Abstract
Background
Periprosthetic infections are devastating for patients and more efficacious preventive strategies are needed. Surface-modified implants using antibacterial coatings represent an option to cope with this problem; however, manufacturing limitations and cytotoxicity have curbed clinical translation. Among metals with antibacterial properties, copper has shown superior in vitro antibacterial performance while maintaining an acceptable cytotoxicity profile. A thin film containing copper could prevent early biofilm formation to limit periprosthetic infections. This pilot study presents the in vitro antibacterial effect, cytotoxicity, and copper ion elution pattern of a thin film of titanium-copper oxide (TiCuO).
Questions/purposes
(1) Do titanium alloy (Ti6Al4V) discs coated with a thin film of TiCuO reduce Staphylococcus epidermidis biofilm and planktonic cell density compared with uncoated discs? (2) Do Ti6Al4V discs coated with a thin film of TiCuO affect normal human osteoblast viability compared with untreated cells? (3) Is copper ion concentration generated by coated discs lower than previously published copper ion concentrations that cause 50% toxicity in similar human cell lines in vitro (TC50)?
Methods
Ninety Ti6Al4V discs (12.5 mm diameter; 1.25 mm thick) were used in this study. Seventy-two Ti6Al4V discs were coated with a thin film of either titanium oxide (TiO) or TiCuO containing 20%, 40%, or 80% copper using high-power impulse magnetron sputtering (HiPIMS). Eighteen Ti6Al4V discs remained uncoated for control purposes. We tested antibacterial properties of S epidermidis grown on discs in wells containing growth medium. After 24 hours, planktonic bacteria as well as biofilms removed by sonication were quantitatively cultured. Annexin/Pi staining was used to quantify in vitro normal human osteoblast cell viability at 24 hours and Day 7, respectively. Copper elution was measured at Days 1, 2, 3, 7, 14, and 28 using an inductively coupled plasma mass spectrometer to analyze aliquots of culture medium. Copper ion concentration achieved at 24 hours was compared with previously published TC50 for gingival fibroblast, a phenotypically similar cell line with available data regarding copper ion exposure.
Results
Discs coated with TiCuO 80% copper showed greater biofilm and planktonic cell density reduction when compared with other tested compositions (analysis of variance [ANOVA]; p < 0.001). Discs coated with TiCuO 80% copper showed mean biofilm and planktonic cell density of 4.0 log10 (SD = 0.4) and 5.7 log10 (SD = 0.2). Discs coated with TiCuO 80% showed a mean difference in biofilm and planktonic cell density of 2.5 log10 (95% confidence interval [CI], 1.9–3.1 log10; p < 0.001) and 1.2 (95% CI, 0.6–1.8; p < 0.001), respectively, when compared with uncoated discs. Normal human osteoblast viability did not differ among all groups at 24 hours (ANOVA; p = 0.2) and Day 7 (ANOVA; p = 0.7). Discs coated with TiCuO 80% copper showed a mean difference (95% CI) in relative cell viability (%) at 24 hours and Day 7 of 31.1 (95% CI, −19.4 to 81.7; p = 0.4) and −5.0 (95% CI, −7.8 to 17.9; p = 0.9), respectively, when compared with untreated cells. For all TiCuO-coated discs, copper ion elution peaked at 24 hours and slowly decreased in a curvilinear fashion to nearly undetectable levels by Day 28. Discs coated with TiCuO 80% copper showed mean copper ion concentration at 24 hours of 269.4 µmol/L (SD = 25.2 µmol/L) and this concentration was lower than previously published TC50 for similar human cell lines at 24 hours (344 µmol/L, SEM = 44 µmol/L).
Conclusions
This pilot study demonstrates a proof of concept that a thin-film implant coating with TiCuO can provide a potent local antibacterial environment while remaining relatively nontoxic to a human osteoblast cell line. Further research in an animal model will be necessary to establish efficacy and safety of this technique and whether it might be useful in the design of implants.
Clinical Relevance
A thin film coating with TiCuO demonstrates high antibacterial activity and low cellular cytotoxicity to human osteoblasts in vitro. Taken together, these properties represent a potential strategy for preventing periprosthetic infection if further work in animal models can confirm these results in vivo.
Introduction
Biofilm-forming bacteria are considered the major cause of periprosthetic infections [32]. Biofilms are created when bacteria adhere to surfaces and secrete an extracellular polymeric matrix. Bacterial adhesion is the first and most important step in this process. Once bacteria establish a biofilm, protection is conferred from both the host immune system and antibiotic therapy, perpetuating the infection [29]. Thus, human host immune cells compete with microorganisms for the implant surface in the so-called “race for the surface” [12]. Staphylococcus epidermidis is recognized as a leading nosocomial pathogen. This microorganism has a strong ability to establish adherent biofilms on artificial surfaces such as central venous catheters, artificial heart valves, and prosthetic joints [36].
Periprosthetic infections are devastating to patients. These infections occur after 0.4% to 2.2% primary THAs and TKAs [24, 25, 46]. For patients undergoing tumor megaprosthetic implantation, the risk is greater, estimated at 4% to 45% [28]. This complication harms patients and strains healthcare systems around the world [7, 17]. In addition, current treatment for periprosthetic infection such as implant extraction, meticulous débridement, antibiotic-loaded cement, and high-dose systemic antibiotic administration adds certain morbidity and can often result in side effects without assuring cure [37].
Several attempts have been made to add antibacterial properties to orthopaedic devices by coating them with metal particles, antibiotics, or antibacterial solutions. Clinical translation has been limited by cytotoxicity concerns, poor adhesive properties, and lack of homogeneity and complexity of coating methods [1, 39, 48]. Silver-coated megaprostheses are currently available in Europe for clinical use in tumor surgery. Infection reduction has been modest with this approach and argyria, a bluish skin decoloration caused by abnormal silver compound deposits, has been reported in up to 20% of patients [9, 15, 23, 45].
The U.S. Environmental Protection Agency has approved over 300 copper alloys as antimicrobial touch surfaces [11] and its public health benefit has been supported by a multicenter study that showed patients treated in intensive care units with copper alloy surfaces had a significantly lower incidence of hospital-acquired infections and/or colonization with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci than did patients treated in standard rooms [33]. Regarding the use of implants containing copper, practically all clinical experience comes from the use of intrauterine contraceptive devices made of copper [21]. Copper is essential for several cellular enzymatic reactions and plays an important role in hydrogen peroxide (H2O2) and highly toxic hydroxyl radical (•OH) generation within phagocytes [2, 14, 30, 34]. This makes copper an interesting material for coating metal implants as a way to prevent periprosthetic infection, especially when considering that copper ions have been shown to have superior antibacterial performance while maintaining biocompatibility in vitro compared with silver, zinc, cobalt, aluminum, and mercury ions [16]. This pilot study explores the value of a thin film of titanium-copper oxide (TiCuO) by describing its antibacterial effect, cytotoxicity, and copper ion elution pattern in vitro.
We asked the following questions: (1) Do titanium alloy (Ti6Al4V) discs coated with a thin film of TiCuO reduce S epidermidis biofilm and planktonic cell density compared with uncoated discs? (2) Do Ti6Al4V discs coated with a thin film of TiCuO affect normal human osteoblast viability compared with untreated cells? (3) Is copper ion concentration generated by coated discs lower than previously published copper ion concentrations that cause 50% toxicity in similar human cell lines in vitro (TC50)?
Materials and Methods
Materials and Coating
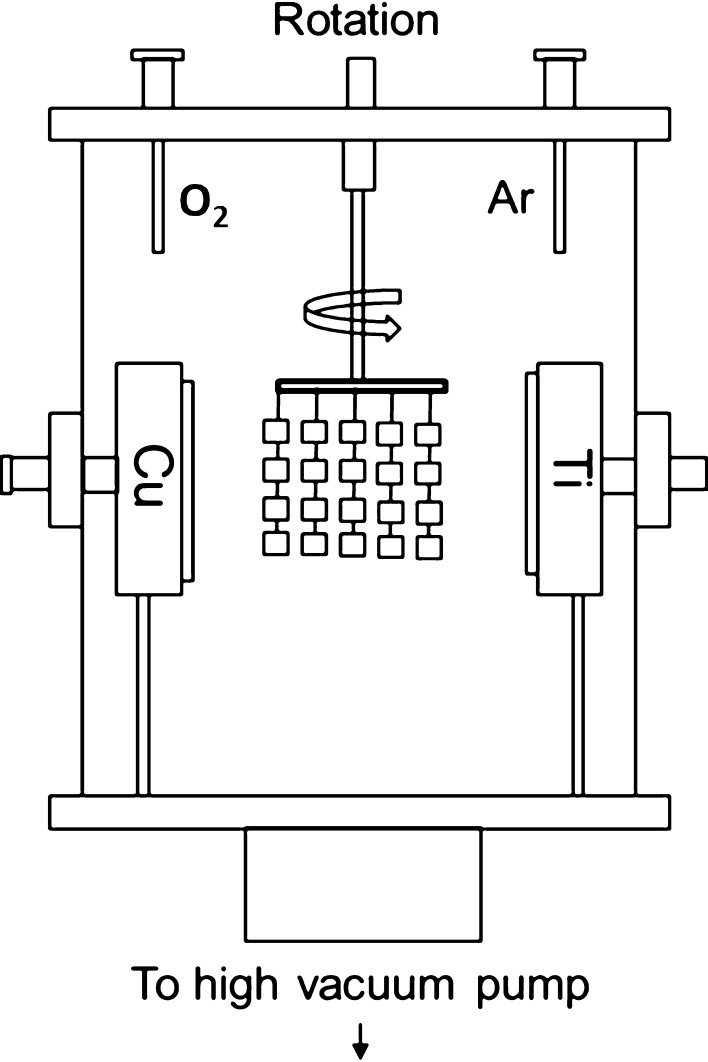
Ti6Al4V discs (12.5 mm diameter × 1.23 mm thick) were waterjet cut from Ti6Al4V sheets (Vincent Metals, Minneapolis, MN, USA). A total of 90 Ti6Al4V discs were manually polished and used in this study. Seventy-two discs were sent to Southwest Research Institute, San Antonio, TX, USA, for coating and 18 discs were left uncoated for control purposes. TiCuO films were deposited in a closed-field unbalanced magnetron sputtering system by reactive sputtering titanium and copper in an argon/oxygen atmosphere (Fig. 1). During the coating process, the substrate holder was rotating constantly in the system at a rotational speed of six revolutions per minute. Titanium targets were powered by a high-power impulse magnetron sputtering (HiPIMS CypriumTM plasma generator; ZpulserTM, Mansfield, MA, USA) and the copper target was powered by pulsed dc magnetron sputtering (Pinnacle plus; Advanced Energy, Fort Collins, CO, USA). Ti6Al4V discs were coated with various ratios of TiO or TiCuO containing 20%, 40%, and 80% copper. The thickness of the film was 450 nm for all coated discs (Fig. 2). Coated discs were randomly analyzed with energy-dispersive x-ray spectroscopy to ensure the films were loaded with the targeted proportion of copper. Coated discs were returned to our institution for further testing. Uncoated and TiO-coated discs were defined as negative controls because we were not expecting an antibacterial effect against S epidermidis. TiCuO-coated discs containing 20%, 40%, and 80% copper, respectively, were defined as experimental discs. All discs underwent flash steam cycle sterilization at 132°C for 3 minutes at 27 to 28 pounds (Getinge Model 433HC, Gothenburg, Sweden) before the experiments. This pilot study combines three experiments testing the following properties of a thin film containing copper: antibacterial activity, cell viability, and elution patterns. We tested five conditions: uncoated discs (Ti6Al4V) and coated discs with TiO, TiCuO 20%, TiCuO 40%, and TiCuO 80% copper. For the antibacterial investigation, we tested all conditions at 24 hours in triplicate (three discs per condition) and we repeated the experiment three times to assess consistency. For cell viability analysis, we tested all conditions at Days 1 and 7 in triplicate and we did not repeat the experiment. For elution pattern assessment, we tested all conditions at Days 1, 2, 3, 7, 14, and 28 in triplicate. Discs were not changed through time and we did not repeat the experiment.
Fig. 1.
This schematic drawing shows the characteristics of the closed-field unbalanced magnetron sputtering system used for TiCuO coating depositions. This deposition system is a cubic chamber (30 inches × 30 inches × 30 inches) that contains two unbalanced magnetrons of reversed magnetic polarities installed vertically to form a closed magnetic field. The substrates were ultrasonically cleaned in acetone and ethyl alcohol for 10 minutes each. After cleaning, the substrates were installed in the deposition chamber on a double-rotation holder. Before all film depositions, a base negative pressure in the deposition system was achieved. The titanium target was powered by HiPIMS (HiPIMS Cyprium™ plasma generator; Zpulser Inc) and the copper target was powered by pulsed dc magnetron sputtering (Pinnacle plus; Advanced Energy Inc). Argon and oxygen gas flows were introduced into the system through ports in the side of the chamber. The chamber was baked to release adsorbed water and gasses at 120°C.
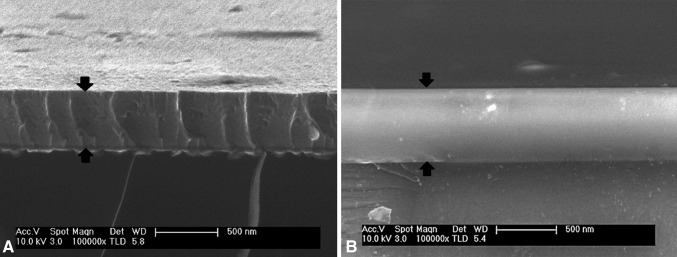
Fig. 2A–B.
The homogeneity of the thin film was confirmed by scanning electron microscopy. The photographs show axial cut and lateral views of TiCuO film containing 40% (A) and 80% (B) of copper. Black arrows define the thickness of the coating.
Antibacterial Activity
Using a previously described method [13, 16], discs were submerged in separate wells containing 2 mL Roswell Park Memorial Institute culture media and 10% fetal calf serum containing 106 colony-forming units per milliliter (CFU/mL) of S epidermidis (IDRL 6061) isolated from a periprosthetic hip infection. After 24 hours at 37°C on an orbital shaker, discs were removed from the wells, dipped into sterile saline to remove planktonic cells, placed into 1 mL sterile saline, vortexed, sonicated, and revortexed. The sonicate fluid was quantitatively cultured and the bacterial load was reported as log10 CFU/mL. In addition, 1 mL of the planktonic cells from each well was collected, the copper ions neutralized with 1 mL of 1.0 g/L sodium thioglycolate and 1.5 g/L sodium thiosulfate, and the solution quantitatively cultured. Bacterial load was reported as log10 CFU/mL. Visual assessment of the amount of biofilm and sonicated fluid was recorded.
Normal Human Osteoblast Viability
Normal human osteoblast cell lines (Lonza, Allendale, NJ, USA) were used for cell viability experiments. Cells were separately seeded in 12-well plates (Corning, Tewksbury, MA, USA) at a concentration of 3 × 104 cells per well. Osteoblast Growth Medium (Lonza), a cell culture medium optimized for osteoid cell lineages, was used as the primary culture medium for normal human osteoblasts. Once cells reached 90% confluence, a Netwell® insert (Corning) was placed in each well to suspend the discs in 2 mL culture medium above the cells (Fig. 3A–B). To simulate normal extracellular fluid turnover, we changed cell medium every 24 hours. Annexin/PI staining was used to determine not only cell death, but also apoptosis at 24 hours and Day 7 of cell exposure. Untreated normal human osteoblast cells were considered the control group.
Fig. 3A–B.
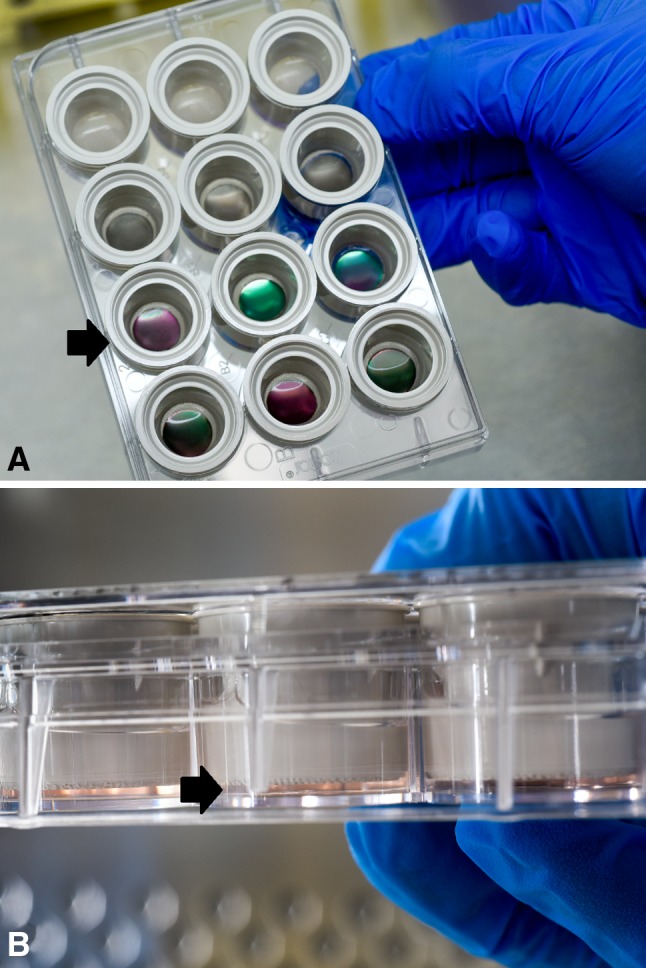
(A) Normal human osteoblast cells (Lonza) were separately seeded in 12-well plates (Corning) at a concentration of 3 × 104 cells per well. OGM medium (Lonza), a cell culture medium optimized for osteoid cell lineages, was used to feed the cells. Once cells reached 90% confluence, a Netwell® insert (Corning) was placed in each well to suspend the discs and avoid direct contact with the cells (black arrow). Two milliliters of culture medium were needed to completely cover the discs. To simulate normal extracellular fluid turnover, cell medium was exchanged every 24 hours. (B) Side view of 12-well plates (Corning). Black arrow is pointing how the cells are separated from the discs.
Elution Pattern and Toxic Concentration of Copper Ions
Copper release rates were measured using an inductively coupled plasma mass spectrometer in dynamic reaction cell mode [4]. The uncoated and coated discs were placed in 2 mL Osteoblast Growth Medium (OGM; Lonza) at 37°C in a humidified atmosphere with 5% CO2. The copper concentration in isolated OGM was measured at Days 1, 2, 3, and 7 for control discs and at Days 1, 2, 3, 4, 7, 14, and 28 days for experimental discs. Medium was changed every 24 hours to simulate physiologic fluid turnover. Different in vitro studies have defined copper ion concentrations that produce 50% of cell population toxicity (TC50) or death (LD50). These concentrations depend mainly on the cell line exposed to copper ions. For gingival fibroblast populations, TC50 at 24 hours has been calculated to be 344 µmol/L (SEM 44 µmol/L) [20]. For neurons and hepatic cells, LD50 range from 17 to 750 µmol/L [35, 42]. In our case, we did not look for TC50 or LD50 of normal human osteoblast expose to copper ions; instead, we exposed cells to TiCuO-coated discs containing different amounts of copper that eluted copper ions. Therefore, we use previously published TC50 for gingival fibroblast, a phenotypically similar human cell line, to compare our results.
Statistical Analysis
All data are presented using summary statistics, including means and SDs for all continuous data. The primary outcomes included biofilm and planktonic cell density (expressed on a log10 scale), relative normal human osteoblast viability (reported as a percentage of untreated cell viability), and copper ion concentration (reported as µmol/L). Separate analyses were conducted for each outcome. Comparisons of the outcomes among the five conditions (described previously) were performed using analysis of variance (ANOVA). When the overall F test was found to be significant, further analysis was conducted using an appropriate multiple comparisons procedure (such as the Ryan-Einot-Gabriel-Welch test) to control the overall experimentwise Type I error rate. To evaluate the effect of the experimental conditions on the release of copper ions over time, a two-factor ANOVA with repeated measures on one factor (time) was used. However, because a significant interaction between time and copper concentration was observed, separate one-factor models were generated to compare the concentration levels separately at each time point. All statistical tests were two-sided, and p values < 0.05 were considered significant. All analysis was conducted using SAS Version 9.3 (SAS Institute Inc, Cary, NC, USA).
Results
Antibacterial Activity
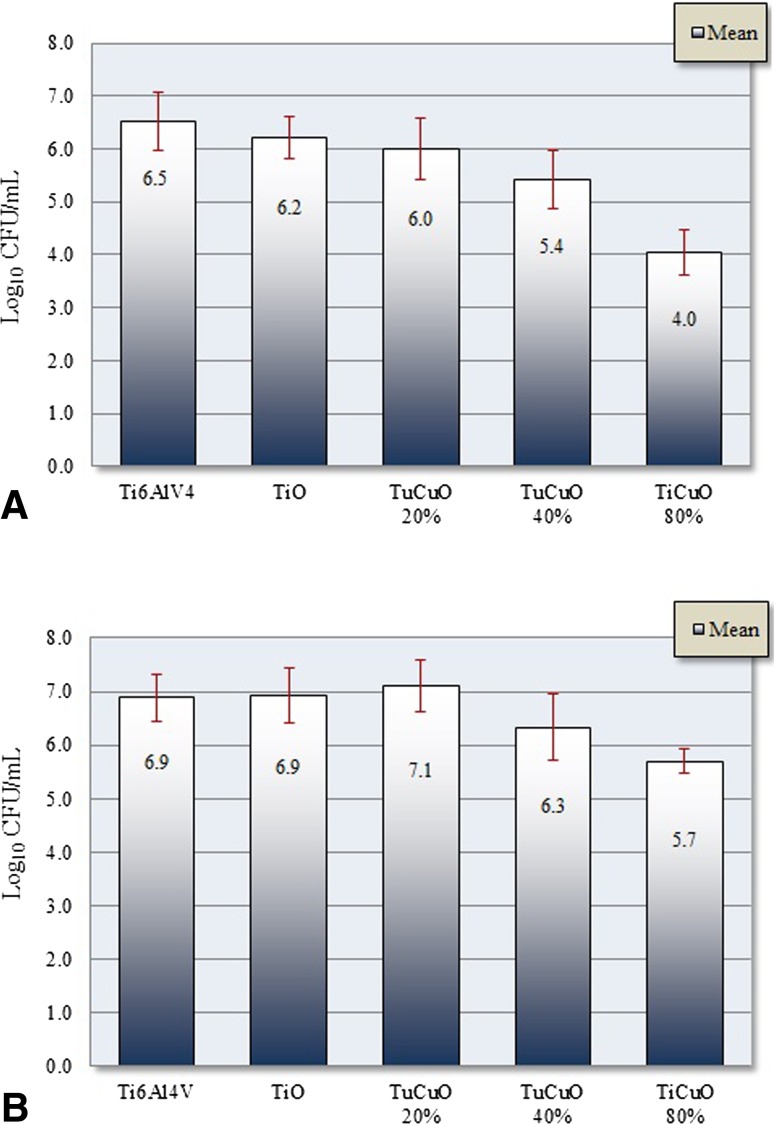
Discs coated with TiCuO 80% copper showed biofilm and planktonic cell density reduction when compared with other tested compositions. Mean (SD) biofilm cell density (log10 CFU/mL) at 24 hours was 6.5 (± 0.7) for Ti6Al4V, 6.2 (± 0.4) for TiO, 6.0 (± 0.7) for TiCuO 20% copper, 5.4 (± 0.7) for TiCuO 40% copper, and 4.0 (± 0.4) for TiCuO 80% copper. Mean planktonic cell density (log10 CFU/mL) at 24 hours was 6.9 (± 0.4) for Ti6Al4V, 6.9 (± 0.5) for TiO, 7.1 (± 0.5) for TiCuO 20% copper, 6.3 (± 0.6) for TiCuO 40% copper, and 5.7 (± 0.2) for TiCuO 80% copper (Fig. 4A–B). Mean difference (95% confidence interval [CI]) in biofilm cell density (log10 CFU/mL) when comparing uncoated discs with discs coated with TiO, TiCuO 20% copper, TiCuO 40% copper, and TiCuO 80% copper was 0.3 (95% CI, −0.2 to 0.8; p = 0.4), 0.5 (95% CI, −0.01 to 1.1; p = 0.1), 1.1 (95% CI, 0.5–1.7; p < 0.001), and 2.5 (95% CI, 1.9–3.1; p < 0.001). Mean difference (95% CI) in planktonic cell density (log10 CFU/mL) when comparing uncoated disc with discs coated with TiO, TiCuO 20% copper, TiCuO 40% copper, and TiCuO 80% copper was −0.03 (95% CI, −0.5 to 0.5; p = 0.9), −0.2 (95% CI, −0.4 to 0.8; p = 0.08), 0.6 (95% CI, −0.01 to 1.1; p = 0.06), and 1.2 (95% CI, 0.6–1.8; p < 0.001). Further statistical analysis showed that mean biofilm and planktonic cell density of uncoated discs, discs coated with TiO, and TiCuO 20% copper were not different from each other. As the copper concentration increased, the amount of biofilm after rinsing became more scant or absent. Visual assessment of planktonic fluid and biofilm sonicate fluid was considerably less turbid when discs coated with TiCuO containing 80% copper were compared with uncoated discs (Fig. 5).
Fig. 4A–B.
These graphs show biofilm (A) and planktonic (B) mean cell density after 24 hours of exposure. Using ANOVA, overall differences among groups were significantly different (p < 0.05); however, only discs coated with TiCuO 80% copper and TiCuO 40% copper demonstrated statistical significance after multiple group comparison analysis (p < 0.05). Discs coated with TiCuO 80% copper achieved 2.5 log10 and 1.2 log10 reduction in biofilm and planktonic cell density compared with uncoated discs (p < 0.001).
Fig. 5A–F.
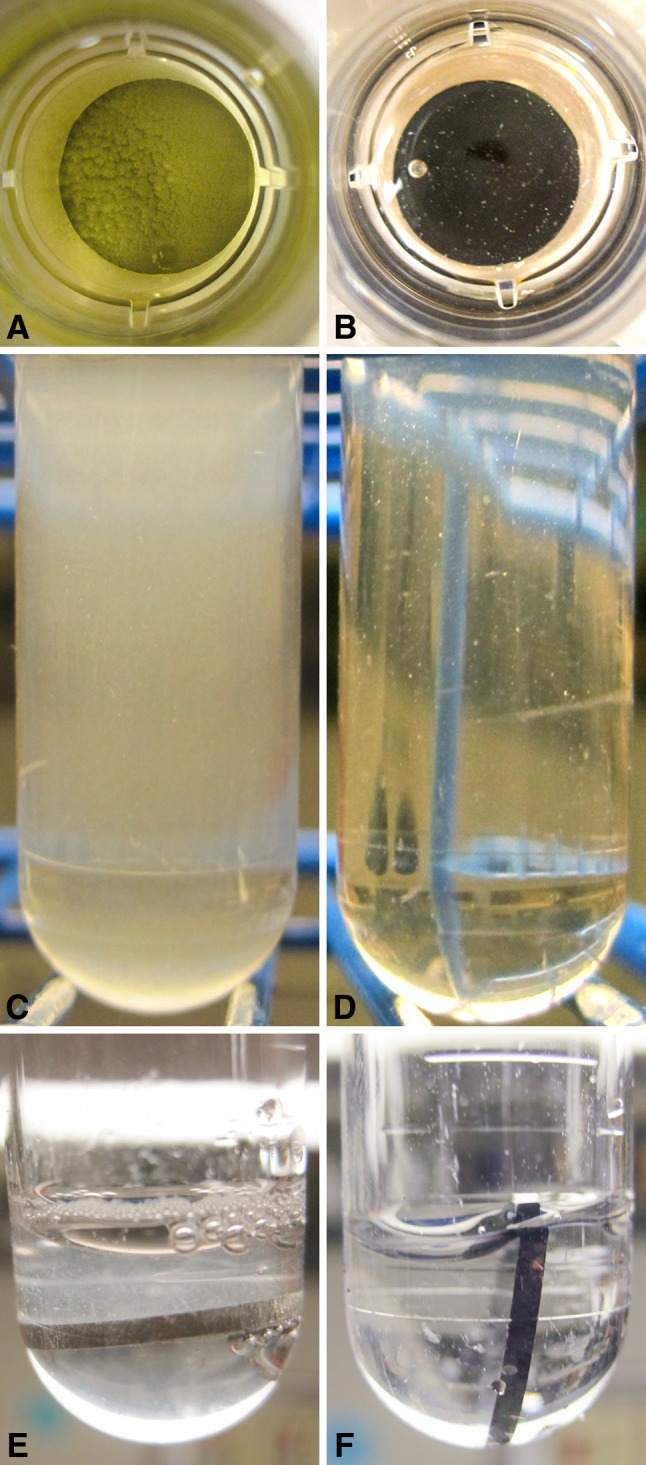
Marked biofilm was present on the top side of uncoated discs (A) compared with scant biofilm seen on discs coated with TiCuO 80% copper (B) after 24 hours. The planktonic fluid from surrounding the uncoated discs was turbid (C) compared with a fairly clear fluid from the wells containing discs coated with TiCuO 80% copper (D). After rinsing, the biofilm was markedly less adherent and easily rinsed off the experimental discs compared with uncoated discs. As the copper concentration increased, the amount of film after rinsing was scant or absent. Marked biofilm was still present on the top side of uncoated discs after rinsing (E) compared with a clear surface of on the top side of discs coated with TiCuO 80% copper (F).
Normal Human Osteoblast Viability
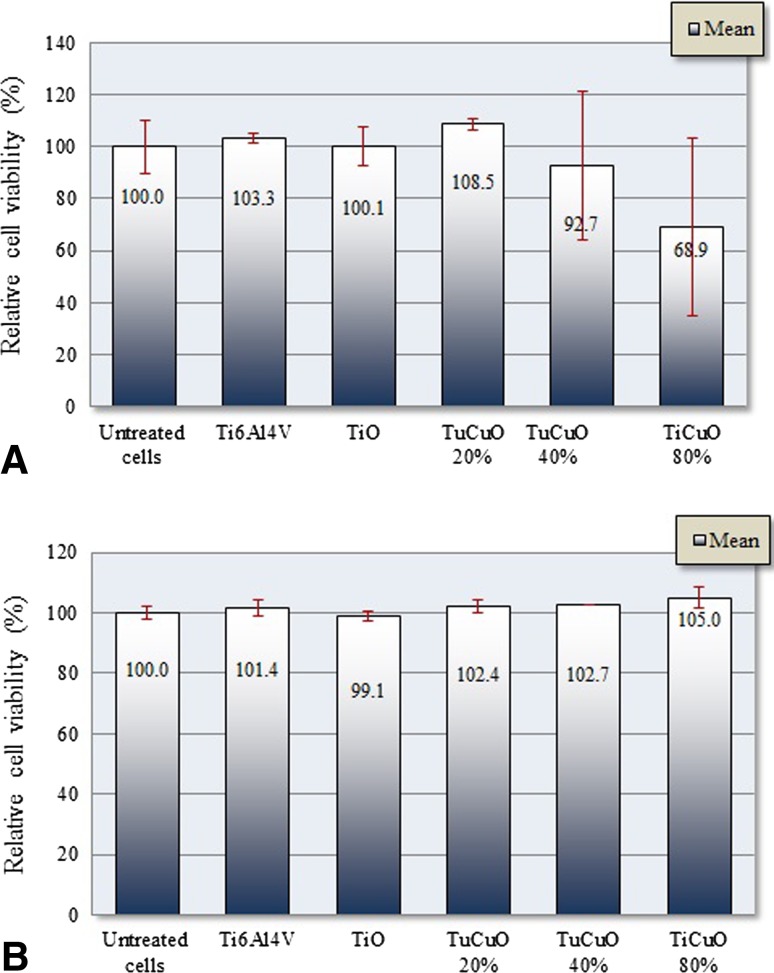
Despite the fact that multiple comparison analysis failed to show differences in cell viability among the groups at 24 hours (p = 0.2) and Day 7 (p = 0.7), discs coated with TiCuO 80% copper considerably affected normal human osteoblast viability at 24 hours. Mean (SD) relative cell viability (%) at 24 hours was 100.3 (± 2.0) for Ti6Al4V, 100.1 (± 7.2) for TiO, 108.5 (± 2.1) for TiCuO 20% copper, 92.7 (± 28.4) for TiCuO 40% copper, and 68.9 (± 34.2) for TiCuO 80% copper. Mean (SD) relative cell viability at Day 7 was 101.4 (± 2.7) for Ti6Al4V, 99.1 (± 1.7) for TiO, 102.4 (± 2.1) for TiCuO 20% copper, 102.7 (± 0.1) for TiCuO 40% copper, and 105.0 (± 3.6) for TiCuO 80% copper. We included one possible outlier in the analysis of discs coated with TiCuO 40% copper (results in triplicates = 109.0, 109.1, 59.9) and one possible outlier in the analysis of discs coated with TiCuO 80% copper (results in triplicates = 89.9, 29.4, 87.4) (Fig. 6A–B). Mean difference (95% CI) in relative cell viability (%) at 24 hours when comparing untreated cells with cells exposed to uncoated discs, discs coated with TiO, TiCuO 20% copper, TiCuO 40% copper, and TiCuO 80% copper was −3.26 (95% CI, −47.3 to 53.8; p = 0.9), −0.1 (95% CI, −50.5 to 50.6; p = 0.9), −8.5 (95% CI, −42.0 to 59.1; p = 0.9), 7.3 (95% CI, −43.2 to 57.9; p = 0.9), and 31.1 (95% CI, −19.4 to 81.7; p = 0.4). Mean difference (95% CI) in relative cell viability (%) at Day 7 when comparing untreated cells with cells exposed to uncoated discs, discs coated with TiO, TiCuO 20% copper, TiCuO 40% copper, and TiCuO 80% copper was −1.4 (95% CI, −11.4 to 14.2; p = 0.9), 1.0 (95% CI, −11.9 to 13.8; p = 0.9), −2.4 (95% CI, −10.5 to 15.2; p = 0.9), −2.7 (95% CI, −10.1 to 15.6; p < 0.9), and −5.0 (95% CI, −7.8 to 17.9; p = 0.9).
Fig. 6A–B.
These graphs compare mean and SD of relative cell viability of normal human osteoblasts (Normal human osteoblast) at 24 hours (A) and Day 7 (B) after of exposure using annexin/PI staining.
Elution Pattern and Toxic Concentration of Copper Ions
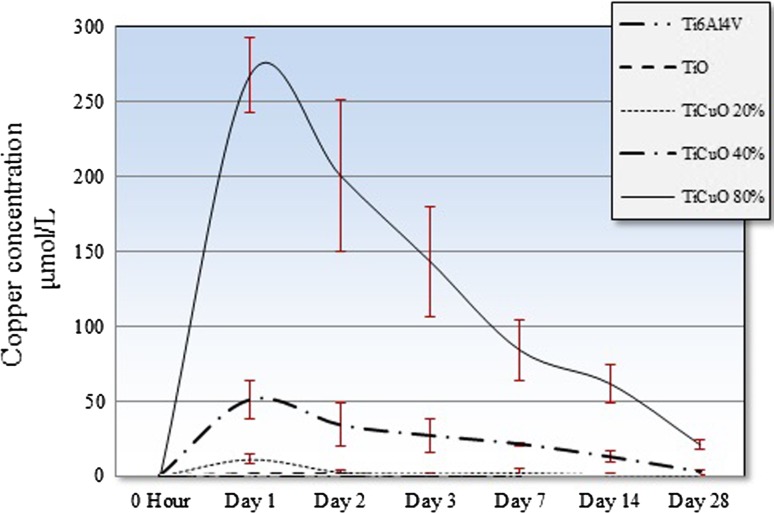
Copper ion concentration that achieved the greatest antibacterial activity at 24 hours was generated by discs coated with TiCuO 80% copper. This concentration was lower than previously described TC50 of copper ions for gingival fibroblasts [20]. Uncoated and TiO-coated discs showed insignificant traces of copper ions in the supernatant over the first 7 days of measurement. All experimental discs had a peak concentration of copper ions at 24 hours with a subsequent curvilinear decrease over time (Fig. 7). Mean (SD) copper ion concentration at 24 hours for discs coated with TiO, TiCuO 20%, 40%, and 80% copper was 1.3 (± 0.9), 11.2 µmol/L (± 2.8), 50.8 µmol/L (± 12.9), and 269.4 µmol/L (± 25.2 µmol/L), respectively. Mean copper ion concentration at Day 28 for discs coated with TiCuO 20%, 40%, and 80% copper was 0.7 µmol/L (SD = 0.3), 2.7 µmol/L (SD = 1.6), and 21.1 µmol/L (SD = 2.3 µmol/L), respectively. Copper ion concentration between each composition was different from each other when compared by separate day (two-way repeated-measures ANOVA p < 0.001) (Table 1).
Fig. 7.
This graph shows the kinetics of copper elution from uncoated discs, TiO-coated discs, and TiCuO-coated discs containing 20% 40%, and 80% copper.
Table 1.
Analysis of copper concentration separate by day
| Day | Coating composition | Mean (SD) µmol/L |
p value | Pairwise comparisons |
|---|---|---|---|---|
| 1 | Uncoated Ti6Al4V | 0.5 (0.1) | < 0.001 | TiCuO 80% > TiCuO 40%, TiCuO 20%, TiO, uncoated Ti6Al4V TiCuO 40% > TiCuO 20%, TiO, uncoated Ti6Al4V |
| TiO | 1.2 (0.9) | |||
| TiCuO 20% | 11.2 (2.8) | |||
| TiCuO 40% | 50.8 (12.9) | |||
| TiCuO 80% | 269.4 (25.2) | |||
| 2 | Uncoated Ti6Al4V | 0.3 (0.1) | < 0.001 | TiCuO 80% > TiCuO 40%, TiCuO 20%, TiO, uncoated Ti6Al4V |
| TiO | 1.0 (0.7) | |||
| TiCuO 20% | 2.8 (0.7) | |||
| TiCuO 40% | 34.0 (14.5) | |||
| TiCuO 80% | 200.8 (50.8) | |||
| 3 | Uncoated Ti6Al4V | 0.3 (0.04) | < 0.001 | TiCuO 80% > TiCuO 40%, TiCuO 20%, TiO, uncoated Ti6Al4V |
| TiO | 0.6 (0.3) | |||
| TiCuO 20% | 1.6 (0.4) | |||
| TiCuO 40% | 26.9 (11.3) | |||
| TiCuO 80% | 143.2 (36.5) | |||
| 7 | Uncoated Ti6Al4V | 0.3 (0.04) | < 0.001 | TiCuO 80% > TiCuO 40%, TiCuO 20% |
| TiO | 0.4 (0.2) | |||
| TiCuO 20% | 2.2 (2.6) | |||
| TiCuO 40% | 21.2 (1.3) | |||
| TiCuO 80% | 83.9 (20.3) | |||
| 14 | Uncoated Ti6Al4V | N/A | < 0.001 | TiCuO 80% > TiCuO 40%, TiCuO 20% |
| TiO | N/A | |||
| TiCuO 20% | 1.0 (0.2) | |||
| TiCuO 40% | 12.6 (3.9) | |||
| TiCuO 80% | 61.5 (13.0) | |||
| 28 | Uncoated Ti6Al4V | N/A | < 0.001 | TiCuO 80% > TiCuO 40%, TiCuO 20% |
| TiO | N/A | |||
| TiCuO 20% | 0.73 (0.34) | |||
| TiCuO 40% | 2.68 (1.56) | |||
| TiCuO 80% | 20.78 (3.23) |
Ti6Al4V = titanium alloy; TiO = titanium oxide; TiCuO 20% = discs coated with titanium copper oxide containing 20% copper; TiCuO 40% = discs coated with titanium copper oxide containing 40% copper; TiCuO 80% = discs coated with titanium copper oxide containing 80% copper; N/A= composition was not tested.
A summary of the antibacterial, cell viability, and elution results of the different coating compositions studied helps to understand how copper content and copper ion concentration are related with better antibacterial effect but increased cytotoxicity, at least at 24 hours (Table 2).
Table 2.
Summary of antibacterial, cell viability, and elution characteristics of different coating compositions at 24 hours
| Composition | Mean (95% CI; p value) biofilm cell density reduction expressed in log10 | Mean (95% CI; p value) planktonic cell density reduction expressed in log10 | Mean (SD) relative normal human osteoblast viability expressed (%) | Mean (SD) copper ion elution expressed (µmol/L) |
|---|---|---|---|---|
| TiO | 0.3 (−0.2 to 0.8; p = 0.4) | −0.03 (−0.5 to 0.5; p = 0.9) | 100.1 (± 7.2) | 1.3 (± 0.9) |
| TiCuO 20% | 0.5 (−0.01 to 1.1; p = 0.1) | − 0.2 (−0.4 to 0.8; p = 0.08) | 108.5 (± 2.1) | 11.2 (± 2.8) |
| TiCuO 40% | 1.1 (0.5–1.7; p < 0.001) | 0.6 (−0.01 to 1.1; p = 0.06) | 92.7 (± 28.4) | 50.8 (± 12.9) |
| TiCuO 80% | 2.5 (1.9–3.1; p < 0.001) | 1.2 (0.6–1.8; p < 0.001) | 68.8 (± 34.2) | 269.4 (± 25.2) |
CI = confident interval; TiO = titanium-oxide; TiCuO 20% = discs coated with titanium-copper-oxide containing 20% copper; TiCuO 40% = discs coated with titanium-copper-oxide containing 40% copper; TiCuO 80% = discs coated with titanium-copper-oxide containing 80% copper.
Discussion
Many orthopaedic societies consider infection prevention one of the most critical issues to address over the next decade [31]. Initiatives to minimize bacterial colonization at the time of insertion have been studied in the past with slow permeation in the clinical setting [1, 39, 48]. Several preclinical and clinical studies support the use of copper alloy touch surfaces to reduce the burden of bacteria in healthcare settings [10, 11, 33], but few studies support the use of copper implants to reduce periprosthetic infections [6, 18, 27, 41]. The ideal antibacterial coating should be biocompatible, thin, dense, and firm with precise decay of the active material. Furthermore, it should display high activity (release) over the first 24 hours and dissipate once the “race for the surface” has been safeguarded. The coating process should not cause physical property changes in the implant and coating homogeneity should be independent of implant shape. Finally, the associated cost with coating should account for a small percentage of total implant cost. Our aim was to describe the antibacterial, cell viability, and elution characteristics of a thin film generated by HiPIMS in the presence of oxygen with different ratios of titanium and copper as a proof-of-concept investigation for use as a strategy to reduce periprosthetic infection.
This study has several limitations. First, in vitro results may not fully recapitulate in vivo performance of copper-coated implants. In this regard, extracellular fluid volumes that surround an implant in vivo greatly differ from the 2 mL fixed volume used in our study to fully submerge the discs in media. Also, complex extracellular fluid turnover in vivo greatly differs from our fixed change of media every 24 hours. Second, there are many ways to engineer the surface of a metal to elute antibacterial copper ions. In this regard, we chose titanium copper oxide coating because while copper ions are eluting, titanium oxide theoretically provided corrosion resistance to the substrate [3]. However, there are many other coatings containing copper that could have similar or potentially even better performance (eg, titanium-copper, copper-nickel, titanium-coper-nitride, titanium-silver-copper) [5, 6, 18, 22, 41, 44]. Also, we chose HiPIMS, a physical vapor deposition method, for coating because it enables deposition of dense, firm, and thin films. However, other advanced methods of coating could have alternative performance (eg, chemical vapor deposition, pulsed laser deposition, and hybrid deposition processes) [26]. Third, although S epidermidis is the most common bacteria involved in periprosthetic infections, there are many other microorganisms that participate in this pathophysiology that remain untested. Additional experiments are needed to determine if these results translate to other microbes of importance in orthopaedics. Lastly, copper is an essential mineral, but excessive copper is toxic. We compared copper ion concentrations generated by discs coated with TiCuO with a previously published TC50 for a similar cell line. Therefore, we cannot assume identical results for normal human osteoblasts. Also, a significant portion of copper toxicity derives from the ability of copper to accept and donate single electrons because it changes oxidation state, which damages DNA and potentially contributes to genotoxicity [19]. We did not test for genotoxicity and this analysis will be mandatory in subsequent animal model testing.
In our study, discs coated with TiCuO 80% copper showed antibacterial activity and greater reduction in biofilm and planktonic cell density. We recorded 2.5 log10 mean reduction in biofilm formation at 24 hours with discs coated with TiCuO 80% copper. Although a small amount of biofilm was still present on the top side of discs coated with TiCuO 80% copper, the biofilm was markedly less adherent compared with Ti6Al4V discs. Upon rinsing, the majority of the biofilm was removed and no longer visible. Hoene et al. [18] presented antibacterial and adverse tissue effects of TiAl6V4 plates coated with titanium-copper using galvanic deposition in vitro. After 24 hours no planktonic or adherent S aureus was found in contrast to the uncoated plates on which 1.05 × 105 CFU/mL of planktonic bacteria was detected in the incubation fluids and 2.45 × 105 CFU/mL adherent bacteria in the rinsed fluids. Stranak et al. [41] presented the antibacterial profile of titanium-copper (Ti-Cu) thin film deposited using different plasma-assisted magnetron sputtering methods. They obtained similar antibacteria effect against S epidermidis compared with our results. They also found an initial cytotoxic effect followed by the growth of osteoblastic cells (MG-63 cell line). Recently, a case report described the use of a bipolar prosthesis coated with titanium-copper-nitride (TiCuN) as a transient spacer for a two-stage septic total hip revision. The patient received 2 weeks of intravenous antibiotics and 2 weeks of oral antibiotics. At 6 weeks, an outpatient miniincision biopsy showed no signs of a persisting infection. At 8 weeks, the patient underwent second-stage total hip revision. Culture from the spacer sonication fluid failed to show microorganism growth [6].
Although statistical analysis showed no difference in cell viability among groups at 24 hours and Day 7 of exposure, discs coated with TiCuO 80% copper showed an expected slightly larger drop in relative cell viability (31%) compared with untreated cells, which correlates with a higher concentration of copper at 24 hours. This last result was highly influenced by a one-data-point possible outlier. At Day 7 minimal variance in relative cell viability among groups was also identified (Fig. 6A–B). A recent study showed less than 20% cytotoxicity when mouse macrophages were exposed to 250 µmol/L copper ions, whereas CuO nanoparticles showed 20% cytotoxicity at concentrations of 125 µmol/L when compared with untreated cells [43]. Another study, using human hepatic liver carcinoma cell lines (HepG2), showed that a concentration of 200 µmol/L for 24 and 48 hours caused 10% and 25% cytotoxicity when compared with untreated cells [38]. Hoene et al. [18] presented the results after intramuscular implantation of two Ti and two Ti-Cu plates into nine rats. Serum copper was elevated until 48 hours and tissue macrophages around implants increased until 72 hours with moderately increased local inflammatory response. In a previously described case report using a bipolar prosthesis coated with titanium copper nitride (TiCuN) as a transient spacer for a two-stage septic total hip revision, patient postoperative serum copper levels at Day 1, Week 1, and Week 6 were all within physiologic range [6]. Copper is an essential trace element with antibacterial properties known since antiquity. Total body copper in adults is approximately 80 mg; the acceptable range of daily intake is 1.3 to 8.0 mg/day [30, 47]. Medical devices containing copper have been used in humans for several decades. Perhaps the most easily recognized is the contraceptive intrauterine device, TCu380A, which has been used for more than 40 years with no reports of copper toxicity [21]. Beyond people with Wilson’s disease, which is a genetic deficiency of ceruloplasmin, there is little evidence to indicate that chronic human exposure to copper results in systemic effects other than liver injury [2]. Although copper is a very weak sensitizer as compared with other metals, it will be necessary to monitor the integrity of periprosthetic soft tissues in the wake of increasing adverse metal reactions to cobalt and chrome implants or other metals such as silver [8].
Copper ion concentration of discs coated with TiCuO 80% copper slightly surpassed 250 µmol/L at 24 hours, decreased to approximately 200 µmol/L at 48 hours, and reached normal physiologic serum levels of copper level at Day 28. Therefore, after a few weeks of copper ion elution, the implant should become copper-free, avoiding potential long-term copper toxicity. Copper ion concentration that has been documented to cause 50% human cell death within 48 hours in vitro ranges from 178 µmol/L to 750 µmol/L depending on cell type [35, 38, 40, 42]. Among cell lines with similar phenotypes to osteoblasts, human gingival fibroblasts had a TC50 of 344 µmol/L [20]. Although concentration of discs coated with TiCuO 80% copper was below the TC50 for gingival fibroblasts, we cannot conclude copper ion concentration achieved by TCuO would not be toxic for normal human osteoblasts. However, our results were below the previously published threshold and furthermore rapidly tapered off from the peak values. Using the parameters determined in this study, a hypothetical 400 mm × 12-mm femoral endomedullary rod implant coated with 450 nm TiCuO containing 80% copper would release nearly 1000 µg of copper during the first day, which is far below the daily intake of 2000 to 3000 µg copper recommended by the World Health Organization [47].
In summary, periprosthetic infection is one of the most devastating complications in orthopaedics, thus necessitating a new generation of implants capable of intrinsic protection from biofilm-forming bacteria. Dense TiCuO coating generated by HiPIMS has shown auspicious antibacterial activity and biocompatibility in vitro; however, validation with an in vivo animal model will be mandatory to further establish the potential of copper-coated implants in humans. As such, this technology may serve as a novel approach for addressing periprosthetic infection. This has particular relevance for tumor prostheses in which infection is a major concern.
Acknowledgments
We thank Andre Terzic MD, PhD, for his constant support, Dirk Larson for his help with the statistical analysis, Paul Stalboerger for his help with cell culture, Frank Secreto for his help with annexin/PI staining, Sarah Cambern for the coordination and analysis of the copper samples by ICP-MS, Eric Sheahan for his help with figure editing, and Jianliang Lin PhD, for his professionalism to handle the coating process at Southwest Research Institute.
Footnotes
The institution of one or more of the authors (GAN, RP, MK, CCW, PJJ, KEB, ADH, RJS) has received funding in excess of USD 100,000 from Codelco (Codelco Lab, Santiago, Chile; FP00076314-01-S01) and in excess of USD 10,000 from the Mayo Clinic. With the exception of one of the authors (RJS), the investigators have not personally received any money related to this study. One of the authors certifies that he (RJS), or a member of his immediate family, has received or may receive payments or benefits, during the study period, in an amount of USD 10,000 to USD 100,000 from Biomet Inc (Warsaw, IN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Antoci V, Jr, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Vancomycin bound to Ti rods reduces periprosthetic infection: preliminary study. Clin Orthop Relat Res. 2007;461:88–95. doi: 10.1097/BLO.0b013e318073c2b2. [DOI] [PubMed] [Google Scholar]
- 2.Brewer GJ. Wilson’s Disease: A Clinician’s Guide to Recognition, Diagnosis, and Management. Boston, MA, USA: Kluwer Academic; 2001. [Google Scholar]
- 3.Buckwalter JA, Einhorn TA, O’Keefe RJ; American Academy of Orthopaedic Surgeons. Orthopaedic Basic Science: Foundations of Clinical Practice. Rosemont, IL, USA: American Academy of Orthopaedic Surgeons; 2007.
- 4.Chan S, Gerson B, Reitz RE, Sadjadi SA. Technical and clinical aspects of spectrometric analysis of trace elements in clinical samples. Clin Lab Med. 1998;18:615–629. [PubMed] [Google Scholar]
- 5.Ehiasarian A, Pulgarin C, Kiwi J. Inactivation of bacteria under visible light and in the dark by Cu films. Advantages of Cu-HIPIMS-sputtered films. Environ Sci Pollut Res Int. 2012;19:3791–3797. doi: 10.1007/s11356-011-0734-7. [DOI] [PubMed] [Google Scholar]
- 6.Ellenrieder M, Haenle M, Lenz R, Bader R, Mittelmeier W. Titanium-copper-nitride coated spacers for two-stage revision of infected total hip endoprostheses. GMS Krankenhaushyg Interdiszip. 2011;6:Doc16. [DOI] [PMC free article] [PubMed]
- 7.Fernandez-Fairen M, Torres A, Menzie A, Hernandez-Vaquero D, Fernandez-Carreira JM, Murcia-Mazon A, Guerado E, Merzthal L. Economical analysis on prophylaxis, diagnosis, and treatment of periprosthetic infections. Open Orthop J. 2013;7:227–242. doi: 10.2174/1874325001307010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furno P, Loubert G, Lambin Y, Maliska P, Patel A, Pilliot J. Acute primary infection due to slow-growing anaerobes after total hip arthroplasty (author’s transl) [in French] Nouv Presse Med. 1982;11:1991–1993. [PubMed] [Google Scholar]
- 9.Glehr M, Leithner A, Friesenbichler J, Goessler W, Avian A, Andreou D, Maurer-Ertl W, Windhager R, Tunn PU. Argyria following the use of silver-coated megaprostheses: no association between the development of local argyria and elevated silver levels. Bone Joint J. 2013;95:988–992. doi: 10.1302/0301-620X.95B7.31124. [DOI] [PubMed] [Google Scholar]
- 10.Gould SWJ, Fielder MD, Kelly AF, Morgan M, Kenny J, Naughton DP. The antimicrobial properties of copper surfaces against a range of important nosocomial pathogens. Ann Microbiol. 2009;59:151–156. doi: 10.1007/BF03175613. [DOI] [Google Scholar]
- 11.Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. Appl Environ Microbiol. 2011;77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gristina A. Biomaterial-centered infection: microbial adhesion versus tissue integration. 1987. Clin Orthop Relat Res. 2004;427:4–12. doi: 10.1097/01.blo.0000145156.89115.12. [DOI] [PubMed] [Google Scholar]
- 13.Haenle M, Fritsche A, Zietz C, Bader R, Heidenau F, Mittelmeier W, Gollwitzer H. An extended spectrum bactericidal titanium dioxide (TiO2) coating for metallic implants: in vitro effectiveness against MRSA and mechanical properties. J Mater Sci Mater Med. 2011;22:381–387. doi: 10.1007/s10856-010-4204-4. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell B, Gutteridge JM. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8:89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- 15.Hardes J, von Eiff C, Streitbuerger A, Balke M, Budny T, Henrichs MP, Hauschild G, Ahrens H. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol. 2010;101:389–395. doi: 10.1002/jso.21498. [DOI] [PubMed] [Google Scholar]
- 16.Heidenau F, Mittelmeier W, Detsch R, Haenle M, Stenzel F, Ziegler G, Gollwitzer H. A novel antibacterial titania coating: metal ion toxicity and in vitro surface colonization. J Mater Sci Mater Med. 2005;16:883–888. doi: 10.1007/s10856-005-4422-3. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Vaquero D, Fernandez-Fairen M, Torres A, Menzie AM, Fernandez-Carreira JM, Murcia-Mazon A, Guerado E, Merzthal L. Treatment of periprosthetic infections: an economic analysis. ScientificWorldJournal. 2013;2013:821650. doi: 10.1155/2013/821650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoene A, Prinz C, Walschus U, Lucke S, Patrzyk M, Wilhelm L, Neumann HG, Schlosser M. In vivo evaluation of copper release and acute local tissue reactions after implantation of copper-coated titanium implants in rats. Biomed Mater. 2013;8:035009. doi: 10.1088/1748-6041/8/3/035009. [DOI] [PubMed] [Google Scholar]
- 19.Iakovidis I, Delimaris I, Piperakis SM. Copper and its complexes in medicine: a biochemical approach. Mol Biol Int. 2011;2011:594529. doi: 10.4061/2011/594529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Issa Y, Brunton P, Waters CM, Watts DC. Cytotoxicity of metal ions to human oligodendroglial cells and human gingival fibroblasts assessed by mitochondrial dehydrogenase activity. Dent Mater. 2008;24:281–287. doi: 10.1016/j.dental.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Kaneshiro B, Aeby T. Long-term safety, efficacy, and patient acceptability of the intrauterine copper T-380A contraceptive device. Int J Womens Health. 2010;2:211–220. doi: 10.2147/IJWH.S6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang DK, Moon SK, Oh KT, Choi GS, Kim KN. Properties of experimental titanium-silver-copper alloys for dental applications. J Biomed Mater Res B Appl Biomater. 2009;90:446–451. doi: 10.1002/jbm.b.31305. [DOI] [PubMed] [Google Scholar]
- 23.Karakasli A, Hapa O, Akdeniz O, Havitcioglu H. Dermal argyria: cutaneous manifestation of a megaprosthesis for distal femoral osteosarcoma. Indian J Orthop. 2014;48:326–328. doi: 10.4103/0019-5413.132528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468:52–56. doi: 10.1007/s11999-009-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindeque B, Hartman Z, Noshchenko A, Cruse M. Infection after primary total hip arthroplasty. Orthopedics. 2014;37:257–265. doi: 10.3928/01477447-20140401-08. [DOI] [PubMed] [Google Scholar]
- 26.Martin PM. Thin film deposition process. In: Couper JER, Erdlac R, Khaladkar P, Lieberman N, Muhlbauer WK, Sherif SA, Dragoon K, Islam R, Kulkarmi V, Martin P, Nee AYC Speight JG, eds. Introduction to Surface Engineering and Functionally Engineered Materials. Hoboken, NJ, USA: Wiley; 2011:39–142.
- 27.Mauerer A, Lange B, Welsch GH, Heidenau F, Adler W, Forst R, Richter RH. Release of Cu2+ from a copper-filled TiO2 coating in a rabbit model for total knee arthroplasty. J Mater Sci Mater Med. 2014;25:813–821. doi: 10.1007/s10856-013-5116-x. [DOI] [PubMed] [Google Scholar]
- 28.Morii T, Morioka H, Ueda T, Araki N, Hashimoto N, Kawai A, Mochizuki K, Ichimura S. Deep infection in tumor endoprosthesis around the knee: a multi-institutional study by the Japanese musculoskeletal oncology group. BMC Musculoskelet Disord. 2013;14:51. doi: 10.1186/1471-2474-14-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nowicka J, Bartoszewicz M, Gosciniak G. Effect of selected properties of Staphylococcus epidermidis to biofilm formation on orthopedic implants [in Polish] Med Dosw Mikrobiol. 2012;64:189–196. [PubMed] [Google Scholar]
- 30.O’Connor JM, Bonham MP, Turley E, McKeown A, McKelvey-Martin VJ, Gilmore WS, Strain JJ. Copper supplementation has no effect on markers of DNA damage and liver function in healthy adults (FOODCUE project) Ann Nutr Metab. 2003;47:201–206. doi: 10.1159/000070486. [DOI] [PubMed] [Google Scholar]
- 31.Parvizi J, Gehrke T. International consensus on periprosthetic joint infection: let cumulative wisdom be a guide. J Bone Joint Surg Am. 2014;96:441. doi: 10.2106/JBJS.N.00023. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro M, Monteiro FJ, Ferraz MP. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter. 2012;2:176–194. doi: 10.4161/biom.22905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salgado CD, Sepkowitz KA, John JF, Cantey JR, Attaway HH, Freeman KD, Sharpe PA, Michels HT, Schmidt MG. Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect Control Hosp Epidemiol. 2013;34:479–486. doi: 10.1086/670207. [DOI] [PubMed] [Google Scholar]
- 34.Samanovic MI, Ding C, Thiele DJ, Darwin KH. Copper in microbial pathogenesis: meddling with the metal. Cell Host Microbe. 2012;11:106–115. doi: 10.1016/j.chom.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schilsky ML, Blank RR, Czaja MJ, Zern MA, Scheinberg IH, Stockert RJ, Sternlieb I. Hepatocellular copper toxicity and its attenuation by zinc. J Clin Invest. 1989;84:1562–1568. doi: 10.1172/JCI114333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schommer NN, Christner M, Hentschke M, Ruckdeschel K, Aepfelbacher M, Rohde H. Staphylococcus epidermidis uses distinct mechanisms of biofilm formation to interfere with phagocytosis and activation of mouse macrophage-like cells 774A.1. Infect Immun. 2011;79:2267–2276. doi: 10.1128/IAI.01142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sener M, Kazimoglu C, Karapinar H, Gunal I, Afsar I. Karatas Sener AG. Comparison of various surgical methods in the treatment of implant-related infection. Int Orthop. 2010;34:419–423. doi: 10.1007/s00264-009-0750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seth R, Yang S, Choi S, Sabean M, Roberts EA. In vitro assessment of copper-induced toxicity in the human hepatoma line, Hep G2. Toxicol In Vitro. 2004;18:501–509. doi: 10.1016/j.tiv.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro IM, Hickok NJ, Parvizi J, Stewart S, Schaer TP. Molecular engineering of an orthopaedic implant: from bench to bedside. Eur Cells Mater. 2012;23:362–370. doi: 10.22203/eCM.v023a28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheline CT, Choi DW. Cu2+ toxicity inhibition of mitochondrial dehydrogenases in vitro and in vivo. Ann Neurol. 2004;55:645–653. doi: 10.1002/ana.20047. [DOI] [PubMed] [Google Scholar]
- 41.Stranak V, Wulff H, Rebl H, Zietz C, Arndt K, Bogdanowicz R, Nebe B, Bader R, Podbielski A, Hubicka Z, Hippler R. Deposition of thin titanium-copper films with antimicrobial effect by advanced magnetron sputtering methods. Mater Sci Eng C Mater Biol Appl. 2011;31:1512–1519. doi: 10.1016/j.msec.2011.06.009. [DOI] [Google Scholar]
- 42.Tchounwou PB, Newsome C, Williams J, Glass K. Copper-induced cytotoxicity and transcriptional activation of stress genes in human liver carcinoma (HepG[2]) cells. Met Ions Biol. 2008;10:285–290. [PMC free article] [PubMed] [Google Scholar]
- 43.Triboulet S, Aude-Garcia C, Armand L, Collin-Faure V, Chevallet M, Diemer H, Gerdil A, Proamer F, Strub JM, Habert A, Herlin N, Van Dorsselaer A, Carriere M, Rabilloud T. Comparative proteomic analysis of the molecular responses of mouse macrophages to titanium dioxide and copper oxide nanoparticles unravels some toxic mechanisms for copper oxide nanoparticles in macrophages. PloS One. 2015;10:e0124496. doi: 10.1371/journal.pone.0124496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vishwakarma V, Josephine J, George RP, Krishnan R, Dash S, Kamruddin M, Kalavathi S, Manoharan N, Tyagi AK, Dayal RK. Antibacterial copper-nickel bilayers and multilayer coatings by pulsed laser deposition on titanium. Biofouling. 2009;25:705–710. doi: 10.1080/08927010903132183. [DOI] [PubMed] [Google Scholar]
- 45.Wafa H, Grimer RJ, Reddy K, Jeys L, Abudu A, Carter SR, Tillman RM. Retrospective evaluation of the incidence of early periprosthetic infection with silver-treated endoprostheses in high-risk patients: case-control study. Bone Joint J. 2015;97:252–257. doi: 10.1302/0301-620X.97B2.34554. [DOI] [PubMed] [Google Scholar]
- 46.Willis-Owen CA, Konyves A, Martin DK. Factors affecting the incidence of infection in hip and knee replacement: an analysis of 5277 cases. J Bone Joint Surg Br. 2010;92:1128–1133. doi: 10.1302/0301-620X.92B8.24333. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization. Copper. Trace Elements in Human Nutrition and Health. Geneva, Switzerland: World Health Organization; 1996:123–139.
- 48.Zhao L, Chu PK, Zhang Y, Wu Z. Antibacterial coatings on titanium implants. J Biomed Mater Res B Appl Biomater. 2009;91:470–480. doi: 10.1002/jbm.b.31463. [DOI] [PubMed] [Google Scholar]