Abstract
Gallid herpesvirus 2 (GaHV-2) causes malignant lymphomas in chickens (Marek’s disease, MD). Although MD is controlled through vaccination efforts, field isolates of GaHV-2 have increased in virulence worldwide and even cause MD in vaccinated chickens. GaHV-2 strains are classified into four categories (mild, virulent, very virulent and very virulent +) based on the virulence exhibited in experimental infection in unvaccinated or MD-vaccinated susceptible chickens. Although MD cases are sporadically reported in Japan, the recent field strains of GaHV-2 in Japan have not been characterized. During isolation of recent field strains by using primary chicken kidney cell cultures, a method classically used for GaHV-2 isolation, vaccine strains were simultaneously isolated. Therefore, it is necessary to separate vaccine strains to characterize the virulence and pathogenicity of the GaHV-2 strains currently distributed in Japan. In this study, we prepared cell suspensions from the spleens of MD-symptomatic chickens, inoculated day-old-chicks and isolated GaHV-2 strains by primary chicken kidney cell cultures at 2−3 weeks post inoculation. The isolated strains were passaged several times on chicken embryo fibroblast cells, and PCR analysis revealed that the isolated strains were not contaminated with vaccine strains. Moreover, the contaminant vaccine strains were completely removed by the purification of plaques observed in chicken kidney cells. These procedures are necessary to isolate GaHV-2 field strains from vaccine strains in order to carry out future studies to characterize these strains and glean insights into GaHV-2 virulence and pathogenicity.
Keywords: chicken, GaHV-2, GaHV-3, Marek’s disease, MeHV-1
Marek’s disease (MD) is a lymphoproliferative disease of chickens and is caused by the cell-associated α-herpesvirus Gallid herpesvirus 2 (GaHV-2) (family: Herpesviridae; subfamily: Alphaherpesvirinae; genus: Mardivirus) [2]. Although MD has previously caused serious economic losses in the poultry industry, it has been well controlled by vaccination [16]. Meleagrid herpesvirus 1 (MeHV-1), which commonly infects turkeys, was developed and used as a MD vaccine candidate [12]. However, the number of field outbreaks increased gradually, and therefore, Gallid herpesvirus 3 (GaHV-3) and attenuated GaHV-2 strains were developed as vaccines against MD [2]. Among them, currently, CVI988 (also known as Rispens), which is an attenuated GaHV-2 strain, is used as the golden standard of MD vaccines worldwide [23]. In recent years, the virulence of GaHV-2 has increased, and MD cases in vaccinated chickens have been sporadically reported [1, 13, 21, 22]. Generally, the virulence of pathogenic GaHV-2 strains is classified as mild (m), virulent (v), very virulent (vv) and very virulent + (vv+) and is determined by the strain’s ability to cause disease in MD-susceptible chickens [22]. The virulence of GaHV-2 has evolved, and field strains of highly virulent GaHV-2 may potentially induce future outbreaks [22].
The viral protein Meq is present only in GaHV-2 strains and is the most important viral protein related to GaHV-2-induced tumorigenicity [5]. Meq is a 339 amino acid protein with an N-terminal basic region leucine zipper (bZIP) domain and a C-terminal transactivation domain [8]. The bZIP domain consists of two stretches of basic residues (basic regions 1 and 2, BR1 and BR2) and a leucine zipper [8]. The transactivation domain is characterized by 2.5 proline-rich repeats (PRRs), which contain several SH3-binding motifs [8]. Meq is considered as a key virulence factor in GaHV-2, since distinct diversity and point mutations were found in the Meq protein, especially in the BR2 and PRR regions, of virulent GaHV-2 strains [9, 14]. We recently showed unique substitutions in the PRRs of the Meq proteins in Japanese GaHV-2 field isolates [11]. In particular, a proline-to-leucine/serine substitution at position 176 in the PRRs in the Meq protein was found in strains recently isolated in Japan, as compared to the GaHV-2 strains found in the US. However, a proline-to-serine substitution at position 193 had also been identified earlier in previous Japanese isolates [11]. Thus, the properties of recent field isolates may be changing. However, no difference was observed between recent and old Japanese isolates with respect to the transactivation activities of the Meq proteins [11]. Therefore, more intense analysis of the characteristics of recent field strains, such as the identification of other viral factors, would be desirable. However, such analysis has yet to be performed with recent field strains of GaHV-2 in Japan, because vaccine strains are widely distributed in the field and often contaminate the isolated viruses. To investigate the pathogenicity and virulence of these strains, methods are needed to effectively separate these strains from vaccine strains.
In this study, we developed such isolation methods using recently collected field isolates of GaHV-2 in Japan, and we were able to completely eliminate vaccine strains. Our study will facilitate future endeavors to investigate the underlying mechanisms of GaHV-2 virulence and pathogenicity without vaccine strain interference. Moreover, the elimination of vaccine strains from field isolates is essential in order to correctly evaluate the protection efficacy of currently available vaccines as well to develop a vaccination program to effectively control MD in the field.
MATERIALS AND METHODS
Sample collection from MD-symptomatic chickens in the field
The spleens and/or kidneys of chickens with MD symptoms living on Japanese poultry farms were collected from 2014 until 2015. In each poultry farm, these samples from 2−7 chickens infected with GaHV-2 strains, named as Kgs-c1, Myz-c1 and Me-c3, were used for the detection and sequencing analysis of the meq gene. Kgs-c1 was used for virus isolation, and day-old-chicks were vaccinated with CVI988, an attenuated GaHV-2 vaccine strain, in the farm from which Kgs-c1 was isolated. Chickens infected with Kgs-c1 presented gross neurological symptoms.
DNA sequencing
Total cellular DNA was extracted from internal organs using SepaGene (Sankojunyaku, Tokyo, Japan) according to the manufacturer’s instructions. Total cellular DNA was used as template for nested PCR using meq-specific primer sets with TAKARA-Taq (TaKaRa, Otsu, Japan). The meq-specific primers used in this study are shown in Table 1. The first round of PCR was performed with the primer set M76S and M6S to amplify a 1,132-bp meq gene fragment. After the amplification, 1 μl of the reaction was used for the second round of PCR. The second round of PCR was performed in a 20 μl reaction mixture containing 1.5 mM MgCl2 and the M10S and M10AS primer set (0.5 μl) to amplify a 1,062-bp meq fragment. Amplification was performed using 35 cycles of 94°C for 45 sec, 57°C for 45 sec and 72°C for 1.5 min. The PCR products were gel-purified and cloned into the pGEM-T easy vector (Promega, Madison, WI, U.S.A.). Plasmids containing inserts were purified by a standard mini-prep method and sequenced using the CEQ 8000 Dye Terminator Cycle Sequencing method and the Quick Start kit (Beckman Coulter, Fullerton, CA, U.S.A.) according to the manufacturer’s instructions. The meq DNA sequences were analyzed using the CEQ 8000 DNA analysis system (Beckman Coulter).
Table 1. Primers used to analyze the nucleotide sequences of the meq gene.
| Primer | Type | Objective | Sequence | Size (bp) |
|---|---|---|---|---|
| M76S | FW | 1st round PCR | 5´-TTCCTAGGCAGGCGTCTCTTG-3´ | 1,132 |
| M6S | RV | 5´-ATGGGGCATAGACGATGTGCT-3´ | ||
| M10S | FW | Nested PCR | 5´-TGTCTCAGGAGCCAGAGCCGGGCGCT-3´ | 1,062 |
| M10AS | RV | 5´-GGGGCATAGACGATGTGCTGCTGAG-3´ | ||
Isolation of GaHV-2 field strains and confirmation of the contamination of vaccine strains
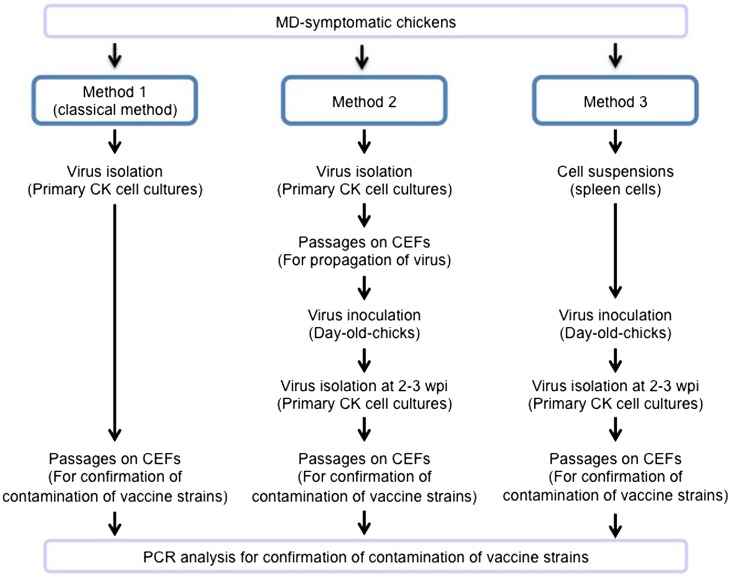
To isolate GaHV-2 field strains of alone, three methods were conducted as outlined in Fig. 1 and described below. A Kgs-c1 isolate, whose meq gene exhibited the most typical sequence found among field strains distributed in Japan, was used for virus isolation.
Fig. 1.
Methods to isolate GaHV-2 field strains. The field strains were isolated by three methods. Method 1 is the classical method using primary CK cell cultures. The second method involved using day-old-chicks. These chicks were inoculated with viruses isolated through primary CK cell cultures. Then, virus isolation was conducted by a primary CK cell culture method at 2−3 wpi. Lastly, in the third method, day-old-chicks were inoculated with cell suspension from the spleens of chickens with MD. The virus isolation was also conducted by a primary CK cell culture at 2−3 wpi. All of the isolated viruses were passaged on CEFs several times, and the contamination of vaccine strains was examined by PCR analysis.
Method 1: Primary chicken kidney (CK) cell cultures were created from kidneys collected from MD-symptomatic chickens. Kidneys were trypsinized and then filtered three times. The trypsinised CK cells were washed with Eagle’s MEM media (Nissui, Tokyo, Japan) and seeded in 6-well plates at 4.0 × 106 cells per well in 2 ml of Eagle’s MEM containing 10% calf serum and incubated at 37°C with 5% CO2. Afterwards, cytopathic effects (CPEs) were observed, and the isolated GaHV-2 strains were then passaged on chicken embryo fibroblasts (CEFs) several times. Then, these strains were inoculated in day-old-chicks (PNP/DO line) to detect contamination with vaccine strains.
Method 2: One-day-old chicks (3 chickens) were housed in an isolater and inoculated intraperitoneally with the isolated viruses from CK cells that were passaged several times on CEFs. Specifically, hatching eggs from PNP/DO chickens were provided by Nagoya University through the National Bio-Resource Project of Japan’s Ministry of Education, Culture, Sports, and Science and Technology. These eggs were hatched in our laboratory. The kidneys were collected from experimentally infected chickens in our laboratory at 2−3 weeks post-inoculation (wpi), and the virus was isolated by primary CK cell cultures and passaged on CEFs as described above.
Method 3: Spleens were collected from poultry farm chickens that developed MD symptoms. The collected spleens were dissociated and passed through a 100 μm mesh cell strainer (BD, Franklin Lakes, NJ, U.S.A.). Day-old-chicks (2 chickens) were housed in an isolater and inoculated intraperitoneally with the obtained cell suspensions. Viruses were isolated by primary CK cell cultures at 2−3 weeks wpi and passaged on CEFs as described above. In addition, the plaques observed on primary CK cells were purified and inoculated in CEFs, and then, 6 and 12 plaques were purified from chickens 1 and 2, respectively. The virus was also passaged on CEFs 4 or 10 times as described above.
For all methods, the contamination of GaHV-2 vaccine strains in all of the samples passaged on CEFs was confirmed by PCR as discussed in the following section. All animal experiments were conducted in accordance with guidelines of the Institutional Animal Care and Use Committee of Hokkaido University, Japan.
Detection of the contaminated vaccine strains by PCR
To confirm the presence of vaccine strains, the GaHV-2, GaHV-3 and MeHV-1 genomes were detected by PCR using primer sets specific for each virus. Total cellular DNA samples were extracted from the passaged viruses on CEFs by using SepaGene (Sankojunyaku) according to the manufacturer’s instructions. Total cellular DNA was used for the PCR with TAKARA-Taq (TaKaRa) and appropriate primer pairs (Table 2). The PCR program was as follows: 94°C for 5 min and 40 cycles of 94°C for 30 sec, 52°C for 30 sec, 72°C for 30 sec and 72°C for 7 min. The amplified fragments were separated on agarose gels (2.0%) and visualized under ultraviolet light after staining with ethidium bromide. The RB1B strain was used as a positive control for virulent GaHV-2. The CVI988 strain, which is a vaccine strain widely used worldwide, was used as a positive control for avirulent GaHV-2. The meq gene of CVI988 contains a 177-bp insertion in the PRRs, and therefore, vaccine strains can be differentiated from virulent strains by amplifying the meq gene [10]. The product sizes were 583 bp and 763 bp, respectively, for virulent and avirulent GaHV-2. SB-1 and Fc126 strains were used as positive controls for GaHV-3 and MeHV-1, respectively.
Table 2. Primers used to detect GaHV-2, GaHV-3 and MeHV-1 genomes.
| Virus | Gene | Type | Sequence | Size (bp) | Reference |
|---|---|---|---|---|---|
| GaHV-2 | meq | FW | 5´-AGTTGGCTTGTCATGAGCCAG-3´ | 583 (763)a) | Murata et al., 2007 [10] |
| RV | 5´-CTGGCTCATGACAAGCCAACT-3´ | ||||
| GaHV-3 | DNA polymerase | FW | 5´-GTCTGCCCTCGTCTTAGC-3´ | 283 | Renz et al., 2006 [15] |
| RV | 5´-ACTCGCTTCCTCCAATTCG-3´ | ||||
| MeHV-1 | sorf1 | FW | 5´-AAGCGCTTGTATGTGTAGG-3´ | 350 | Islam et al., 2006 [6] |
| RV | 5´-TATGGACGTCATGCAGTTGG-3´ |
a) Expected sizes are shown for meq, and the L-meq is in parentheses.
RESULTS
Distinct diversity of Meq from 2014−2015 GaHV-2 field isolates in Japan
To investigate the distinct diversity of the Meq protein in GaHV-2 field strains during 2014−2015, the nucleotide sequences of the meq gene were determined, and their deduced amino acid sequences were established. The nucleotide sequences of the meq genes from different chickens collected at the same farms completely matched each other. Collected amino acid sequences indicated several differences at positions 77 and 80 in BR2 and positions 176 and 217 in the PRRs when compared with that of RB1B reference strain (Table 3) [17]. A proline-to-serine/leucine substitution at position 176, found previously in Japanese GaHV-2 field isolates [11], was also found in the isolates analyzed in this study (Table 3). Thus, GaHV-2 strains, which encode a Meq protein carrying a proline-to-serine/leucine substitution at position 176, seem to be widely distributed in Japan as reported previously [11]; yet we also noted distinct diversity in the 2014−2015 collected isolates.
Table 3. Meq amino acid substitutions in the GaHV-2 field strains analyzed in this study.
| Strain | Amino acid sequence |
Virulence, country and year | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 77 | 80 | 153a) | 174 | 176a) | 193 | 217/276a, b) | 280/339b) | |||
| PPPP | PPPP | PPPP | ||||||||
| Kgs-c1 | E | Y | P | T | S | P | A | V | Japan, 2014−2015 | This study |
| Myz-c1 | E | Y | P | T | L | P | A | V | Japan, 2014−2015 | |
| Me-c3 | E | Y | P | T | L | P | A | G | Japan, 2014−2015 | |
| MD239 | E | Y | P | N | P | S | A | V | Japan, Early 1980s | Murata et al., 2013 [11] |
| MD242 | E | Y | P | T | P | S | A | V | Japan, Early 1980s | |
| Nr-c1 | E | Y | P | T | S | P | A | V | Japan, 2004 | Murata et al., 2013 [11] |
| Me-c1 | E | Y | P | T | L | P | P | V | Japan, 2004 | |
| Nig-c1 | K | D | P | T | P | P | A | V | Japan, 2004 | |
| Tkc-1 | K | D | P | T | P | P | A | V | Japan, 2004 | |
| New | K | D | Q | T | A | P | A | V | vv+, U.S.A. | Shamblin et al., 2004 [17] |
| W/Md5 | K | D | P | T | P | P | A | V | vv+/vv, U.S.A. | |
| RB1B/GA | K | D | P | T | P | P | P | V | vv/v, U.S.A. | |
| BC-1 b) | A | D | P | T | P | P | P | V | v, U.S.A. | |
| CU-2 b) | E | D | P | T | P | P | P | V | m, U.S.A. | |
a) Interruptions at position 2 of the direct proline repeats. b) BC-1 and CU-2 contain 59 a.a. proline–rich amplification.
Virus isolation by a primary CK culture line
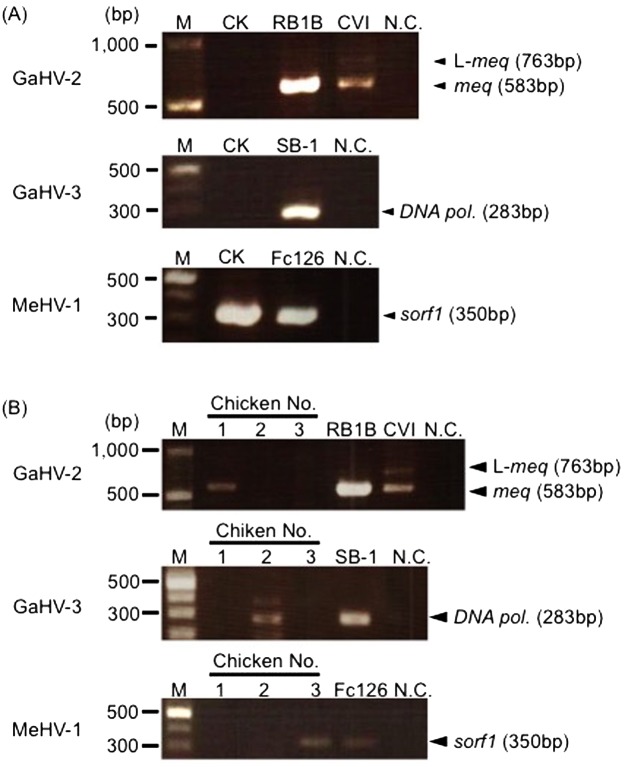
To isolate recent GaHV-2 field strains, we firstly developed a primary CK culture, which is a classical method to isolate GaHV-2 [2] (Fig. 1, Method 1). In this study, we chose the Kgs-c1 strain for the virus isolation, since its Meq amino acid sequence was common among Japanese field strains [11]. The kidney cells prepared from MD-symptomatic chickens were cultured, and a few days later, CPEs were observed on these CK cells. The plaque sizes of the viruses were relatively large, and the virus propagated rapidly throughout several passages on CEFs. These results suggest that the majority of isolated virus were vaccine strains adapted to in vitro culture. To confirm the contamination of vaccine strains in the isolated virus, PCR analysis was conducted. The sorf1 gene of MeHV-1 was detected, even though the MD-symptomatic chickens used for virus isolation were vaccinated with CVI988, whereas the meq gene of GaHV-2 was not detected (Fig. 2A). Thus, the virus-isolation by a classical CK culture method caused the contamination of vaccine strains, and the vaccine strains seem to be widely distributed in the field.
Fig. 2.
Virus isolation through a primary CK cell culture and from chickens inoculated with the isolated viruses on CK cells. (A) Viruses were isolated by a primary CK cell culture and passaged on CEFs 10 times. (B) Day-old-chicks were inoculated with viruses isolated via primary CK cell cultures. Then, viruses were isolated again by a primary CK cell culture at 2−3 wpi and passaged on CEFs 10 times. The GaHV-2, GaHV-3 and MeHV-1 genomes were detected in DNA samples from the passaged viruses. RB1B was used as a positive control for virulent GaHV-2, and CVI988 was used as a positive control for the attenuated GaHV-2 vaccine strain. SB-1 and Fc126 were used as positive controls for GaHV-3 and MeHV-1, respectively.
Virus isolation from chickens inoculated with the isolated viruses on CK cells
Although the propagation rates of vaccine strains are usually superior to those of virulent field strains in vitro, the propagation rates are not different in vivo [7, 10]. To remove the vaccine strains from the viruses isolated through a primary CK culture, we inoculated the isolated virus in three chicks after a few passages on CEFs (Fig. 1, Method 2). The contamination of vaccine strains was examined by PCR analysis. The meq gene was detected in chicken 1, but vaccine strains were not detected (Fig. 2B). In chickens 2 and 3, however, GaHV-3 or MeHV-1 was detected, respectively, indicating that vaccine strains could not be completely removed via this method.
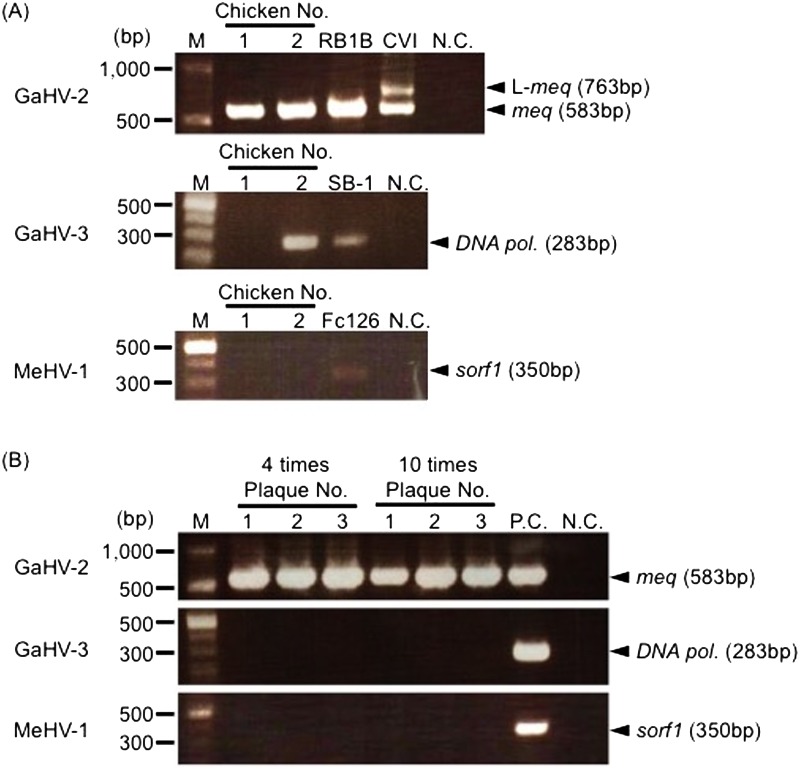
Virus isolation from chickens inoculated with spleen cell suspensions from MD chickens: To suppress the propagation of vaccine strains by passaging isolates in vitro, we prepared cell suspensions from the spleens of MD chickens and inoculated two chicks. PCR analysis revealed that the meq gene was detected in chickens 1 and 2, but GaHV-3 was also detected in chicken 2 (Fig. 3A). These results suggest that the contamination of MeHV-1 was considerably suppressed by excluding the in vitro culture procedure. To completely differentiate the field GaHV-2 strain from vaccine strains, we purified the plaques observed on the primary CK cells from chickens 1 and 2. All of the purified plaques were passaged at 4 or 10 times on CEFs to examine the contamination of vaccine strains by PCR. PCR analysis revealed that GaHV-2, but not GaHV-3 and MeHV-1, was detected in all plaque-purified samples even when the primary CK cells (chicken 2) were contaminated with GaHV-3 (Fig. 3B), indicating that GaHV-2 was completely separated from vaccine strains.
Fig. 3.
Virus isolation from chickens inoculated with cell suspensions from MD chickens. (A) Day-old-chicks were inoculated with cell suspensions from the spleens of chickens with MD. Viruses were isolated by a primary CK cell culture at 2-3 wpi and passaged on CEFs 10 times. (B) Six and 12 plaques observed on CK cells from chickens 1 and 2 were purified and inoculated in CEFs, and the virus was passaged on CEFs 4 or 10 times. The picture indicates the detection of the GaHV-2, GaHV-3 and MeHV-1 genomes in DNA samples from 3 passaged viruses from CK cells of chicken 2. RB1B was used as a positive control for virulent GaHV-2, and CVI988 was used as a positive control for the attenuated GaHV-2 vaccine strain. SB-1 and Fc126 were used as positive controls for GaHV-3 and MeHV-1, respectively.
DISCUSSION
GaHV-2 field strains in various countries have increased in virulence over time [22]. Among several viral factors that could cause the enhanced virulence of GaHV-2, the distinct diversity of point mutations in the Meq protein was notable, since Meq is important in GaHV-2 oncogenicity and has been previously shown to correlate with virulence levels [11, 14, 17]. In the Meq protein sequences of recent field isolates in Japan, similar substitutions were found, but the substitutions at position 176 in the PRRs were unique as compared to those found in other countries [11]. Thus, the properties of the field isolates in Japan seem to be changing. However, the amino acid substitutions at position 176 in Meq from Japanese isolates could not affect the transactivation activities, one of the important functions of the Meq protein [11]. Therefore, the molecular characterization of field GaHV-2 strains in Japan, including the influence of other viral proteins on the pathogenicity and virulence, is required. However, until this study, GaHV-2 strains from Japan could not be fully characterized, because vaccine strains frequently contaminated virus isolates by primary CK cell cultures. In this study, we inoculated cell suspensions prepared from the spleens of chickens with MD and isolated the GaHV-2 field strains by a CK cell culture in order to remove contaminating vaccine strains, and we used tissues from MD-symptomatic chickens infected with Kgs-c1, whose Meq protein carries a proline-to-serine substitution at position 176, as the material for the virus isolation.
Classically, a primary CK cell culture is performed to easily isolate the GaHV-2 strains [2], or the isolation is conducted by inoculating cell suspensions from tumor lesions or peripheral blood mononuclear cells (PBMCs) from infected chickens on CK cells or CEFs [2]. However, in our study, the virus isolation through a primary CK culture resulted in the contamination of vaccine strains. These results suggest that vaccine strains are predominantly propagated, and thereby, the majority of the virus population. MD vaccines are typically prepared by propagating the vaccine strains on CEFs; therefore, the vaccine strains have become acclimated to the in vitro culture environment. Thus, the use of a primary CK culture for virus isolation makes it difficult to eliminate contamination by vaccine strains acclimated to the in vitro environment.
To propagate the field GaHV-2 strains predominantly, we inoculated day-old-chicks with viruses obtained using materials of chickens with MD and propagated the viruses in vivo; we then used primary CK cells and spleens as the inoculum. The targets for the latent infection and the transformation by GaHV-2 are mainly CD4+ T lymphocytes [2]; therefore, the spleen may serve as a reservoir of infected cells even when it is difficult to grossly find obvious tumor lesions. The inoculation of the viruses isolated through a primary CK cell culture or cell suspension from the spleens of MD-symptomatic chickens failed to completely remove vaccine strains in our study. However, field strains of GaHV-2 could be occasionally isolated without contamination of vaccine strains. In addition, the purification of the plaques observed on primary CK cells resulted in the complete removal of vaccine strains. Virulent GaHV-2 strains are predominantly propagated in vivo in the acute infection phase, compared to vaccine strains [7, 10]. In addition, vaccination does not sufficiently suppress the propagation of a co-infected virulent strain [10], although the propagation rate of virulent strains in vaccinated chickens was lower than that in chickens infected with a virulent strain alone [7]. Thus, the inoculation of viruses obtained from MD-symptomatic chicken spleens to day-old chicks would be an appropriate method to separate field strains from vaccine strains.
The inoculation of cell suspensions from tumor lesions or PBMCs from infected chickens on CK cells or CEFs as sources for the virus isolation was not conducted in this study. Primary culture cells (CK cells or CEFs) have to be prepared to isolate GaHV-2 strains, but consistent preparation is difficult and depends on laboratory scales. In addition, the viability of infected cells has to be maintained during the transfer from the poultry farms to the laboratory, because GaHV-2 is a cell-associated virus. However, the viability of chicken lymphocytes has been difficult to maintain due to the lack of an appropriate in vitro chicken lymphocyte culture system, and thereby, the virus titer will decrease during the transfer.
In vitro serial passage results in the attenuation of GaHV-2 strains [3, 4, 24], although the mechanisms underlying the attenuation have yet to be fully elucidated. At passages 40−60, a vv+ strain, 648a [24], was moderately oncogenic, and the oncogenicity was lost after passage 70 [2, 24]. Previously, expansion of 132-bp repeats, direct repeat sequences within the inverted repeat regions flanking the unique long region of the GaHV-2 genome, during serial passage had been considered to be the cause of the GaHV-2 attenuation [18]. However, recombinant viruses lacking the 132-bp repeats were attenuated by serial passage in vitro, indicating that this expansion is not the direct cause of GaHV-2 attenuation [18]. Several other candidates, such as UL5, related to the attenuation of GaHV-2 attenuation by serial passage were recently identified [4, 19]. Thus, in vitro passage carries the risk that the properties of GaHV-2 might be changed from those of the original field strain. However, in the case of in vivo passage of GaHV-2 strains, the genetic background and the immunological pressure may affect the properties of the strains. It is important to pay attention to the presence of maternal antibodies, vaccine history and susceptibility of the day-old chicks used for passaging. The best way to maintain the properties of field GaHV-2 strains may be to construct a cloned bacterial artificial chromosome (BAC) for the GaHV-2 genome to reduce the potential for mutation of the virus genome during in vitro passage; additionally, the BAC clones have to be prepared using the infected cells at earlier passages, before critical mutations affecting virus characteristics can be introduced.
Several differences were reported between the nucleotide sequences of highly virulent strains distributed in the US, such as Md5 and Md11 and CVI988 [20]. Among them, we previously determined and compared the nucleotide sequences of viral genes such UL49 that encodes tegument protein and a short deletion was found in CVI988, and MDV074 that the deletion was found in some attenuated strains, in recent field isolates in Japan. However, distinct diversity and point mutations were not found in those genes (Murata, unpublished observations). Therefore, to identify the viral factors crucial to the pathogenicity and virulence of GaHV-2 distributed in Japan, the Kgs-c1 strain isolated in this study will be subjected to whole genome sequencing. In addition, the protection efficacy of currently available vaccines against the Kgs-c1 strains should be evaluated to control MD more effectively in the field.
Acknowledgments
This research was supported in part by Grants-in-Aid for Young Scientists (B: 25850194) and Scientific Research (B: 26292147) from Japan Society for the Promotion of Science.
REFERENCES
- 1.Barrow A., Venugopal K.1999. Molecular characteristics of very virulent European MDV isolates. Acta Virol. 43: 90–93. [PubMed] [Google Scholar]
- 2.Calnek B. W., Witter R. L.1997. Marek’s disease. pp. 369−413. In: Diseases of Poultry, 10th ed. (Calnek, B. W., Barnes, H. J., Beard, C. W., McDougald, Y. M. and Saif, Y. M. eds.), Iowa State University Press, Ames. [Google Scholar]
- 3.Gimeno I. M., Witter R. L., Hunt H. D., Reddy S. M., Neumann U.2001. Differential attenuation of the induction by Marek’s disease virus of transient paralysis and persistent neurological disease: a model for pathogenesis studies. Avian Pathol. 30: 397–409. doi: 10.1080/03079450120066403 [DOI] [PubMed] [Google Scholar]
- 4.Hildebrandt E., Dunn J. R., Perumbakkam S., Niikura M., Cheng H. H.2014. Characterizing the molecular basis of attenuation of Marek’s disease virus via in vitro serial passage identifies de novo mutations in the helicase-primase subunit gene UL5 and other candidates associated with reduced virulence. J. Virol. 88: 6232–6242. doi: 10.1128/JVI.03869-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones D., Lee L., Liu J. L., Kung H. J., Tillotson J. K.1992. Marek disease virus encodes a basic-leucine zipper gene resembling the fos/jun oncogenes that is highly expressed in lymphoblastoid tumors. Proc. Natl. Acad. Sci. U.S.A. 89: 4042–4046. doi: 10.1073/pnas.89.9.4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Islam A., Cheetham B. F., Mahony T. J., Young P. L., Walkden-Brown S. W.2006. Absolute quantitation of Marek’s disease virus and Herpesvirus of turkeys in chicken lymphocyte, feather tip and dust samples using real-time PCR. J. Virol. Methods 132: 127–134. doi: 10.1016/j.jviromet.2005.10.009 [DOI] [PubMed] [Google Scholar]
- 7.Lee S. I., Ohashi K., Morimura T., Sugimoto C., Onuma M.1999. Re-isolation of Marek’s disease virus from T cell subsets of vaccinated and non-vaccinated chickens. Arch. Virol. 144: 45–54. doi: 10.1007/s007050050484 [DOI] [PubMed] [Google Scholar]
- 8.Liu J. L., Kung H. J.2000. Marek’s disease herpesvirus transforming protein MEQ: a c-Jun analogue with an alternative life style. Virus Genes 21: 51–64. doi: 10.1023/A:1008132313289 [DOI] [PubMed] [Google Scholar]
- 9.Lupiani B., Lee L. F., Cui X., Gimeno I., Anderson A., Morgan R. W., Silva R. F., Witter R. L., Kung H. J., Reddy S. M.2004. Marek’s disease virus-encoded Meq gene is involved in transformation of lymphocytes but is dispensable for replication. Proc. Natl. Acad. Sci. U.S.A. 101: 11815–11820. doi: 10.1073/pnas.0404508101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murata S., Chang K. S., Lee S. I., Konnai S., Onuma M., Ohashi K.2007. Development of a nested polymerase chain reaction method to detect oncogenic Marek’s disease virus from feather tips. J. Vet. Diagn. Invest. 19: 471–478. doi: 10.1177/104063870701900503 [DOI] [PubMed] [Google Scholar]
- 11.Murata S., Hashiguchi T., Hayashi Y., Yamamoto Y., Matsuyama-Kato A., Takasaki S., Isezaki M., Onuma M., Konnai S., Ohashi K.2013. Characterization of Meq proteins from field isolates of Marek’s disease virus in Japan. Infect. Genet. Evol. 16: 137–143. doi: 10.1016/j.meegid.2012.12.032 [DOI] [PubMed] [Google Scholar]
- 12.Okazaki W., Purchase H. G., Burmester B. R.1970. Protection against Marek’s disease by vaccination with a herpesvirus of turkeys. Avian Dis. 14: 413–429. doi: 10.2307/1588488 [DOI] [PubMed] [Google Scholar]
- 13.Raja A., Dhinakar Raj G., Bhuvaneswari P., Balachandran C., Kumanan K.2009. Detection of virulent Marek’s disease virus in poultry in India. Acta Virol. 53: 255–260. doi: 10.4149/av_2009_04_255 [DOI] [PubMed] [Google Scholar]
- 14.Renz K. G., Cooke J., Clarke N., Cheetham B. F., Hussain Z., Fakhrul Islam A. F., Tannock G. A., Walkden-Brown S. W.2012. Pathotyping of Australian isolates of Marek’s disease virus and association of pathogenicity with meq gene polymorphism. Avian Pathol. 41: 161–176. doi: 10.1080/03079457.2012.656077 [DOI] [PubMed] [Google Scholar]
- 15.Renz K. G., Islam A., Cheetham B. F., Walkden-Brown S. W.2006. Absolute quantification using real-time polymerase chain reaction of Marek’s disease virus serotype 2 in field dust samples, feather tips and spleens. J. Virol. Methods 135: 186–191. doi: 10.1016/j.jviromet.2006.03.017 [DOI] [PubMed] [Google Scholar]
- 16.Schat K. A.1987. Marek’s disease: a model for protection against herpesvirus-induced tumours. Cancer Surv. 6: 1–37. [PubMed] [Google Scholar]
- 17.Shamblin C. E., Greene N., Arumugaswami V., Dienglewicz R. L., Parcells M. S.2004. Comparative analysis of Marek’s disease virus (MDV) glycoprotein-, lytic antigen pp38- and transformation antigen Meq-encoding genes: association of meq mutations with MDVs of high virulence. Vet. Microbiol. 102: 147–167. doi: 10.1016/j.vetmic.2004.06.007 [DOI] [PubMed] [Google Scholar]
- 18.Silva R. F., Reddy S. M., Lupiani B.2004. Expansion of a unique region in the Marek’s disease virus genome occurs concomitantly with attenuation but is not sufficient to cause attenuation. J. Virol. 78: 733–740. doi: 10.1128/JVI.78.2.733-740.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spatz S. J.2010. Accumulation of attenuating mutations in varying proportions within a high passage very virulent plus strain of Gallid herpesvirus type 2. Virus Res. 149: 135–142. doi: 10.1016/j.virusres.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 20.Spatz S. J., Petherbridge L., Zhao Y., Nair V.2007. Comparative full-length sequence analysis of oncogenic and vaccine (Rispens) strains of Marek’s disease virus. J. Gen. Virol. 88: 1080–1096. doi: 10.1099/vir.0.82600-0 [DOI] [PubMed] [Google Scholar]
- 21.Sung H. W.2002. Recent increase of Marek’s disease in Korea related to the virulence increase of the virus. Avian Dis. 46: 517–524. doi: 10.1637/0005-2086(2002)046[0517:RIOMSD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 22.Witter R. L.1997. Increased virulence of Marek’s disease virus field isolates. Avian Dis. 41: 149–163. doi: 10.2307/1592455 [DOI] [PubMed] [Google Scholar]
- 23.Witter R. L.1998. Control strategies for Marek’s disease: a perspective for the future. Poult. Sci. 77: 1197–1203. doi: 10.1093/ps/77.8.1197 [DOI] [PubMed] [Google Scholar]
- 24.Witter R. L.2002. Induction of strong protection by vaccination with partially attenuated serotype 1 Marek’s disease viruses. Avian Dis. 46: 925–937. doi: 10.1637/0005-2086(2002)046[0925:IOSPBV]2.0.CO;2 [DOI] [PubMed] [Google Scholar]