Abstract
Canine pancreatitis is a relatively common disorder, and its mortality rate remains high. However, prognostic factors for pancreatitis based on evidence are limited. Moreover, the relationship between changes in C-reactive protein (CRP) concentration—an important prognostic factor for human patients with acute pancreatitis—and the prognosis of dogs with pancreatitis has not been widely studied. Therefore, we examined prognostic factors for canine pancreatitis during the first medical examination and evaluated the usefulness of serial CRP measurements during hospitalization. Sixty-five dogs met the inclusion criteria, including 22 that were hospitalized and treated. In Study 1, a multivariate analysis revealed that three factors— decreased platelet count and a marked (greater than 1,000 µg/l) elevation of specific canine pancreatic lipase (Spec cPL) concentration at the first medical examination, as well as elevated blood urea nitrogen (BUN) and/or creatinine (CRE) level—were significantly different between the survivors and nonsurvivors. Moreover, CRP concentrations on the third and fourth days were significantly different between the two groups in Study 2. An evaluation of the decreased platelet count, remarkable elevation of Spec cPL concentration at the first medical examination, elevation of BUN and/or CRE as well as serial CRP concentration measurements may be useful for predicting the prognosis of canine pancreatitis.
Keywords: canine, CRP, pancreatitis, Spec cPL
Canine pancreatitis is a relatively common disorder that can result in a wide range of clinical signs, as well as multiple organ failure in severe cases. The mortality rate among dogs with pancreatitis remains high, ranging from 27% to 58% [9, 18]. The clinical condition of dogs with pancreatitis varies from mild to severe, and requires an accurate diagnosis and an appropriate treatment immediately. However, canine pancreatitis lacks specific treatment because almost all cases of canine pancreatitis are idiopathic, which contributes to a higher mortality rate. According to some studies, the mean survival time for severe cases from admission to discharge or death was 3.2 days (range, 1 to 13 days) and 5.0 days (range, 1 to 10 days) [7, 10]. These studies show that clinicians have to judge whether a case is severe or mild appropriately, and start intensive treatment and hospitalization immediately if it is determined to be severe. However, evidence on prognostic factors for canine pancreatitis is scarce. Only three studies have been published, and the indicators that should be used are not defined precisely [10, 13, 14]. In these studies, organ score (evaluation of multiple organ failure), severity score (scoring system of systemic conditions), hypothermia and metabolic acidosis were identified as the prognostic factors for pancreatitis [10, 13, 14]. However, those studies did not assess specific lipases for pancreas (Spec cPL [IDEXX], FUJI DRI-CHEM (FDC)-v-LIP [FUJIFILM]) as prognostic factors. Spec cPL is now widely used for the diagnosis of pancreatitis, and reports have shown that they have high specificity and sensitivity for pancreatitis [19, 21]. On the other hand, FDC-v-LIP was recently developed bedside (Point-of Care) test and reported to have a high correlation with Spec cPL [8]. Furthermore, a multivariate analysis, usually used for identifying prognostic factors to adjust for confounding variables, was not conducted in those studies.
In human medicine, C-reactive protein (CRP)—one of the acute-phase proteins produced in the liver—is considered to be one of the most important prognostic factors for pancreatitis and is a factor for determining the severity score according to the Japanese guidelines for the management of acute pancreatitis [20]. Several reports have indicated that the prognosis of pancreatitis patients can be evaluated by measuring CRP concentrations [16, 17]. In dogs, elevated CRP concentration is known to be associated with various inflammatory diseases [1, 3, 11]. In addition, some studies have identified the usefulness of serial CRP concentration measurements in dogs with several diseases, such as idiopathic polyarthritis and acute abdomen syndrome, to evaluate the response to treatments and the prognosis [6, 12]. The CRP concentration in dogs with pancreatitis is known to be elevated and is significantly high than in control dogs [15]. The relationship between CRP concentration and prognosis of dogs with pancreatitis has been examined, revealing that the CRP concentration in samples obtained within two days from the onset of clinical signs had a significant correlation with the outcome [10]. However, no significant difference was reported between prognosis and the serial CRP concentration measured during the first medical examination in that study.
The objective of this study was to determine the factors that may help predict short-term (15 days) prognosis in dogs with pancreatitis at the first medical examination and during hospitalization.
MATERIALS AND METHODS
Study design
This was a retrospective study. We reviewed the medical records of dogs that were referred to the Veterinary Medical Center of the University of Tokyo (Japan) from April 2014 to April 2015, and included dogs that were diagnosed with pancreatitis. Pancreatitis was diagnosed when the following two criteria were fulfilled: (1) compatible clinical signs (anorexia, lethargy, diarrhea, vomiting, abdominal pain or a combination of these) and (2) an increase in Spec cPL concentration (Spec cPL 400 µg/l or greater). We conducted two studies using these criteria. In order to focus on the prediction of short-term prognosis, included dogs were divided into two groups: Group S included dogs that survived for more than 15 days after the first medical examination; Group N included dogs that died within those 15 days.
The serum Spec cPL concentration was measured at IDEXX Laboratories Japan (Tokyo, Japan). Other biochemical parameters including FDC-v-LIP [8] and CRP were measured using a dry chemistry analyzer (FUJI DRI-CHEM 5000V; FUJIFILM Corporation, Tokyo, Japan) [5, 21].
Study 1. Evaluation of prognostic factors at the first medical examination
Dogs that fulfilled the inclusion criteria mentioned above were included in Study 1. The medical history, physical examination, hematology and biochemical analysis, and abdominal ultrasonography results (diffuse enlargement of the pancreas, hyperechoic mesentery surrounding the pancreas and variable pancreatic hypoechogenicity) were obtained from the medical records of the dogs. We adopted the values measured at the first medical examination—body temperature, heart rate, breathing rate, hematocrit (Ht), white blood cell (WBC), platelet, total protein (TP), albumin (ALB), blood urea nitrogen (BUN), creatinine (CRE) and liver enzyme (alkaline phosphatase [ALP], alanine aminotransferase [ALT]), glucose (GLU) and CRP levels, and pancreas-specific lipases (Spec cPL and FDC-v-LIP)—as the factors to be evaluated. The differences in these values between Group S and Group N were statistically evaluated using univariate and multivariate analyses to detect prognostic factors for pancreatitis.
Study 2. Evaluation of usefulness of serial measurements of CRP and other biomarkers
Of the dogs that met the inclusion criteria, those that were hospitalized for treatment were included in Study 2. We evaluated the relationship between serial biomarker measurements and the prognosis of dogs hospitalized to receive treatment. CRP, Spec cPL and FDC-v-LIP were adopted as the factors to be evaluated. We tested the relationship of CRP concentrations measured on the first to fifth days after admission with prognosis of the dogs. As for Spec cPL and FDC-v-LIP, the relationship among the values measured on the first day, third day and fifth day from admission was analyzed.
Statistical analysis
All statistical analyses were performed using commercially available software (JMP Pro, version 11.2.0, for Mac, SAS Institute Inc., Cary, NC, U.S.A.).
The relationship between the values measured and the outcome was tested with Fisher’s exact test or the multivariable logistic regression model (to test CRP and body temperature). A reference range was used as a cutoff for each value. An upper limit of measurement was used as a cutoff if or few dogs had values within the reference range. Multivariable analyses were performed for measurements with a univariate analysis of significance of P≤0.1. The multivariable logistic regression model was used for the values in the multivariate analysis. The relationship between serial concentrations of CRP, Spec cPL and FDC-v-LIP during hospitalization and the outcome was tested using the Wilcoxon rank sum test. Significance was set at P<0.05 for all tests.
RESULTS
Cases
Sixty-five dogs fulfilled the criteria and were included in Study 1. Of those dogs, 22 were hospitalized and included in Study 2. The most commonly affected breeds were Miniature Dachshunds (n=20), Shih Tzus (n=5) and Toy Poodles (n=4). Of the 65 dogs, 31 were male (15 intact and 16 castrated), and 34 were female (6 intact and 28 spayed). The median age of the dogs was 11 years (range, 2–16 years).
Clinical signs that were compatible with pancreatitis were reported, including anorexia (n=51, 78%), lethargy (n=50, 77%), vomiting (n=38, 58%), diarrhea (n=24, 37%) and abdominal pain (n=11, 17%). Ultrasonographic findings suggesting pancreatitis (diffuse enlargement of the pancreas, hyperechoic mesentery surrounding the pancreas and variable pancreatic hypoechogenicity) were observed in 24 dogs.
Study 1. Evaluation of prognostic factors at the first medical examination
Table 1 shows the results of a comparison of values at the first medical examination between Group S and Group N, by univariate analysis. The breathing rate could not be analyzed, because most of the dogs were panting, and no significant difference was found between the presence of panting and the outcome (P=0.5200). P values derived from the univariate analysis were as follows: BUN, P=0.0019; CRE, P=0.0167; platelets, P=0.0525; Spec cPL, P=0.0692; GLU, P=0.0824; heart rate, P=0.0913; CRP (0.7 mg/dl to 7.0 mg/dl), P=0.8351; CRP (greater than 7.0 mg/dl), P=0.1147; WBC, P=0.1247; body temperature (lower than 38°C), P=0.1809; body temperature (higher than 39°C), P=6354; FDC-v-LIP, P=0.1951; Ht, P=0.1982; TP, P=0.6714; ALP, P=0.7565; ALB, P=1.000; and ALT, P=1.000.
Table 1. A comparison of variables measured during the first medical examination between survivors and nonsurvivors by univariate analysis.
| Variables | No. Survivors | No. Nonsurvivors | P-value | |
|---|---|---|---|---|
| BUN (mg/dl) | ≤29.2 | 44 | 6 | − |
| >29.2 | 7 | 8 | 0.0019 a) | |
| CRE (mg/dl) | ≤1.4 | 49 | 10 | − |
| >1.4 | 2 | 4 | 0.0167 a) | |
| Platelet (×104/μl) | ≥20 | 44 | 9 | − |
| <20 | 6 | 5 | 0.0525 | |
| Spec cPL (μg/l) | ≤1,000 | 30 | 4 | − |
| >1,000 | 21 | 10 | 0.0692 | |
| GLU (mg/dl) | ≤128 | 39 | 8 | − |
| >128 | 9 | 6 | 0.0824 | |
| Heart rate (/min) | ≤160 | 46 | 10 | − |
| >160 | 5 | 4 | 0.0913 | |
| CRP (mg/dl) | ≤0.7 | 12 | 2 | − |
| >0.7, ≤7.0 | 30 | 6 | 0.8351 | |
| >7.0 | 9 | 6 | 0.1147 | |
| WBC (/μl) | ≤17,000 | 24 | 3 | − |
| >17,000 | 26 | 11 | 0.1247 | |
| Body temperature (°C) | 38−39 | 39 | 9 | − |
| <38 | 2 | 2 | 0.1809 | |
| >39 | 9 | 3 | 0.6354 | |
| FDC-v-LIP (U/l) | ≤1,000 | 37 | 7 | − |
| >1,000 | 14 | 7 | 0.1951 | |
| Ht (%) | ≥37 | 35 | 7 | − |
| <37 | 14 | 7 | 0.1982 | |
| TP (g/dl) | ≥5.0 | 42 | 13 | − |
| <5.0 | 7 | 1 | 0.6714 | |
| ALP (U/l) | ≤3,500 | 35 | 9 | − |
| >3,500 | 16 | 5 | 0.7565 | |
| ALB (g/dl) | ≥2.5 | 24 | 5 | − |
| <2.5 | 16 | 4 | 1.0000 | |
| ALT (U/l) | ≤78 | 16 | 4 | − |
| >78 | 35 | 10 | 1.0000 | |
a) P<0.05.
A multivariable logistic regression model was used for values that had significant differences between Groups S and N in the univariate analysis (P<0.1), including the BUN, CRE, Spec cPL, GLU, platelet count and heart rate measurements. In this analysis, we integrated the elevations of BUN and CRE into one value, because both BUN and CRE measurements are used for the evaluation of renal function. The multivariate analyses identified three factors—elevation of BUN and/or CRE, decreased platelet count and remarkable elevation of Spec cPL concentration—as the significantly different parameters between the two groups (Table 2).
Table 2. Prognostic factors for canine pancreatitis analyzed by the multivariable logistic regression model. Spec cPL is manufactured by IDEXX.
| Variables | No. Survivors | No. Nonsurvivors | Odds ratio (95% CI) | P-value | |
|---|---|---|---|---|---|
| BUN or/and CRE | Normala) | 43 | 6 | − | − |
| Abnormalb) | 8 | 8 | 20.08 (3.5–182.8) | 0.0005 c) | |
| Platelet (×104/μl) | ≥20 | 44 | 9 | − | − |
| <20 | 6 | 5 | 25.35 (3.5–283.8) | 0.0009 c) | |
| Spec cPL (μg/l) | ≤1,000 | 30 | 4 | − | − |
| >1,000 | 21 | 10 | 6.23 (1.2–43.1) | 0.0269 c) | |
| GLU (mg/dl) | ≤128 | 39 | 8 | − | − |
| >128 | 9 | 6 | 2.68 (0.3–22.5) | 0.3399 | |
| Heart rate (/min) | ≤160 | 46 | 10 | − | − |
| >160 | 5 | 4 | 1.63 (0.2–12.3) | 0.8784 | |
a) Normal means they are within the reference range. b) Abnormal blood urea nitrogen (BUN) and creatinine (CRE) level means elevated BUN and/or CRE. GLU, Glucose. c) P<0.05.
Study 2. Evaluation of the usefulness of serial measurements of CRP and other biomarkers
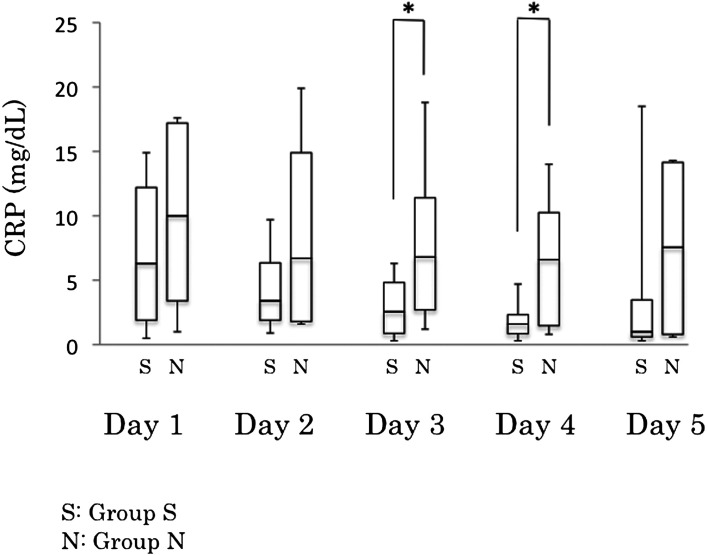
The median (range) CRP concentration for the two groups (Group S vs Group N) for days 1 to 5 was 6.3 mg/dl vs. 10 mg/dl (0.5–14.9 mg/dl vs. 1.0–17.6 mg/dl), 3.4 mg/dl vs. 6.7 mg/dl (0.9–9.7 mg/dl vs. 1.6–19.9 mg/dl), 2.55 mg/dl vs. 6.8 mg/dl (0.3–6.3 mg/dl vs. 1.2–18.8 mg/dl), 1.6 mg/dl vs. 6.6 mg/dl (0.3–4.7 mg/dl vs. 0.8–14.0 mg/dl) and 1.0 mg/dl vs. 7.55 mg/dl (0.3–18.5 mg/dl vs. 0.6–14.3 mg/dl), respectively (Table 3 and Fig. 1). The median CRP concentration for Group N on the third and fourth days was significantly higher than for Group S (P=0.0252, P=0.00438, respectively). No significant differences were found between the two groups regarding the median CRP concentration on the first, second and fifth days. A receiver operating characteristic (ROC) curve analysis revealed a CRP concentration of 6.5 mg/dl as the cutoff with high specificity and sensitivity. Using this cutoff value, the included dogs were divided into two groups and analyzed using Fisher’s exact test (Table 4). Significant CRP concentration differences were found between these two groups (P=0.0048 and P=0.0048, respectively).
Table 3. Time-course (days 1 to 5) change of C-reactive protein (CRP) concentration in survivors and nonsurvivors.
| Days | Median value (range), mg/dl |
P-value | |
|---|---|---|---|
| Survivors | Nonsurvivors | ||
| Day 1 | 6.3 (0.5−14.9) | 10 (1.0−17.6) | 0.3592 |
| Day 2 | 3.4 (0.9−9.7) | 6.7 (1.6−19.9) | 0.3081 |
| Day 3 | 2.55 (0.3−6.3) | 6.8 (1.2−18.8) | 0.0252 a) |
| Day 4 | 1.6 (0.3−4.7) | 6.6 (0.8−14) | 0.0438 a) |
| Day 5 | 1.0 (0.3−18.5) | 7.55 (0.6−14.3) | 0.3948 |
a) P<0.05.
Fig. 1.
Serial C-reactive protein (CRP) concentrations of survivors (S) and nonsurvivors (N) on days 1 to 5. *P<0.05.
Table 4. Differences in the number of survivors and nonsurvivors on days 3 and 4, categorized by C-reactive protein (CRP) concentration (cutoff 6.5 mg/dl).
| Days | Groups | No. |
No. |
P-value |
|---|---|---|---|---|
| CRP ≥ 6.5 mg/dl | CRP < 6.5 mg/dl | |||
| Day 3 | Survivors | 0 | 15 | 0.0048 a) |
| Nonsurvivors | 4 | 3 | ||
| Day 4 | Survivors | 0 | 15 | 0.0048 a) |
| Nonsurvivors | 4 | 3 |
a) P<0.05.
Statistical analyses of Spec cPL concentrations during hospitalization could not be performed, because only six dogs had Spec cPL recorded. As for FDC-v-LIP, the relationship between the outcome and FDC-v-LIP concentrations on the third and fifth days was analyzed. The mean FDC-v-LIP concentration of Group S (n=13) vs. Group N (n=6) on the third day was 726 U/l vs. 643.5 U/l (range, 143–1,000 U/l vs. 238–1,000 U/l). No significant difference was found between the two groups (P=0.8595). The mean FDC-v-LIP concentration for Group S (n=8) vs. Group N (n=4) on the fifth day was 895 U/l vs. 1,000 U/l (range, 203–1,000 U/l vs. 69–1,000 U/l), which was not significantly different between the two groups (P=0.3786).
DISCUSSION
This study has identified decreased platelet count and a remarkable elevation of Spec cPL concentration, as well as elevated BUN and/or CRE level, as the prognostic factors for canine pancreatitis at first diagnosis. Moreover, the median CRP concentrations measured on the third and fourth days of hospitalization were significantly different between the survivors and non-survivors, indicating that CRP concentration is a good biomarker to monitor hospitalized dogs with pancreatitis. .
The results of this study are consistent with those of a previous study, wherein the elevation of BUN and/or CRE was included in the “organ score” and correlated with the outcome [14]. The results of this study, along with the previous study, indicate that the elevation of BUN and/or CRE is useful for evaluating the prognosis of dogs with pancreatitis.
To our knowledge, this is the first study to report the possibility that a decreased platelet count is a prognostic factor for canine pancreatitis. Previous studies that examined the prognostic factors for pancreatitis did not find a significant difference between coagulation disorders and the prognosis of patients [10]. However, several studies have reported that in dogs with severe pancreatitis, disseminated intravascular coagulation (DIC) tends to occur [4, 10]. Pápa et al. reported that three of 80 dogs with pancreatitis developed DIC and had a poor prognosis [13]. In this study, 11 dogs had a platelet count below the reference range. Five of those dogs died within 15 days, and 1 of the 5 dogs developed DIC. Based on the results of these studies, a decreased platelet count could be a prognostic factor for canine pancreatitis, because severe cases of pancreatitis tend to result in DIC, which can contribute to a poor prognosis.
Pancreas-specific lipases have not been assessed as prognostic factors in previous studies. This study revealed that a remarkable elevation of Spec cPL concentration correlated with short-term prognosis in dogs with pancreatitis. It is possible that this is attributed to a large amount of lipase leaking from the pancreas in severe cases because of severe damage caused by increased inflammation. There is a possibility that the conventional lipase assessment cannot detect this change in amount along with the degree of inflammation [14], whereas Spec cPL assessment can. One interesting finding is that FDC-v-LIP—one of the specific lipases for pancreatitis that is reported to have a strong correlation with Spec cPL—did not have the same result as Spec cPL. This may be due to the differences in the way they are measured: Spec cPL is measured by immunoassays, and FDC-v-LIP is measured by a dry chemistry analyzer using the FUJI DRI-CHEM lipase slide, which uses triolein as the reaction substrate. The CRP concentration on the third and fourth days was significantly different between the two groups, although no significant difference was found between them regarding the CRP concentration measured during the first medical examination in Study 2. The finding that no differences existed between the two groups regarding the CRP concentration at the first medical examination is consistent with that of a previous report. This is because most dogs with pancreatitis have elevated CRP concentrations (51 of 65 dogs in this study), which makes it difficult to predict a prognosis using only one CRP concentration measurement. In contrast, the CRP concentration on the third and fourth days was significantly different between the surviving and non-surviving groups. This indicates that severe pancreatitis cases did not immediately respond to intensive treatment in the hospital; CRP concentrations did not decrease until the third or fourth day. The result suggests that CRP concentrations should be measured daily in hospitalized dogs to assess the prognosis of pancreatitis as well as other infections and immune-mediated diseases [2]. Some studies in humans have found that the CRP concentrations measured during the hospitalization of patients with severe pancreatitis were significantly higher than those in patients with the mild form. The current study found the same result in dogs. In addition, a CRP concentration of 6.5 mg/dl was detected to be a good cutoff to divide dogs into survivors and non-survivors (Table 4). However, further work is required to evaluate the usefulness of this value for predicting the prognosis of dogs with pancreatitis during hospitalization. The CRP concentration measured on the fifth day did not have a significant correlation with the outcome, because the number of cases decreased (2 dogs died within 4 days), the biggest one, and 1 dog had a high CRP concentration (18.5 mg/dl) on the fifth day and died on the 27th day.
In this retrospective study, we assume that no clear difference existed in treatment between the two groups. For instance, prednisolone, which is controversial for treating pancreatitis, was administered to 21 of 22 dogs. Nevertheless, significant differences in CRP concentration were found in surviving and non-surviving groups, indicating that the dogs which did not respond well to the treatments including prednisolone showed poor prognosis. In our study, however, matching of the treatment was not conducted, because it was difficult due to its retrospective study design.
Limitations that were considered to be important to this study were that the records were collected retrospectively and the number of included dogs was limited. Moreover, the current study adopted two inclusion criteria for pancreatitis: (1) compatible clinical signs (anorexia, lethargy, diarrhea, vomiting, abdominal pain or a combination of these) and (2) an increase in Spec cPL concentration (Spec cPL 400 µg/l or greater). These criteria did not include abnormal findings on ultrasonography to detect pancreatitis and were different from the criteria used in other studies. This leads to the difference seen in the proportion of clinical signs compared with other reports. However, assessing the ultrasonographic findings is difficult for a retrospective study, because evaluating the ultrasonography performed by only one clinician using the same criteria is not possible. In addition, the treatment protocols applied to each case were not controlled. This is also one of the major limitations.
In conclusion, this study has identified that the elevation of BUN and/or CRE, decreased platelet count and remarkable elevation of Spec cPL concentration are the prognostic factors for canine suspected pancreatitis. Moreover, the median CRP concentrations on the third and fourth days of hospitalization were significantly different between the survivors and non-survivors. Hence, this study suggested dogs with pancreatitis should be evaluated at least for three factors at the first medical examination to evaluate whether intensive treatment and hospitalization are needed. Further evaluation of prognosis via serial CRP concentration measurements is recommended, if intensive treatment is needed in hospital. Clinicians should choose appropriate treatments for each case and suggest to owners the possibility of discharge from the hospital.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology, Japan (No.26292159).
REFERENCES
- 1.Ceron J. J., Eckersall P. D., Martýnez-Subiela S.2005. Acute phase proteins in dogs and cats: current knowledge and future perspectives. Vet. Clin. Pathol. 34: 85–99. doi: 10.1111/j.1939-165X.2005.tb00019.x [DOI] [PubMed] [Google Scholar]
- 2.Cerón J. J., Martinez-Subiela S., Ohno K., Caldin M.2008. A seven-point plan for acute phase protein interpretation in companion animals. Vet. J. 177: 6–7. doi: 10.1016/j.tvjl.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 3.Eckersall P. D., Bell R.2010. Acute phase proteins: Biomarkers of infection and inflammation in veterinary medicine. Vet. J. 185: 23–27. doi: 10.1016/j.tvjl.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 4.Feldman B. F., Madewell B. R., O’Neill S.1981. Disseminated intravascular coagulation: antithrombin, plasminogen, and coagulation abnormalities in 41 dogs. J. Am. Vet. Med. Assoc. 179: 151–154. [PubMed] [Google Scholar]
- 5.Flatland B., Breickner L. C., Fry M. M.2014. Analytical performance of a dry chemistry analyzer designed for in-clinic use. Vet. Clin. Pathol. 43: 206–217. doi: 10.1111/vcp.12122 [DOI] [PubMed] [Google Scholar]
- 6.Galezowski A. M., Snead E. C., Kidney B. A., Jackson M. L.2010. C-reactive protein as a prognostic indicator in dogs with acute abdomen syndrome. J. Vet. Diagn. Invest. 22: 395–401. doi: 10.1177/104063871002200308 [DOI] [PubMed] [Google Scholar]
- 7.Hess R. S., Saunders H. M., Van Winkle T. J., Shofer F. S., Washabau R. J.1998. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in dogs with fatal acute pancreatitis: 70 cases (1986−1995). J. Am. Vet. Med. Assoc. 213: 665–670. [PubMed] [Google Scholar]
- 8.Ishioka K., Hayakawa N., Nakamura K., Terashima K.2011. Patient-side assay of lipase activity correlating with pancreatic lipase immunoreactivity in the dog. J. Vet. Med. Sci. 73: 1481–1483. doi: 10.1292/jvms.11-0166 [DOI] [PubMed] [Google Scholar]
- 9.Mansfield C.2012. Acute pancreatitis in dogs: advances in understanding, diagnostics, and treatment. Top. Companion Anim. Med. 27: 123–132. doi: 10.1053/j.tcam.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 10.Mansfield C. S., James F. E., Robertson I. D.2008. Development of a clinical severity index for dogs with acute pancreatitis. J. Am. Vet. Med. Assoc. 233: 936–944. doi: 10.2460/javma.233.6.936 [DOI] [PubMed] [Google Scholar]
- 11.Nakamura M., Takahashi M., Ohno K., Koshino A., Nakashima K., Setoguchi A., Fujino Y., Tsujimoto H.2008. C-reactive protein concentration in dogs with various diseases. J. Vet. Med. Sci. 70: 127–131. doi: 10.1292/jvms.70.127 [DOI] [PubMed] [Google Scholar]
- 12.Ohno K., Yokoyama Y., Nakashima K., Setoguchi A., Fujino Y., Tsujimoto H.2006. C-reactive protein concentration in canine idiopathic polyarthritis. J. Vet. Med. Sci. 68: 1275–1279. doi: 10.1292/jvms.68.1275 [DOI] [PubMed] [Google Scholar]
- 13.Pápa K., Máthé A., Abonyi-Tóth Z., Sterczer A., Psáder R., Hetyey C., Vajdovich P., Vörös K.2011. Occurrence, clinical features and outcome of canine pancreatitis (80 cases). Acta Vet. Hung. 59: 37–52. doi: 10.1556/AVet.59.2011.1.4 [DOI] [PubMed] [Google Scholar]
- 14.Ruaux C. G., Atwell R. B.1998. A severity score for spontaneous canine acute pancreatitis. Aust. Vet. J. 76: 804–808. doi: 10.1111/j.1751-0813.1998.tb12331.x [DOI] [PubMed] [Google Scholar]
- 15.Tvarijonaviciute A., García-Martínez J. D., Caldin M., Martínez-Subiela S., Tecles F., Pastor J., Ceron J. J.2015. Serum paraoxonase 1 (PON1) activity in acute pancreatitis of dogs. J. Small Anim. Pract. 56: 67–71. doi: 10.1111/jsap.12297 [DOI] [PubMed] [Google Scholar]
- 16.Uchikov P. A., Sirakova I. P., Murdjeva M. A., Uchikov A. P.2000. Changes in plasma levels of acute phase proteins in pancreatitis. Folia Med. (Plovdiv) 42: 23–30. [PubMed] [Google Scholar]
- 17.Viedma J. A., Pérez-Mateo M., Agulló J., Domínguez J. E., Carballo F.1994. Inflammatory response in the early prediction of severity in human acute pancreatitis. Gut 35: 822–827. doi: 10.1136/gut.35.6.822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson P.2015. Pancreatitis in dogs and cats: definitions and pathophysiology. J. Small Anim. Pract. 56: 3–12. doi: 10.1111/jsap.12293 [DOI] [PubMed] [Google Scholar]
- 19.Xenoulis P. G., Steiner J. M.2012. Canine and feline pancreatic lipase immunoreactivity. Vet. Clin. Pathol. 41: 312–324. doi: 10.1111/j.1939-165X.2012.00458.x [DOI] [PubMed] [Google Scholar]
- 20.Yokoe M., Takada T., Mayumi T., Yoshida M., Isaji S., Wada K., Itoi T., Sata N., Gabata T., Igarashi H., Kataoka K., Hirota M., Kadoya M., Kitamura N., Kimura Y., Kiriyama S., Shirai K., Hattori T., Takeda K., Takeyama Y., Hirota M., Sekimoto M., Shikata S., Arata S., Hirata K.2015. Japanese guidelines for the management of acute pancreatitis: Japanese Guidelines 2015. J. Hepatobiliary Pancreat. Sci. 22: 405–432. doi: 10.1002/jhbp.259 [DOI] [PubMed] [Google Scholar]
- 21.Yuki M., Hirano T., Nagata N., Kitano S., Imataka K., Tawada R., Shimada R., Ogawa M.2016. Clinical Utility of Diagnostic Laboratory Tests in Dogs with Acute Pancreatitis: A Retrospective Investigation in a Primary Care Hospital. J. Vet. Intern. Med. 30: 116–122. doi: 10.1111/jvim.13660 [DOI] [PMC free article] [PubMed] [Google Scholar]