Abstract
Reduction in glomerular filtration rate (GFR) is a common complication in advanced stages of heart failure (HF). The convenient and precise assessment for GFR would be useful for early detection of renal impairment in HF dogs. Our hypothesis of this study was the GFR would be reduced in advanced stages of HF from chronic mitral valvular insufficiency (CMVI), as indicated by renal markers including serum cystatin-C (Cys-C) and symmetric dimethylarginine (SDMA) concentrations. Forty-three client-owned dogs consisting of 33 dogs with different stages of HF from CMVI and 10 age-matched healthy dogs were enrolled in this study. Serum Cys-C and SDMA concentrations along with other renal (i.e., urea nitrogen and creatinine) and echocardiographic markers were evaluated in healthy and CMVI dogs. Serum Cys-C concentrations were 1.4 ± 0.4 mg/l in control, 2.1 ± 0.9 mg/l in ISACHC I, 2.9 ± 0.8 mg/l in ISACHC II and 3.6 ± 0.6 mg/l in ISACHC III dogs, whereas serum SDMA concentrations were 8 ± 2 µg/dl in control, 14 ± 3 µg/dl in ISACHC I, 18 ± 6 µg/dl in ISACHC II and 22 ± 7 µg/dl in ISACHC III dogs. There was close correlation of serum Cys-C and SDMA concentrations to serum creatinine, urea nitrogen and the severity of HF. Our study demonstrated that the GFR was decreased in dogs with CMVI having earlier stages of HF.
Keywords: biomarker, chronic mitral valvular disease, cystatin-C, heart failure, symmetric dimethylarginine
Cardiorenal syndrome (CRS) is a well-known complication from heart failure (HF) in humans and is defined as concomitant disorders of the heart and kidneys, whereby “acute or chronic dysfunction in one organ may induce acute or chronic dysfunction of the other” [24]. One human study demonstrated that renal failure is very common in patients suffering from congestive HF, observed in one-third of all admissions [27]. Low cardiac output, elevation of both intra-abdominal and central venous pressures, and neurohormonal and inflammatory activation are involved in pathophysiology of CRS. Renal dysfunction, secondary to poor cardiac function, has been documented in humans and dogs [5, 22, 23].
Chronic mitral valvular insufficiency (CMVI) is the most common cause of HF in small breed dogs and is characterized by progressive myxomatous degeneration of the atrioventricular valves [4]. Long-standing or acute HF causes poor tissue perfusion and may induce ischemic injury to vital organs, including the kidneys [6].
Cystatin-C or cystatin 3 (Cys-C) is a recently developed biomarker of kidney function and has recently been studied for its role in predicting new-onset or deteriorating kidney diseases [9, 18, 25]. Cys-C has a low molecular weight (approximately 13.3 kilodaltons), and it is removed from the bloodstream by glomerular filtration in the kidneys. Serum levels of Cys-C are a more precise test of kidney function (as represented by the glomerular filtration rate, GFR) than serum creatinine (CRE) levels [18, 25]. Several studies in dogs and cats found Cys-C was a good marker for detecting renal injuries [1, 2, 12, 17, 20], although serum Cys-C levels had been increased in dogs with various non-renal diseases (e.g. immune-mediated, endocrine, dermatologic, cardiologic and neoplastic) [2, 9, 17, 28]. Symmetric dimethylarginine (SDMA) is a recently developed renal biomarker which is closely correlated with GFR in dogs [21] and cats [7], because it is only excreted by the kidneys after degradation. Similar to CRE, SDMA is specific to renal function and is not increased by other systemic diseases including hepatic and endocrine diseases. Recent studies found the elevation of SDMA was noticed earlier than that of CRE in animals with chronic kidney disease [14].
The aim of this study was to evaluate the serum Cys-C and SDMA concentrations in dogs with CMVI at varying degrees of HF.
MATERIALS AND METHODS
Study population
Prior to this study, we obtained the approval of the animal ethics committee of Kangwon National University to obtain blood samples from healthy dogs for serum Cys-C and SDMA assays. Informed written consent for sample collection, including information pertinent to our investigation, were obtained from the dog owners, prior to the commencement of our study. Our study population consisted of two groups. The normal control group consisted of 10 healthy, age-matched small-breed dogs (2.7–7.9 kg), between 7 and 14 years of age with no evidence of cardiovascular or other systemic disease apparent on physical examination, complete blood count, chemistry panel, chest radiography and echocardiography. The CMVI group included 33 small breed dogs (2.4–8.7 kg) aged between 7–15 years. All dogs with CMVI had been presented for a cardiology consultation, because of previous identification of a heart murmur, because of the presence of clinical signs indicating cardiovascular disease or both. All dogs underwent physical examination, echocardiography, complete blood count and blood chemistry evaluation. We excluded dogs that had other clinically relevant systemic diseases, such as renal failure and hypertension, by routine renal examinations including urinalysis, renal ultrasound and blood pressure measurement. The diagnosis of CMVI was made on the basis of clinical signs, chest radiography and echocardiographic findings, according to published guidelines for the diagnosis of CMVI in dogs [4]. The dogs with CMVI were divided by the criteria proposed by the International Small Animal Cardiac Health Council (ISACHC) for the functional classification of HF. Some dogs with symptomatic CMVI were being medicated for heart disease, depending on its severity, with drugs, such as enalapril, furosemide, spironolactone, pimobendan, digoxin and amlodipine.
Analysis of serum Cys-C and SDMA concentrations
To minimize the influence of feeding, all dogs were fasted for 12 hr before the collection of blood samples. Whole blood was withdrawn from either the cephalic or jugular veins for determination of serum levels of Cys-C and SDMA. Blood samples were drawn directly into sterile vacutainer® tubes (BD, Franklin Lakes, NJ, U.S.A.) and then centrifuged at 1,500 × g for 10 min at 4°C. The supernatants were stored at −80°C or dry ice for shipping or testing. Serum Cys-C levels were measured using a commercial ELISA based kit (Dog Cystatin C ELISA kit, MyBioSourse, San Diego, CA, U.S.A.), according to the manufacturer’s recommendation. The Cys-C ELISA kit applies the competitive enzyme immunoassay technique utilizing a monoclonal anti-Cys-C antibody and a Cys-C-HRP conjugate. Prior to a study, the test kit and method were completely validated for use with dog serum. We also tested serial dilutions of the samples in duplicate. N-terminal pro brain natriuretic peptide (NT-proBNP) and SDMA concentrations were determined by reference laboratory (IDEXX Laboratories, Westbrook, ME, U.S.A.). Concentrations of serum urea nitrogen (UN) and CRE were determined with an automated biochemistry analyzer (VetScan VS2, Abaxis, Union city, CA, U.S.A.).
Echocardiography
Vertebral heart score (VHS) was determined using a lateral thoracic radiograph of each dog, as described in elsewhere [10], prior to echocardiography. Echocardiographic examinations were conducted in accordance with recommended standards for dogs. M-mode, Doppler and 2-dimensional echocardiography were performed in left and right lateral recumbency using a ultrasound machine with a 3–9 MHz phase transducer (Acuson X-300, Siemens, Mountain view, CA, U.S.A.). M-mode echocardiography was used to measure left ventricular dimension in systole (LVIDs) and diastole (LVIDd). 2-D echocardiography was used to measure left atrium (LA) and proximal aortic (Ao) diameter from the right parasternal short axis at the aortic valve level. These measurements were used to determine the LA to proximal Ao diameter and LVIDd to Ao diameter ratios (LA/Ao and LVIDd/Ao, respectively).
Statistical analysis
Statistical analyses were performed using commercially available statistical software (SPSS 15.0 for Windows, IBM, New York, NY, U.S.A.). Descriptive statistics were calculated for quantitative variables by study group and analyzed for normality using the Kolmogorov-Smirnov test. One-way ANOVA or Kruskal-Wallis testing was used to compare mean concentrations of renal markers among study group. When differences among the groups were detected, Tukey’s multiple comparison test was used as a posthoc analysis to identify groups that differed significantly (P<0.05). Pearson’s coefficient of bivariate correlation analysis was used to test the strength of the association between renal markers (e.g. UN, CRE, Cys-C and SDMA) and echocardiographic markers used for evaluation of disease severity. In all comparisons, a probability value of P<0.05 was considered statistically significant, unless stated otherwise.
RESULTS
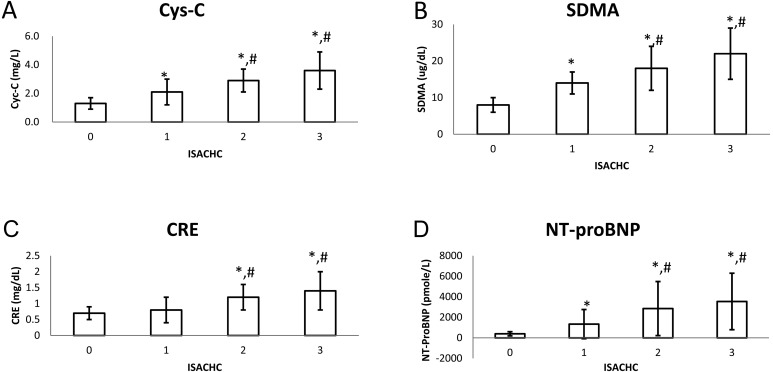
The mean serum Cys-C concentrations were 1.4 ± 0.4 mg/l in control, 2.1 ± 0.9 mg/l in ISACHC I, 2.9 ± 0.8 mg/l in ISACHC II and 3.6 ± 0.6 mg/l in ISACHC III (Fig. 1A), whereas the mean serum SDMA concentrations were 8 ± 2 µg/dl in control, 14 ± 3 µg/dl in ISACHC I, 18 ± 6 µg/dl in ISACHC II and 22 ± 7 µg/dl in ISACHC III (Table 1, Fig. 1B). The mean serum UN concentrations were 18 ± 7 mg/dl in control, 19 ± 8 mg/dl in ISACHC I, 34 ± 19 mg/dl in ISACHC II and 46 ± 35 mg/dl in ISACHC III, whereas the mean serum CRE concentrations were 0.7 ± 0.2 mg/dl in control, 0.8 ± 0.4 mg/dl in ISACHC I, 1.2 ± 0.4 mg/dl in ISACHC II and 1.4 ± 0.6 mg/dl in ISACHC III (Table 1, Fig. 1C). The mean serum NT-proBNP concentrations were 396 ± 200 pmole/l in control, 1,337 ± 1,422 pmole/l in ISACHC I, 2,853 ± 2,634 pmole/l in ISACHC II and 3,546 ± 2,754 pmole/l in ISACHC III (Table 1, Fig. 1D). A statistically significant difference in serum Cys-C, SDMA and NT-proBNP concentrations was found between the control and the HF groups (ISACHC I-III) (P<0.05) and between asymptomatic (ISACHC I) and symptomatic (ISACHC II and III) groups (P<0.05).
Fig. 1.
Concentrations of cystatin C (Cys-C), symmetric dimethylarginine (SDMA), creatinine (CRE) and N-terminal pro brain natriuretic peptide (NT-proBNP) in this study group. 0: healthy control (n=10), 1: ISACHC I (n=8), 2: ISACHC II (n=10), 3: ISACHC III (n=15). ISACHC (international small animal cardiac health council). * P<0.05 Control vs ISACHC I, II and III. #P<0.05 ISACHC I vs. ISACHC II and III.
Table 1. Mean ± standard deviation values of test parameters in this study population.
| ISACHC | Age |
BW |
UN |
CRE |
VHS | LA: Ao | LVID:Ao | Cys-C |
SDMA |
NT-ProBNP |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| yrs | kg | mg/dl | mg/dl | mg/l | μg/dl | pmole/l | |||||
| 0 | Mean | 8.0 | 5.0 | 18 | 0.7 | 8.8 | 1.1 | 1.4 | 1.3 | 8 | 396 |
| SD | 2.4 | 2.7 | 7 | 0.2 | 0.4 | 0.1 | 0.1 | 0.4 | 2 | 200 | |
| 1 | Mean | 10.7 | 3.9 | 19 | 0.8 | 9.0 | 1.5a) | 1.8a) | 2.1a) | 14a) | 1,337a) |
| SD | 3.1 | 2.3 | 8 | 0.4 | 0.0 | 0.3 | 0.3 | 0.9 | 3 | 1,422 | |
| 2 | Mean | 12.1 | 5.0 | 34a,b) | 1.2a,b) | 10.5a,b) | 1.9a,b) | 2.1a,b) | 2.9a,b) | 18a,b) | 2,853a,b) |
| SD | 2.8 | 2.2 | 19 | 0.4 | 0.7 | 0.3 | 0.3 | 0.8 | 6 | 2,634 | |
| 3 | Mean | 11.3 | 4.9 | 46a,b) | 1.4a,b) | 11.9a,b) | 2.6a,b) | 2.8a,b) | 3.6a,b) | 22a,b) | 3,546a,b) |
| SD | 1.9 | 1.4 | 35 | 0.6 | 1.1 | 0.7 | 0.6 | 1.3 | 7 | 2,754 | |
ISACHC (international small animal cardiac health council), BW (body weight), UN (urea nitrogen), CRE (creatinine), VHS (vertebral heart scale), left atrium to aorta ratio (LA/Ao), left ventricular diastolic dimension to aorta ratio (LVID/Ao), cystatin-C (Cys-C), Symmetric dimethylarginine (SDMA), N-terminal pro brain natriuretic peptide (NT-proBNP). a) Statistically significance between control and heart failure groups, P<0.01. b) Statistically significance between asymptomatic and symptomatic heart failure groups, P<0.01.
Upper limit of UN, CRE, Cys-C and SDMA set at 25 mg/dl, 1.4 mg/dl, 1.4 mg/l and 14 µg/dl, respectively, based on veterinary references [1, 8, 19]. Numbers of dogs having higher value than upper limit of UN were 1/10 in control, 3/8 in ISACHC I, 7/10 in ISACHC II and 9/15 in ISACHC III, whereas those of CRE were 0/10 in control, 1/8 in ISACHC I, 5/10 in ISACHC II and 6/15 in ISACHC III. In addition, numbers of dogs having higher value than upper limit of Cys-C were 2/10 in control, 5/8 in ISACHC I, 10/10 in ISACHC II and 15/15 in ISACHC III, whereas those of SDMA were 0/10 in control, 6/8 in ISACHC I, 9/10 in ISACHC II and 14/15 in ISACHC III.
Using univariate analysis, close correlations among renal markers (i.e., Cys-C, SDMA, CRE and UN) were found (Table 2). Serum Cys-C and SDMA concentrations were not correlated to age or body weight, but closely correlated to the severity of HF (i.e., ISACHC) and echocardiographic markers (i.e., LA/Ao and LVID/Ao). Serum SDMA concentration was weakly correlated to serum NT-proBNP concentration and VHS, while serum Cys-C concentration was not correlated to these two variables (Table 2).
Table 2. Correlation of heart failure stage, SDMA and cystatin-C concentration to other variables.
| ISACHC |
SDMA |
Cys-C |
||||
|---|---|---|---|---|---|---|
| R | P | R | P | R | P | |
| Age | 0.292 | 0.07 | 0.301 | 0.054 | 0.299 | 0.07 |
| BW | 0.05 | 0.751 | 0.117 | 0.455 | 0.297 | 0.059 |
| ISACHC | - | - | 0.732 | 0 | 0.696 | 0 |
| UN | 0.461 | 0.002 | 0.766 | 0 | 0.616 | 0 |
| CRE | 0.555 | 0 | 0.648 | 0 | 0.641 | 0 |
| VHS | 0.716 | 0 | 0.494 | 0.001 | 0.401 | 0.009 |
| LA/Ao | 0.793 | 0 | 0.666 | 0 | 0.481 | 0.001 |
| LVID/Ao | 0.801 | 0 | 0.49 | 0.001 | 0.369 | 0.017 |
| Cys-C | 0.696 | 0 | 0.766 | 0 | - | - |
| SDMA | 0.732 | 0 | - | - | 0.766 | 0 |
| NTproBNP | 0.514 | 0 | 0.424 | 0.005 | 0.296 | 0.06 |
ISACHC (international small animal cardiac health council), BW (body weight), UN (urea nitrogen), CRE (creatinine), VHS (vertebral heart scale), left atrium to aorta ratio (LA/Ao), left ventricular diastolic dimension to aorta ratio (LVID/Ao), cystatin-C (Cys-C), symmetric dimethylarginine (SDMA), N-terminal pro brain natriuretic peptide (NT-proBNP).
DISCUSSION
Azotemia and renal impairment increase with the severity of HF and are frequent findings in dogs with CMVI [22]. Therefore, the early detection of renal impairment associated with HF potentially plays a key role in long-term management of HF in dogs. The current tests available for detecting renal impairment in dogs, involve measuring GFR, urine concentrating ability and detecting proteinuria and abnormal urinary sediment [8]. Because significant correlation between GFR and severity of renal failure was found, indirect measurement of GFR using CRE and UN concentration is more widely used for early detection of renal diseases in dogs [8]. Several studies found that the Cys-C is an ideal maker for reduction in GFR, because of constant production and plasma concentration in the absence of GFR impairment, low intraindividual variability, and absence of plasma protein binding, tubular secretion, tubular reabsorption without catabolism and extrarenal clearance [26]. Furthermore, one human study found the Cys-C is a better marker for detecting renal dysfunction than serum CRE [11]. Recent veterinary studies also found the SDMA is strongly correlated with GFR in dogs [21] and cats [7] and is elevated from earlier stage of chronic kidney diseases, compared to serum CRE [14]. Unlike CRE, the SDMA was not affected by lean body mass and is more precisely estimated in aged and diseased dogs and cats [16].
This study clearly found progressive elevation in renal markers in dogs with advancing heart disease, suggesting that the reduction of GFR might be worsened with the progression of cardiac dysfunction. According to a recent canine study, the SDMA started to rise from ~40% loss of nephron in dogs [14], while the creatinine did from ~70% loss of nephron [8]. In this study, the SDMA was higher than reference range in 9/10 and 14/15 dogs with ISACHC II and III, respectively, while the CRE was higher in 5/10 and 6/15 dogs, respectively. This finding suggested the SDMA could detect renal impairment earlier than the CRE in dogs. Although the Cys-C is a promising renal marker for canine kidney disease, test standardization and reference range have yet been clearly established in dogs. One study found the mean Cys-C concentration in the healthy dogs and the dogs with renal failure was 1.08 ± 0.16 mg/l (range 0.76–1.44 mg/l) and 4.37 ± 1.79 mg/l (range 1.12–9.13 mg/l), respectively [1]. Based on this reference, we set upper normal limit at 1.44 mg/l in the Cys-C test of this study. With this reference range, all dogs with ISACHC II and III had higher levels of Cys-C than upper normal limit. With assumption of SDMA >14 µg/dl indicating renal impairment, the statistical analysis found >2.34 mg/l of Cys-C indicated the evidence of renal impairment. With this new reference range, the Cys-C was higher in 7/10 and 13/15 dogs with ISACHC II and III, respectively. This finding suggested the Cys-C could also detect renal impairment earlier than the CRE.
The increased levels of Cys-C and SDMA in dogs with advanced stage HF (i.e., ISACHC II and III) in this study group suggested the risk of renal impairment to be higher in dogs with more advanced heart disease, failure and its treatment. Reduction in GFR and the presence of azotemia are well known complications to advanced HF and its therapy, in the dog [22]. In addition, serum Cys-C and SDMA found to be well correlated with GFR in dogs [1, 12, 14, 16, 17, 19]. One study found the GFR was lower in NYHA class III-IV dogs than in class I to II dogs [22]. Although serum CRE and UN tests are widely used to detect a reduction in GFR in dogs [8], those tests in dogs are often influenced by breed, age, diet and exercise [8]. Unlike serum CRE and UN, the SDMA does not appear to be influenced by age, body weight or gender [15], although recent studies found that the Cys-C concentration was influenced by age and body weight [3, 9]. However, our study strongly suggested that the Cys-C was not influenced by age or body weight.
Interestingly, 6/8 dogs with asymptomatic HF (i.e., ISACHC I) in this study had higher SDMA levels than reference range, while 3/8 and 1/8 dogs had higher UN and CRE levels, respectively. This result suggested many dogs with asymptomatic HF could suffer the reduction of GFR from reduction of renal perfusion, although they are clinically asymptomatic. This is particularly important to clinicians, as we may be overlooking the risk of renal injuries in dogs with asymptomatic HF. Therefore, early intervention for preventing renal injuries is necessary for successful management of heart failure in dogs.
The serum Cys-C and SDMA concentrations in this study varied widely in dogs with CMVI. This might be due to the fact that the reduction of GFR might vary based on the variable degree of neurohormonal response to a fall in cardiac output, the type of cardiac medication being utilized, and/or the degree of pre-existing and on-going renal injuries. In some dogs in which, kidney function has stabilized after acute injury, the GFR can be increased by compensatory mechanisms, and serum Cys-C and SDMA concentrations may return towards normal. Therefore, the type of renal injury (e.g., ischemia) and the compensatory response influence the serum Cys-C and SDMA concentrations.
There are several limitations in this study. Firstly, although serum CRE concentration was found to be less influenced by long-term enalapril administration in dogs with CMVI, the therapy with enalapril and furosemide tended to increase the UN [13]. Because our dogs with advanced HF had many cardiac medications, specifically or secondarily reducing the GFR, the influence of drugs on serum Cys-C and SDMA levels still warrants investigation. Secondly, furosemide can reduce the GFR and thus can influence the levels of renal markers in dogs having higher dose of furosemide. Because the levels of SDMA and Cys-C were still higher in asymptomatic HF dogs having no furosemide than control dogs, our study findings is less likely influenced by furosemide administration, although a further study is required to clarify the influence of furosemide in serum Cys-C and SDMA levels. Thirdly, although recent studies have found that serum Cys-C was well correlated with the reduction of GFR in dogs [1], the methodology and the accuracy for serum Cys-C in dogs have yet been clearly established. Lastly, the study population was small and may not have provided sufficient statistical power to adequately reflect the correlation of serum Cys-C and SDMA to the severity of HF in CMVI dogs.
In conclusion, our study demonstrated that the GFR was decreased in dogs with CMVI having earlier stages of HF, based on results from serum Cys-C and SDMA tests, and thus, earlier intervention for preventing renal injuries even in asymptomatic HF dogs is necessary for successful long-term management of HF in dogs.
REFERENCES
- 1.Almy F. S., Christopher M. M., King D. P., Brown S. A.2002. Evaluation of cystatin C as an endogenous marker of glomerular filtration rate in dogs. J. Vet. Intern. Med. 16: 45–51. doi: 10.1111/j.1939-1676.2002.tb01605.x [DOI] [PubMed] [Google Scholar]
- 2.Antognoni M. T., Siepi D., Porciello F., Fruganti G.2005. Use of serum cistatin C determination as a marker of renal function in the dog. Vet. Res. Commun. 29Suppl 2: 265–267. doi: 10.1007/s11259-005-0058-5 [DOI] [PubMed] [Google Scholar]
- 3.Antognoni M. T., Siepi D., Porciello F., Rueca F., Fruganti G.2007. Serum cystatin-C evaluation in dogs affected by different diseases associated or not with renal insufficiency. Vet. Res. Commun. 31Suppl 1: 269–271. doi: 10.1007/s11259-007-0044-1 [DOI] [PubMed] [Google Scholar]
- 4.Atkins C., Bonagura J., Ettinger S., Fox P., Gordon S., Haggstrom J., Hamlin R., Keene B., Luis-Fuentes V., Stepien R.2009. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J. Vet. Intern. Med. 23: 1142–1150. doi: 10.1111/j.1939-1676.2009.0392.x [DOI] [PubMed] [Google Scholar]
- 5.Bongartz L. G., Cramer M. J., Doevendans P. A., Joles J. A., Braam B.2005. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur. Heart J. 26: 11–17. doi: 10.1093/eurheartj/ehi020 [DOI] [PubMed] [Google Scholar]
- 6.Borgarelli M., Häggström J.2010. Canine degenerative myxomatous mitral valve disease: natural history, clinical presentation and therapy. Vet. Clin. North Am. Small Anim. Pract. 40: 651–663. doi: 10.1016/j.cvsm.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 7.Braff J., Obare E., Yerramilli M., Elliott J., Yerramilli M.2014. Relationship between serum symmetric dimethylarginine concentration and glomerular filtration rate in cats. J. Vet. Intern. Med. 28: 1699–1701. doi: 10.1111/jvim.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun J. P., Lefebvre H. P.2008. Kidney function and damage. pp. 485–528. In: Clinical Biochemistry of Domestic Animals, 6th ed. (Kaneko, J. J., Harvey, J. W. and Bruss, M. L. eds.), Elsevier, London. [Google Scholar]
- 9.Braun J. P., Perxachs A., Pechereau D., De La Farge F.2002. Plasma cystatin C in the dog: Reference values and variations with renal failure. Comp. Clin. Pathol. 11: 44–49. doi: 10.1007/s580-002-8081-2 [DOI] [Google Scholar]
- 10.Buchanan J. W., Bücheler J.1995. Vertebral scale system to measure canine heart size in radiographs. J. Am. Vet. Med. Assoc. 206: 194–199. [PubMed] [Google Scholar]
- 11.Dharnidharka V. R., Kwon C., Stevens G.2002. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am. J. Kidney Dis. 40: 221–226. doi: 10.1053/ajkd.2002.34487 [DOI] [PubMed] [Google Scholar]
- 12.Gonul R., Kayar A., Or M. E., Kahraman I.2004. Assessment of renal function in dogs with renal disease using serum cystatin C. Indian Vet. J. 81: 872–874. [Google Scholar]
- 13.Häggström J., Hansson K., Karlberg B. E., Kvart C., Madej A., Olsson K.1996. Effects of long-term treatment with enalapril or hydralazine on the renin-angiotensin-aldosterone system and fluid balance in dogs with naturally acquired mitral valve regurgitation. Am. J. Vet. Res. 57: 1645–1652. [PubMed] [Google Scholar]
- 14.Hall J. A., Yerramilli M., Obare E., Yerramilli M., Jewell D. E.2014. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J. Vet. Intern. Med. 28: 1676–1683. doi: 10.1111/jvim.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall J. A., Yerramilli M., Obare E., Yerramilli M., Melendez L. D., Jewell D. E.2015. Relationship between lean body mass and serum renal biomarkers in healthy dogs. J. Vet. Intern. Med. 29: 808–814. doi: 10.1111/jvim.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall J. A., Yerramilli M., Obare E., Yerramilli M., Yu S., Jewell D. E.2014. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in healthy geriatric cats fed reduced protein foods enriched with fish oil, L-carnitine, and medium-chain triglycerides. Vet. J. 202: 588–596. doi: 10.1016/j.tvjl.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 17.Jensen A. L., Bomholt M., Moe L.2001. Preliminary evaluation of a particle-enhanced turbidimetric immunoassay (PETIA) for the determination of serum cystatin C-like immunoreactivity in dogs. Vet. Clin. Pathol. 30: 86–90. doi: 10.1111/j.1939-165X.2001.tb00263.x [DOI] [PubMed] [Google Scholar]
- 18.Kos J., Stabuc B., Cimerman N., Brünner N.1998. Serum cystatin C, a new marker of glomerular filtration rate, is increased during malignant progression. Clin. Chem. 44: 2556–2557. [PubMed] [Google Scholar]
- 19.Miyagawa Y., Takemura N., Hirose H.2009. Evaluation of the measurement of serum cystatin C by an enzyme-linked immunosorbent assay for humans as a marker of the glomerular filtration rate in dogs. J. Vet. Med. Sci. 71: 1169–1176. doi: 10.1292/jvms.71.1169 [DOI] [PubMed] [Google Scholar]
- 20.Monti P., Benchekroun G., Berlato D., Archer J.2012. Initial evaluation of canine urinary cystatin C as a marker of renal tubular function. J. Small Anim. Pract. 53: 254–259. doi: 10.1111/j.1748-5827.2012.01198.x [DOI] [PubMed] [Google Scholar]
- 21.Nabity M. B., Lees G. E., Boggess M. M., Yerramilli M., Obare E., Yerramilli M., Rakitin A., Aguiar J., Relford R.2015. SDMA assay validation, stability, and evaluation as a marker for early detection of chronic kidney disease in dogs. J. Vet. Intern. Med. 29: 1036–1044. doi: 10.1111/jvim.12835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolle A. P., Chetboul V., Allerheiligen T., Pouchelon J. L., Gouni V., Tessier-Vetzel D., Sampedrano C. C., Lefebvre H. P.2007. Azotemia and glomerular filtration rate in dogs with chronic valvular disease. J. Vet. Intern. Med. 21: 943–949. doi: 10.1111/j.1939-1676.2007.tb03047.x [DOI] [PubMed] [Google Scholar]
- 23.Ronco C., Haapio M., House A. A., Anavekar N., Bellomo R.2008. Cardiorenal syndrome. J. Am. Coll. Cardiol. 52: 1527–1539. doi: 10.1016/j.jacc.2008.07.051 [DOI] [PubMed] [Google Scholar]
- 24.Ronco C., McCullough P., Anker S. D., Anand I., Aspromonte N., Bagshaw S. M., Bellomo R., Berl T., Bobek I., Cruz D. N., Daliento L., Davenport A., Haapio M., Hillege H., House A. A., Katz N., Maisel A., Mankad S., Zanco P., Mebazaa A., Palazzuoli A., Ronco F., Shaw A., Sheinfeld G., Soni S., Vescovo G., Zamperetti N., Ponikowski P. and Acute Dialysis Quality Initiative (ADQI) consensus group. 2010. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur. Heart J. 31: 703–711. doi: 10.1093/eurheartj/ehp507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roos J. F., Doust J., Tett S. E., Kirkpatrick C. M.2007. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children--a meta-analysis. Clin. Biochem. 40: 383–391. doi: 10.1016/j.clinbiochem.2006.10.026 [DOI] [PubMed] [Google Scholar]
- 26.Séronie-Vivien S., Delanaye P., Piéroni L., Mariat C., Froissart M., Cristol J. P. and SFBC “Biology of renal function and renal failure” working group. 2008. Cystatin C: current position and future prospects. Clin. Chem. Lab. Med. 46: 1664–1686. doi: 10.1515/CCLM.2008.336 [DOI] [PubMed] [Google Scholar]
- 27.Tang W. H., Mullens W.2010. Cardiorenal syndrome in decompensated heart failure. Heart 96: 255–260. doi: 10.1136/hrt.2009.166256 [DOI] [PubMed] [Google Scholar]
- 28.Wehner A., Hartmann K., Hirschberger J.2008. Utility of serum cystatin C as a clinical measure of renal function in dogs. J. Am. Anim. Hosp. Assoc. 44: 131–138. doi: 10.5326/0440131 [DOI] [PubMed] [Google Scholar]