Abstract
Salvinorin A is a kappa opioid agonist and the principal psychoactive constituent of the Salvia divinorum plant, which has been used for hallucinogenic effects. Previous research on salvinorin A pharmacokinetics likely underestimated plasma levels typically resulting from the doses administered due to inefficient vaporization and not collecting samples during peak drug effects. Six healthy adults inhaled a single high dose of vaporized salvinorin A (n=4, 21 mcg/kg; n=2, 18 mcg/kg). Participant- and monitor-rated effects were assessed every 2 min for 60 min post-inhalation. Blood samples were collected at 13 time points up to 90 min post-inhalation. Drug levels peaked at 2 min and then rapidly decreased. Drug levels were significantly, positively correlated with participant and monitor drug effect ratings. Significant elevations in prolactin were observed beginning 5 min post-inhalation and peaking at 15 min post-inhalation. Cortisol showed inconsistent increases across participants. Hormonal responses were not well correlated with drug levels. This is the first study to demonstrate a direct relationship between changes in plasma levels of salvinorin A and drug effects in humans. The results confirm the efficacy of an inhalation technique for salvinorin A.
Keywords: Salvia divinorum, salvinorin A, pharmacokinetics, prolactin, cortisol, endocrine
Introduction
The plant Salvia divinorum (a member of the mint family) has been used historically in shamanic practices of the Mazatec people of Oaxaca, Mexico for at least several hundred years (Ott, 1995; Valdés et al., 1983), although it was not botanically described until the 1960s (Epling and Jativa, 1962). Within the past 15 years S. divinorum has gained increased popularity as a psychoactive drug in non-traditional contexts (Perron et al., 2012; Wu et al., 2011). In non-traditional use, products containing S. divinorum leaves, sometimes infused with S. divinorum extract in order to increase drug effects, are typically smoked (Baggot et al., 2010; Gonzolez et al., 2006). Salvinorin A, the primary psychoactive compound in S. divinorum, is a kappa opioid agonist hallucinogen that is not active at the 5-HT2A receptor, the primary site of activity for classic hallucinogens such as LSD and psilocybin (Cunningham et al., 2011; Prisinzano, 2005; Roth et al., 2002). Although S. divinorum and salvinorin A have not been controlled at the federal level in the US, at the time of this writing at least 35 states within the US and 27 nations have enacted various levels of restriction for S. divinorum (Siebert, 2015).
Understanding the effects of salvinorin A, including its pharmacokinetic profile, in humans is important for understanding recreational use of S. divinorum. Laboratory research has not found evidence of persisting psychotic-type episodes resulting from salvinorin A (Addy, 2012; Johnson et al., 2011; MacLean et al., 2013; Ranganathan et al., 2012). Cases of persisting psychotic-type episodes have been reported in association with recreational use, although the causal role of S. divinorum remains unclear (Vandrey et al., 2013). In addition, the dissociative and perceptual effects resulting from salvinorin A (Addy, 2012; Johnson et al., 2011; MacLean et al., 2013; Ranganathan et al., 2012), could potentially result in dangerous behavior in an unsupervised environment. Therefore, studying the pharmacokinetic profile of salvinorin A may inform the understanding of potential adverse reactions observed in recreational S. divinorum use. Examining human salvinorin A effects is also important because salvinorin A or derivative compounds may serve as therapeutic agents for neurological (e.g., Alzheimer’s disease), pain, mood, personality, gastrointestinal, and cocaine-use disorders (Cunningham et al., 2011; Kivell and Prisinzano, 2010; Mello and Negus, 2000; Morani et al., 2009; Sheffler and Roth, 2003; Tejeda et al., 2012). While a study of inhaled salvinorin A would primarily model inhaled recreational use of S. divinorum, its results may also have limited relevance for potential therapeutic applications. Regardless of whether potential therapeutic applications would deliver the drug via vaporization, examining the relation between plasma drug levels and resulting subjective effects in the vaporized route may inform the more general relation between plasma drug levels and subjective effects at play in potential therapeutic applications.
Studies of intraperitoneally injected salvinoirin A in rats, and intravenously injected salvinorin A in rhesus monkeys, have provided basic pharmacokinetic data (Schmidt et al. 2005; Teksin et al., 2009). However, cross-species differences and differences between routes of administration may limit the implications of these findings for the pharmacokinetics of inhaled salvinorin A in humans. One previous study assessed the pharmacokinetic profile of salvinorin A in humans (Ranganathan et al., 2012). That study showed increases in salvinorin A, prolactin, and cortisol resulting from inhaled administration of the drug. However, there are several issues that remain unexamined. First the previous study did not examine the relation between individual salvinorin A pharmacokinetic and psychoactive effects. Second, Ranganathan and colleagues examined plasma levels of salvinorin A at only 3 timepoints post-inhalation (15, 20, and 30 min.), with the first assessment occurring substantially after the time at which peak participant-rated effects were observed in our research (2 min. post-inhalation) (Johnson et al., 2011; MacLean et al., 2013), suggesting that pharmacokinetic analysis of peak drug effects was missed. Third, Ranganathan and colleagues used a commercial vaporizer to deliver a maximum dose (12 mg) that was approximately eight to twelve time higher than the maximum doses administered in studies that used a glass pipe to vaporize salvinorin A (Johnson et al., 2011; MacLean et al., 2013; Maqueda et al., in press), and a study that had participants smoke S. divinorum leaves infused with additional salvinorin A (Addy, 2012; Addy et al., 2015). Substantial differences with the previously reported pharmacokinetic study regarding dose and delivery system warrant a pharmacokinetic analysis of our study.
In the present study, we examined the time course of salvinorin A plasma levels after inhalation of a high dose, delivered via a relatively efficient vaporization system. Blood was drawn at relatively frequent post-inhalation timepoints in order to accurately describe plasma levels surrounding the relatively rapid peak drug effects of salvinorin A. This frequent sampling allowed us to analyze the correspondence between drug levels and subjective effects throughout the drug time course. In addition, we examined levels of prolactin and cortisol, which are both sensitive to kappa agonist administration (Ur et al., 1997). In order to address one aspect of the efficiency of the delivery system, residual salvinorin A from the glass pipe was assayed for each session.
Methods
Participants
Participants were 6 individuals who participated in a previous study assessing the effects of inhaled salvinorin A in the laboratory (Johnson et al., 2011; MacLean et al., 2013). The sample size was judged sufficient for examining pharmacokinetic data because robust significant subject-rated effects were observed with fewer participants (Johnson et al., 2011). Participants had taken part in up to 20 previous sessions (16 salvinorin A doses in ascending order and 4 intermixed placebo sessions under blind conditions) that did not involve collecting blood samples. Two individuals (1 female, 1 male) whose subjective and cognitive data were included in our previous sample of 8 participants (MacLean et al., 2013) did not participate in the final salvinorin A administration session, which was the only session involving blood draws. In the case of the male, the participant decided not to participate in the blood draw session upon considering several subjectively intense sessions previously in the study. In the case of the female, the investigators decided not to continue her onto the blood draw session due to excessive spontaneous arm movements in previous sessions, which may have interfered with the blood draws.
For the 6 participants reported here, mean age was 25 years (range: 21–35). They reported using S. divinorum on a mean of 11 previous occasions (range: 1–40), with their reported first use at a mean age of 21 years (range: 16–31). They reported using classic hallucinogens on a mean of 32 previous occasions (range: 5–111). Study staff who were present during drug administration had established rapport with participants during previous preparatory sessions and lower dose and placebo sessions as described previously (Johnson et al., 2011).
Procedure
Each participant inhaled a single high dose of vaporized salvinorin A. The dose administered was the highest tolerated dose of salvinorin A in previous sessions. For four participants this dose was 21.0 mcg/kg, which was the maximal dose in the dose run-up. For the other two this dose was 18.0 mcg/kg because they replied “yes” to a question asking them if they would refuse to receive the same or higher doses at the conclusion of a 19.5 mcg/kg session. As described previously (MacLean et al., 2013) subjective drug strength and monitor-rated effects (drug strength, distance from usual daily reality, unresponsiveness, psychological distress, paranoia, anxiety/fear, motor activity, joy/peace and physical distress) and physiology measures (systolic and diastolic blood pressure, heart rate) were assessed every 2 min for 60 min after inhalation. Blood samples were collected at 13 time points (baseline, 1, 2, 3, 4, 5, 10, 15, 20, 25, 30, 60, 90) and cold centrifuged to obtain plasma. Plasma samples were purified by solid phase extraction and analyzed in triplicate via liquid chromatography-tandem mass spectrometry (LC-MS/MS) using a +5 mass analogue of salvinorin A as internal standard (Caspers et al., 2013). Analyses of prolactin and cortisol were performed with ELISA kits (Calbiotech, Spring Value, CA) and were run in triplicate. The average of these three assays was used in analyses. Residual salvinorin A in the glass pipe was determined for each session. Specifically, dichloromethane (1mL in 3 separate washes) was used to wash the inner surfaces of the glass pipe. The combined 3 ml of resulting solution was then dried under a stream of nitrogen. Three separate samples from the resulting residue were dissolved into mobile phase and subjected to LC-MS/MS for analysis.
Data Analysis
For each participant, we calculated Pearson’s correlations between each hormonal assay (prolactin, and cortisol) and subjective and monitor ratings of drug effects, using only the 7 time points when both blood and ratings were collected (baseline, 2, 4, 10, 20, 30 and 60 min post-inhalation). Correlations were also conducted for each participant between salvinorin A and prolactin levels, between salvinorin A and cortisol levels, and between prolactin and cortisol levels. Correlations between drug and hormones used the 7 common timepoints indicated above, while correlations among hormones used 13 common timepoints. Because a delayed hormonal response (relative to drug levels) might obscure a relationship between drug and hormonal levels, the same pairs of correlations were also conducted at the group level using peak values (i.e., single maximal value across the time course for each participant) for drug and hormonal levels.
Repeated measures regression (SAS PROC MIXED, AR(1) covariance structure) was used to model the relationship between salvinorin A plasma level and participant and monitor ratings of drug effects from baseline to 60 min post-administration. As with the correlations, this analysis only used the 7 timepoints common to both blood draws and drug strength ratings. Statistical significance was defined as p < .05.
The percent of the intended dose that remained as residual salvinorin A in the glass pipe was calculated using the salvinorin A residual mass for each participant (i.e., mean of the triplicate LC-MS/MS assays) and the prepared absolute salvinorin A dose for each participant (i.e., taking bodyweight into account).
Results
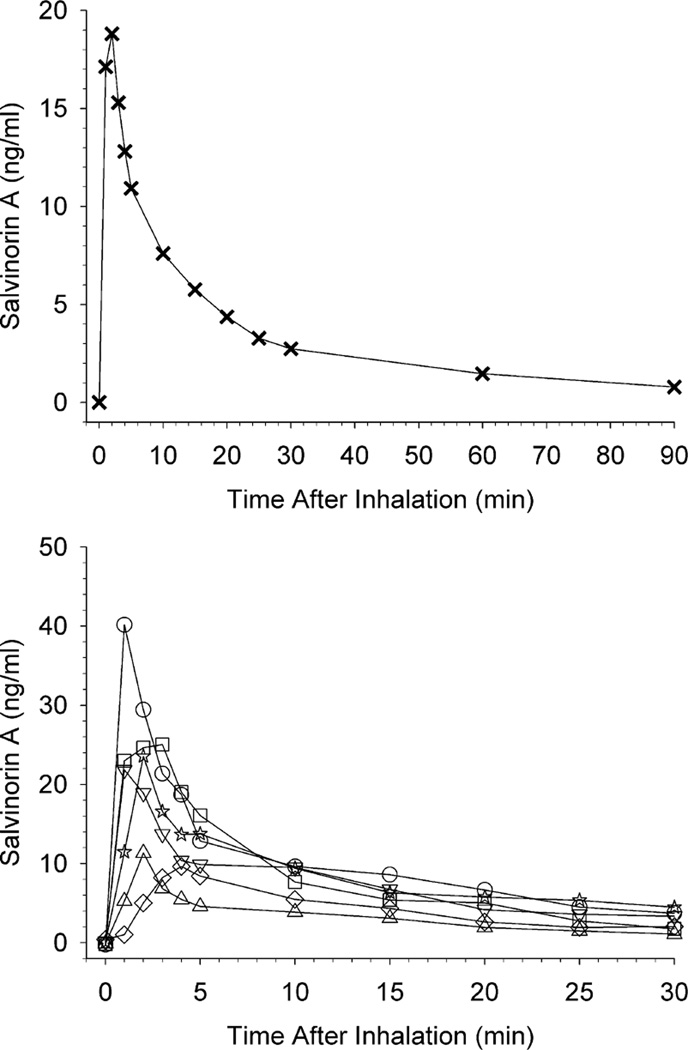
Samples were collected and assayed for salvinorin A level at all timepoints. For prolactin and cortisol, sample volumes were insufficient to obtain results for two timepoints for 1 participant (at the 1 and 10 min timepoints). Coefficients of variation (CV) for plasma samples (in triplicate) and standards (in duplicate) were < 10%. The upper panel of Fig. 1 shows mean salvinorin A levels at all blood collection time points (up to 90 min post-inhalation). In order to show individual variability contributing to mean levels, the lower panel shows individual participant salvinorin A levels at each time point up to 30 min post-inhalation. The upper panel shows that mean peak salvinorin A levels occurred at 2 min post-inhalation, followed by rapid reductions and then more gradual reductions until the final time point at 90 min post-infusion, at which time salvinorin A levels were close to baseline (zero). Although these trends were generally observed at the individual participant level (lower panel), notable variations occurred, with peak effects occurring as early at 1 min to as late as 4 min post-inhalation.
Fig. 1.
The upper panel shows mean salvinorin A levels at all blood collection time points. The lower panel shows individual participant salvinorin A levels at each time point up to 30 min post-inhalation; individual participants are designated by different symbols. In both panels, the pre-inhalation assessment timepoint is shown at 0 min.
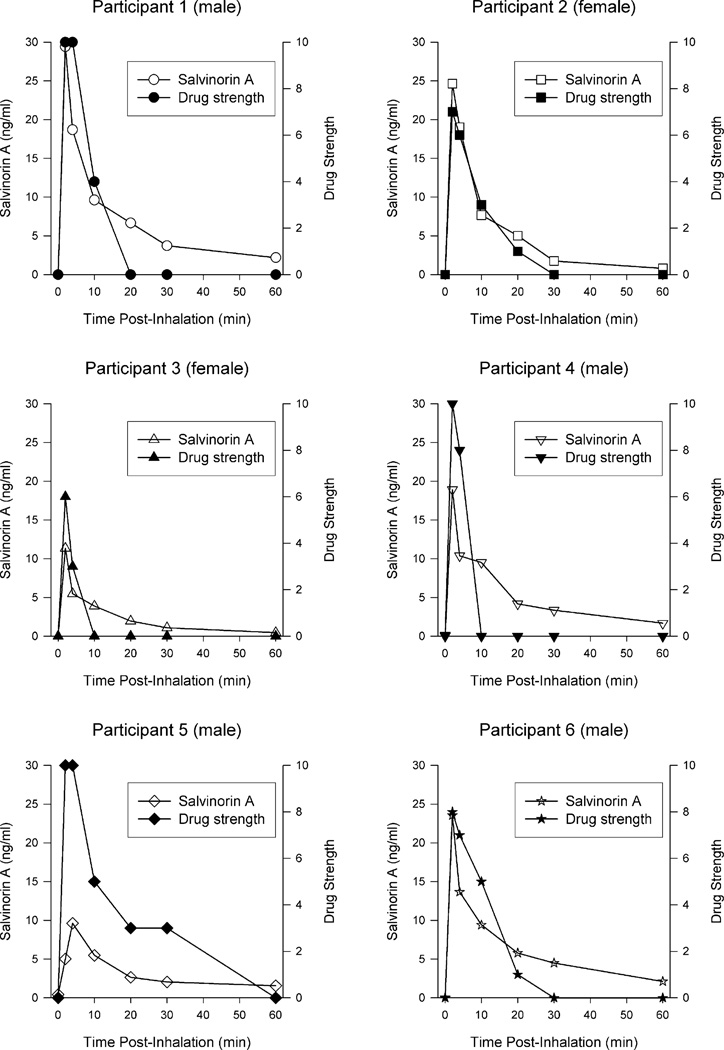
To illustrate the relationship between drug blood levels and subjective drug strength, each panel of Fig. 2 shows an individual participant’s salvinorin A plasma levels and subjective drug strength. Ratings of drug strength were closely associated with plasma levels. The median Pearson correlation between plasma levels and drug strength across individuals was r = .93 (range: .88–.99; all significant).
Fig. 2.
Each panel shows individual participant salvinorin A plasma levels (left axis) and subjective rating of drug strength (right axis) for all time points in which both measures were assessed. Individual participants are designated by the same symbols shown in Fig 1. The pre-inhalation assessment timepoint is shown at 0 min.
Repeated measures regression showed that salvinorin A level significantly increased participant (F(1,35) = 74.08, p < .0001) and monitor (F(1,35) = 29.14, p < .0001) ratings of drug strength, and monitor ratings of distance from usual daily reality (F(1,35) = 15.41, p < .001), unresponsiveness (F(1,35) = 19.82, p < .0001), psychological distress (F(1,35) = 21.26, p < .0001) and paranoia (F(1,35) = 11.87, p = .002). The effect of salvinorin A level was not significant for the remaining monitor ratings (anxiety/fear, motor activity, joy/peace and physical distress). The effect of salvinorin A level was also not significant for physiology measures (systolic and diastolic blood pressure, heart rate). Results remained unchanged after controlling for lifetime use of hallucinogens and lifetime use of S. divinorum.
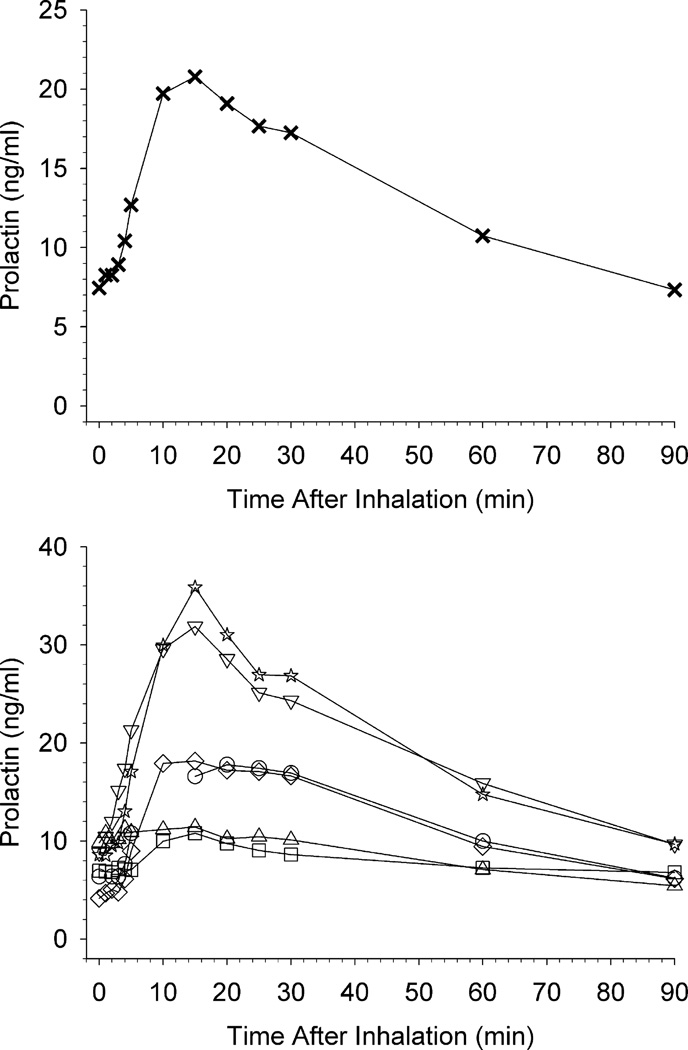
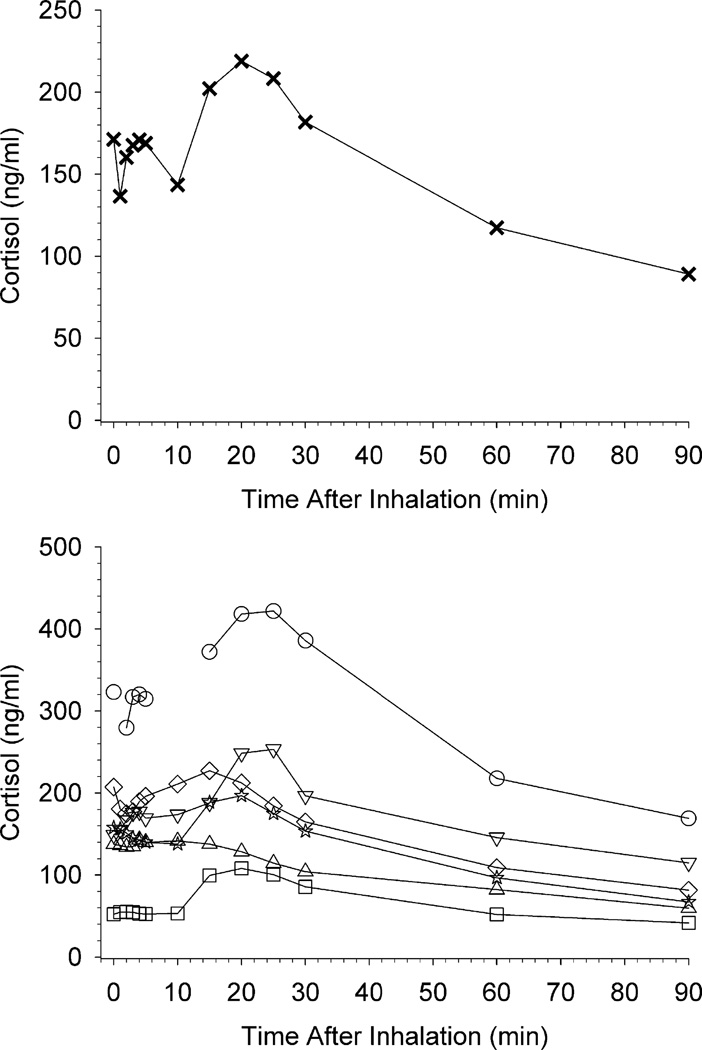
Fig. 3 shows the effects of salvinorin A administration on plasma prolactin. The upper panel shows mean prolactin levels, and the lower panel shows prolactin levels in individual participants. Mean peak effects occurred at 15 min post-inhalation and gradually decreased through 90 min. However, individual participant data show a plateau of peak prolactin levels from 10 to 30 min post-inhalation for some individuals. Fig. 4 shows the effects of salvinorin A administration on plasma cortisol. The upper panel shows mean cortisol levels, and the lower panel shows cortisol levels in individual participants. The mean cortisol time course resembled that of prolactin. However, there was substantial individual variability with little evidence of a cortisol response observed in some participants. There were no significant correlations between cortisol or prolactin levels and drug-effect ratings within individual participants. No individual participant correlations between hormone levels and physiological measures were significant with the exception of 1 positive correlation between pulse and cortisol, and 1 negative correlation between systolic blood pressure and prolactin. Salvinorin A levels were not significantly correlated with either cortisol or prolactin levels within any individual participant. Levels of prolactin and cortisol were positively correlated within each participant across the 13 timepoints (1 participant with 11 timepoints due to missing data) (Pearson r range: .36 to.92; significant for 4 of 6 participants). In correlations at the group level, no significant relation was detected between salvinorin A and prolactin levels (p = .82), between salvinorin A and cortisol levels (p = .15), or between prolactin and cortisol levels (p = .68).
Fig. 3.
The upper panel shows mean prolactin levels at all blood collection time points. The lower panel shows individual participant prolactin levels at each time point up to 30 min post-inhalation; individual participants are designated by the same symbols shown in Fig 1; unconnected data points indicate a missing timepoint. In both panels, the pre-inhalation assessment time-point is shown at 0 min.
Fig. 4.
The upper panel shows mean cortisol levels at all blood collection time points. The lower panel shows individual participant cortisol levels at each time point up to 30 min post-inhalation; individual participants are designated by the same symbols shown in Fig 1; unconnected data points indicate a missing time-point. In both panels, the pre-inhalation assessment timepoint is shown at 0 min.
Coefficients of variation for the triplicates of residual salvinorin A assays for each participant were <4%. The mean mass of salvinorin A residue in the glass pipe across participants was 57.1 mcg (SD= 24.3 mcg), representing a mean of 4.21% (SD=2.25%) of the prepared absolute dose.
Discussion
This study is unique in that it examined the time course (including frequent, early timepoints) of salvinorin A plasma levels after salvinorin A inhalation, delivered via a relatively efficient vaporization system. The present study resulted in novel information relevant to three domains: drug delivery, time course of drug levels, and time course and magnitude of hormonal effects.
Drug delivery
The present study showed substantially higher salvinorin A plasma levels compared to the previous study of inhaled salvinorin A pharmacokinetics (Ranganathan et al., 2012). The previous study found a mean salvinorin A level of approximately 0.9 to 1.0 ng/ml resulting from 8 and 12 mg salvinorin A (with little difference between those two doses). In contrast, in the present study, at doses ~8 times lower (18.0 and 21.0 mcg/kg, which equate to ~1.26 and 1.47 mg for a 70 kg bodyweight person), resulted in a mean of 18.8 ng/ml at peak effects. These data suggest the present study used a substantially more efficient delivery method. Differences in efficiency could involve multiple factors including temperature and air flow topography. Moreover, the analysis showing only a small percentage of residual salvinorin A in the glass pipe highlights the efficiency of the delivery system.
Time course of salvinorin A blood levels
The present study found strong correspondence between salvinorin A levels and ratings of drug strength throughout the time course. Unlike the previous study of salvinorin A pharmacokinetics (Ranganathan et al., 2012), this study was able to demonstrate this relationship due to more frequent drug effect rating assessments and blood draws. The present results indicate that subjective effects of salvinorin A are a direct function of concurrent plasma levels of the drug. This finding is consistent with a study of intravenous salvinorin A in rhesus monkeys reporting overt sedation-like behavior effects generally overlapping with the period of detected plasma levels of salvinorin A (e.g., within ~15 min. post-injection) (Schmidt et al. 2005).
Time course and magnitude of hormonal response
Similar to Ranganathan et al. (2012), the present study showed increases in prolactin and, less consistently, cortisol following salvinorin A administration. Due to infrequent sampling, the previous study did not have the ability to determine how closely hormone levels and salvinorin A levels were related in time. By showing rapid increases in salvinorin A levels that match the rapid subjective effects of the drug, the present study had the potential to demonstrate a strong correspondence between drug and hormone levels. However, the present study showed that prolactin and cortisol responses to salvinorin A administration followed a more delayed and prolonged time course than the drug itself.
Conclusion
This study provides important information regarding the pharmacokinetics of a relatively novel drug used for its hallucinogenic effects. It confirmed that a relatively efficient vaporization method resulted in substantially higher drug plasma levels compared to a previous study of salvinorin A pharmacokinetics (Ranganathan et al., 2012). Moreover, this study showed strong correlations between salvinorin A blood levels and drug strength ratings across the time course of drug effects, suggesting that subjective effects are a product of concurrent blood levels. This study also showed that salvinorin A generally increased prolactin, although it followed a more delayed and prolonged time course than the drug itself. Cortisol showed inconsistent increases across participants. Because smoking and vaporization both involve inhalation, the results of this study may be relevant to the recent use of S. divinorum in non-traditional contexts.
Acknowledgments
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Drug Abuse (NIDA) through R01DA003889, T32DA007209, and R01DA018151. Analysis of prolactin and cortisol was supported by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins with grants P30 CA006973 from the National Institutes of Health (NIH) and UL1 RR025005 from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research. Manuscript contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. The project described was also supported in part by Grant Number UL1 RR025005.
Footnotes
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- Addy PH. Acute and post-acute behavioral and psychological effects of salvinorin A in humans. Psychopharmacology. 2012;220:195–204. doi: 10.1007/s00213-011-2470-6. [DOI] [PubMed] [Google Scholar]
- Addy PH, Garcia-Romeu A, Metzger M, Wade J. The subjective experience of acute, experimentally-induced Salvia divinorum inebriation. Journal of Psychopharmacology. 2015;29(4):426–435. doi: 10.1177/0269881115570081. [DOI] [PubMed] [Google Scholar]
- Baggott MJ, Erowid E, Erowid F, Galloway GP, Mendelson J. Use patterns and self-reported effects of Salvia divinorum: an internet-based survey. Drug and Alcohol Dependence. 2010;111:250–256. doi: 10.1016/j.drugalcdep.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Baggott MJ, Erowid E, Erowid F, Mendelson JE. Use of Salvia divinorum, an unscheduled hallucinogenic plant: A web-based survey of 500 users. Clinical Pharmacology and Therapeutics. 2004;75:72. [Google Scholar]
- Caspers MJ, Williams TD, Lovell KM, Lozama A, Butelman ER, Kreek MJ, Johnson M, Griffiths R, Maclean K, Prisinzano TE. LC-MS/MS quantification of salvinorin A from biological fluids. Analytical Methods. 2013;5(24):7042–7048. doi: 10.1039/C3AY40810H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CW, Rothman RB, Prisinzano TE. Neuropharmacology of the naturally occurring kappa-opioid hallucinogen salvinorin A. Pharmacological Reviews. 2011;63:316–347. doi: 10.1124/pr.110.003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epling C, Jativa MC. A new species of Salvia from Mexico. Botanical Museum Leaflets, Harvard University. 1962;20:75–76. [Google Scholar]
- Gonzalez D, Riba J, Bouso JC, Gomez-Jarabo G, Barbanoj MJ. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug and Alcohol Dependence. 2006;85:157–162. doi: 10.1016/j.drugalcdep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Johnson MW, MacLean KA, Reissig CJ, Prisinzano TE, Griffiths RR. Human psychopharmacology and dose-effects of salvinorin A, a kappa opioid agonist hallucinogen present in the plant Salvia divinorum. Journal of Drug and Alcohol Dependence. 2011;115:150–155. doi: 10.1016/j.drugalcdep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivell B, Prisinzano TE. Kappa opioids and the modulation of pain. Psychopharmacology. 2010;210:109–119. doi: 10.1007/s00213-010-1819-6. [DOI] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, Reissig CJ, Griffiths RR, Prisinzano TE. Dose-related effects of salvinorin A in humans: Dissociative, hallucinogenic, and memory effects. Psychopharmacology. 2013;226(2):381–392. doi: 10.1007/s00213-012-2912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqueda AE, Valle M, Addy PH, Antonijoan RM, Puntes M, Coimbra J, Ballester MR, Garrido M, González M, Claramunt J, Barker S, Johnson MW, Griffiths RR, Riba J. Salvinorin-A induces intense dissociative effects, blocking external sensory perception and modulating interoception and sense of body ownership in humans. International Journal of Neuropsychopharmacology. doi: 10.1093/ijnp/pyv065. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS : Interactions between kappa opioid agonists and cocaine. Preclinical studies. Annals of the New York Academy of Sciences. 2000;909:104–132. doi: 10.1111/j.1749-6632.2000.tb06678.x. [DOI] [PubMed] [Google Scholar]
- Morani AS, Kivell B, Prisinzano TE, Schenk S. Effect of kappa- opioid receptor agonists U69593, U50488H, spiradoline and salvinorin A on cocaine-induced drug-seeking in rats. Pharmacology Biochemistry and Behavior. 2009;94:244–249. doi: 10.1016/j.pbb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J. Ethnopharmacognosy and human pharmacology of Salvia divinorum and salvinorin A. Curare. 1995;18:103–129. [Google Scholar]
- Perron BE, Ahmedani BK, Vaughn MG, Glass JE, Abdon A, Wu LT. Use of Salvia divinorum in a nationally representative sample. American Journal of Drug and Alcohol Abuse. 2012;38:108–113. doi: 10.3109/00952990.2011.600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisinzano TE. Psychopharmacology of the hallucinogenic sage Salvia divinorum. Life Sciences. 2005;78:527–531. doi: 10.1016/j.lfs.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, Schnakenburg A, Skosnik PD, Cohen BM, Pittman B, Sewell RA, D’Souza DC. Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the κ opioid agonist salvinorin A in humans. Biological Psychiatry. 2012;72:871–879. doi: 10.1016/j.biopsych.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MD, Schmidt MS, Butelman ER, Harding WW, Tidgewell K, Murry DJ, Kreek MJ, Prisinzano TE. Pharmacokinetics of the plant-derived kappa-opioid hallucinogen salvinorin A in nonhuman primates. Synapse. 2005;58:208–210. doi: 10.1002/syn.20191. [DOI] [PubMed] [Google Scholar]
- Sheffler DJ, Roth BL. Salvinorin A: the “magic mint” hallucinogen finds a molecular target in the kappa opioid receptor. Trends in Pharmacological Sciences. 2003;24:107–109. doi: 10.1016/S0165-6147(03)00027-0. [DOI] [PubMed] [Google Scholar]
- Siebert D. The Legal Status of Salvia divinorum. Sagewisdom.org. 2015 Aug 12th; Retrieved October 24th, 2015, from http://www.sagewisdom.org/legalstatus.html.
- Tejeda HA, Shippenburg TS, Henriksson R. The dynorphin/κ- opioid receptor system and its role in psychiatric disorders. Cellular and Molecular Life Sciences. 2012;69:857–896. doi: 10.1007/s00018-011-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teksin ZS, Lee IJ, Nemieboka NN, et al. Evaluation of the transport, in vitro metabolism and pharmacokinetics of Salvinorin A, a potent hallucinogen. Eur. J. Pharm. Biopharm. 2009;72(2):471–477. doi: 10.1016/j.ejpb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ur E, Wright DM, Bouloux PM, Grossman A. The effects of spiradoline (U-62066E), a kappa-opioid receptor agonist, on neuroendocrine function in man. British Journal of Pharmacology. 1997;120:781–784. doi: 10.1038/sj.bjp.0700971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés L, Díaz J, Paul A. Ethnopharmacology of ska María Pastora (Salvia divinorum, Epling and Játiva M.) Journal Of Ethnopharmacology. 1983;7(3):287–312. doi: 10.1016/0378-8741(83)90004-1. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Johnson MW, Johnson PS, Khalil MA. Novel drugs of abuse: A snapshot of an evolving marketplace. Adolescent Psychiatry. 2013;3(2):123–134. doi: 10.2174/2210676611303020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Woody GE, Yang C, Li JH, Blazer DG. Recent national trends in Salvia divinorum use and substance-use disorders among recent and former Salvia divinorum users compared with nonusers. Journal of Substance Abuse and Rehabilitation. 2011;2011:53–68. doi: 10.2147/SAR.S17192. [DOI] [PMC free article] [PubMed] [Google Scholar]