Abstract
Background
Compressive osseointegration is as an alternative to traditional intramedullary fixation. Two- to 10-year survivorship and modes of failure have been reported; however, as a result of relatively small numbers, these studies are limited in their ability to identify risk factors for failure.
Questions/purposes
(1) What is survivorship free from aseptic mechanical and survivorship free from overall failure of compressive osseointegration fixation? (2) What patient factors (age, sex, body mass index [BMI], anatomic location of reconstruction, indication for reconstruction, radiation, chemotherapy) are associated with increased risk of failure?
Methods
Between 2006 and 2014, surgeons at one center treated 116 patients with 137 Compress® implants for lower extremity oncologic reconstructions, revision arthroplasty, and fracture nonunion or malunion. One hundred sixteen implants were available for review with a minimum of 2-year followup (mean, 4 years; range, 2–9 years). Kaplan-Meier survival plots were produced to examine survivorship and Cox regression modeling was used to generate hazard ratios (HRs) for potential risk factors for failure. Patient factors (age, sex, BMI, anatomic location of reconstruction, indication for reconstruction, radiation, chemotherapy) were obtained from chart review and an institutional database.
Results
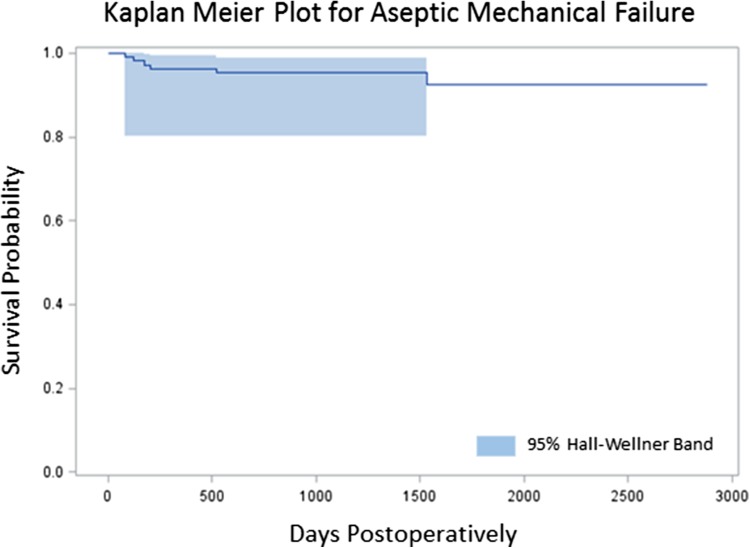
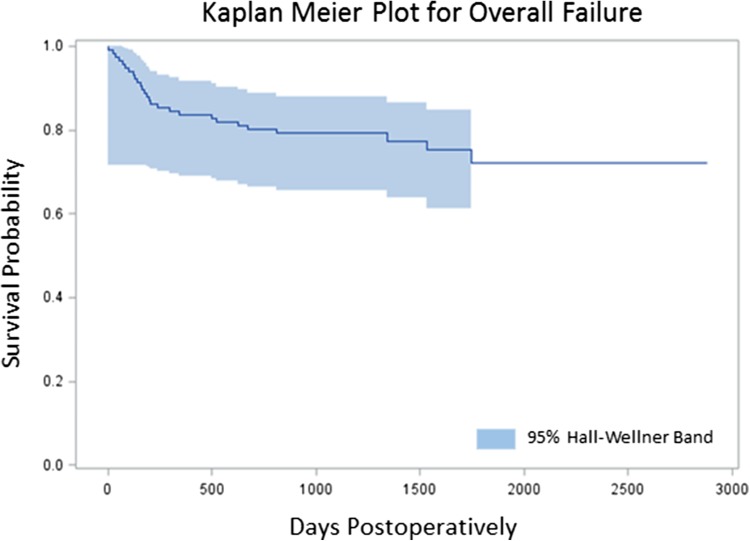
Survivorship free from aseptic mechanical failure was 95% (95% confidence interval [CI], 91%–99%) at 18 months and 93% (95% CI, 86%–99%) at 4 years. Survivorship free from overall failure was 82% (95% CI, 75%–89%) at 18 months and 75% (95% CI, 66%–84%) at 4 years. Risk of overall failure was increased with reconstruction of the proximal tibia (HR, 4.42; 95% CI 0.98–19.9) and distal femur (HR, 1.74; 95% CI, 0.50–6.09) compared to the proximal femur (HR, 1; referent; p = 0.049). Risk of aseptic mechanical failure was increased with reconstruction of the proximal tibia (HR, 1; referent) and distal femur (HR, 0.37; 95% CI, 0.08–1.77) compared with the proximal femur (HR, 0, p = 0.048). Radiation was associated with increased risk of overall failure (HR, 3.85; 95% CI, 1.84–8.02; p < 0.003), but not aseptic mechanical failure. Age, sex, BMI, chemotherapy, and surgical indication were not associated with increased risk of aseptic or overall failure.
Conclusions
This study questions the use of age as a contraindication for the use of this technology and suggests this technology may be considered in proximal femoral reconstruction and for patients with indications other than primary oncologic reconstructions. Future research should establish long-term survivorship data to compare this approach with conventional intramedullary stems and to evaluate the potential benefits of preventing stress shielding and preserving bone stock in revision situations.
Level of Evidence
Level III, therapeutic study.
Introduction
Compressive osseointegration fixation offers an alternative to traditional cemented or noncemented intramedullary fixation stems. This technology creates a stable, high-pressure bone-implant interface that theoretically avoids stress shielding [3, 6, 10]. The continuous force at the bone-implant interface causes hypertrophy with bony ingrowth into the porous surface of the component. This has the potential to decrease the rate of aseptic mechanical failure, allow for stable short-segment fixation, and offers relative ease of prosthetic revision with preservation of bone stock [3, 4, 11].
Aseptic mechanical failure of compressive osseointegration fixation occurs when the implant does not develop stable bony integration at the bone-implant interface in the absence of infection. This may lead to implant loosening at the spindle or fracture about the anchor plug [2, 4, 7, 12, 13, 15]. Other modes of failure for this implant are periprosthetic fracture around a stable implant, infection leading to revision of the implant or amputation, and progression of oncologic disease leading to removal of the implant [4, 7, 11, 12]. However, to our knowledge, these studies were limited by their relatively small numbers and mainly evaluated patients who had undergone primary oncologic reconstructions. It is unknown whether patients undergoing revision arthroplasty, fracture nonunion, or malunion would have similar survivorship and modes of failure.
Only a limited number of studies involving a relatively small number of implants are available for reconstructions of the proximal femur and proximal tibia [4, 11, 12]. In fact, to our knowledge, available reports include a total of only nine reconstructions of the proximal femur with this approach [4, 11]. Because of differences in mechanical forces across the hip and the knee and differences in bone quality at the different locations, survivorship may differ [14]. No previous study has been able to establish if location of anatomic reconstruction would be a risk factor for failure. Chemotherapy has been shown to decrease the rate of cortical hypertrophy at the bone-implant interface and there was a trend toward reduced prosthetic survivorship in one study [2]. However, other clinical studies do not show reduced survivorship in patients who receive chemotherapy or radiation, leaving unclear if chemotherapy or radiation is a risk factor for failure [4, 11]. Previous authors have suggested that older than 50 years of age is a relative contraindication to the use of this technology; however, there are very limited published reports of the use of this technology in patients older than 50 years of age, and no clinical series has shown an increased risk of failure in older patients [11].
We therefore asked: (1) What is survivorship free from aseptic mechanical and survivorship free from overall failure of compressive osseointegration fixation? (2) What patient factors (age, sex, body mass index [BMI], anatomic location of reconstruction, indication for reconstruction, radiation, chemotherapy) are associated with increased risk of failure?
Patients and Methods
Between 2006 and 2014, surgeons at one center treated 116 patients with 137 Compress® (Biomet, Warsaw, IN, USA) implants for lower extremity reconstructions. During that time, the general indications for use of this implant were primary oncologic reconstructions, revision arthroplasty with massive bone loss, and fracture nonunion or malunion requiring endoprosthetic reconstruction. Alternative treatments sometimes used in those situations included traditional intramedullary fixation stems; these were performed in an additional 41 patients, who were not studied here. Over the study period, the use of intramedullary fixation stems sharply declined and only eight intramedullary fixation stems were used in the last 5 years for the same indications. Of the 116 patients, 23 patients (20% with 26 implants) died and 20 patients (17% with 23 implants) had failed before 2 years. One hundred sixteen implants were available with a minimum of 2-year followup for analysis. Patients without recent followup in the clinic, defined as radiographic and clinical followup in the last year, were contacted by phone. Two patients (2%, with two implants) were unable to be reached by phone after completing a minimum of 2 years of clinical and radiographic follow up and censored to the date of most recent followup. We include data from those who failed before 2 years and those accounted for beyond that point; mean followup in this series was 4 years (range, 2–9 years). Endpoints were evaluated by chart review. Risk factors including age, sex, indication for use, anatomic location for reconstruction, BMI, chemotherapy, and radiation therapy were evaluated to see whether any were associated with increased risk of failure.
Institutional review board approval was obtained for the study. In general, indications for the use of compressive osseointegration fixation were reconstruction of the proximal femur, distal femur, and proximal tibia where there was massive bone loss requiring endoprosthetic reconstruction. Patients were considered for this technology if they had previous failed arthroplasty, fracture nonunions, malunions, or required a reconstruction after an oncologic resection. Older age was not considered a contraindication for use. Inclusion criteria were treatment with a Compress® with a minimum clinical and radiographic followup of 2 years. Exclusion criteria were patients treated with a different implant for similar indications. The compression force applied was determined based on the cortical thickness of the bone, measured intraoperatively, and preference for 800 lb/square inch was given if the cortical bone was sufficient. The spindle surface type (hydroxyapatite or porous titanium) was determined by the availability of the implants. Antirotation pins were not routinely used. The Compress® device was used in all cases. For all proximal femoral reconstructions, the decision to perform hemiarthroplasty versus THA and the choice of specific acetabular and bearing surface components were determined individually by the operating surgeon based on the patient age and preexisting arthritis. For all distal femoral and proximal tibial reconstructions, the Biomet Orthopaedic Salvage System (OSS™) rotating hinge knee arthroplasty components were used.
All patients were instructed to follow a strict touch-down weightbearing protocol for 6 weeks followed by progression to weightbearing as tolerated. Postoperative followup was performed at 2 weeks, 6 weeks, 3 months, and then every 3 to 12 months depending on individual patient factors.
Operative reports, implant records, clinic notes, and radiographs for each patient were reviewed. Survivorship free from aseptic mechanical failure was defined as patients without failure of osseointegration at the bone-implant interface requiring revision surgery in the absence of apparent infection. Survivorship free from overall failure was all patients without a revision of the bone-implant spindle for any reason. Patients who underwent reoperation for exchange of a femoral head, acetabular liner, or another modular component were not considered a failure of compressive osseointegration. Demographic data were recorded for every patient. The indication for surgery was categorized as primary oncologic reconstruction, revision arthroplasty, or fracture nonunion or malunion. Patients treated for a failed primary oncologic reconstruction were classified into the revision arthroplasty group. Patients who received chemotherapy and radiation therapy were noted, although we were unable to subdivide this group to determine who received chemotherapy preoperatively, postoperatively, or both. Operative details, including the use of antirotation pins, compression force, and spindle surface type (hydroxyapatite or porous titanium), were recorded. As a result of the limited use of antirotation pins in this cohort and the preference for 800 lbs/square inch compression force, these were not considered as risk factors for failure in this analysis. Spindle size and shape were determined based on the individual patient’s anatomy and were not considered a risk factor in this analysis. A similar resection length could end at a different level of bone such as the diaphysis in a larger patient or metaphysis in a smaller patient and thus was not considered in the analysis.
Statistical Analysis
Kaplan-Meier [9] survival plots with Hall-Wellner bands were produced to examine survival time of the Compress® free from aseptic mechanical failure and free from overall failure. Cox regression modeling with sandwich variance estimation (to account for within-subject correlation) was used to generate hazard ratios (HRs) and 95% confidence intervals (CIs) for potential risk factors for failure. Extended Cox regression was used to evaluate anatomic location of reconstruction because the proportional hazard assumption was not met for that variable. All statistical analysis was performed in SAS 9.4® software (Cary, NC, USA). Clinically meaningful categories were selected for age and BMI was dichotomized around the mean of our population at 30 kg/m2.
Results
The Kaplan-Meier survivorship free from aseptic mechanical failure was 95% (95% CI, 91%–99%) at 18 months and 93% (95% CI, 86%–99%) at 4 years (Fig. 1). Survivorship free from overall failure was 82% (95% CI, 75%–89%) at 18 months and 75% (95% CI, 66%–84%) at 4 years (Fig. 2). Risk of overall failure was increased with reconstruction of the proximal tibia (HR, 4.42; 95% CI 0.98–19.9) and distal femur (HR, 1.74; 95% CI, 0.50–6.09) compared to the proximal femur (HR, 1; referent; p = 0.049). Risk of aseptic mechanical failure was increased with reconstruction of the proximal tibia (HR, 1; referent) and distal femur (HR, 0.37; 95% CI, 0.08–1.77) compared with the proximal femur (HR, 0, p = 0.048) (Tables 1, 2). The extended Cox regression indicated that the increased hazard for the proximal tibia group was at the beginning of followup (< 180 days) with no failures after this point. Seventeen revisions were done for infection, six revisions were done for aseptic mechanical failure, two revisions were done for periprosthetic fractures around an anchor plug, one revision was done for local progression of oncologic disease, and one implant was removed for a dysvascular leg.
Fig. 1.
Kaplan-Meier plot demonstrating survivorship of compressive osseointegration fixation. Survivorship for aseptic mechanical failure was 96%.
Fig. 2.
Kaplan-Meier plot demonstrating survivorship of compressive osseointegration fixation. Overall survivorship was 80%.
Table 1.
Patient and surgical characteristics: mechanical failures
| Patient or surgical characteristic | All patients | Aseptic mechanical failure | Hazard ratio (95% CI) |
|---|---|---|---|
| Total patients | 114 | 6 | |
| Age at surgery (years) | p = 0.22 | ||
| < 40 | 29 | 2 | 1.00 (referent) |
| 40–54 | 28 | 1 | 0.45 (0.05–3.88) |
| 55–69 | 38 | 3 | 1.05 (0.19–5.8) |
| > 70 | 19 | 0 | 0 |
| p = 0.99 | |||
| < 55 | 57 | 3 | 1.00 (referent) |
| > 55 | 57 | 3 | 0.99 (0.22–4.34) |
| Sex | p = 0.94 | ||
| Male | 58 | 3 | 1.00 (referent) |
| Female | 56 | 3 | 1.07 (0.22–5.14) |
| BMI (kg/m2) | p = 0.25 | ||
| < 30 | 66 | 2 | 1.00 (referent) |
| > 30 | 48 | 4 | 2.68 (0.52–13.8) |
| Location of reconstruction | p = 0.048 | ||
| Proximal femur | 37 | 0 | 0 |
| Distal femur | 64 | 4 | 0.37 (0.08–1.77) |
| Proximal tibia | 13 | 2 | 1.00 (referent) |
| Indication for reconstruction | p = 0.22 | ||
| Primary oncologic | 40 | 1 | 1.00 (referent) |
| Revision arthroplasty | 69 | 5 | 2.86 (0.33–25.1) |
| Fracture non-/malunion | 5 | 0 | 0 |
| Radiation | p = 0.08 | ||
| No | 101 | 6 | 1.00 (referent) |
| Yes | 13 | 0 | 0 |
| Chemotherapy | p = 0.63 | ||
| No | 61 | 4 | 1.00 (referent) |
| Yes | 53 | 2 | 0.68 (0.14–3.43) |
CI = confidence interval; BMI = body mass index.
Table 2.
Patient and surgical characteristics: overall failures
| Patient or surgical characteristic | All patients | Overall failures | Hazard ratio (95% CI) |
|---|---|---|---|
| Total patients | 116 | 27 | |
| Age at surgery (years) | 0.51 | 1.00 (referent) | |
| < 40 | 29 | 9 | 0.73 (0.24–2.23) |
| 40–54 | 28 | 7 | 0.71 (0.30–1.70) |
| 55–69 | 38 | 9 | 0.30 (0.06–1.46) |
| > 70 | 21 | 2 | |
| p = 0.26 | |||
| < 55 | 57 | 16 | 1.00 (referent) |
| > 55 | 59 | 11 | 0.66 (0.33–1.35) |
| Sex | p = 0.68 | ||
| Male | 59 | 15 | 1.00 (referent) |
| Female | 57 | 12 | 0.85 (0.40–1.80) |
| BMI (kg/m2) | p = 0.35 | ||
| < 30 | 66 | 13 | 1.00 (referent) |
| > 30 | 50 | 14 | 1.43 (0.68–2.99) |
| Location of reconstruction | p = 0.049 | ||
| Proximal femur | 39 | 7 | 1.00 (referent) |
| Distal femur | 64 | 16 | 1.74 (0.50–6.09) |
| Proximal tibia | 13 | 4 | 4.42 (0.98-19.9) |
| Indication for reconstruction | p = 0.52 | ||
| Primary oncologic | 41 | 7 | 1.00 (referent) |
| Revision arthroplasty | 70 | 18 | 1.48 (0.58–3.73) |
| Fracture non-/malunion | 5 | 2 | 2.22 (0.54–9.11) |
| Radiation | p = 0.003 | ||
| No | 103 | 20 | 1.00 (referent) |
| Yes | 13 | 7 | 3.85 (1.84–8.02) |
| Chemotherapy | p = 0.17 | ||
| No | 62 | 12 | 1.00 (referent) |
| Yes | 54 | 15 | 1.67 (0.81–3.44) |
CI = confidence interval; BMI = body mass index.
Radiation was associated with increased risk of overall failure (HR, 3.85; 95% CI, 1.84–8.02; p = 0.003) (Table 2) but not aseptic failure (HR, 0; p = 0.08) (Table 1). Age, sex, BMI, indication for reconstruction, and chemotherapy were not associated with increased risk of aseptic or overall failure (Tables 1, 2) with the numbers available.
Discussion
Compressive osseointegration fixation has the potential advantages of decreasing aseptic failure rates as a result of prevention of stress shielding, allowing for short-segment fixation, and preservation of bone stock [3, 4, 11]. Previous studies have been limited by their relatively small numbers, which limited the ability of those series to identify risk factors for failure [4, 7, 11, 12]. To our knowledge, this study is the largest published series on this topic. Our survivorship free from aseptic mechanical failure was 95% and survivorship free from overall failure was 80%. We identified reconstructions of the proximal femur as having a decreased risk for both aseptic mechanical and overall failure and radiation as risk factors for overall failure.
This study had a number of limitations. This was a retrospective study leading to the potential for selection bias; 41 patients were treated with traditional intramedullary stem endoprostheses during this study period and may have been candidates for compressive osseointegration fixation. However, the use of traditional intramedullary stem fixation sharply decreased and only eight were performed in the last 5 years. During the study period, no patients were lost to followup before 2 years for reasons other than death. This was a single-center patient cohort and the results may not be representative of a broader population. Our center is a tertiary referral center and our patients come from various urban, rural, and socioeconomic backgrounds, and so our results may apply best to others in similar settings. Functional outcomes data were not available in this review. Although those are important, the study’s intent was to analyze survivorship and identify risk factors for failure of compressive osseointegration fixation and not to assess functional outcome measures. The number of patients available precluded a multivariate analysis to determine if comparisons were matched in other diagnosis. Although this is a limitation, this still represents the largest series of patients treated with this technology to our knowledge. We did not have a control group of patients undergoing traditional intramedullary fixation, although previous studies have found no differences in survivorship with the numbers available when the Compress® has been compared with noncemented and cemented intramedullary fixation endoprosthesis [5, 13].
Our overall and aseptic mechanical failure is comparable to other survivorship studies using compressive osseointegration fixation, which report overall survivorship of 67% to 85% and aseptic mechanical failure rates of 4% to 12% [5, 7, 11–13]. Our results also compare favorably to a recent large multicenter review of 2174 traditional intramedullary stems, which found overall survivorship of 75% and aseptic survivorship of 88% [8]. Our cohort included patients treated with indications other than oncologic reconstructions in young patients suggesting this technology provides similar results for the indications of revision arthroplasty and fracture nonunion or malunion.
We found that in reconstructions involving the hip, the proximal femur group had a decreased risk of aseptic mechanical and overall failure. To our knowledge, no other study has evaluated this endpoint in implants using compressive osseointegration. Our finding may be explained by the different forces acting across the hip than in the more constrained knee [14]. Our cohort included 47 patients with proximal femoral reconstructions. The next largest cohort of proximal femoral reconstructions reported on six [4]. Thus, having greater numbers allowed us to make the comparisons between anatomic locations of reconstruction and failure.
Previous studies have used age older than 50 years, systemic medical conditions thought to impair bone healing, and history of prior radiation to the bone as relative contraindications to the use of compressive osseointegration fixation [11]. These factors were not considered relative contraindications by our senior authors. We found no difference in risk of failure between age groups, and our population had an older mean age than previous survivorship studies, which have mean ages between 18 and 30 years [2, 4, 5, 7, 11, 12]. Our finding that age was not a risk factor for aseptic mechanical or overall failure calls into question the appropriateness of using age older than 50 years as a relative contraindication to use of the Compress®. Future studies should evaluate bone quality or decreased bone mineral density as a potential risk factor for failure and should continue to follow patients to determine long-term results of this technology in older patients. Our results are in line with other survivorship studies, which have failed to show chemotherapy as a risk factor for failure [4, 11]. Although chemotherapy has been shown to decrease the rate of cortical hypertrophy at the bone-implant interface, our study supports that this imaging finding is not clinically relevant [2]. We found that radiation was a risk factor for overall but not aseptic mechanical failure, suggesting that this is a function of the increased risk of infection in radiated tissue. Previous studies have not found radiation to be a risk factor for failure, although they were limited by their relatively small numbers to evaluate this effect [4, 11, 13]. Biomechanically, the use of antirotation pins has been shown to improve the rotational stability of the Compress®; however, clinical studies have not shown any difference in survival for patients with and without antirotation pins [1, 4, 11]. Our overall survivorship was similar to previous studies and only three patients had antirotation pins, further questioning the clinical importance of their use.
In conclusion, this study questions many of the previously described relative contraindications to the use of compressive osseointegration fixation, which were based on expert opinion and do not have sufficient clinical evidence to support them [11]. Our findings suggest that this technology may be considered in proximal femoral reconstruction and for patients with indications other than primary oncologic reconstructions. As far as we are aware, this is the first study to identify anatomic location of reconstruction as a risk factor for aseptic mechanical and overall failure. Longer followup is needed to compare this technology with conventional intramedullary stems and to establish the potential benefits of preventing stress shielding and preserving bone stock. Future research should establish long-term survivorship data to compare this approach with conventional intramedullary stems and to evaluate the potential benefits of preventing stress shielding and preserving bone stock in revision situations.
Acknowledgments
We thank Sabina Blizzard BS, and Shannon Hiratzka MPH, for their assistance with statistical analysis and Marie Kane MSME, for her editing assistance.
Footnotes
One of the authors (JH), or a member of his immediate family, has or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000, from Biomet® (Warsaw, IN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Avedian RS, Chen T, Lindsey D, Palanca A, Mohler D. Antirotation pins improve stability of the Compress limb salvage implant: a biomechanical study. Clin Orthop Relat Res. 2014;472:3982–3986. doi: 10.1007/s11999-014-3899-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avedian RS, Goldsby RE, Kramer MJ, O’Donnell RJ. Effect of chemotherapy on initial compressive osseointegration of tumor endoprostheses. Clin Orthop Relat Res. 2007;459:48–53. doi: 10.1097/BLO.0b013e3180514c66. [DOI] [PubMed] [Google Scholar]
- 3.Bini SA, Johnston JO, Martin DL. Compliant prestress fixation in tumor prostheses: interface retrieval data. Orthopedics. 2000;23:707–711; discussion 711–712. [DOI] [PubMed]
- 4.Calvert GT, Cummings JE, Bowles AJ, Jones KB, Wurtz LD, Randall RL. A dual-center review of compressive osseointegration for fixation of massive endoprosthetics: 2- to 9-year followup. Clin Orthop Relat Res. 2014;472:822–829. doi: 10.1007/s11999-013-2885-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farfalli GL, Boland PJ, Morris CD, Athanasian EA, Healey JH. Early equivalence of uncemented press-fit and Compress femoral fixation. Clin Orthop Relat Res. 2009;467:2792–2799. doi: 10.1007/s11999-009-0912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frost HM. Wolff’s Law and bone’s structural adaptations to mechanical usage: an overview for clinicians. Angle Orthod. 1994;64:175–188. doi: 10.1043/0003-3219(1994)064<0175:WLABSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Healey JH, Morris CD, Athanasian EA, Boland PJ. Compress knee arthroplasty has 80% 10-year survivorship and novel forms of bone failure. Clin Orthop Relat Res. 2013;471:774–783. doi: 10.1007/s11999-012-2635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, Windhager R, Kotz RI, Mercuri M, Funovics PT, Hornicek FJ, Temple HT, Ruggieri P, Letson GD. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418–429. doi: 10.2106/JBJS.J.00834. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 10.Kramer MJ, Tanner BJ, Horvai AE, O’Donnell RJ. Compressive osseointegration promotes viable bone at the endoprosthetic interface: retrieval study of Compress implants. Int Orthop. 2008;32:567–571. doi: 10.1007/s00264-007-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monument MJ, Bernthal NM, Bowles AJ, Jones KB, Randall RL. What are the 5-year survivorship outcomes of compressive endoprosthetic osseointegration fixation of the femur? Clin Orthop Relat Res. 2015;473:883–890. doi: 10.1007/s11999-014-3724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Donnell RJ. Compressive osseointegration of tibial implants in primary cancer reconstruction. Clin Orthop Relat Res. 2009;467:2807–2812. doi: 10.1007/s11999-009-0986-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedtke AC, Wustrack RL, Fang AS, Grimer RJ, O’Donnell RJ. Aseptic failure: how does the Compress® implant compare to cemented stems? Clin Orthop Relat Res. 2012;470:735–742. doi: 10.1007/s11999-011-2159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor SJ, Walker PS, Perry JS, Cannon SR, Woledge R. The forces in the distal femur and the knee during walking and other activities measured by telemetry. J Arthroplasty. 1998;13:428–437. doi: 10.1016/S0883-5403(98)90009-2. [DOI] [PubMed] [Google Scholar]
- 15.Tyler WK, Healey JH, Morris CD, Boland PJ, O’Donnell RJ. Compress® periprosthetic fractures: interface stability and ease of revision. Clin Orthop Relat Res. 2009;467:2800–2806. doi: 10.1007/s11999-009-0946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]