Abstract
Background
The proximal tibia is one of the most challenging anatomic sites for extremity reconstructions after bone tumor resection. Because bone tumors are rare and large case series of reconstructions of the proximal tibia are lacking, we undertook this study to compare two major reconstructive approaches at two large sarcoma centers.
Questions/purposes
The purpose of this study was to compare groups of patients treated with endoprosthetic replacement or osteoarticular allograft reconstruction for proximal tibia bone tumors in terms of (1) limb salvage reconstruction failures and risk of amputation of the limb; (2) causes of failure; and (3) functional results.
Methods
Between 1990 and 2012, two oncologic centers treated 385 patients with proximal tibial resections and reconstruction. During that time, the general indications for those types of reconstruction were proximal tibia malignant tumors or bone destruction with articular surface damage or collapse. Patients who matched the inclusion criteria (age between 15 and 60 years old, diagnosis of a primary bone tumor of the proximal tibia treated with limb salvage surgery and reconstructed with endoprosthetic replacement or osteoarticular allograft) were included for analysis (n = 149). In those groups (endoprosthetic or allograft), of the patients not known to have reached an endpoint (death, reconstructive failure, or limb loss) before 2 years, 85% (88 of 104) and 100% (45 of 45) were available for followup at a minimum of 2 years. A total of 88 patients were included in the endoprosthetic group and 45 patients in the osteoarticular allograft group. Followup was at a mean of 9.5 (SD 6.72) years (range, 2–24 years) for patients with endoprosthetic reconstructions, and 7.4 (SD 5.94) years for patients treated with allografts (range, 2–21 years). The following variables were compared: limb salvage reconstruction failure rates, risk of limb amputation, type of failures according to the Henderson et al. classification, and functional results assessed by the Musculoskeletal Tumor Society system.
Results
With the numbers available, after competitive risk analysis, the probability of failure for endoprosthetic replacement of the proximal tibia was 18% (95% confidence interval [CI], 10.75–27.46) at 5 years and 44% (95% CI, 31.67–55.62) at 10 years and for osteoarticular allograft reconstruction was 27% (95% CI, 14.73–40.16) at 5 years and 32% (95% CI, 18.65–46.18) at 10 years. There were no differences in terms of risk of failures at 5 years (p = 0.26) or 10 years (p = 0.20) between the two groups. Fifty-one of 88 patients (58%) with proximal tibia endoprostheses developed a reconstruction failure with mechanical causes being the most prevalent (32 of 51 patients [63%]). A total of 19 of 45 osteoarticular allograft reconstructions failed (42%) and nine of 19 (47%) of them were caused by early infection. Ten-year risk of amputation after failure for endoprosthetic reconstruction was 10% (95% CI, 5.13–18.12) and 11% (95% CI, 4.01–22.28) for osteoarticular allograft with no difference between the groups (p = 0.91). With the numbers available, there were no differences between the groups in terms of the mean Musculoskeletal Tumor Society score (26.58, SD 2.99, range, 19–30 versus 27.52, SD 1.91, range, 22–30; p = 0.13; 95% CI, −2,3 to 0.32). Mean extension lag was more severe in the endoprosthetic group than the osteoarticular allograft group: 13.56° (SD 18.73; range, 0°–80°) versus 2.41° (SD 5.76; range, 0°–30°; p < 0.001; 95% CI, 5.8–16.4).
Conclusions
Reconstruction of the proximal tibia with either endoprosthetic replacement or osteoarticular allograft appears to offer similar reconstruction failures rates. The primary cause of failure for allograft was infection and for endoprosthesis was mechanical complications. We believe that the treating surgeon should have both options available for treatment of patients with malignant or aggressive tumors of the proximal tibia. (S)he might consider an allograft in a younger patient to achieve better extensor mechanism function, whereas in an older patient or one with a poorer prognosis where return to function and ambulation quickly is desired, an endoprosthesis may be advantageous.
Level of Evidence
Level III, therapeutic study.
Introduction
Limb salvage surgery is the standard practice for most malignant or locally aggressive bone tumors, of which approximately 50% arise around the knee [25]. However, the proximal tibia is a challenging anatomic site for extremity reconstructions after bone tumor resection and it is associated with a high incidence of surgical complications [6, 18, 20, 22–24]. Functional reconstructive options for large defects in the proximal tibia include biological reconstruction (transplantation of a structural allograft), endoprosthetic replacement, and composite reconstruction with use of an allograft and a knee prosthesis.
Benefits and complications have been described for the different reconstruction procedures [1, 4, 6, 8–10]. Endoprosthetic reconstruction of the proximal tibia has the advantages of full weightbearing in the first weeks postoperatively, giving the patient relatively rapid functional restoration [6, 8–10]. On the other hand, the use of biological reconstruction gives the chance to restore the bone stock and reconstruct the extensor mechanism [1, 2, 4]. However, any of the reconstruction techniques described for proximal tibia resections have shown a higher index of complications compared with the distal femur and have failure rates reported from 27% to 55% [1, 2, 4, 6, 8–10]. The ideal method of reconstruction for this anatomic area remains unresolved.
The aim of this study was to directly compare outcomes between patients treated with endoprosthetic replacement or osteoarticular allograft for proximal tibia bone tumors in two different oncologic centers in terms of (1) limb salvage reconstruction failure and risk of amputation of the limb; (2) causes of failure; and (3) functional results.
Patients and Methods
A retrospective study from the longitudinally maintained oncology databases of two institutions was done for all patients with a primary bone tumor of the proximal tibia treated by resection and limb salvage surgery. Between 1990 and 2012, 385 patients were treated in the two centers involved in the study: Oncology Unit 1 (Royal Orthopaedic Hospital, Birmingham, UK) exclusively used endoprosthetic replacement for bone tumor reconstruction at the time of the study (150 endoprosthetic reconstruction per year) and Unit 2 (Italian Hospital of Buenos Aires, Buenos Aires, Argentina) specialized in allograft reconstruction for bone defects (723 massive allograft reconstructions between 1980 and 2012). During that time, the general indications for those types of reconstructions were proximal tibia malignant tumors or bone destruction with articular surface damage or collapse. Patients who matched the inclusion criteria (age between 15 and 60 years old, diagnosis of a primary bone tumor of the proximal tibia treated with limb salvage surgery and reconstructed with endoprosthetic replacement or osteoarticular allograft) were included for analysis (n = 149). All patients with metastasis at diagnosis were excluded. In those groups (endoprosthetic or allograft), of the patients not known to have reached an endpoint (death, reconstructive failure, or limb loss) before 2 years, 85% (88 of 104) and 100% (45 of 45) were available for followup at a minimum of 2 years. A total of 88 patients were included in the endoprosthetic group and 45 patients in the osteoarticular allograft group. Followup was at a mean of 9.5 (SD 6.72) years (range, 2–24 years) for patients with endoprosthetic reconstructions and 7.4 (SD 5.94) years for patients treated with allografts (range, 2–21 years). The following variables were compared: limb salvage reconstruction failure rates, risk of limb amputation, type of failures according to the Henderson et al. classification, and functional results assessed by the Musculoskeletal Tumor Society system. A total of 88 patients were included in the endoprosthetic group and 45 patients in the osteoarticular allograft group. No differences were found between the two groups in terms of age, gender, use of chemotherapy, and followup, although the allograft group had more giant tumors of bone and the endoprosthetic group had more “other” tumor indications (Table 1).
Table 1.
Demographic characteristics of the 133 patients of the series
| Demographic characteristic | Endoprotheses | Osteoarticular allograft | p value (95% CI) |
|---|---|---|---|
| Age (years) | 26, SD 12.36 (range, 15–60) | 25, SD 9.83 (range, 15–56) | 0.6 (−3.1 to 5.2) |
| Gender (%) | 62 Males (70%) 16 Female (30%) |
26 Males (58%) 19 Females (42%) |
−0.14 |
| Type of tumor (number) | Osteosarcoma: 55 | Osteosarcoma: 26 | 0.20 |
| Chondrosarcoma: 10 | Chondrosarcoma: 2 | 0.21 | |
| Ewing’s sarcoma: 4 | Ewing’s sarcoma: 5 | 0.16 | |
| GCT: 4 | GCT: 14 | 0.001 | |
| Other*: 15 | Other†: 1 | 0.001 | |
| Chemotherapy (%) | 68 (77%) | 28 (62%) | 0.1 |
| Followup (years) | 9.5, SD 6.72 (range, 2–24) | 7.4, SD 5.94 (range, 2–21) | 0.081 (−0.2 to 4.4) |
* Spindle cell sarcoma (9), leiomyosarcoma (3), fibrosarcoma (1), aneurysmal bone cyst (1), desmoplastic fibroma (1), adamantinoma (1); †chondroblastoma (1); CI = confidence interval; GCT = giant cell tumor.
The following variables were analyzed in the two groups and compared: (1) limb salvage reconstruction failure with revision surgery as the endpoint; (2) failures of limb salvage reconstruction based on the modified Henderson et al. classification (2014) for endoprostheses or allograft failures [11]; and (3) functional results. The modified classification for limb salvage for endoprosthetic and allografts Types 1 to 5 defines Types 1, 2, and 3 as mechanical failures and Types 4 and 5 as nonmechanical failures: Type 1—soft tissue failures (1A: failure of function/1B: failure of cover; Type 2–aseptic loosening for endoprosthetics (2A: early < 2 years after implantation/2B: late > 2 years after implantation) or graft–host nonunion for allografts (2A: hypertrophic nonunion/2B: atrophic nonunion); Type 3–structural failure (3A: implant or fixation/3B: bone or graft); Type 4–infection (4A: early < 2 years after implantation/4B: late > 2 years after implantation); and Type 5–tumor progression (5A: soft tissue/5B: bone). The functional evaluation of the patients was performed with the use of the revised 30-point functional classification system established by the International Society of Limb Salvage and the Musculoskeletal Tumor Society and active ROM was evaluated at the last followup [7].
Surgical Techniques
Osteoarticular Allograft
The nonirradiated allografts were harvested under sterile conditions and stored frozen in the bone bank at the study institution. No attempt was made to preserve the viability of the articular cartilage, and bacteriologic and viral tests available at the time were performed in accordance with the recommendations of the American Association of Tissue Banks.
Through an extended anteromedial approach to the knee, we released the extensor mechanism by sectioning the host patellar tendon and resected the tumor at the proximal tibia with an adequate margin of normal tissue according to preoperative staging studies. A transverse osteotomy was used in every case. At the time of allograft implantation, rigid fixation of the host-donor junction was always obtained. After internal fixation was achieved, soft tissues from the allograft were attached to corresponding host tissues to obtain the greatest possible stability. Soft tissue reconstructions included repair of the posterior capsule, the anterior and posterior cruciate ligaments, and the medial collateral ligament. Allograft tissue flaps overlapped the corresponding host tissues and were sutured to restore knee stability. We attempted to preserve the host meniscus and reattach them to the osteoarticular allograft tissue. The extensor mechanism then was reconstructed to the corresponding tissue of the allograft. A medial gastrocnemius flap was performed in all patients to provide soft tissue coverage to the proximal tibia allograft [18].
Proximal Tibia Endoprosthetic Replacement
All of the prostheses were custom-made, designed and manufactured at the Department of Biomedical Engineering of the Institute of Orthopaedics of University College, London (now known as Stanmore Implants Worldwide, Royal National Orthopaedic Hospital Trust, Stanmore, Middlesex, UK). A Stanmore type-knee with a rotating hinge was used in an attempt to absorb rotational stresses and reduce wear on the bushes. Since 1992, all of the tibial components have had a hydroxyapatite (HA) collar at the bone-prosthesis interface to encourage ingrowth of bone into the collar and decrease the risk of loosening (80 of 88). All the operations were performed in a clean-air operating room. Resection of the tumor was carried out following oncological principles, endeavoring to achieve a wide margin of resection. A rotation flap of the medial head of gastrocnemius was done in all patients for soft tissue coverage of the defect. The patella ligament was attached directly to the transposed gastrocnemius to restore the extensor mechanism [20].
Statistical Analysis
Demographic differences between groups were assessed using Student’s t-test. The reconstruction failure rates were calculated by competitive risk analysis method with limb salvage reconstruction censored at the time of failure or last followup. The differences between groups were compared by log-rank test. The statistical analysis was performed using the R programming language (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as p < 0.05 [21].
Results
With the numbers available, after competitive risk analysis, the probability of failure for endoprosthetic replacement of the proximal tibia was 18% (95% confidence interval [CI], 10.75–7.46) at 5 years and 44% (95% CI, 31.67–55.62) at 10 years and for osteoarticular allograft reconstructions was 27% (95% CI, 14.73–40.16) at 5 years and 32% (95% CI, 18.65–46.18) at 10 years. There were no differences between the groups in terms of risk of failure at 5 (p = 0.26) or 10 years (p = 0.20).
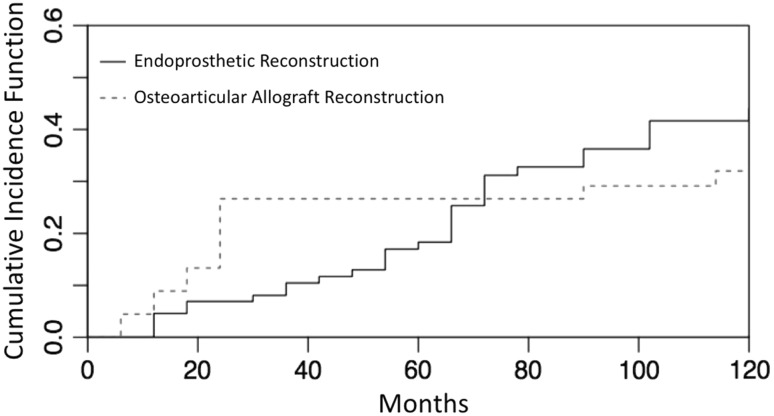
Although for proximal tibia endoprosthetic reconstructions, the risk of revision increased gradually in 10 years, proximal tibia osteoarticular allografts presented a higher risk of failure in the first 24 months (Fig. 1). Interestingly, the failure curve for the allografts appears to plateau, whereas the trajectory for the endoprostheses continues an upward trend (Fig. 1). Ten-year risk of amputation after failure for endoprosthetic reconstruction was 10% (95% CI, 5.13–18.12) and 11% (95% CI, 4.01–22.28) for osteoarticular allograft with no difference between the groups (p = 0.91).
Fig. 1.
This image shows the cumulative risk functions for endoprosthetic and osteoarticular allograft reconstructions of the proximal tibia.
Failures of Limb Salvage Reconstruction
In the group of patients with proximal tibia endoprosthetic replacement, 58% (51 of 88) of limb salvage reconstructions failed. One patient in this group with a postoperative lower limb ischemia that resulted in an above-knee amputation could not be classified by the Henderson classification. The remaining 50 failures were classified according to Henderson classification as mechanical in 32 patients and nonmechanical in 18 of the patients. Infection represented 10% (nine of 88) of all endoprosthetic reconstruction failures with five patients defined as having early infection and four patients as having late infection (Table 2).
Table 2.
Modified Henderson classification for limb salvage reconstruction failure
| Failures | Endoprosthetic reconstruction (n = 88) |
Osteoarticular allograft (n = 45) |
|
|---|---|---|---|
| Total failures | 51 (58%) | 19 (42%) | |
| Overall mechanical failures (%) | 32 (36.5%) | 3 (7%) | |
| Henderson Type 1 | A | 2 | 0 |
| (soft tissue failure) | B | 0 | 0 |
| Henderson Type 2 | A | 0 | 0 |
| (aseptic loosening or graft-host nonunion | B | 5 | 0 |
| Henderson Type 3 | A | 22 | 0 |
| (structural failure) | B | 2 | 3 |
| Overall nonmechanical failures | 18 (20.5%) | 14 (31%) | |
| Henderson Type 4 | A | 5 | 9 |
| (infection) | B | 4 | 0 |
| Henderson Type 5 | A | 3 | 0 |
| (tumor progression) | B | 6 | 5 |
| Failures that could not be classified by Henderson classification | 1*(1%) | 2†(4%) |
Henderson et al. classification: Type 1: soft tissue failures (1A: failure of function/1B: failure of cover; Type 2: aseptic loosening for endoprosthetics (2A: early < 2 years after implantation/2B: late > 2 years after implantation) or graft–host nonunion for allografts (2A: hypertrophic no union/2B: atrophic nonunion); Type 3: structural failure (3A: implant or fixation/3B: bone or graft); Type 4: infection (4A: early < 2 years after implantation/4B: late > 2 years after implantation); Type 5: tumor progression (5A: soft tissue/5B: bone); * one patient in endoprosthetic reconstruction group had a reconstruction failure (acute ischemia of limb) that could not be classified by Henderson classification; †two patients in the osteoarticular allograft group had a reconstruction failure (osteoarthritis + joint collapse) that could not be classified by Henderson classification.
Nineteen patients in the endoprosthetic group with mechanical failures were treated with one-stage revision, 11 patients underwent wear-related exchange of a bushing or other articulating component, and two patients needed soft tissue reconstruction of the extensor mechanism. Nonmechanical failures were treated with two-stage revision surgeries in eight patients and above-knee amputations in 10 of them.
Forty-two percent (19 of 45) of the patients treated with an osteoarticular allograft reconstruction failed (Table 2). Two patients in this group who developed severe osteoarthritis resulting in revision surgery to allograft-composite prostheses for pain relief could not be classified by the Henderson classification. The remaining 17 failures were classified according to the Henderson classification as mechanical in three patients and nonmechanical in 14 patients. Nonmechanical failures represented 74% (14 of 19) and early infection (n = 9) was the most prevalent followed by local recurrences (n = 5). Five patients with nonmechanical failures were treated with two-stage revision (three alloprosthetic composites and two proximal tibia endoprosthetic replacements), six needed to be treated with an above-knee amputation, and three with knee arthrodesis. Structural failure of allografts occurred in three patients (fracture through the graft) and all of them were revised to a new osteoarticular allograft. Forty-seven percent (nine of 19) of the patients with more than 7 years of followup after biological reconstruction presented with severe osteoarthritis on radiographs, although only two presented with joint collapse and needed to be revised to allograft composite knee prostheses for pain relief.
Functional Outcomes
With the numbers available, there were no differences between patients treated with endoprosthetic replacement and those treated with allograft in terms of the mean Musculoskeletal Tumor Society (MSTS) score (26.58, SD 2.99, range, 19–30 versus 27.52, SD 1.91, range, 22–30; p = 0.13; 95% CI, −2.3 to 0.32).
Patients with allograft reconstruction presented with better active ROM. The mean extension lag was more severe in the endoprosthetic reconstruction group than the osteoarticular allograft group: 13.56° (SD 18.73; range, 0°–80°) versus 2.41° (SD 5.76; range, 0°–30°; p < 0.001; 95% CI, 5.8–16.4).
Discussion
The proximal tibia is the second most common site for primary bone tumors after the distal femur; however, the results in this group of patients are much worse compared with distal femur reconstructions [10, 17, 19]. Several reconstruction techniques have been described: complete biological reconstruction, endoprosthetic replacement, or a combination of both with alloprosthetic composites [1, 4, 6, 9, 10]. Benefits and disadvantages have been reported for all of them [1, 4, 6, 9, 18, 20]. Given the lack of outcome data from directly comparing these kinds of reconstructions, we wished to compare the experience of two oncology units who specialized in endoprostheses and osteoarticular reconstructions [4, 26].
Our study has certain limitations. First, we recognize that the retrospective design of this study and the selection bias for the patients who were treated in two different countries by two different groups, Oncology Unit 1 (Royal Orthopaedic Hospital, Birmingham, UK) exclusively at the time of the study used endoprosthetic replacement and Unit 2 (Italian Hospital of Buenos Aires, Buenos Aires, Argentina) exclusively (or nearly exclusively) used allograft reconstruction after primary bone tumor resections. Second, the number of patients not known to have reached an endpoint (death, reconstructive failure, or limb loss) before 2 years and lost to followup was higher in the endoprosthetic group compared with the osteoarticular allograft group (16 of 104 [15%] versus zero of 45 [0%]). Third, the duration of followup was different in the groups; mean followup was 2 years longer in patients treated with prosthetic reconstruction (9.5 versus 7.4 years) and this will tend to make prosthetic reconstructions look worse, because it has more time to accrue failures and complications. Because this study goes back several decades, it is possible that a patient who had surgery early in the study and had 2-year followup but was subsequently lost to followup is not accounted for recently. Such a patient, if not known to have died, is likely to have been revised. We cannot determine what proportion of our patients have not been seen in the last 5 years in each group or if that proportion was different between the groups. This differential loss to followup might have resulted in underreporting of complications and failures. Fourth, the group has some inherent heterogeneity in terms of diagnosis, the amount of soft tissue resection, extent of internal fixation, and extent of resection, which could affect the incidence of failures, complications, and functional outcomes. The inclusion of some patients with benign tumors that did not receive chemotherapy or other adjuvants and the apparent imbalance of tumors such as giant cell tumor of bone between the centers is a further limitation. To our knowledge, this is the largest comparative study of alternative methods for proximal tibia reconstruction after primary bone tumors resections and allows us to have adequate numbers to analyze this site of reconstruction.
There was no difference in 5- and 10-year reconstruction failure rates between both groups (Fig. 1). The failure rate of endoprosthetic reconstructions rose gradually over time. This probably has to do with the fact that the main cause of failure in this group of patients is related to mechanical complications. Wunder et al. compared the complications and functional outcomes associated with the use of an irradiated allograft-implant composite or a modular tumor prosthesis for replacement of the knee after resection of a bone sarcoma and found that prostheses presented better functional outcomes with a minor number of failures [26]. However, only 11 patients were included in the allograft group and proximal tibia reconstructions represented 25% of the complete series (19 of 75). Another important consideration refers to the fact that nonunion, bushing changes, and reconstruction of the extensor mechanism were not considered a failure (as Henderson et al. classification does). Myers et al. reported a series of 194 proximal tibial replacements (95 being a fixed-hinge design and 99 a rotating-hinge with a HA collar). The risk of revision for aseptic loosening in the fixed-hinge knees was 46% at 10 years and was reduced to 3% in the rotating-hinge knee with a HA collar [20].
Twenty-four of the endoprosthetic reconstruction failures were classified as Henderson Type 3 (Table 2), defined as structural failures, and included in this group was wear-related exchange of a bushing or other articulating component [11]. The longer the prosthesis is in situ, the greater the risk that a replacement of bushings will be called for [6, 10, 19]. For osteoarticular reconstruction, the most critical period was the first 24 months. The majority of osteoarticular allograft failures occurred within 24 months with infection being the most prevalent as had been reported previously [15, 16]. It has been reported that the introduction of gastrocnemius flaps for soft tissue coverage after a proximal tibia resection has led to a decline in postoperative infection rates [13, 20]. Three patients with limb salvage reconstruction failure could not be classified by Henderson classification: one patient (endoprosthetic group) with a postoperative lower limb ischemia that resulted in an above-knee amputation and two patients (osteoarticular allograft group) who developed severe osteoarthritis resulting in revision surgery to allograft-composite prostheses. If changing the bushing for endoprosthetic reconstructions is considered as mechanical failure (Henderson et al. Type 3A), using a knee prosthesis as a result of osteoarthritis in an osteoarticular allograft reconstruction should be also classified as a failure. We consider that this should be revised in the future. Henderson Type 5 failures are defined as “failure of limb salvage due to a recurrent tumor as a consequence of contamination at the time of the endoprosthetic reconstruction” [11]. From our point of view, all local recurrences (soft tissue or bone) that affect limb salvage reconstruction should be considered as a failure, not only the ones that presented after positive margins. Only one of nine local recurrences in endoprosthetic reconstructions and one of five in osteoarticular allografts were after contaminated margins during surgery.
Functional results analyzed by MSTS showed no overall differences between the groups despite there being some differences between the functional abilities of the two groups. Reconstruction of the extensor mechanism after proximal tibia tumor resection is a major concern and its success has shown some relationship with functional outcomes [2, 3, 5]. Reconstruction with allogeneic tissue from the proximal tibia allograft sutured to the recipient’s remnant patellar tendon can restore and stabilize active knee extension, whereas the suture of the medial gastrocnemius flap to the extensor mechanism after endoprosthetic reconstruction has shown acceptable results [12–14]. We found that patients treated with allograft had better active extension with less extensor lag than attaching the patella ligament directly to the transposed gastrocnemius flap. We allowed patients who underwent endoprosthetic reconstruction to weightbearing immediately after surgery so we consider endoprostheses to be a better option for older adult patients and those with poorer prognosis (patients with metastases on diagnosis, patients with poor response to chemotherapy) where longevity is less of a concern. On the other hand, osteoarticular allografts provide restoration of bone stock and reliable reattachment of the extensor mechanism to the graft with a mean recovery period to full weightbearing of 23 weeks and should be strongly considered for young patients with a good response to chemotherapy or benign bone tumors.
In conclusion, reconstruction of the proximal tibia with either endoprosthetic replacements or osteoarticular allografts appears to offer similar reconstruction failure rates. The primary cause of failure for osteoarticular allograft was infection and for endoprosthetic reconstruction was mechanical complications. We believe that the treating surgeon should have both options available for treatment of patients with malignant or aggressive tumors of the proximal tibia. (S)he might consider an allograft in a younger patient to achieve restoration of the bone stock and better extensor mechanism function, whereas in an older patient or one with a poorer prognosis where return to function and ambulation quickly is desired, an endoprosthetic reconstruction may be advantageous.
Footnotes
One of the authors certifies that he (LAA-T) or a member of his immediate family, has or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 from Stryker Americas (Miramar, FL, USA). One of the authors certifies that he (SRC) or a member of his immediate family, has or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 from Biomet (Warsaw, IN, USA). One of the authors certifies that he (LMJ) or a member of his immediate family, has or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 Biomet, Stanmore (Elstree, UK), and Implantcast (Buxtehude, Germany).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the reporting of this case report, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Royal Orthopaedic Hospital, Birmingham, UK, and Italian Hospital of Buenos Aires, Argentina.
References
- 1.Abed YY, Beltrami G, Campanacci DA, Innocenti M, Scoccianti G, Capanna R. Biological reconstruction after resection of bone tumors around the knee: long-term follow-up. J Bone Joint Surg Br. 2009;91:1366–1372. doi: 10.1302/0301-620X.91B10.22212. [DOI] [PubMed] [Google Scholar]
- 2.Ayerza MA, Aponte-Tinao LA, Abalo E, Muscolo DL. Continuity and function of patellar tendon host-donor suture in tibial allograft. Clin Orthop Relat Res. 2006;450:33–38. doi: 10.1097/01.blo.0000229291.21722.b5. [DOI] [PubMed] [Google Scholar]
- 3.Bickels J, Wittig JC, Kollender Y, Neff RS, Kellar-Graney K, Meller I, Malawer MM. Reconstruction of the extensor mechanism after proximal tibia endoprosthetic replacement. J Arthroplasty. 2001;16:856–862. doi: 10.1054/arth.2001.25502. [DOI] [PubMed] [Google Scholar]
- 4.Brien EW, Terek RM, Healey JH, Lane JM. Allograft reconstruction after proximal tibial resection for bone tumors. An analysis of function and outcome comparing allograft and prosthetic reconstructions. Clin Orthop Relat Res. 1994;303:116–127. [PubMed] [Google Scholar]
- 5.Capanna R, Scoccianti G, Campanacci DA, Beltrami G, De Biase P. Surgical technique: extraarticular knee resection with prosthesis-proximal tibia-extensor apparatus allograft for tumors invading the knee. Clin Orthop Relat Res. 2011;469:2905–2914. doi: 10.1007/s11999-011-1882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckhardt JJ, Matthews JG, Eilber FR. Endoprosthetic reconstruction after bone tumor resections of the proximal tibia. Orthop Clin North Am. 1991;22:149–160. [PubMed] [Google Scholar]
- 7.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 8.Gebhardt MC, Flugstad DI, Springfield S, Mankin HJ. The use of bone allografts for limb salvage in high grade extremity osteosarcoma. Clin Orthop Relat Res. 1991;270:181–196. [PubMed] [Google Scholar]
- 9.Gilbert NF, Yasko AW, Oates SD, Lewis VO, Cannon CP, Lin PP. Allograft-prosthetic composite reconstruction of the proximal part of the tibia. An analysis of the early results. J Bone Joint Surg Am. 2009;91:1646–1656. [DOI] [PubMed]
- 10.Grimer RJ, Carter SR, Tillman RM, Sneath RS, Walker PS, Unwin PS, Shewell PC. Endoprosthetic replacement of the proximal tibia. J Bone Joint Surg Br. 1999;81:488–494. doi: 10.1302/0301-620X.81B3.9234. [DOI] [PubMed] [Google Scholar]
- 11.Henderson ER, O’Connor MI, Ruggieri P, Windhager R, Funovics PT, Gibbons CL, Guo W, Hornicek FJ, Temple HT, Letson GD. Classification of failure of limb salvage after reconstructive surgery for bone tumors: a modified system including biological and expandable reconstructions. Bone Joint J. 2014;96:1436–1440. doi: 10.1302/0301-620X.96B11.34747. [DOI] [PubMed] [Google Scholar]
- 12.Holzapfel BM, Rechl H, Lehner S, Pilge H, Gollwitzer H, Steinhauser E. Alloplastic reconstruction of the extensor mechanism after resection of tibial sarcoma. Sarcoma. 2011:545104. [DOI] [PMC free article] [PubMed]
- 13.Jentzsch T, Erschbamer M, Seeli F, Fuchs B. Extensor function after medial gastrocnemius flap reconstruction of the proximal tibia. Clin Orthop Relat Res. 2013;471:2333–2339. doi: 10.1007/s11999-013-2851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kendall SJ, Singer GC, Briggs TW, Cannon SR. A functional analysis of massive knee replacement after extra-articular resections of primary bone tumors. J Arthroplasty. 2000;15:754–760. doi: 10.1054/arth.2000.8104. [DOI] [PubMed] [Google Scholar]
- 15.Lord CF, Gebhardt MC, Tomford WW, Mankin HJ. Infection in bone allografts. Incidence, nature, and treatment. J Bone Joint Surg Am. 1988;70:369–376. doi: 10.2106/00004623-198870030-00008. [DOI] [PubMed] [Google Scholar]
- 16.Loty B, Tomeno B, Evrard J, Postel M. Infection in massive bone allografts sterilized by radiation. Int Orthop (SICOT). 1994;18:164–171. doi: 10.1007/BF00192473. [DOI] [PubMed] [Google Scholar]
- 17.Muscolo DL, Ayerza MA, Aponte-Tinao LA, Ranalletta M. Use of distal femoral osteoarticular allografts in limb salvage surgery. J Bone Joint Surg Am. 2005;87:2449–2455. doi: 10.2106/JBJS.D.02170. [DOI] [PubMed] [Google Scholar]
- 18.Muscolo DL, Ayerza MA, Farfalli G, Aponte-Tinao LA. Proximal tibia osteoarticular allografts in tumor limb salvage surgery. Clin Orthop Relat Res. 2010;468:1396–1404. doi: 10.1007/s11999-009-1186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumors: long-term results. J Bone Joint Surg Br. 2007;89:521–526. doi: 10.1302/0301-620X.89B4.18631. [DOI] [PubMed] [Google Scholar]
- 20.Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. The long-term results of endoprosthetic replacement of the proximal tibia for bone tumors. J Bone Joint Surg Br. 2007;89:1632–1637. doi: 10.1302/0301-620X.89B12.19481. [DOI] [PubMed] [Google Scholar]
- 21.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria; 2014. Available at: http://www.R-project.org. Accessed March 4, 2016.
- 22.Racano A, Pazionis T, Farrokhyar F, Deheshi B, Ghert M. High infection rate outcomes in long-bone tumor surgery with endoprosthetic reconstruction in adults: a systematic review. Clin Orthop Relat Res. 2013;471:2017–2027. doi: 10.1007/s11999-013-2842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramseier LE, Dumont CE, Exner GU. Rotationplasty (Borggreve/Van Nes and modifications) as an alternative to amputation in failed reconstructions after resection of tumors around the knee joint. Scand J Plast Reconstr Surg Hand Surg. 2008;42:199–201. doi: 10.1080/02844310802069434. [DOI] [PubMed] [Google Scholar]
- 24.Robert RS, Ottaviani G, Huh WW, Palla S, Jaffe N. Psychosocial and functional outcomes in long-term survivors of osteosarcoma: a comparison of limb-salvage surgery and amputation. Pediatr Blood Cancer. 2010;54:990–999. doi: 10.1002/pbc.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sim FH, Beauchamp CP, Chao EY. Reconstruction of musculoskeletal defects about the knee for tumor. Clin Orthop Relat Res. 1987;221:188–201. [PubMed] [Google Scholar]
- 26.Wunder JS, Leitch K, Griffin AM, Davis AM, Bell RS. Comparison of two methods of reconstruction for primary malignant tumors at the knee: a sequential cohort study. J Surg Oncol. 2001;77:89–99. doi: 10.1002/jso.1076. [DOI] [PubMed] [Google Scholar]