Abstract
In order to empower patients as decision makers, physicians must educate them about their treatment options in a factual, non-biased manner. We propose that site-specific availability of treatment options may be a novel source of bias, whereby physicians describe treatments more positively when they are available. We performed a content analysis of physicians’ descriptions of robotic prostatectomy within 252 appointments at four Veterans Affairs medical centers where robotic surgery was either available or unavailable. We coded how physicians portrayed robotic versus open prostatectomy across specific clinical categories and in the appointment overall. We found that physicians were more likely to describe robotic prostatectomy as superior when it was available [F(1, 42) = 8.65, p = .005]. We also provide initial qualitative evidence that physicians may be shaping their description of robotic prostatectomy in an effort to manage patients’ emotions and demand for the robotic technology. To our knowledge, this is the first study to provide empirical evidence that treatment availability influences how physicians describe the advantages and disadvantages of treatment alternatives to patients during clinical encounters, which has important practical implications for patient empowerment and patient satisfaction.
Keywords: patient-physician communication, robotics, prostatectomy, veterans, prostatic neoplasms
Patients need accurate, non-biased information about treatment alternatives in order to become fully informed, empowered decision-makers. For example, men diagnosed with clinically localized prostate cancer must choose between surgery, radiation, or active surveillance. Because each of these treatment options is associated with a unique combination of risks and benefits, the “right” treatment choice for a patient depends on how he personally values the pros and cons. Patients and physicians must work together in a process of shared decision making to identify the treatment that best matches patients’ preferences (Thompson et al., 2007). Patient education is at the heart of shared decision making (Barry & Edgman-Levitan, 2012). Although patients may seek educational materials outside of the clinical encounter (Mirkin et al., 2012), the information they receive from their physicians remains particularly influential (Davison & Breckon, 2012). Patients trust their physicians to present all treatment options and to educate them in a factual, non-biased manner about their treatment options so that they can make informed choices.
Unfortunately, physicians do not always provide patients with unbiased information. Physicians’ specialties can be a source of bias; for example, surgeons are more likely to recommend surgery whereas radiation oncologists are more likely to recommend radiation for men with early stage prostate cancer (Fowler et al., 2000). Similarly, orthopedic surgeons are biased towards surgical versus non-surgical interventions (Hudak, Clark, & Raymond, 2011). Physicians may also be influenced by direct financial incentives and relationships with industry (Jagsi, 2007).
In this study, we explore another potential source of bias: the availability of treatment options (in this case, robotic prostatectomy). We perform a content analysis of physicians’ descriptions of robotic prostatectomy in order to determine whether the availability of robotic surgery influenced whether physicians discussed robotic prostatectomy and, if discussed, how they described robotic prostatectomy. The appointments were recorded as part of a larger study of prostate cancer decision-making with the Veterans Affairs (VA) medical system. By chance, robotic surgery was available at some (but not all) of our treatment sites. Because the physicians (primarily residents) in our study were salaried employees within the VA system, they did not have a direct financial incentive to describe robotic prostatectomy in a particular manner. Thus, we are able to examine whether physicians’ descriptions of robotic prostatectomy were influenced by treatment availability even though there was no direct financial incentive to do so.
Method
Human subjects approval
This study was approved by the Institutional Review Boards at each of the participating sites; all participants were provided written informed consent and guarantees of confidentiality. All personal identifiers have been removed or disguised so the persons described are not identifiable and cannot be identified through the details of the story.
Procedure
We analyzed 252 clinical appointments that were recorded and transcribed as part of a trial of prostate cancer decision-making. Appointments were recorded from 2008–2012 at four geographically dispersed VA medical centers, one of which did not offer robotic surgery. The recordings capture the clinical appointments during which physicians and patients first discuss prostate cancer treatment options. Further details on the trial are published elsewhere (Holmes-Rovner et al., 2015).
Data analysis
We uploaded all transcribed appointments into Dedoose (Version 4.5, 2013), a qualitative data analysis software program. A team of five coders (4 research assistants and the lead researcher) identified all discussions of robotic prostatectomy. We used a directed content analytic approach (Elo and Kyngäs, 2008; Hsieh and Shannon, 2005) to capture how physicians described robotic prostatectomy. Based on current literature, we developed an a priori list of categories across which physicians might compare robotic to open prostatectomy (e.g., intraoperative blood loss and risk of impotence). Using a development set of randomly selected transcripts, two coders identified comparative statements within each transcript; they used the a priori list of categories as a guide but marked all comparative statements. Coders assessed whether the physician described robotic prostatectomy as inferior, equivalent, or superior to open prostatectomy. If physicians made multiple comparative comments within a single category, coders’ scores took all comments into account. We used an iterative process to refine the coding system until we reached thematic saturation (i.e., no new categories were being identified) and had clarified the boundaries between types of descriptions (inferior, equivalent, and superior).
Coders also assigned a global score to each conversation to capture whether the physician portrayed robotic prostatectomy as inferior, equivalent, or superior to open prostatectomy in the appointment as a whole. Coders only assigned a global score if there was enough information within the conversation to make this judgment. Again, we used an iterative process to refine these boundaries.
We trained coders on the finalized coding system, and two coders (randomly selected from our pool of five coders) independently analyzed each transcript. We coded the development transcripts at the end to minimize carryover from the development period. Discrepancies were resolved through group discussion with the coders who coded the transcript and led by the lead researcher (K.S.). If any uncertainty remained, the senior researcher was consulted.
The above analyses capture if and how physicians discussed robotic prostatectomy; however, they do not explain why physicians described robotic prostatectomy in a particular manner. Based on existing literature and our broad reading of transcripts during the development period, we hypothesized (post-hoc) that physicians’ descriptions may have been influenced by patients’ interest in robotic surgery. To explore this question, coders identified all discussions about the availability and marketing of robotic surgery. Through careful, repeated reading of the identified excerpts, K.S. used an inductive content analysis approach (Elo and Kyngäs, 2008) to develop thematic sub-categories: unprompted patient inquiries about robotic surgery, physician references to patient interest in robotic surgery, and patient reactions to learning that robotic surgery was (un)available. K.S. also identified several unique cases that offered insights into why physicians may be describing robotic prostatectomy in a particular manner. It is important to note that the results in this section of the paper reflect an exploratory content analysis rather than a rigorous in-depth analysis method such as grounded theory. The results of this analysis should be interpreted as one potential reason for our findings rather than a comprehensive answer to this research question.
Statistical analysis
Because there was a relatively small frequency of comparative statements within some categories, we collapsed comparative statements across all categories and used a chi-square test to evaluate whether there was a relationship between the availability of robotic surgery and how physicians described robotic prostatectomy. First, we ran a 2 (availability: available vs. unavailable) × 3 (robot portrayal: inferior vs. equivalent vs. superior) chi-square test. We then ran follow-up 2 × 2 chi-square tests to evaluate whether there was a relationship between the availability of robotic surgery and whether physicians described robotic prostatectomy as superior versus equivalent, superior versus inferior, and equivalent versus inferior. Due to the categorical nature of the data, we were not able to control for the impact of repeated measures from a single physician across multiple encounters.
We used the qualitative analysis method of magnitude coding to transform the global scores into quantitative values appropriate for statistical analyses (Saldaña, 2012). We assigned values in the following manner: robotic prostatectomy portrayed as inferior = −1, equivalent = 0, and superior = +1. We averaged the global scores for all appointments associated with a particular physician to create an average “robotic portrayal score” for each physician; this alleviated the concern of repeated measures from a single physician. We then used SPSS version 21.0 to conduct a one-way ANOVA to determine whether physicians’ robotic portrayal scores differed when robotic surgery was available versus unavailable. We excluded 3 global scores from this analysis because we did not know the physician’s identification numbers (Nunavailable = 2, Navailable = 1).
Results
Physicians’ discussion of robotic prostatectomy
Overall, physicians discussed robotic prostatectomy in 46.8% of appointments (118/252). Physicians more frequently discussed robotic prostatectomy when robotic surgery was available versus unavailable [73.6% (89/122) vs. 22.1% (29/130) of appointments; χ2 (1) = 64.8, p < .001]. Results below only include appointments in which physicians discussed robotic prostatectomy.
Patient and physician characteristics
Overall, 77% of physicians (34/44) discussed robotic prostatectomy in at least one appointment. When robotic surgery was available, 13 physicians discussed robotic prostatectomy in 29 appointments. At the three sites where robotic surgery was unavailable, 6, 7, and 8 physicians discussed robotic prostatectomy in 17, 20, and 52 appointments, respectively. On average, 3.4 appointments were analyzed per physician. Tables 1 and 2 display patient and physician characteristics, respectively, for appointments in which robotic prostatectomy was discussed. There were more non-white patients [χ2 (1) = 13.42, p < .001] and physicians were older [F(1,28) = 8.91, p < .01] at sites where robotic surgery was available. No other patient or physician demographic variables differed.
Table 1.
Patient demographics when robotic surgery was available versus unavailable for appointments in which robotic prostatectomy was discussed.
| Characteristic | Robotic Surgery | Total (n = 118) |
p | |
|---|---|---|---|---|
| Available (n = 89)a |
Unavailable (n = 29) |
|||
| Age (M, SD) | 62.8 (5.6) | 61.7 (3.8) | 62.5 (5.2) | .361 |
| Race (%) | < .001 | |||
| White | 93 | 56 | 82 | |
| Black | 7 | 42 | 33 | |
| Native American | 0 | 2 | 2 | |
| Education (%) | .209 | |||
| High school or less | 27 | 14 | 24 | |
| Tumor histology | .741 | |||
| Gleason 6b | 52 | 48 | 51 | |
All demographic information is missing for one patient. Gleason score is missing from one additional patient.
All other patients had Gleason 7 tumor histology (by study design).
P-values compare whether each demographic characteristic differed across sites where robotic surgery was available versus unavailable. Age was compared using a one-way ANOVA; all other categories were compared using a Pearson Chi-Squared test. The chi-square test for race compared White vs. Non-White (which included Black and Native American).
Table 2.
Physician demographics when robotic surgery was available versus unavailable for appointments in which robotic prostatectomy was discussed.
| Characteristic | Robotic Surgery | Overall (n = 34) |
p | |
|---|---|---|---|---|
| Available (n = 21)a |
Unavailable (n = 13)b |
|||
| Age (M, SD) | 34.6 (6.0) | 28.7 (3.8) | 32.6 (5.7) | .006 |
| Race (%) | .202 | |||
| White | 74 | 50 | 66 | |
| Black | 0 | 10 | 3 | |
| Asian | 21 | 40 | 28 | |
| Other | 5 | 0 | 3 | |
| Gender | .729 | |||
| Female (%) | 15 | 20 | 17 | |
All demographic information is missing for one physician; race is missing for one additional physician.
All demographic information missing for three physicians.
P-values compare whether each demographic characteristic differs across sites where robotic surgery was available versus unavailable. Age was compared using a one-way ANOVA; all other categories were compared using a Pearson Chi-Squared test. The chi-square test for race compared White vs. Non-White (which included Black, Asian, and Other).
Physicians’ portrayal of robotic prostatectomy
Physicians compared robotic to open prostatectomy across 15 categories within 5 broad clinical areas: 1) Intraoperative: blood loss, risk of bowel injury, incisions, visualization of the surgical field, operative time, and haptic (tactile) sense; 2) Post-operative: hospital stay, pain, and recovery; 3) Oncologic: cancer cure; 4) Quality of Life: risk of impotence and risk of incontinence; and 5) Miscellaneous: availability of longitudinal outcome data and cost. Table 3 displays exemplars of physician portrayals of robotic prostatectomy within specific categories. Due to space constraints, we only provide exemplars for the 9 categories in which physicians compared robotic to open prostatectomy in more than 10% of appointments (all data were used in analyses below).
Table 3.
Exemplars of physician portrayals of robotic prostatectomy within specific clinical categories.a
| Clinical Category | Portrayal of robotic prostatectomy
|
|
|---|---|---|
| Equivalent | Superior | |
| Blood loss | The risks for surgery are both pretty similar. There’s risk of infection, risk of bleeding. | The advantage of the robot is there is less blood loss. |
| Incisions b | You do have some smaller incisions with the robotic, but if you added up all the incisions from all the ports and from the incision to remove the prostate itself, it ends up equaling about the same incision length. | The advantage to [robotic surgery] is small incisions and not cutting belly muscle. |
| Visualization | n/a | One of the aspects about robotic surgery is that the visualization is tremendous. It’s all in high definition; it’s three dimensional in my screen where I’m looking. |
| Hospital stay | You’re in the hospital for the same amount of time either way. | There is a shorter hospital stay by about twelve hours [with robotic surgery]. |
| Recovery | They haven’t even been able to show a faster recovery time for the robotic surgery. | The benefit of [robotic surgery] is that there’s quicker recovery after surgery. |
| Pain | Post-operative pain, that sort of thing, is kind of a wash. | There is less pain with a robot. |
| Cancer cure | Cancer outcomes: Is there a difference in margin rates, getting the prostate cancer out? No. | n/a |
| Impotence | The erections after [robotic surgery] are not any better. | We’re probably doing a better job sparing the nerves [with robotic surgery], which is what’s important for preserving erections. |
| Incontinence | I’ll be honest, there’s no difference in the outcomes in urinary control, having the open surgery versus having the robot. | I think I’ve really seen a difference in the urine control and when that comes [earlier with the robot]. |
Note. n/a indicates that physicians never portrayed robotic prostatectomy in that manner.
Due to space constraints, the table only includes exemplars from categories that physicians compared in >10% of the appointments that discussed robotic prostatectomy.
In the category of incisions, the physician described robotic prostatectomy as inferior in two appointments (e.g. “If you added up all the holes for the robotic surgery, it adds up to probably more than that [from open surgery] by the time that you get all the little cuts put together.”) For ease of presentation, this was omitted from the table.
Physicians’ portrayal of robotic prostatectomy within specific clinical categories differed as function of the robot’s availability (χ2 (2) =11.22, p = .004; see Figure 1). When robotic surgery was available, physicians more frequently described robotic prostatectomy as superior versus equivalent (χ2 (1) = 6.23, p = .013) and superior versus inferior (χ2 (1) = 8.06, p = .005). Physicians did not differ in the frequency with which they described robotic prostatectomy as equivalent versus inferior (χ2 (1) = 1.65, p = .20).
Figure 1.
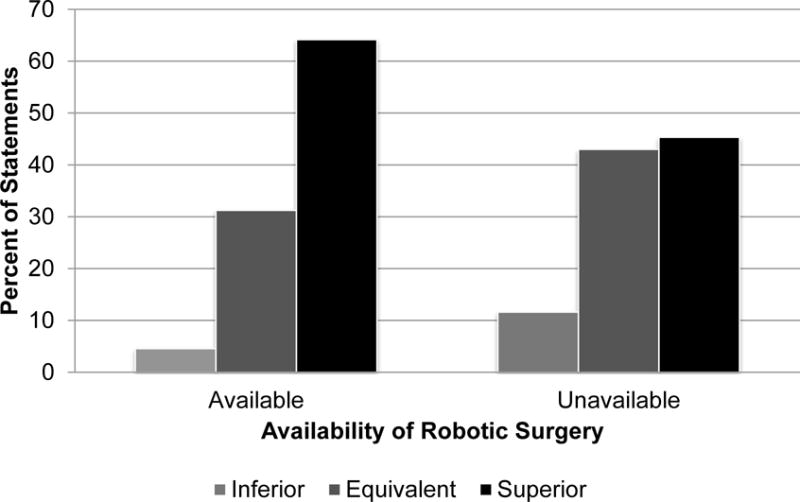
Physicians’ portrayal of robotic prostatectomy within specific clinical categories. The percentage of statements in which the physician portrayed robotic prostatectomy as inferior, equivalent, or superior to open prostatectomy when robotic surgery was available versus unavailable. There was a significant relationship between how physicians described robotic prostatectomy and the availability of robotic surgery (χ2 (2) = 11.22, p = .004). When robotic surgery was available, physicians more frequently described robotic prostatectomy as superior versus equivalent (χ2 (1) = 6.23, p = .013) and superior versus inferior (χ2 (1) = 8.06, p = .005). Physicians did not differ in their likelihood of describing robotic prostatectomy as equivalent versus inferior as a function of the robot’s availability (χ2 (1) = 1.65, p = .20).
Physicians’ global portrayal of robotic prostatectomy also differed as a function of the robot’s availability. Physicians’ robotic portrayal scores ranged from −1.0 to 1.0 (M = .49, SD = .48, SE = .07). Physicians portrayed robotic prostatectomy in a more positive manner when robotic surgery was available (Mavailable = .61, SD = .48, SE = .08) versus unavailable (Munavailable = .17, SD = .33, SE = .10; F (1, 42) = 8.65, p = .005; Figure 2).
Figure 2.
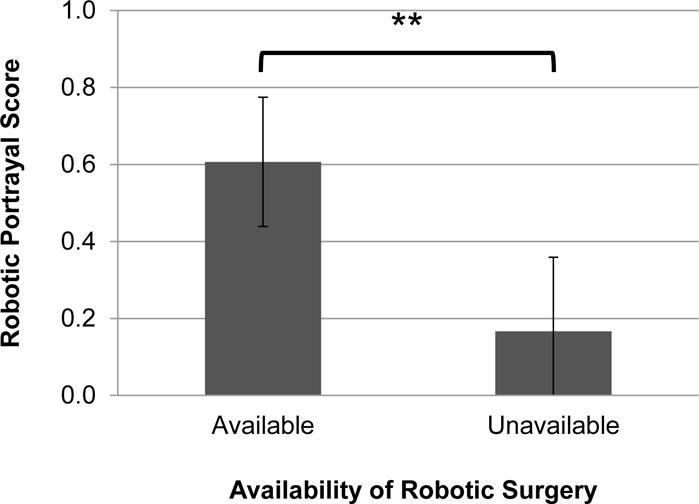
Physicians’ global portrayal of robotic prostatectomy when robotic surgery was available versus unavailable, where −1, 0, and 1 indicate that the physician portrayed robotic prostatectomy as inferior, equivalent, or superior, respectively, in the appointment as a whole. Physicians portrayed robotic prostatectomy more positively when robotic surgery was available versus unavailable (Mavailable = .61 vs. Munavailable = .17; F(1, 42) = 8.65, p = .005). Error bars represent ± 2 standard errors.
Physicians’ motivation for portraying robotic prostatectomy in a particular manner
Our exploratory analysis revealed preliminary evidence that physicians may have tailored their portrayal of robotic prostatectomy in response to (or in anticipation of) patients’ positive (negative) emotions when robotic surgery was available (unavailable), and that these emotions were driven by patients’ interest in robotic surgery. Both patients and physicians made comments that indicated patients have an interest in robotic surgery. Patients asked if robotic surgery was available (“Do you do robot here?”), and physicians noted that their patients often asked about robotic surgery (“I’m just bringing [robotic prostatectomy] up because a lot of people say, ‘What about the robot?’”). Some patients expressed explicit preferences for robotic prostatectomy (“My preference is robotic surgery, based on what I read, with the recovery period being shorter with the robotic da Vinci.”), and physicians also noted that they had treated patients who preferred robotic prostatectomy (“We’ve had some people who’ve been diagnosed with prostate cancer and they one-hundred percent want a robotic approach and then they go somewhere else and have their surgery.”). Interestingly, both patients and physicians referred to the advertising of robotic prostatectomy, suggesting that this may have contributed to patients’ interest in the procedure (patient: “I keep seeing these commercials about the da Vinci system.” and physician: “They often talk about robotic surgery in the news.”).
Physicians’ response to this interest depended on the availability of robotic surgery. When robotic surgery was unavailable, physicians may have portrayed robotic prostatectomy as equivalent in order to decrease patients’ negative emotion associated with the unavailability. Some patients verbally expressed anger upon learning that robotic surgery was unavailable (“You see? That is what’s so stupid about the VA.”), and physicians noted that they had treated patients who were concerned about the unavailability of robotic surgery (“Are you making sure I’m getting the best because I’m over at the VA?”). In response to this negative emotion, physicians tried to assure patients that they were not receiving inferior treatment (“If you look at long term outcomes related to cancer and cancer recurrence and issues long term, there has really been no difference. That’s why the VA system has not really invested in the robot.”). Alternatively, when robotic surgery was available, physicians may have portrayed robotic prostatectomy as superior in order to increase patients’ positive emotion associated with the availability. Some patients verbally expressed excitement upon learning that robotic surgery was available (“Good.”), and physicians noted that patients were lucky to be at a site that offered robotic surgery (“Fortunately we have a robot, which most VA’s don’t.”).
Interestingly, when one physician found out that robotic surgery was medically contraindicated for a particular patient and thus was effectively “unavailable” to that patient, he shifted his description of robotic surgery from one that emphasized the robot’s superiority to one that emphasized its equivalence. Early in the appointment, the physician described robotic surgery in a positive manner: “[With robot], you don’t have a big incision, but you have six little poke holes about your belly…After robotic surgery you usually stay [in the hospital] about one day.”. After hearing the physicians’ full description, the patient stated, “I’d rather go with robotic [surgery],” and the physician supported his decision, stating, “I think it’s a good idea.”. Later, however, as the physician prepared to schedule the robotic surgery, he discovered that the patient had a history of abdominal surgery, which is a contraindication for robotic prostatectomy. The physician then stated, “I don’t think I can offer you a robotic surgery with all this stuff [surgery] you had down there. It would just be too dangerous, just trying to get the robot in.” It was only at this point that the physician told the patient, “We can do the open surgery, though,” and assured the patient that he would not be receiving an inferior treatment:
“There’s a lot of marketing that goes on for the robotic surgery: you see billboards and advertisements all over the place, and people boast about the really good results and everything. Basically the literature, though, the scientific articles about it, looking how people do [with] the robotic versus the open, there’s really very little difference. They haven’t even been able to show a faster recovery time for the robotic surgery. People tell you that it’s faster recovery, but nobody’s been able to show that in scientific studies.”
Discussion
In this study, we found that physicians were more likely to discuss robotic prostatectomy and to portray it as superior when robotic surgery was available versus unavailable. We demonstrated this trend both in how physicians compared robotic to open prostatectomy across specific clinical categories and how they portrayed robotic prostatectomy overall. To our knowledge, this is the first study to empirically demonstrate that physicians’ descriptions of treatment options may be biased by treatment availability. Additionally, we found qualitative evidence that physicians may be shaping their descriptions of robotic surgery in response to (or in anticipation of) patients’ emotions associated with their demand for robotic surgery. It is important for physicians to be aware that their treatment descriptions may be influenced by treatment availability, as this has important practical implications for patient empowerment and treatment outcome satisfaction.
From a patient empowerment perspective, it is concerning that patients received different information about the advantages and disadvantages of robotic prostatectomy (or even whether it existed as an option) as a result of where they received their care. Ideally, physicians’ treatment descriptions should not differ as a function of non-clinical aspects such as their medical specialty, financial incentives, industry relationships, or treatment availability. Although one might argue that it would be unethical to discuss unavailable treatment options, many patients do have the option to seek treatment at another medical facility. Even within the VA system, “roughly 80 percent of patients have private insurance—or are covered through Medicare and Medicaid—and are thus able to seek care from private sector physicians and hospitals” (Klein, 2011). Patients should at least be informed that robotic surgery is an option so that they can decide if they would like to seek treatment elsewhere. Alternatively, when robotic surgery is available, a more balanced presentation of the advantages and disadvantages of robotic prostatectomy may lead patients to choose to receive open surgery in order to avoid the extra cost associated with robotic prostatectomy (Bolenz et al., 2010).
We acknowledge, however, that it may be difficult for physicians to present robotic prostatectomy in a non-biased manner regardless of availability, particularly given patients’ emotions that may stem from their demand for robotic surgery (Gomes, 2011). Addressing patients’ emotional needs is an important part of clinical encounters (Fuertes et al., 2007) with important downstream consequences such as patient satisfaction and treatment adherence (Street, Makoul, Arora, and Epstein, 2009). Our findings point to an understudied source of patient emotion, the differential availability of desired treatment options. We believe that future research is needed to explore how physicians can address patients’ emotional concerns while providing them with a balanced, non-biased explanation of treatment options regardless of their availability.
From a treatment satisfaction perspective, physicians should be careful not to inappropriately raise patient expectations by describing robotic prostatectomy in an overly positive manner when evidence on the benefits is equivocal. Unrealistically high patient expectations for robotic prostatectomy is thought to be the driving factor behind the finding that patients who underwent robotic (vs. open) prostatectomy were 3–4 times more likely to be regretful and dissatisfied with their treatment despite similar functional outcomes. Previous research has found that websites often present overly positive, non-evidence-based information about robotic prostatectomy (Mirkin et al., 2012), and it is important that physicians do not exacerbate this problem.
Limitations
Although our study offers important insights, it has a number of limitations. First, we recorded appointments from 2008 to 2012, during which time additional studies were published about robotic and open prostatectomy (e.g. Berryhill et al., 2008; Schroeck et al., 2008; Yu et al., 2012). Although it is theoretically possible that the variability we found in how physicians portray robotic prostatectomy was influenced in part by the changing nature of this evidence, this would not explain the systematic differences in descriptions across sites.
Second, we cannot rule out that the differences we observed were a result of other site-specific characteristics that varied systematically with availability rather than physicians’ attempts to manage patients’ emotions. For example, our results could potentially reflect actual differences in outcomes because when robotic surgery is available, physicians will have more experience with the procedure, and increased experienced is associated with improved outcomes (Herrmann et al., 2007). However, we do not believe this explains our findings for two reasons. First, the majority of physicians framed their descriptions of robotic prostatectomy as a reflection of underlying differences in the procedures themselves rather than a reflection of physicians’ experience with the procedures. In fact, physicians only mentioned that physicians’ experience can influence the outcomes of robotic prostatectomy in 7.6% of appointments (9/118). Second, because most physicians at the VA site where robotic surgery was unavailable performed robotic surgery at their affiliated academic center, physicians’ experience with robotic surgery likely did not vary drastically as a function of the robot’s availability. Again, this supports our hypothesis that the differences we observed in physicians’ descriptions of robotic prostatectomy were a reflection of their attempt to manage patients’ emotions rather than differences in experience with the technology.
Third, there may be other reasons that physicians portrayed robotic prostatectomy differently when it was available versus unavailable in addition to an attempt to manage patients’ emotions. For example, physicians may have perceived it as futile (or even unethical) to portray unavailable treatment options in a positive manner. Alternatively, physicians at sites where robotic prostatectomy was available may have felt pressured to justify the institution’s investment in such an expensive technology.
Finally, our findings may not be generalizable to other settings. First, our study included only one site where robotic surgery was unavailable versus three where it was available. Second, our study was conducted within the VA medical system, which differs from the general medical system in a number of ways. For example, patients in the VA are poorer, older, sicker, and more likely to have social problems and mental illnesses (Oliver, 2007). This may have exacerbated patients’ negative emotions when robotic prostatectomy was unavailable, increasing differences in physicians’ descriptions when robotic surgery was available versus unavailable. Thus, it is possible our findings may not generalize to non-VA settings. Third, the physicians in our study were primarily residents. Because residents’ approaches to clinical care are shaped by the approaches of their attending physicians (Feldman, Skeel Williams, Knox, & Coates, 2012), we believe that our findings may have been equivalent if the physicians in our study were primarily attending physicians. However, we cannot be certain of this, nor can we assume that our findings would translate to other types of medical professionals. In general, it will be important to replicate our findings with larger, more representative samples.
Conclusion
Physicians were more likely to discuss robotic prostatectomy when it was available. Furthermore, when they did discuss it, they were more likely to describe robotic prostatectomy as superior to open prostatectomy when robotic surgery was available, suggesting that treatment availability is a novel source of bias during clinical appointments. Our study offers important insights into how physicians may frame the advantages and disadvantages of treatment options depending on their availability. These findings are particularly important because new technologies such as proton beam therapy are following a similar trajectory as robotic surgery: there is differential availability of the technology, patient demand associated with direct-to-consumer advertising (Shah, Paly, Efstathiou, & Bekelman, 2013), and contradictory evidence of what (if any) advantage the new technology offers (Konski, Speier, Hanlon, Beck, & Pollack, 2007). Within this context, physicians may be particularly likely to shape their descriptions of treatment options as function of their availability. Future research is needed to examine if physicians’ descriptions of other treatments are influenced by their availability and to explore the potential downstream consequences of this phenomenon from a patient, physician, and institutional perspective.
Acknowledgments
We would like to thank the many residents and attending urologists who participated in this study, especially Jeffrey Montgomery, Edward McGuire, Ted Skolarus, Christopher Kane, Kirsten Green, and Philip Walther. We would also like to thank our dedicated and hard-working staff who contributed significantly to the success of the project: Rosemarie K. Pitsch, Julie A. Tobi, Gregory Greene, Peninah Kaniu, Maria Granata, Patricia Hartwell, Hollis Weidenbacher, Natalie Atyeo, Kelly Davis, Haley Miller, Margaret Oliver, Elizabeth Reiser, and Biqi Zhang. Special thanks to Natalie Atyeo for her help with manuscript preparation. Financial support for this study was provided by an IIR Merit Award from U.S. Department of Veterans Affairs (IIR 05-283) to Dr. Fagerlin and Federal Grant T32 GM007171 to Karen Scherr as part of the Medical Scientist Training Program.
Footnotes
Lillie D. Williamson is now at the Department of Communication, University of Illinois at Urbana-Champaign.
Contributor Information
Karen A. Scherr, Fuqua School of Business and School of Medicine, Duke University
Angela Fagerlin, Departments of Internal Medicine and Psychology, Center for Bioethics and Sciences in Medicine, University of Michigan Ann Arbor, The Ann Arbor VA Venter for Clinical Management Research, Ann Arbor, Michigan.
John T. Wei, Department of Urology, University of Michigan
Lillie D. Williamson, Fuqua School of Business, Duke University
Peter A. Ubel, Fuqua School of Business, School of Medicine and Sanford School of Public Policy, Duke University
References
- Barry MJ, Edgman-Levitan S. Shared decision making — The pinnacle of patient-centered care. The New England Journal of Medicine. 2012;366:780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- Berryhill R, Jhaveri J, Yadav R, Leung R, Rao S, El-Hakim A, Tewari A. Robotic prostatectomy: A review of outcomes compared with laparoscopic and open approaches. Urology. 2008;72:15–23. doi: 10.1016/j.urology.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Bolenz C, Gupta A, Hotze T, Ho R, Cadeddu JA, Roehrborn CG, Lotan Y. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. European Urology. 2010;57:453–458. doi: 10.1016/j.eururo.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Davison BJ, Breckon E. Factors influencing treatment decision making and information preferences of prostate cancer patients on active surveillance. Patient Education and Counseling. 2012;87:369–374. doi: 10.1016/j.pec.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Dedoose Version 4.5, web application for managing, analyzing, and presenting qualitative and mixed method research data. Los Angeles, CA: SocioCultural Research Consultants, LLC; 2013. [Google Scholar]
- Elo S, Kyngäs H. The qualitative content analysis process. Journal of Advanced Nursing. 2008;62:107–115. doi: 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- Feldman L, Skeel Williams K, Knox M, Coates J. Influencing controlled substance prescribing: Attending and resident physician use of a state prescription monitoring program. Pain Medicine. 2012;13:908–914. doi: 10.1111/j.1526-4637.2012.01412.x. [DOI] [PubMed] [Google Scholar]
- Fowler FJ, Jr, Collins MM, Albertsen PC, Zietman A, Elliott DB, Barry MJ. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA. 2000;283:3217–3222. doi: 10.1001/jama.283.24.3217. [DOI] [PubMed] [Google Scholar]
- Fuertes JN, Mislowack A, Bennett J, Paul L, Gilbert TC, Fontan G, Boylan LS. The physician–patient working alliance. Patient Education and Counseling. 2007;66:29–36. doi: 10.1016/j.pec.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Gomes P. Surgical robotics: Reviewing the past, analysing the present, imagining the future. Robotics and Computer-Integrated Manufacturing. 2011;27:261–266. doi: 10.1016/j.rcim.2010.06.009. [DOI] [Google Scholar]
- Herrmann TR, Rabenalt R, Stolzenburg JU, Liatsikos EN, Imkamp F, Tezval H, Burchardt M. Oncological and functional results of open, robot-assisted and laparoscopic radical prostatectomy: Does surgical approach and surgical experience matter? World journal of urology. 2007;25:149–160. doi: 10.1007/s00345-007-0164-9. [DOI] [PubMed] [Google Scholar]
- Holmes-Rovner M, Montgomery JS, Rovner DR, Scherer L, Whitfield J, Kahn VC, Fagerlin A. Informed decision making: Assessment of the quality of physician communication about prostate cancer diagnosis and treatment. Medical Decision Making. 2015 doi: 10.1177/0272989X15597226. [DOI] [PubMed] [Google Scholar]
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qualitative Health Research. 2005;15:1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- Hudak PL, Clark SJ, Raymond G. How surgeons design treatment recommendations in orthopaedic surgery. Social Science & Medicine. 2011;73:1028–1036. doi: 10.1016/j.socscimed.2011.06.061. [DOI] [PubMed] [Google Scholar]
- Jagsi R. Conflicts of interest and the physician-patient relationship in the era of direct-to-patient advertising. Journal of Clinical Oncology. 2007;25:902–905. doi: 10.1200/JCO.2006.08.7122. [DOI] [PubMed] [Google Scholar]
- Klein S. The Veterans Health Administration implementing patient-centered medical homes in the nation’s largest integrated delivery system. New York: The Commonwealth Fund; 2011. (Publication No. 1537). [Google Scholar]
- Konski A, Speier W, Hanlon A, Beck JR, Pollack A. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? Journal of Clinical Oncology. 2007;25:3603–3608. doi: 10.1200/JCO.2006.09.0811. [DOI] [PubMed] [Google Scholar]
- Mirkin JN, Lowrance WT, Feifer AH, Mulhall JP, Eastham JE, Elkin EB. Direct-to-consumer Internet promotion of robotic prostatectomy exhibits varying quality of information. Health Affairs. 2012;31:760–769. doi: 10.1377/hlthaff.2011.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver A. The Veterans Health Administration: An American success story? The Milbank Quarterly. 2007;85:5–35. doi: 10.1111/j.1468-0009.2007.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldaña J. The Coding Manual for Qualitative Researchers. 14. Sage; 2012. [Google Scholar]
- Schroeck FR, Krupski TL, Sun L, Albala DM, Price MM, Polascik TJ, Moul JW. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. European Urology. 2008;54:785–793. doi: 10.1016/j.eururo.2008.06.063. [DOI] [PubMed] [Google Scholar]
- Shah A, Paly JJ, Efstathiou JA, Bekelman JE. Physician evaluation of internet health information on proton therapy for prostate cancer. International Journal of Radiation Oncology* Biology* Physics. 2013;85:e173–e177. doi: 10.1016/j.ijrobp.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street RL, Makoul G, Arora NK, Epstein RM. How does communication heal? Pathways linking clinician–patient communication to health outcomes. Patient Education and Counseling. 2009;74:295–301. doi: 10.1016/j.pec.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Forman JD, Tangen CM. Guideline for the management of clinically localized prostate cancer : 2007 update. The Journal of Urology. 2007;177:2106–2131. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Nguyen PL, Hu JC. Hospital volume, utilization, costs and outcomes of robot-assisted laparoscopic radical prostatectomy. The Journal of Urology. 2012;187:1632–1638. doi: 10.1016/j.juro.2011.12.071. [DOI] [PubMed] [Google Scholar]


