2013). Shaw and colleagues (2013) followed a cohort of 92 participants with childhood ADHD who received a structural MRI at baseline (average age 10) and again in young adulthood (average age 24). Of those with childhood ADHD, 37 had persistent ADHD while 55 remitted. They found that in the young adults, the number of ADHD symptoms was positively correlated with cortical thinning in frontal (cingulate cortex, medial PFC, paracentral gyrus), parietal (precuneus, postcentral gyrus), and fusiform (Shaw et al., 2013) regions. In a similar study, Proal and colleagues (2011) found that males diagnosed with childhood ADHD that persisted into adulthood (n=17) demonstrated thinner cortex in frontal (precentral, middle frontal, frontal pole, ACC) and occipital regions compared to controls and those with remitted ADHD (n=26). Although this cohort was followed through age 25, the neuroimaging analysis was conducted when the cohort was age 41. Thus, like most of the cross-sectional studies, results may be specific to middle-adulthood. In summary, both longitudinal studies suggest that structural abnormalities observed in childhood ADHD are seen in persistent adult ADHD, especially in frontal (Proal et al., 2011; Shaw et al., 2013) and parietal (Shaw et al., 2013) regions.
Despite the aforementioned comorbidity between ADHD and SUD, it is notable that most studies examining the impact of adult ADHD on brain structure did not exclude for SUD (Almeida et al., 2010; Almeida Montes et al., 2013; Biederman et al., 2008; Hesslinger et al., 2002; Makris et al., 2007; Proal et al., 2011; Seidman et al., 2006, 2011). Others that did exclude for SUD, but did not examine the potential impact of frequent CAN or binge drinking exposure on brain structure (Ahrendts et al., 2011; Amico et al., 2011; Depue et al., 2010; Frodl et al., 2010; Perlov et al., 2008; Onnink et al., 2014). Only one study to our knowledge (Proal et al., 2011) statistically examined the impact of alcohol use disorders (AUD) and SUD (a mixed variable primarily including CAN use disorders) on brain structure (this investigation did not yield a significant relation between AUD/SUD and brain structure). Importantly, Proal et al. did not specifically examine how frequency of CAN or binge drinking might influence brain structure- an important variable when examining neurocognitive effects (Lorenzetti et al., 2014). As a result, it is impossible to rule out the potential impact of CAN, especially given the average age of CAN use initiation is between 15–16 (Degenhardt et al., 2008) and CAN is the most commonly used illicit drug in individuals with ADHD (Lee et al., 2011; Molina et al., 2013).
Disruption of the endogenous endocannabinoid system by exogenous CAN exposure may be particularly concerning in youth with ADHD, who may already demonstrate a neurodevelopmental lag (e.g., Castellanos et al., 2002; Shaw et al., 2014, 2007). The major psychoactive ingredient of CAN, THC (delta-9-tetrahydrocannabinol), produces its effects through attaching to the cannabinoid 1 receptor (CB1) in the brain. In humans, CB1 receptors are localized on both axons and glial cells (Mackie, 2005) and demonstrate high density in the PFC, parietal, limbic, and striatal regions (Terry et al., 2009). Daily young adult CAN users have demonstrated significant downregulation of the CB1 density throughout the cortex, cingulate, insula, hippocampus, and parahippocampal gyrus (Hirvonen et al., 2012). The endogenous endocannabinoid system undergoes developmental changes during the adolescence, when the CB1 density peaks (Belue et al., 1995; Howlett et al., 2002) and some have argued that the endocannabinoid system plays a direct role in neurodevelopment, moderating neurotransmitter release, neurogenesis, and regulating glial cell activity (Viveros et al., 2005). Indeed, studies have shown that the adolescent brain may be particularly sensitive to CAN effects; preclinical research has reported increased cellular changes associated with THC (delta-9-tetrahydrocannabinol; the major psychoactive component of CAN) exposure during adolescence compared to adulthood (Cha et al., 2006; Kang-Park et al., 2007; Quinn et al., 2008; Rubino and Parolaro, 2008; Schneider and Koch, 2003). For example, THC exposure in adolescence resulted in reduced hippocampal synaptic connections and cognitive impairment that lasted into adulthood (Rubino et al., 2009). Further, given that the endocannabinoid system interacts with the adrenergic system, especially in the PFC (Cathel et al., 2014), regular CAN use in youth with ADHD may result in further disruption of the adrenergic attentional system, worsening the neurodevelopmental trajectory in youth with ADHD.
Regular CAN use in youth has been linked with neurocognitive abnormalities (see Batalla et al., 2013; Lisdahl et al., 2014), especially in those with an early age of CAN use onset (see Lisdahl, 2013, 2014 for reviews). For example, individuals with an adolescent CUO (before the age of 15–18 depending on the study) were more likely to demonstrate cognitive problems, including lowered IQ and poorer attention, verbal memory, visual search, verbal fluency, and executive function (Ehrenreich et al., 1999; Gruber et al., 2012; Medina et al., 2007; Pope et al., 2003; Wilson et al., 2000) and abnormalities in brain function and structure (Becker et al., 2010a, 2010b; Churchwell et al., 2010; Gruber et al., 2011; Jager et al., 2010; Lopez-Larson et al., 2011; Meier et al., 2012; Wilson et al., 2000).
With few exceptions (Tzilos et al., 2005), investigations have reported structural abnormalities in regular CAN-using youth in the frontal cortex (ACC, OFC, insula, paracentral gyrus), lingual temporal, inferior and superior parietal cortex, hippocampus, amygdala, nucleus accumbens, and cerebellum (Ashtari et al., 2011; Churchwell et al., 2010; Demirakca et al., 2011; Jacobus et al., 2014; Jarvis et al., 2008; Kumra et al., 2012; Lopez-Larson et al., 2011; Lorenzetti et al., 2015; Mata et al., 2010; McQueeny et al., 2011; Medina et al., 2009, 2010, 2007; Schacht et al., 2012; Yücel et al., 2008; Cousijn et al., 2012; Price et al., 2015), with the most prominent findings in the PFC and hippocampus (Lorenzetti et al., 2014)- areas that have high CB1 receptors density (Terry et al., 2009). Most studies in adult or young adult samples have demonstrated decreased volumes across brain regions in association with CAN use (see Lisdahl et al., 2014). In contrast, within younger adolescent samples (e.g., 16–18 years), CAN use is often related to increased volumes and thickness (e.g., Medina et al., 2009, 2010; McQueeny et al., 2011; Lopez-Larson et al., 2011), suggesting CAN during early adolescent years may disrupt the healthy pruning process (Lisdahl et al., 2014). However, at least one study found thinner cortices in adolescent users (Jacobus et al., 2014). Further, another study found that abnormal OFC structures predicted the initiation of CAN use (Cheetham et al., 2012), making it difficult to determine causal relationships. In sum, both age of CAN use onset and current CAN use in youth are associated with brain structure abnormalities, although the direction of findings and causal relationships need to be confirmed.
Given this high comorbidity (Pingault et al., 2013), examining the unique and interactive effects of both ADHD and CAN use and age of onset on brain structure in young adults is of great interest. This study utilized neuroimaging data collected as part of the Multimodal Treatment of Attention-Deficit/Hyperactivity Disorder (MTA) study, a longitudinal study following children with ADHD and a local normative comparison group from ages 7 to 9 (baseline) into young adulthood (average age 24; see Tamm et al., 2013 for details). For our first aim, we examined the independent and interactive effects of childhood ADHD and regular CAN use on whole brain cortical thickness and subcortical (caudate, nucleus accumbens, hippocampus, amygdala) and cerebellar gray matter volumes. For our secondary aims, we assessed whether persistent versus remitted ADHD diagnostic status predicted structural brain differences after controlling for CAN use status.
We also investigated whether adolescent age of CUO significantly predicted brain morphometry in the ADHD group. We hypothesized that both ADHD and CAN use status would each significantly predict reduced cortical thickness and subcortical volumes, whereas the subgroup with comorbid ADHD and CAN use would demonstrate the greatest reductions in cortical thickness (including prefrontal, parietal regions) and subcortical (amygdala, hippocampus, nucleus accumbens, caudate) and cerebellar gray matter volumes. We also hypothesized that those with persistent ADHD would demonstrate greater structural reductions compared with remitters and controls (Proal et al., 2011; Shaw et al., 2013). Finally, we hypothesized that early CUO would be associated with thicker cortex in prefrontal regions and greater gray matter volumes in the amygdala and nucleus accumbens areas, perhaps due to delayed gray matter neuromaturation and increased reward-center dendritic branching due to early cannabis exposure (Kolb et al., 2006; Gilman et al., 2014).
2. METHODS
The study was approved by each of the six MTA site’s Institutional Review Boards (University of Pittsburgh, Universities of California, Irvine and Berkeley, New York University, Duke University, and Columbia University). Informed consent was obtained from all participants prior to initiating the study sessions.
2.1 Participants
Participants for the current neuroimaging study were recruited from the longitudinal follow-up of the multi-site MTA (either after 14 or 16 years after study enrollment in childhood) (see Tamm et al., 2013 for further description). Original MTA participants included 579 children aged 7.0 to 9.9 years diagnosed in childhood with ADHD Combined Type, plus age- and neighborhood-matched children in a local normative comparison group (LNCG, n=289), recruited two years later. ADHD and LNCG participants were followed longitudinally with visits at 3, 6, 8, 10, 12, 14, and 16 years after baseline assessment of the ADHD group. Details regarding the MTA procedures for initial diagnosis, demographic information, and treatment specifics have been described previously ("A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD," 1999). For the current neuroimaging study, participants were brought in for an MRI and neuropsychological evaluation at one of six sites (Newman et al., 2015).
Inclusion Criteria: A participant was classified as a CAN User if he or she reported using CAN monthly or more often during the previous year, and as a CAN Non-user (NU) if he or she had used CAN fewer than 4 times during the previous year. Exclusion Criteria: Exclusionary criteria included magnetic resonance imaging (MRI) contraindications (e.g., orthodontic braces, claustrophobia), neurologic injury or a history of traumatic brain injury with loss of consciousness or that occurred in the past year, and current use of psychotropic medications other than for ADHD. Although psychiatric diagnoses were not exclusionary, the sample had very low rates of comorbid psychiatric disorder diagnoses [no participants met criteria for past year anorexia, bulimia, dysthymia, mania, generalized anxiety disorder, social phobia, panic disorder, and schizophrenia; the rest of the diagnostic counts were: conduct disorder (n=2), major depression (n=1), agoraphobia (n=1), obsessive compulsive disorder (n=1), post-traumatic stress disorder (n=1)]. Participants were also excluded if they self-reported binge drinking (drinking ≥5 drinks in a single session) ≥1 time/week, as well as monthly or greater recreational use of other substances (e.g., cocaine, narcotics, hallucinogens, etc.). Abstinence from CAN was required for 36 hours before the MRI scan. Using these selection procedures, 81 ADHD (37 CAN and 44 NU) and 39 LNCG (18 CAN and 21 NU) were enrolled, totaling 119 participants for the primary group analyses. Participants ranged in age from 21–27 years and were 80% male (see Table 1 for more details).
Table 1.
Participant Demographic and Drug Use Characteristics by Group.
| LNCG Non-user (n=21) Mean (SD) or % |
LNCG CAN User (n=18) Mean (SD) or % |
ADHD Non-user (n=44) Mean (SD) or % |
ADHD CAN User (n=37) Mean (SD) or % |
|
|---|---|---|---|---|
| Age* | 23.4 (1.5) | 23.6 (1.5) | 24.6 (1.4) | 24.3 (1.3) |
| % Male | 67% | 89% | 77% | 92% |
| % Caucasian | 57% | 78% | 62% | 49% |
| IQ | 105.9 (23.4) | 110.9 (22.8) | 103.5 (16.8) | 101.9 (13.6) |
| % Current ADHD meds | 0% | 0% | 5% | 12% |
| Cannabis use onset (CUO) age | - | 17.0 (2.8) range 13–23 |
- | 15.3 (2.9) range 10–22 |
| % Early CUO (<16 yo) | - | 31% | - | 47% |
| % Past year daily cannabis use* | 0% | 50% | 0% | 62% |
| Days used cannabis past month* | 0.1 (0.3) | 19.9 (9.2) | 0.1 (0.4) | 15.2 (11.6) |
| % 0 yrs regular cannabis use | 81% | 11% | 76% | 14% |
| % 2> yrs regular cannabis use | 19% | 50% | 5% | 65% |
| % Smoke Cigarettes | 19% | 33% | 26% | 57% |
| Age Regularly Drank Alcohol | 19.0 (1.8) | 18.4 (1.9) | 19.1 (2.5) | 18.0 (2.8) |
| Past Year Binge Episodes* | 2.6 (2.1) | 4.9 (2.4) | 3.9 (2.4) | 4.0 (2.7) |
Notes:
p<.05, see text for details.
2.2 Design and Procedure
As described in Tamm and colleagues (2013), potential participants were identified based on participant responses to the Substance Use Questionnaire (Molina et al., 2013; Molina and Pelham, 2003) obtained at the year 14 or 16 MTA follow-up visit. The study was described to participants and additional screening for inclusion/exclusion criteria was conducted (e.g., brain injury screen; Bogner and Corrigan, 2009). Eligible participants returned for a single session during which neuropsychological measures (see Tamm et al., 2013) were completed, followed by an MRI scan. All participants observed a minimum of 24-hour abstinence period for drugs and alcohol, a 1-hour abstinence period for nicotine and caffeine, and a 24-hour abstinence period for over the counter and prescription medications prior to the cognitive testing.
2.3 Measures
2.3.1 Persistence of ADHD Symptoms
Participants who were diagnosed with ADHD in childhood were classified in young adulthood as either “persistent” or “desistent” based on self and/or parent-report data from the Conners’ Adult ADHD Rating Scale (CAARS; Conners et al., 2002) at the 12, 14, or 16 year follow-up assessment of the primary MTA study. ADHD was considered “persistent” if they had either a self-report or a parent-report (or both) of at least 4 symptoms in at least one domain (i.e., inattentive, hyperactive/impulsive) that were endorsed as either occurring “often” or “very frequently”. ADHD participants were classified as “desistent” if both self-report and a parent-report included 3 or fewer symptoms that were rated as occurring “often” or “very frequently”. Further details regarding this persistence definition can be found in Sibley et al. (under review).
2.3.2 Substance Use Questionnaire and Substance Use Recency Questionnaire (SUQ, SURQ; Molina et al., 2013; Molina and Pelham, 2003)
The SUQ assesses past 12-month use of alcohol, tobacco products, CAN, and other drugs. It was administered throughout the longitudinal study beginning at the 2 year follow-up and, therefore, also prospectively measured age of regular (weekly) use onset for CAN and alcohol. Age of regular CAN Use Onset (CUO) was calculated only in those who reported at least one time-point of weekly CAN use during their 14–16 years of participation in the longitudinal MTA study. A modified version of the SUQ, the SURQ, administered for the current study (MRI protocol), measured the number of days the participant used CAN, alcohol, nicotine, and other drugs in the past 30 days (e.g., Tamm et al., 2013). From this measure, a binary nicotine use variable indicated whether or not participants reported current cigarette smoking. These measures were modeled after similar substance use measures that rely on confidential youth self-report (Molina et al., 2013). An NIH Certificate of Confidentiality was obtained to strengthen assurance of privacy.
2.4 MRI Data Acquisition and Pre-Processing
2.4.1 MRI Acquisition
High-resolution anatomical MPRAGE T1-weighted images (TR/TE/TI=2170/5.56/1100ms, 160 sagittal slices, TH=1.2mm, in-plane resolution=1×1mm) were acquired along with T2-weighted images (TR/TE=6440/67ms) co-planar to the functional acquisitions. Pulse sequence parameters used across scanner manufacturers and models were optimized for equivalence in contrast properties and consistency in image-derived quantitative measures.
2.4.2 Structural MRI Pre-processing
High-resolution anatomical images for each subject were processed using the FreeSurfer’s (http://surfer.nmr.mgh.harvard.edu) semi-automated surface-based analysis: (1) images are pre-processed for spatial (Talairach) and signal intensity normalization; (2) brain tissues are segmented by labeling white matter, gray matter, and subcortical and cerebellar regional volumes are calculated (Dale et al., 1999); (3) outer gray matter and white matter boundaries are identified to define the cortical surface and converted to a mesh of over 150,000 tessellated vertices to allow point-to-point surface measures; and (4) cortical thickness (in millimeters) is measured as the distance between corresponding vertices of the white matter and gray matter surfaces (Fischl and Dale, 2000). Trained MRI technicians inspected all images to assess for editing needs as described by the FreeSurfer workflow (see https://surfer.nmr.mgh.harvard.edu/fswiki/FreeSurferWiki). Manual interventions were made by putting in control points to distinguish gray matter/white matter boundaries when errors occurred. Editing the subcortical segmentation was performed when a voxel was incorrectly labeled, which occurred exclusively in the cerebellar cortex. To control for type I error, a Monte Carlo simulation was performed for each voxel-wise analysis to determine the number of voxels exceeding the statistical threshold that is required to protect against family-wise error at p=.05 (Smith et al., 2006).
2.5 Data Analysis
All dependent variables were normally distributed and there was no evidence of multicollinearity in any of the analyses. All preliminary demographic and subcortical analyses were conducted using SPSS v21; ANOVAs and Chi-square tests were run to examine potential demographic and drug use differences between groups. For all subcortical analyses run in SPSS, corrections for False Discovery Rate (FDR), using the Benjamini and Hochberg method, were conducted for each hemisphere. Finally, all whole-brain cortical thickness analyses were run in Freesurfer; monte carlo simulations (1000 iterations) were run to perform a cluster-wise correction at p=.05.
2.5.1 Aim One Analyses
Standard least squares multiple regressions were used to examine whether ADHD group, CAN group, and CAN*ADHD interactions significantly predicted subcortical volumes. Age, gender, total brain volume and group status were in block one. Block two included CAN*ADHD, binge drinking and nicotine use status. Block two was interpreted if any covariates were significant, otherwise Block one results are reported. To determine whether ADHD group and CAN use status influence cortical thickness, a voxel-wise ANCOVA was conducted in Freesurfer qdec modeling cortical thickness with ADHD group, CAN group, and CAN*ADHD interactions as predictors, while covarying gender and age. The cluster threshold size needed to achieve a cluster-wise p value of .05 was 771 mm^2. Significantly different regions were then exported into SPSS to examine whether past year binge drinking or nicotine use status affected results.
2.5.2 Aim Two Analyses
To examine whether Aim One results were influenced by persistence of ADHD symptoms, five MANCOVAs were conducted to examine whether ADHD persistence status (controls, desisters, persisters), CAN group, and CAN*ADHD persistence interactions significantly predicted subcortical volumes after controlling for total brain volume, gender, age, past-year binge-drinking episodes, and nicotine use status. To determine whether ADHD persistence status and CAN use status affected cortical thickness, a voxel-wise ANCOVA was conducted in Freesurfer qdec modeling cortical thickness with ADHD persistence status, CAN group, and CAN*ADHD persistence interactions as predictors, covarying gender and age. The cluster threshold size needed to achieve a cluster-wise p value of .05 was 756 mm^2. Significantly different regions were exported into SPSS to examine whether past year binge drinking and nicotine use status affected results.
Finally, we conducted a series of multiple regressions to examine whether early CUO (younger than 16 vs. 17 and older) significantly predicted subcortical volumes after covarying total brain volume, gender, age (block one) and age of regular alcohol use onset and nicotine use status (block two). To determine whether early CUO affected cortical thickness, we conducted a voxel-wise ANCOVA in Freesurfer, modeling cortical thickness with CUO as the primary predictor, while covarying age and gender. The cluster threshold size needed to achieve a cluster-wise p value of .05 was 770 mm^2. Follow-up regressions in SPSS were conducted to ensure results were not affected by age of regular alcohol use onset and nicotine use status.
3. RESULTS
3.1 Demographic and Drug Use Information
3.1.1 Demographics by CAN and ADHD Groups (N=120)
ANOVAs and chi-square tests revealed that groups did not differ significantly with respect to gender [x2(1)7.0, p=.07], ethnicity [x2(18)18.3, p=.44], or baseline IQ [F(3,118)=1.03, p=.38]. Groups did significantly differ in terms of age [F(3,118)=4.3, p=.006]: the LNCG groups were approximately one year younger than the ADHD groups at the follow-up MRI scan. See Table 1. Given the differences in age and marginally significant differences in gender, these variables were statistically controlled in all analyses.
3.1.2 Drug Use by CAN and ADHD Groups
The CAN group had an average length of abstinence from CAN of 24.9 days (SD=105; range 1–731). The groups did not significantly differ in age of CUO [F(3,60)=1.3, p=.28], age of regular alcohol use onset [F(3,100)=1.3, p=.28], or past-month binge-drinking episodes [F(3,118)=0.8, p=.51]. They significantly differed in terms of past-year use of CAN [F(3,118)=5.05, p<.001], days of CAN use in the past month [F(3,118)=51.9, p<.001], number of assessments reporting CAN exposure [F(3,118)=22.3, p<.001], past-year binge-drinking episodes [F(3,118)=2.68, p=.05], and nicotine use status [x2(3)11.7, p=.008]. As expected, NU groups (LNCG-NU and ADHD-NU) reported significantly less CAN use than both the CAN user groups (LNCG-CAN and ADHD-CAN). The LNCG-NU group demonstrated significantly less past-year binge drinking compared to the LNCG-CAN group (ADHD subgroups did not significantly differ). Past-year binge-drinking episodes were covaried in all subsequent analyses.
3.1.3 Demographics by Persistence (n=52) and Desistence (n=23) ADHD vs. LNCG (n=39)
ANOVAs and chi-square tests revealed that persisters, desisters, and LNCG groups did not significantly differ with respect to gender [x2(2)1.6, p=.45], ethnicity [x2(10)=5.2, p=.88], or baseline IQ [F(2,120)=1.13, p=.33]. Groups did significantly differ in terms of age [F(2,120)=6.69, p=.002].
3.1.3.1 Drug Use by Persistence vs. Desistence ADHD
Groups did not differ by age of CUO [F(2,59)=.75, p=.48], past-year CAN use [F(2,120)=.57, p=.57], number of assessments reporting CAN exposure [F(2,120)=.93, p=.40], age of regular alcohol use onset [F(2,103)=.59, p=.56], past-month binge-drinking episodes [F(2,120)=.29, p=.75], past-year binge-drinking episodes [F(2,120)=.44, p=.64], and nicotine smoking status [x2(2)=3.8, p=.15]. They marginally differed on past-month CAN use [F(2,120)=2.51, p<.09], with LNCG group reporting less than desisters.
3.1.4 Demographics by CUO within ADHD Group (n=41)
ANOVAs and chi-square tests revealed that in the ADHD groups, early and late CUO subgroups did not differ in gender [x2(1)=.03, p=.96], ethnicity [x2(5)9.2, p=.11], age [F(1,40)=.43, p=.52], or baseline IQ [F(1,40)=1.03, p=.32].
3.1.4.1 Drug Use by CUO
Groups did not differ in past-year CAN use [F(1,40)=.54, p=.47], past month CAN use [F(1,40)=1.1, p<.29], past-month binge-drinking episodes [F(1,40)=1.2, p=.28], past-year binge-drinking episodes [F(1,39)=.03, p=.86], or nicotine use status [x2(1).27, p=.61]. As expected, groups did differ on age of CUO [F(1,40)=70.3, p<.001], number of assessments reporting CAN exposure [F(1,40)=.6.5, p=.02], and age of regular alcohol use onset [F(1,40)=4.2, p=.05], with early CUO demonstrating earlier age of regular CAN and alcohol use onset and greater number of assessments with CAN use reported.
3.2 Brain Morphometry Findings
Prior to analyzing the primary aims, we confirmed that MRI site did not significantly predict subcortical volumes or cortical thickness (p’s>.10); this is consistent with other multi-site MRI studies demonstrating low between-scanner variability in cortical thickness (Han et al., 2006; Dewey et al., 2010; Jovicich et al., 2006) as well as a previous analysis utilizing the current sample (Newman et al., 2015).
3.2.1 Subcortical Volumes: ADHD and CAN Group
After controlling for age, gender, total brain volume, CAN group status, binge drinking and nicotine use, childhood ADHD did not significantly predict brain structure. CAN users demonstrated significantly smaller left hippocampal volumes [beta=−.18, p=.04; FDR corrected p=.20]. Increased past-year binge drinking significantly predicted smaller left caudate [beta=−.22, p=.008], right caudate [beta=−.18, p=.03], and right nucleus accumbens [beta=−.22, p=.02] volumes. Nicotine use status did not predict subcortical or cerebellar structure in this sample.
3.2.1.1 Cortical Thickness: CAN and ADHD Group
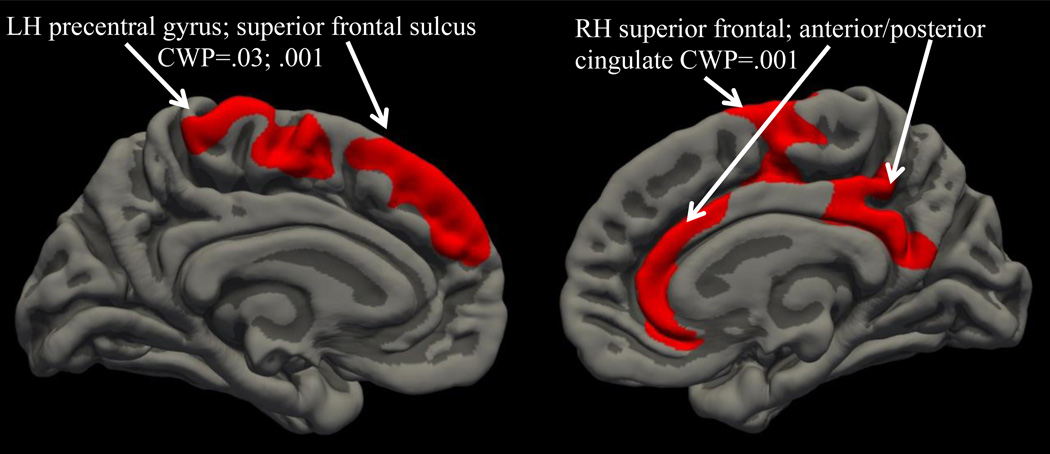
Childhood ADHD diagnosis did not significantly predict cortical thickness. However, CAN users had reduced cortical thickness in a right hemisphere region that included the superior frontal sulcus, anterior and posterior cingulate [cluster 1: size 3649 mm2, location MNIX 5.1, MNIY −46, MNIZ 24.2; cluster-wise p value (CWP)=.001]. CAN users also demonstrated thinner left hemisphere superior frontal sulcus and precentral gyrus (cluster 1: size 870 mm2, location MNIX 47.2, MNIY 23.1, MNIZ 19.4; CWP=.03) and superior frontal sulcus (cluster 2: size 1256 mm2, location MNIX 52.1, MNIY −8, MNIZ 21.9; CWP=.001; see Figure 1). Binge drinking and nicotine use status did not significantly predict these clusters.
Figure 1.
Whole brain cluster-corrected analysis examining impact of ADHD and cannabis use on cortical thickness; red color indicates cortical thickness is reduced in cannabis users compared to non-using controls (medial and inferior views).
3.2.2 Subcortical Volumes: Persistence of ADHD
MANCOVAs revealed no differences in subcortical structures between the ADHD persister vs. desister subgroups [pillai’s trace= .46, p=.77].
3.2.2.1 Cortical Thickness: Persistence of ADHD
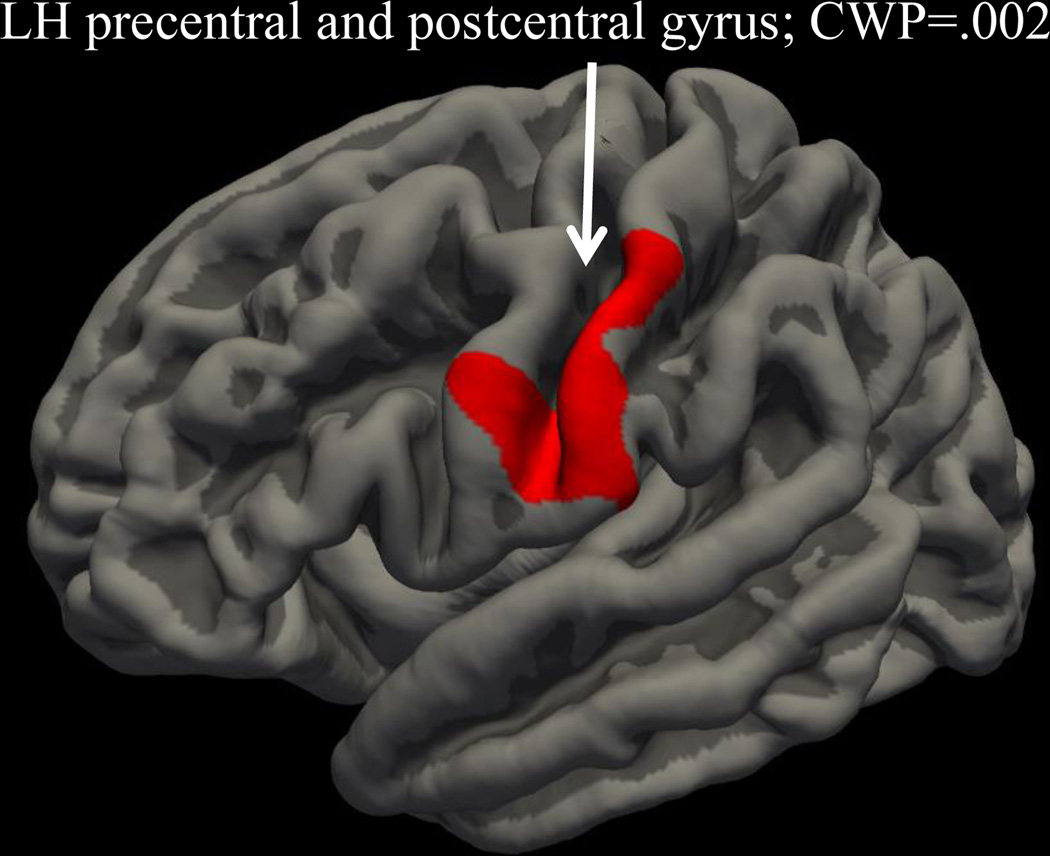
Whole-brain cortical thickness, correcting for family-wise error, revealed that after controlling for age, gender, and CAN use, those with persistent ADHD demonstrated significantly thinner left precentral/postcentral (cluster 1: size 1191 mm2, location MNIX 12.6, MNIY 2.4, MNIZ 44.7; CWP=.002) cortical thickness compared to the LNCG group (see Figure 2). Binge drinking and nicotine use did not significantly predict this cluster.
Figure 2.
Whole brain cluster-corrected analysis examining impact of ADHD persistence on cortical thickness; red color indicates cortical thickness is reduced in persistent ADHD group compared to LNCG group (lateral view).
3.2.3 Subcortical Volumes: CUO
After controlling for age, gender, total brain volume, and age of regular alcohol use onset, and nicotine use status, early CUO was associated with significantly larger left nucleus accumbens volume [beta=−.34, p=.02; FDR corrected p=.10] and marginally larger right nucleus accumbens volume [beta=−.17, p=.10; FDR corrected p=.50]. Nicotine use status [beta=.35, p=.02] and later age of alcohol use onset [beta=.35, p=.02] also significantly predicted larger left nucleus accumbens volume.
3.2.3.1 Cortical Thickness: CUO
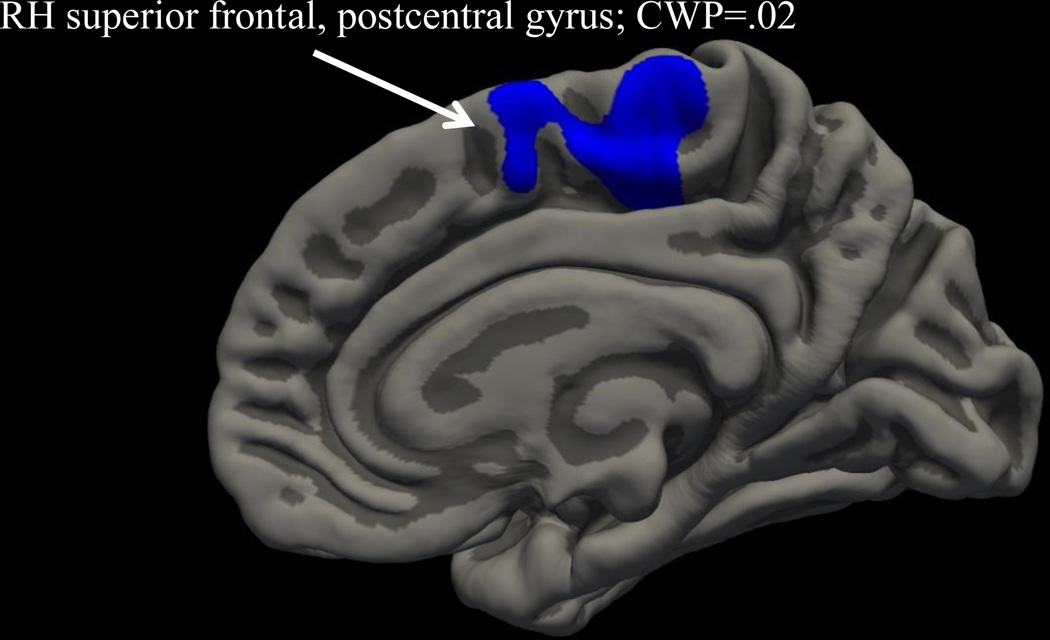
Whole-brain cortical thickness analysis, correcting for family-wise error, revealed that after controlling for age and gender, early CUO in those with ADHD predicted greater right hemisphere superior frontal and postcentral (Cluster 1: size 883 mm2, location MNIX 4.7, MNIY −27.4, MNIZ 65.9; CWP=.02) cortical thickness compared to late onset CUO (see Figure 3). (Age of onset of regular alcohol use and nicotine use status did not predict cortical thickness in these clusters).
Figure 3.
Whole brain cluster-corrected analysis examining impact of cannabis use onset (CUO) on cortical thickness; blue color indicates cortical thickness is greater in early onset CUO compared to late onset (medial view).
4. DISCUSSION
We examined structural neuroimaging data collected as part of the MTA longitudinal study following children with ADHD and a local comparison group from ages 7 to 9.9 into young adulthood (see Tamm et al., 2013 for details). The goal was to examine the impact of ADHD diagnosis (childhood and current), CAN use (frequency and age of onset), and their interaction on subcortical and cerebellar volumes and cortical thickness. Controlling for demographics, gender, binge drinking, nicotine and CAN use, we found that childhood ADHD diagnosis did not predict any brain morphometry measures, although individuals who had persistent ADHD into young adulthood had significantly thinner left precentral and postcentral cortical thickness compared to the LNCG group. Furthermore, after controlling for demographics, binge drinking, nicotine use, and ADHD diagnosis, CAN users had reduced cortical thickness in bilateral superior frontal sulcus, right anterior and posterior cingulate, and left precentral gyrus. Additionally, early CUO was associated with significantly thicker right superior frontal gyrus and postcentral gyrus compared to later CUO. These findings highlight the need to screen for CAN and binge drinking in youth with ADHD, as regular use of these substances may worsen their neurodevelopmental trajectory.
Although childhood diagnosis of ADHD did not predict morphometry after our rigorous statistical control, those individuals who demonstrated persistent symptoms of ADHD into young adulthood had significantly thinner left precentral and postcentral cortical thickness compared to desisters and LNCG groups. The persistent diagnosis was based on a prospectively refined phenotype of 4-plus symptoms at year 14–16 follow-up (based on either self or parent report and prior studies supporting these methods (Barkley et al., 2002; Sibley et al., 2012). Precentral and postcentral cortical areas have been implicated in inhibitory control (Ma et al., 2012; Pliszka et al., 2006) and working memory load (Jaeggi et al., 2003). This study lends further evidence that several abnormalities observed in childhood ADHD may mature by young adulthood (e.g., Castellanos et al., 2002; Nakao et al., 2011; Shaw et al., 2007), especially in those who experience remission of their symptoms. Still, those with persistent ADHD into young adulthood continued to demonstrate structural abnormalities in regions underlying inhibitory control and working memory load. Additional large-scale longitudinal studies examining neurocognitive development in youth with ADHD are needed to replicate these findings.
These findings are not consistent with previous research that has implicated structural abnormalities in young adults with ADHD, including the superior frontal gyrus, cingulate cortex, precentral gyrus, postcentral gyrus, precuneus, hippocampus, caudate, amygdala, and nucleus accumbens (Amico et al., 2011; Almeida et al., 2010; (Almeida Montes et al., 2013; Biederman et al., 2008; Frodl et al, 2010; Makris et al., 2007; Onnink et al., 2014; Proal et al., 2011; Seidman et al., 2006). However, in our sample, past-year binge alcohol and CAN use predicted abnormalities in these same regions. Therefore, inconsistencies in the literature regarding ongoing structural abnormalities in young adults with ADHD may relate to inadequate statistical control of comorbid substance use in past research (Pingault et al., 2013), as only one study to date reported statistically controlling for SUD (Proal et al., 2011) and no studies have controlled for recent exposure or age of regular use onset. Future studies examining the trajectory of brain development in youth with ADHD will need to closely measure and control for frequency and quantity of substance use exposure.
After controlling for ADHD diagnosis, age, gender, and binge drinking, we found that CAN users had smaller left hippocampal volumes. However, this finding did not survive FDR correction and the effect size was small. Still, these findings are consistent with previous animal models (e.g., Rubino et al., 2009) and studies demonstrating abnormal hippocampal volumes in regular CAN users (Ashtari et al., 2011; Demirakca et al., 2011; Medina et al., 2007; Schacht et al., 2012; for review see Lorenzetti et al., 2014), including a sample of male CAN users who did not have significant comorbid alcohol use (Lorenzetti et al., 2015). CAN users also demonstrated thinner bilateral superior frontal sulcus, right anterior and posterior cingulate, and left precentral gyrus. This pattern is consistent with that of Lopez-Larson (2011), who found reduced cortical thickness in bilateral superior frontal cortices in adolescent CAN users, although they also found abnormalities in the insula, lingual gyrus, superior temporal, inferior and superior parietal, and left paracentral regions. A lack of significant findings in these regions in the current sample may relate to our sample’s older age (24 vs. 17 years), less recent CAN use exposure (approximately half the use), and shorter duration of use in the current cohort compared to the Lopez-Larson (2011) sample. Further, abnormalities found by Lopez-Larson (2011) in paracentral and parietal regions, as outlined below, may be driven by the early age of CUO in their sample (15.7 years old). Still, overall, the current findings are consistent with reviews demonstrating CAN-related abnormalities in the frontolimbic network (Lisdahl et al., 2014), regions that have dense CB1 receptors (Terry et al., 2009).
This report also adds further evidence to the hypothesis that early onset of regular CAN use is associated with worse neurocognitive outcomes in youth with ADHD (Lisdahl et al., 2013; Rubino et al., 2008; Tamm et al., 2013). This may be due to disruption in endocannabinoid-mediated neurodevelopment (i.e., disrupted pruning and myelination) and abnormal neuromodulation of the adrenergic attentional system (Viveros et al., 2005; Cathel et al., 2014). Specifically, we found that youth with ADHD who began using CAN use early (age 16 and younger) had larger left nucleus accumbens and thicker right superior frontal gyrus and postcentral gyrus compared to later CAN use onset. Although the nucleus accumbens finding was only marginally significant after correction of multiple comparisons due to a small effect size, this is consistent with animal findings suggesting enhanced dendritic branching in the reward center following drug exposure in this region (McDonald et al., 2005) and human findings of abnormal left nucleus accumbens shape in regular CAN users (Gilman et al., 2014). Still, due to the small effect size, this finding needs to be replicated. Taken together, these studies support the theory that CAN use in adolescence may sensitize the reward network to drugs of abuse (Churchwell et al., 2012; De Bellis et al., 2013), increasing risk for CAN use disorders (Winters and Lee, 2008). Consistent with the observed PFC abnormalities, our group (Tamm et al., 2013) previously reported that in a similar sample, individuals with an early CUO also demonstrated poorer executive functioning (decision-making, working memory, response inhibition). Interestingly, studies in adults with ADHD also report abnormalities in these regions (Almeida et al., 2010; Almeida Montes et al., 2013; Biederman et al., 2008; Makris et al., 2007; Proal et al., 2011; Seidman et al., 2006). Therefore, additional research is needed to examine how early onset of regular CAN use impacts the trajectory of brain development in youth with ADHD.
Although binge drinking was not the primary focus of the current study, it is important to note that past-year binge drinking frequency significantly predicted reduced bilateral caudate, left amygdala, and right nucleus accumbens volumes after controlling for CAN use, age, gender, and ADHD status. Further, although binge drinking did not predict the significant clusters in this study, it is important to note that we did not conduct a whole-brain cortical thickness analysis with binge drinking as the primary predictor. Several studies have now reported structural abnormalities associated with binge drinking in youth, including reduced bilateral cerebellar volumes (Lisdahl et al., 2013), poorer white matter integrity (Bava et al., 2013; McQueeny et al., 2009), and abnormal prefrontal and cingulate cortical thickness (Squeglia et al., 2012). High dose of alcohol exposure has been linked with reduced cholinergic and dopaminergic neurotransmitter gene signaling, upregulation of neuronal death, atrophy and reduced synaptic refinement (Coleman et al., 2011; Pascual et al., 2007; Vallés et al., 2004). We did not find significant reductions in cerebellar volume linked to binge drinking, despite previous findings in teens (Lisdahl et al., 2013). Differences in outcomes may be due to an older cohort (average age 24 vs. 18) and combined effects of alcohol and CAN, which may have opposing effects on cerebellar volumes (Medina et al., 2010). Future studies will need to focus on the combined, and independent, effects of binge drinking, CAN, and ADHD on brain structure throughout adolescence into young adulthood.
Limitations of this study are important to consider. First, subgroup sample sizes for the secondary analyses examining the impact of age of CAN use onset (n=41), persistence (n=52), and desistence (n=23) were relatively small. Further, the current study was not able to examine the potential impact of ADHD medication on brain structure, as only 5% of ADHD non-users and 12% of ADHD CAN users reported current medication use. Second, length of abstinence was not confirmed with toxicology testing and only a minimum of 24 hours of abstinence was expected of participants. Future studies will need to examine the impact of CAN on brain structure in youth with ADHD following a two week abstinence period to rule-out any influence of withdrawal or acute effects. Third, although ADHD diagnosis was clearly characterized in a longitudinal design, neuroimaging was not conducted prior to the onset of CAN, nicotine, and alcohol use. Although these were measured over time, enabling the accurate classification of groups, we were not able to control for baseline differences in brain morphometry. Therefore, it remains difficult to disentangle the impact of preexisting differences versus direct effects of binge drinking, nicotine and CAN exposure on brain structure (e.g., Hanson et al., 2010; Hill et al., 2007; Ridenour et al., 2009). Therefore, a large-scale longitudinal study following individuals with and without ADHD prior to the initiation of substance use and after significant substance use exposures that includes careful cumulative substance use measurement is needed to clearly examine causal relationships and replicate findings.
In conclusion, we found that although childhood ADHD did not predict brain structure after controlling for substance use, individuals who demonstrated persistent ADHD symptoms into young adulthood had continued abnormalities in brain regions underlying working memory and inhibitory control (precentral and postcentral cortices). In addition, CAN users (with and without ADHD) had significantly thinner superior frontal sulcus, anterior and posterior cingulate, and precentral gyrus. Although the hippocampal finding had a small effect size, this structural abnormality has been reported across multiple CAN studies (see Lorenzetti et al., 2014). In those with ADHD, early age of CAN use onset was associated with thicker superior frontal gyrus, and postcentral gyrus as well as previously demonstrated poorer executive functioning (Tamm et al., 2013). Notably, the current study lends additional evidence suggesting that early onset of regular CAN use may disrupt neuromaturation, especially in reward and executive function networks. These results highlight the necessity to screen youth with ADHD for regular CAN use and binge drinking, as use of these substances may further disrupt brain development and executive functioning (Tamm et al., 2013) in already vulnerable individuals. Finally, additional longitudinal studies are needed to study the causal impact of CAN use on brain development trajectories in youth with and without ADHD.
Table 2.
Demographic and Drug Use Characteristics by Persistent and Desistent ADHD and LNCG Groups.
| LNCG (n=39) Mean (SD) or % |
Desistent ADHD (n=23) Mean (SD) or % |
Persistent ADHD (n=52) Mean (SD) or % |
||
|---|---|---|---|---|
| Age* | 23.5 (1.4) | 24.6 (1.2) | 24.3 (1.3) | |
| % Male | 76% | 91% | 81% | |
| % Caucasian | 67% | 61% | 58% | |
| IQ | 108.2 (23.0) | 104.6 (15.5) | 102.6 (15.3) | |
| % Current ADHD meds | 0% | 4% | 8% | |
| Cannabis use onset (CUO) age |
16.6 (2.9) | 15.4 (3.6) | 15.6 (2.8) | |
| % Early CUO (<16 yo) | 40% | 50% | 48% | |
| % Past year daily cannabis use |
26% | 26% | 25% | |
| Days used cannabis past month |
9.3 (11.7) | 3.6 (6.3) | 8.4 (12.0) | |
| % 0 yrs regular cannabis use |
49% | 56% | 42% | |
| % 2> yrs regular cannabis use |
15% | 13% | 21% | |
| % Smoke Cigarettes | 26% | 30% | 44% | |
| Age Regularly Drank Alcohol |
18.7 (1.9) | 19.0 (2.1) | 18.3 (2.9) | |
| Past Year Binge Episodes | 1.7 (2.8) | 4.5 (2.5) | 3.6 (2.5) | |
Notes:
p<.05, see text for details.
Highlights.
-
◦
Little is known about the effects of both ADHD and cannabis use on brain structure.
-
◦
Persistent ADHD was linked with abnormalities in frontoparietal structure.
-
◦
Cannabis users had abnormal frontolimbic brain structure.
-
◦
Adolescent onset cannabis users demonstrated unique structural abnormalities.
-
◦
Prospective longitudinal studies are needed to clarify causal relationships.
Acknowledgments
Role of Funding Source:
Data collection and sharing for this project was funded by the NIDA MTA Neuroimaging Study (National Institute on Drug Abuse Grant Contract #: HHSN271200800009C). NIDA played a role in the HHSN271200800009C protocol development. The funding institute did not play a role in the data analysis, manuscript preparation or submission of this manuscript.
Manuscript preparation was supported by NIH/NIDA (R01 DA030354; PI: Lisdahl).
Dr. Tamm receives research grant funding from NIH/NIMH, NIDA, & NICHD.
Dr. Epste receives research grant funding from NIH/NIMH, NIDA & NICHD.
Dr. Jernigan receives grant funding from NIH/NIDA.
Dr. Molina receives grant funding from NIH/NIMH (N01MH12010) and NIDA (DA-8-5553).
Dr. Hinshaw receives research grant funding from NIH/NIMH (U01 MH050461; N01MH12009) & NIDA (DA-8-5550).
Dr. Swanson has received research support from NIH/NIMH (U01 MH050440; N01MH12011), Alza, Richwood, Shire, Celgene, Novartis, Celltech, Gliatech, Cephalon, Watson, CIBA, Janssen, and McNeil; has been on the advisory board for Alza, Rich-wood, Shire, Celgene, Novartis, Celltech, UCB, Gliatech, Cephalon, McNeil, and Lilly; has been on speaker’s bureau for Alza, Shire, Novartis, Celltech, UCB, Cephalon, CIBA, Janssen, and McNeil; and has consulted to Alza, Richwood, Shire, Celgene, Novartis, Celltech, UCB, Gliatech, Cephalon, Watson, CIBA, Janssen, McNeil, and Lilly.
We thank the participants of this study and the numerous staff involved with the coordination of visits.
Appendix A
9The Multimodal Treatment Study of Children with ADHD (MTA) was a National Institute of Mental Health (NIMH) cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and finally under a National Institute on Drug Abuse (NIDA) contract. Collaborators from NIMH: Benedetto Vitiello, M.D. (Child & Adolescent Treatment and Preventive Interventions Research Branch), Joanne B. Severe, M.S. (Clinical Trials Operations and Biostatistics Unit, Division of Services and Intervention Research), Peter S. Jensen, M.D. (currently at REACH Institute and Mayo Clinic), L. Eugene Arnold, M.D., M.Ed. (currently at Ohio State University), Kimberly Hoagwood, Ph.D. (currently at Columbia); previous contributors from NIMH to the early phases: John Richters, Ph.D. (currently at National Institute of Nursing Research); Donald Vereen, M.D. (currently at NIDA). Principal investigators and co-investigators from the sites are: University of California, Berkeley/San Francisco: Stephen P. Hinshaw, Ph.D. (Berkeley), Glen R. Elliott, Ph.D., M.D. (San Francisco); Duke University: Karen C. Wells, Ph.D., Jeffery N. Epstein, Ph.D. (currently at Cincinnati Children's Hospital Medical Center), Desiree W. Murray, Ph.D.; previous Duke contributors to early phases: C. Keith Conners, Ph.D. (former PI); John March, M.D., M.P.H.; University of California, Irvine: James Swanson, Ph.D., Timothy Wigal, Ph.D.; previous contributor from UCLA to the early phases: Dennis P. Cantwell, M.D. (deceased); New York University: Howard B. Abikoff, Ph.D.; Montreal Children's Hospital/ McGill University: Lily Hechtman, M.D.; New York State Psychiatric Institute/Columbia University/Mount Sinai Medical Center: Laurence L. Greenhill, M.D. (Columbia), Jeffrey H. Newcorn, M.D. (Mount Sinai School of Medicine). University of Pittsburgh: Brooke Molina, Ph.D., Betsy Hoza, Ph.D. (currently at University of Vermont), William E. Pelham, Ph.D. (PI for early phases, currently at Florida International University). Follow-up phase statistical collaborators: Robert D. Gibbons, Ph.D. (University of Illinois, Chicago); Sue Marcus, Ph.D. (Mt. Sinai College of Medicine); Kwan Hur, Ph.D. (University of Illinois, Chicago). Original study statistical and design consultant: Helena C. Kraemer, Ph.D. (Stanford University). Collaborator from the Office of Special Education Programs/US Department of Education: Thomas Hanley, Ed.D. Collaborator from Office of Juvenile Justice and Delinquency Prevention/Department of Justice: Karen Stern, Ph.D. Additional investigators for Neuroimaging Substudy: Leanne Tamm, Ph.D., PI (Cincinnati Children's Hospital Medical Center), James Bjork, Ph.D. (Department of Psychiatry, Virginia Commonwealth University), Daniel Mathalon, M.D., Ph.D. (UC San Francisco), Allen Song, Ph.D. (Duke), Bradley Peterson, M.D. (Columbia), Steven Potkin, M.D. & Claudia Buss, Ph.D. (UC Irvine), Katerina Velanova, Ph.D. (Pittsburgh), Neuroimaging Consultants: Susan Tapert, Ph.D. & Joshua Kuperman, Ph.D. (UC San Diego), BJ Casey, Ph.D. & Leah Sommerville, Ph.D. (Sackler Institute, Cornell), Krista Lisdahl, Ph.D. (University of Wisconsin-Milwaukee). Neuroimaging Analysis and Interpretation: Terry Jernigan, Ph.D. & Anders Dale, Ph.D. (UC San Diego), F. Xavier Castellanos, M.D. & Clare Kelly, Ph.D. (New York University).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: Drs. Lisdahl, Tamm, Epstein, Jernigan, Molina, Hinshaw, Swanson, Kelly and Bjork assisted with study design and protocol development. Dr. Tamm assisted with data management and computed summaries of diagnostic comorbidities. Dr. Lisdahl, in consultation with Drs. Tamm, Epstein, Hinshaw, Kellly, and Molina, determined the CAN and ADHD group membership and drug use cut-offs. Dr. Lisdahl managed the literature searches and summaries of previous related work, with contributions by Drs. Tamm, Epstein, Molina, Hinshaw, Swanson and Bjork. Dr. Lisdahl undertook the statistical analysis and received consultation from Drs. Tamm, Epstein, Jernigan, Molina, Hinshaw, Swanson, Newman, Kelly and Bjork on selection of covariates and analytic plan. Dr. Lisdahl wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest:
Dr. Lisdahl has nothing to declare.
Dr. Tamm has nothing to declare.
Dr. Epstein has nothing to declare.
Dr. Jernigan has nothing to declare.
Dr. Molina has nothing to declare.
Dr. Hinshaw has nothing to declare.
Dr. Newman has nothing to declare.
Dr. Kelly has nothing to declare.
Dr. Bjork has nothing to declare.
REFERENCES
- Ahrendts J, Rüsch N, Wilke M, Philipsen A, Eickhoff SB, Glauche V, Perlov E, Hennig J, van Elst LT. Visual cortex abnormalities in adults with ADHD: a structural MRI study. World J. Biol. Psychiatry. 2011;12:260–270. doi: 10.3109/15622975.2010.518624. [DOI] [PubMed] [Google Scholar]
- Almeida LG, Ricardo-Garcell J, Prado H, Barajas L, Fernández-Bouzas A, Avila D, Martínez RB. Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: a cross-sectional study. J. Psychiatr. Res. 2010;44:1214–1223. doi: 10.1016/j.jpsychires.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Almeida Montes LG, Prado Alcántara H, Martínez García RB, De La Torre LB, Avila Acosta D, Duarte MG. Brain cortical thickness in ADHD: age, sex, and clinical correlations. J. Atten. Disord. 2013;17:641–654. doi: 10.1177/1087054711434351. [DOI] [PubMed] [Google Scholar]
- Almeida Montes LG, Ricardo-Garcell J, Barajas De La Torre LB, Prado Alcántara H, Martínez García RB, Fernández-Bouzas A, Avila Acosta D. Clinical correlations of grey matter reductions in the caudate nucleus of adults with attention deficit hyperactivity disorder. J. Psychiatry Neurosci. 2010;35:238–246. doi: 10.1503/jpn.090099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico F, Stauber J, Koutsouleris N, Frodl T. Anterior cingulate cortex gray matter abnormalities in adults with attention deficit hyperactivity disorder: a voxel-based morphometry study. Psychiatry Res. 2011;191:31–35. doi: 10.1016/j.pscychresns.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, Gee J, Sevy S, Kumra S. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J. Psychiatr. Res. 2011;45:1055–1066. doi: 10.1016/j.jpsychires.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J. Abnorm. Psychol. 2002;111:279–289. [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Batalla A, Bhattacharyya S, Yücel M, Fusar-Poli P, Crippa JA, Nogué S, Torrens M, Pujol J, Farre M, Martin-Santos R. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8(2):e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal changes in white matter integrity among adolescent substance users. Alcohol. Clin. Exp. Res. 2013;37(Suppl. 1):E181–E189. doi: 10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J. Altered parahippocampal functioning in cannabis users is related to the frequency of use. Psychopharmacology (Berl.) 2010a;209:361–374. doi: 10.1007/s00213-010-1805-z. [DOI] [PubMed] [Google Scholar]
- Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J. The impact of early-onset cannabis use on functional brain correlates of working memory. Prog. Neuropsychopharmacol. Biol Psychiatry. 2010b;34:837–845. doi: 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Belue RC, Howlett AC, Westlake TM, Hutchings DE. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol. Teratol. 1995;17:25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- Biederman J, Makris N, Valera EM, Monuteaux MC, Goldstein JM, Buka S, Boriel DL, Bandyopadhyay S, Kennedy DN, Caviness VS, Bush G, Aleardi M, Hammerness P, Faraone SV, Seidman LJ. Towards further understanding of the co-morbidity between attention deficit hyperactivity disorder and bipolar disorder: a MRI study of brain volumes. Psychol. Med. 2008;38:1045–1056. doi: 10.1017/S0033291707001791. [DOI] [PubMed] [Google Scholar]
- Bogner J, Corrigan JD. Reliability and predictive validity of the Ohio State University TBI identification method with prisoners. J. Head Trauma Rehabil. 2009;24:279–291. doi: 10.1097/HTR.0b013e3181a66356. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn. Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sharp WS, Gottesman RF, Greenstein DK, Giedd JN, Rapoport JL. Anatomic brain abnormalities in monozygotic twins discordant for attention deficit hyperactivity disorder. Am. J. Psychiatry. 2003;160:1693–1696. doi: 10.1176/appi.ajp.160.9.1693. [DOI] [PubMed] [Google Scholar]
- Cathel AM, Reyes BA, Wang Q, Palma J, Mackie K, Van Bockstaele EJ, Kirby LG. Cannabinoid modulation of alpha2 adrenergic receptor function in rodent medial prefrontal cortex. Eur. J. Neurosci. 2014;40:3202–3214. doi: 10.1111/ejn.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacol. Biochem. Behav. 2006;83:448–455. doi: 10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50:9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons JG, Yücel M, Lubman DI. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol. Psychiatry. 2012;71:684–692. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Cherkasova MV, Hechtman L. Neuroimaging in attention-deficit hyperactivity disorder: beyond the frontostriatal circuitry. Can. J. Psychiatry. 2009;54:651–664. doi: 10.1177/070674370905401002. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Carey PD, Ferrett HL, Stein DJ, Yurgelun-Todd DA. Abnormal striatal circuitry and intensified novelty seeking among adolescents who abuse methamphetamine and cannabis. Dev. Neurosci. 2012;34:310–317. doi: 10.1159/000337724. doi: 000337724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Lopez-Larson M, Yurgelun-Todd DA. Altered frontal cortical volume and decision making in adolescent cannabis users. Front. Psychol. 2010;1:225. doi: 10.3389/fpsyg.2010.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerkin SM, Schulz KP, Berwid OG, Fan J, Newcorn JH, Tang CY, Halperin JM. Thalamo-cortical activation and connectivity during response preparation in adults with persistent and remitted ADHD. Am. J. Psychiatry. 2013;170:1011–1019. doi: 10.1176/appi.ajp.2013.12070880. [DOI] [PubMed] [Google Scholar]
- Coleman LG, He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol. Clin. Exp. Res. 2011;35:671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59:3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Wang L, Bergman SR, Yaxley RH, Hooper SR, Huettel SA. Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder. Drug Alcohol Depend. 2013;133:134–145. doi: 10.1016/j.drugalcdep.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, Bruffaerts R, de Girolamo G, Gureje O, Huang Y, Karam A, Kostyuchenko S, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5:e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirakca T, Sartorius A, Ende G, Meyer N, Welzel H, Skopp G, Mann K, Hermann D. Diminished gray matter in the hippocampus of cannabis users: possible protective effects of cannabidiol. Drug Alcohol Depend. 2011;114:242–245. doi: 10.1016/j.drugalcdep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Depue BE, Burgess GC, Bidwell LC, Willcutt EG, Banich MT. Behavioral performance predicts grey matter reductions in the right inferior frontal gyrus in young adults with combined type ADHD. Psychiatry Res. 2010;182:231–237. doi: 10.1016/j.pscychresns.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey J, Hana G, Russell T, Price J, McCaffrey D, Harezlak J, Sem E, Anyanwu JC, Guttmann CR, Navia B, Cohen R, Tate DF HIV Neuroimaging Consortium. Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. Neuroimage. 2010;51:1334–1344. doi: 10.1016/j.neuroimage.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl.) 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Doyle A, Murray K, Petty C, Adamson JJ, Seidman L. Neuropsychological studies of late onset and subthreshold diagnoses of adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2006;60:1081–1087. doi: 10.1016/j.biopsych.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr. Scand. 2012;125:114–126. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- Frodl T, Stauber J, Schaaff N, Koutsouleris N, Scheuerecker J, Ewers M, Omerovic M, Opgen-Rhein M, Hampel H, Reiser M, Moller HJ, Meisenzahl E. Amygdala reduction in patients with ADHD compared with major depression and healthy volunteers. Acta Psychiatr. Scand. 2010;121:111–118. doi: 10.1111/j.1600-0447.2009.01489.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Kuster JK, Lee S, Lee MJ, Kim BW, Makris N, van der Kouwe A, Blood AJ, Breiter HC. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J. Neurosci. 2014;34:5529–5538. doi: 10.1523/JNEUROSCI.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H, James AC. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn. 2010;72:6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychol. Addict. Behav. 2012;26:496–506. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Silveri MM, Dahlgren MK, Yurgelun-Todd D. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Exp. Clin. Psychopharmacol. 2011;19:231–242. doi: 10.1037/a0023034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, Tapert SF. Hippocampal volumes in adolescents with and without a family history of alcoholism. Am. J. Drug Alcohol Abuse. 2010;36:161–167. doi: 10.3109/00952991003736397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF. Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology. 2004;18:485–503. doi: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- Hesslinger B, Tebartz van Elst L, Thiel T, Haegele K, Hennig J, Ebert D. Frontoorbital volume reductions in adult patients with attention deficit hyperactivity disorder. Neurosci. Lett. 2002;328:319–321. doi: 10.1016/s0304-3940(02)00554-2. [DOI] [PubMed] [Google Scholar]
- Hill SY, Kostelnik B, Holmes B, Goradia D, McDermott M, Diwadkar V, Keshavan M. fMRI BOLD response to the eyes task in offspring from multiplex alcohol dependence families. Alcohol. Clin. Exp. Res. 2007;31:2028–2035. doi: 10.1111/j.1530-0277.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Heustis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Sorg SF, Nguyen-Louie TT, Tapert SF. Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. J. Stud. Alcohol Drugs. 2014;75:729–743. doi: 10.15288/jsad.2014.75.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Seewer R, Nirkko AC, Eckstein D, Schroth G, Groner R, Gutbrod K. Does excessive memory load attenuate activation in the prefrontal cortex? Load-dependent processing in single and dual tasks: functional magnetic resonance imaging study. Neuroimage. 2003;19:210–225. doi: 10.1016/s1053-8119(03)00098-3. [DOI] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(6):561–572. 572.e561–572.e561. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis K, DelBello MP, Mills N, Elman I, Strakowski SM, Adler CM. Neuroanatomic comparison of bipolar adolescents with and without cannabis use disorders. J. Child Adolesc. Psychopharmacol. 2008;18:557–563. doi: 10.1089/cap.2008.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. nstitute for Social Research. Ann Arbor: The University of Michigan; 2015. Monitoring The Future National Survey Results On Drug Use: 1975–2014: Overview, Key Findings On Adolescent Drug Use. [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kang-Park MH, Wilson WA, Kuhn CM, Moore SD, Swartzwelder HS. Differential sensitivity of GABA A receptor-mediated IPSCs to cannabinoids in hippocampal slices from adolescent and adult rats. J. Neurophysiol. 2007;98:1223–1230. doi: 10.1152/jn.00091.2007. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Limebeer CL, Parker LA. Chronic treatment with Delta-9-tetrahydrocannabinol alters the structure of neurons in the nucleus accumbens shell and medial prefrontal cortex of rats. Synapse. 2006;60:429–436. doi: 10.1002/syn.20313. [DOI] [PubMed] [Google Scholar]
- Kumra S, Robinson P, Tambyraja R, Jensen D, Schimunek C, Houri A, Reis T, Lim K. Parietal lobe volume deficits in adolescents with schizophrenia and adolescents with cannabis use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:171–180. doi: 10.1016/j.jaac.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol. Rev. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J. Abnorm. Psychol. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front. Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Wright NE, Kirchner-Medina C, Maple KE, Shollenbarger S. Considering cannabis: the effects of regular cannabis use on neurocognition in adolescents and young adults. Curr. Addict. Rep. 2014;1:144–156. doi: 10.1007/s40429-014-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav. Brain Res. 2011;220:164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Solowij N, Fornito A, Lubman DI, Yucel M. The association between regular cannabis exposure and alterations of human brain morphology: an updated review of the literature. Curr. Pharm. Des. 2014;20:2138–2167. doi: 10.2174/13816128113199990435. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Solowij N, Whittle S, Fornito A, Lubman DI, Pantelis C, Yücel M. Gross morphological brain changes with chronic, heavy cannabis use. Br. J. Psychiatry. 2015;206:77–78. doi: 10.1192/bjp.bp.114.151407. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Multimodal Treatment Study of Children with ADHD. Arch. Gen. Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Ma J, Lei D, Jin X, Du X, Jiang F, Li F, Zhang Y, Shen X. Compensatory brain activation in children with attention deficit/hyperactivity disorder during a simplified Go/No-go task. J. Neural Transm. 2012;119:613–619. doi: 10.1007/s00702-011-0744-0. [DOI] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb. Exp. Pharmacol. 2005;168:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb. Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Mata I, Perez-Iglesias R, Roiz-Santiañez R, Tordesillas-Gutierrez D, Pazos A, Gutierrez A, Vazquez-Barquero JL, Crespo-Facorro B. Gyrification brain abnormalities associated with adolescence and early-adulthood cannabis use. Brain Res. 2010;1317:297–304. doi: 10.1016/j.brainres.2009.12.069. [DOI] [PubMed] [Google Scholar]
- Mattfeld AT, Gabrieli JD, Biederman J, Spencer T, Brown A, Kotte A, Kagan E, Whitfield-Gabrieli S. Brain differences between persistent and remitted attention deficit hyperactivity disorder. Brain. 2014;137:2423–2428. doi: 10.1093/brain/awu137. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Chua SE, Oosterlaan J, Hung SF, Tang CP, .Lee CC, Kwong SL, Ho TP, Cheung C, Suckling J, Leung PW. Age-related grey matter volume correlates of response inhibition and shifting in attention-deficit hyperactivity disorder. Br. J. Psychiatry. 2009;194:123–129. doi: 10.1192/bjp.bp.108.051359. [DOI] [PubMed] [Google Scholar]
- McDonald CG, Dailey VK, Bergstrom HC, Wheeler TL, Eppolito AK, Smith LN, Smith RF. Periadolescent nicotine administration produces enduring changes in dendritic morphology of medium spiny neurons from nucleus accumbens. Neurosci. Lett. 2005;385:163–167. doi: 10.1016/j.neulet.2005.05.041. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Padula CB, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behav Brain Res. 2011;224:128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alcohol. Clin. Exp. Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J. Int. Neuropsychol. Soc. 2007;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict. Biol. 2009;14:457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Res. 2010;182:152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol. Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, McDonald K, Ward A, Poulto R, Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Eugene Arnold L, Swanson JM, Pelham WE, Hechtman L, Hoza B, Epstein JN, Wigal T, Abikoff HB, Greenhill LL, Jensen PS, et al. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52:250–263. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Pelham WE. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J. Abnorm. Psychol. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Molina BS, Pelham WE, Gnagy EM, Thompson AL, Marshal MP. Attention-deficit/hyperactivity disorder risk for heavy drinking and alcohol use disorder is age specific. Alcohol. Clin. Exp. Res. 2007;31:643–654. doi: 10.1111/j.1530-0277.2007.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am. J. Psychiatry. 2011;168:1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Newman E, Jernigan TL, Lisdahl KM, Tamm L, Tapert SF, Potkin SG, Mathalon D, Molina B, Bjork J, Castellanos FX, Swanson J, Kuperman JM, et al. Go/No Go task performance predicts cortical thickness in the caudal inferior frontal gyrus in young adults with and without ADHD. Brain Imaging Behav. 2015 doi: 10.1007/s11682-015-9453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onnink AM, Zwiers MP, Hoogman M, Mostert JC, Kan CC, Buitelaar J, Franke B. Brain alterations in adult ADHD: effects of gender, treatment and comorbid depression. Eur. Neuropsychopharmacol. 2014;24:397–409. doi: 10.1016/j.euroneuro.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Logan GD, Sergeant JA. Response inhibition in AD/HD, CD, comorbid AD/HD + CD, anxious, and control children: a meta-analysis of studies with the stop task. J. Child Psychol. Psychiatry. 1998;39:411–425. [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Miñarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur. J. Neurosci. 2007;25:541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Pastura G, Mattos P, Gasparetto EL, Araújo AP. Advanced techniques in magnetic resonance imaging of the brain in children with ADHD. Arq. Neuropsiquiatr. 2011;69:242–252. doi: 10.1590/s0004-282x2011000200020. [DOI] [PubMed] [Google Scholar]
- Peng X, Lin P, Zhang T, Wang J. Extreme learning machine-based classification of ADHD using brain structural MRI data. PLoS One. 2013;8:e79476. doi: 10.1371/journal.pone.0079476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlov E, Philipsen A, Tebartz van Elst L, Ebert D, Henning J, Maier S, Bubl E, Hesslinger B. Hippocampus and amygdala morphology in adults with attention-deficit hyperactivity disorder. J. Psychiatry Neurosci. 2008;33:509–515. [PMC free article] [PubMed] [Google Scholar]
- Pingault JB, Côté SM, Galéra C, Genolini C, Falissard B, Vitaro F, Tremblay RE. Childhood trajectories of inattention, hyperactivity and oppositional behaviors and prediction of substance abuse/dependence: a 15-year longitudinal population-based study. Mol. Psychiatry. 2013;18:806–812. doi: 10.1038/mp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingault JB, Tremblay RE, Vitaro F, Carbonneau R, Genolini C, Falissard B, Côté SM. Childhood trajectories of inattention and hyperactivity and prediction of educational attainment in early adulthood: a 16-year longitudinal population-based study. Am. J. Psychiatry. 2011;168:1164–1170. doi: 10.1176/appi.ajp.2011.10121732. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Glahn DC, Semrud-Clikeman M, Franklin C, Perez R, Xiong J, Liotti M. Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. Am. J. Psychiatry. 2006;163:1052–1060. doi: 10.1176/ajp.2006.163.6.1052. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am. J. Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Polderman TJ, Boomsma DI, Bartels M, Verhulst FC, Huizink AC. A systematic review of prospective studies on attention problems and academic achievement. Acta Psychiatr. Scand. 2010;122:271–284. doi: 10.1111/j.1600-0447.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Price JS, McQueeny T, Shollenbarger S, Browning EL, Wieser J, Lisdahl KM. Effects of marijuana use on prefrontal and parietal volumes and cognition in emerging adults. Psychopharmacology (Berl.) 2015 doi: 10.1007/s00213-015-3931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, Lerch JP, He Y, Zijdenbos A, Kelly C, Milham MP, Castellanos FX. Brain gray matter deficits at 33-year follow-up in adults with attention-deficit/hyperactivity disorder established in childhood. Arch. Gen. Psychiatry. 2011;68:1122–1134. doi: 10.1001/archgenpsychiatry.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, Thompson MR, Dawson B, Mallet PE, Kashem MA, Matsuda-Matsumoto H, Iwazaki T, McGregor IS. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33:1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- Ridenour TA, Tarter RE, Reynolds M, Mezzich A, Kirisci L, Vanyukov M. Neurobehavior disinhibition, parental substance use disorder, neighborhood quality and development of cannabis use disorder in boys. Drug Alcohol Depend. 2009;102:71–77. doi: 10.1016/j.drugalcdep.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Mol. Cell. Endocrinol. 2008;286:S108–S113. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, Guidali C, Pinter M, Sala M, Bartesaghi R, Parolaro D. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Hutchison KE, Filbey FM. Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharmacology. 2012;37:2368–2376. doi: 10.1038/npp.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Liang L, Valera EM, Monuteaux MC, Brown A, Kaiser J, Spencer T, Faraone SV, Makris N. Gray matter alterations in adults with attention-deficit/hyperactivity disorder identified by voxel based morphometry. Biol. Psychiatry. 2011;69:857–866. doi: 10.1016/j.biopsych.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Gush G, Aleardi M, Faraone SV, Biederman J. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol. Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Shaw P, De Rossi P, Watson B, Wharton A, Greenstein D, Raznahan A, Sharp W, Lerch JP, Chakravarty MM. Mapping the development of the basal ganglia in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53:780.e711–789.e711. doi: 10.1016/j.jaac.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W. Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2013;74:599–606. doi: 10.1016/j.biopsych.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BS, Gnagy EM, Waxmonsky JG, Waschbusch DA, Derefinko KJ, Wymbs BT, Garefino AC, Babinski DE, Kuriyan AB. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J. Consult Clin. Psychol. 2012;80(December (6)):1052–1061. doi: 10.1037/a0029098. http://dx.doi.org/10.1037/a0029098 Epub 2012 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage. 2014;92:356–368. doi: 10.1016/j.neuroimage.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MA, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev. Med. Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology (Berl.) 2012;220:529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MA, Rudnik-Levin F. Attention deficit/hyperactivity disorder and substance abuse. Diagnostic and therapeutic considerations. Ann. N. Y. Acad. Sci. 2001;931:251–270. doi: 10.1111/j.1749-6632.2001.tb05783.x. [DOI] [PubMed] [Google Scholar]
- Tamm L, Epstein JN, Lisdahl KM, Molina B, Tapert S, Hinshaw SP, Arnold LE, Velanoav K, Abikoff H, Swanson JM MTA Neuroimaging Group. Impact of ADHD and cannabis use on executive functioning in young adults. Drug Alcohol Depend. 2013;133:607–614. doi: 10.1016/j.drugalcdep.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry GE, Liow JS, Zoghbi SS, Hirvonen J, Farris AG, Lerner A, Tauscher JT, Schaus JM, Phebus L, Felder CC, Morse CL, Hong JS, Halldin C, Pike VW, Innis RB. Quantitation of cannabinoid CB1 receptors in healthy human brain using positron emission tomography and an inverse agonist radioligand. Neuroimage. 2009;48:362–370. doi: 10.1016/j.neuroimage.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzilos GK, Cintron CB, Wood JB, Simpson NS, Young AD, Pope HG, Yurgelun-Todd DA. Lack of hippocampal volume change in long-term heavy cannabis users. Am. J. Addict. 2005;14:64–72. doi: 10.1080/10550490590899862. [DOI] [PubMed] [Google Scholar]
- Vallés SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 2004;14:365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Moreno E, Marco EM. Behavioural and neuroendocrine effects of cannabinoids in critical developmental periods. Behav. Pharmacol. 2005;16:353–362. doi: 10.1097/00008877-200509000-00007. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol. Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, Loo SK, Carlson CL, McBurnett K, Lahey BB. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J. Abnorm. Psychol. 2012;121:991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J. Addict. Dis. 2000;19:1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- Winters KC, Lee CY. Likelihood of developing an alcohol and cannabis use disorder during youth: association with recent use and age. Drug Alcohol Depend. 2008;92:239–247. doi: 10.1016/j.drugalcdep.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Arch. Gen. Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]