Abstract
Craniodental morphology of the Eurasian otter (Lutra lutra) in the Korean Peninsula, Japanese islands and Kinmen Island (Taiwan) was studied using geometric morphometrics to identify the skull variations between the populations. Forty adult skulls were examined (29 specimens from the Korean Peninsula, six from Shikoku, Honshu and Hokkaido of Japan, and five from Kinmen Island). Images of the dorsal and ventral views of the skull and the right lateral view of the mandible were analyzed. Specimens from the Korean Peninsula were larger than those from the Japanese islands and Kinmen Island. However, no correlation was observed between the shape variations in the three populations and the centroid size of the skull. The Mann-Whitney U-test showed that relative warps (RWs) RW1, RW2 and RW4 of the dorsal view and RW2 of the ventral view of the skull differed significantly between the populations. Some craniodental differences between the populations were seen in the dorsal and ventral views of the skull, mostly at the snout and parietal regions. The MANOVA test revealed significant differences between the specimens from the Japanese islands and Korean Peninsula and between the specimens from the Korean Peninsula and Kinmen Island. RWs plots showed an overlap of all three populations. In conclusion, the comparisons of the three examined populations revealed significant differences in their craniodental morphology.
Keywords: craniodental variation, geometric morphometrics, Japan, Korea, Taiwan
The Eurasian otter (L. lutra) is distributed across the Palaearctic from Ireland to Japan. Despite the fact that it has the widest distribution among otter species, it has been listed as near threatened on the red list of the International Union for Conservation of Nature (IUCN). Since the year 2004, its population has been continuously decreasing [13, 22]. Seven subspecies are recognized by IUCN: L. l. lutra, L. l. nair, L. l. monticola, L. l. kutab, L. l. aurobrunnea, L. l. barang and L. l. chinensis. L. l. lutra is the subspecies found in Korea and L. l. chinensis, in Taiwan [22]. There is some controversy surrounding the classification of the Japanese otter as it might be classified as a subspecies of L. lutra or a distinct species [5, 10, 21, 24]. The IUCN will not recognize it as a distinct species until an additional review clarifies its taxonomic status [22].
Imaizumi and Yoshiyuki [10] have concluded, on the basis of their morphological study, that the Japanese river otter L. nippon is different from L. lutra. The L. l. whitleyi specimens from Hokkaido have been reported as subspecies of L. lutra. Some molecular genetic studies were carried out by different researchers to identify its taxonomic status. Suzuki et al. [21] have reported that the Japanese river otter should be categorized as a species distinct from L. nippon. Geographically, L. nippon is the species of otter found in Honshu, Kyushu and Shikoku. Endo et al. [5] have studied skull specimens of the Japanese river otter from Shikoku Island and have revealed that their skull morphology is different from that of the Chinese population. Recently, Waku et al. [24] have evaluated the phylogenetic status of the Japanese river otter using two specimens from Honshu and Shikoku. The specimens have different divergence times; the individual from Honshu might be a member of L. lutra and the specimen from Shikoku, a distinct species. Both species have been reported to be extinct in the Japanese islands [2, 4]. The Red List of the Ministry of the Environment of Japan cited the Japanese river otter as extinct in 2012, after the last sighting of the animal in 1979 [17].
The Eurasian otter is designated as a Korean National Natural Monument, and the species is considered endangered in Korea [11] (Fig. 2). Ando et al. [1] have studied the status of the Eurasian otter in Southern Korea and have reported sightings of these animals in the coastal areas and some main river systems. However, a general survey has concluded that the population is declining in Korea [4]. A phylogeographic study has suggested that the Korean subspecies is separate from the European L. l. lutra and is closer to the Far-eastern Asian species. However, further studies, including specimens from the Amur region and Northeast China, are necessary to confirm its taxonomic status [12].
Fig. 2.
Eurasian otter in the wild, Korea, an image courtesy of Young-Jun Kim.
The species is also found in Taiwan [20], and it has been categorized as endangered by the Wildlife Conservation Act of Taiwan [15]. Foster-Turley et al. [6] have reported no recent status update for the species in Taiwan. Some studies have shown that the Kinmen Island (Fig. 1) is the only area in Taiwan with a stable population of Eurasian otters [9, 15]. However, this population is now declining [9, 15].
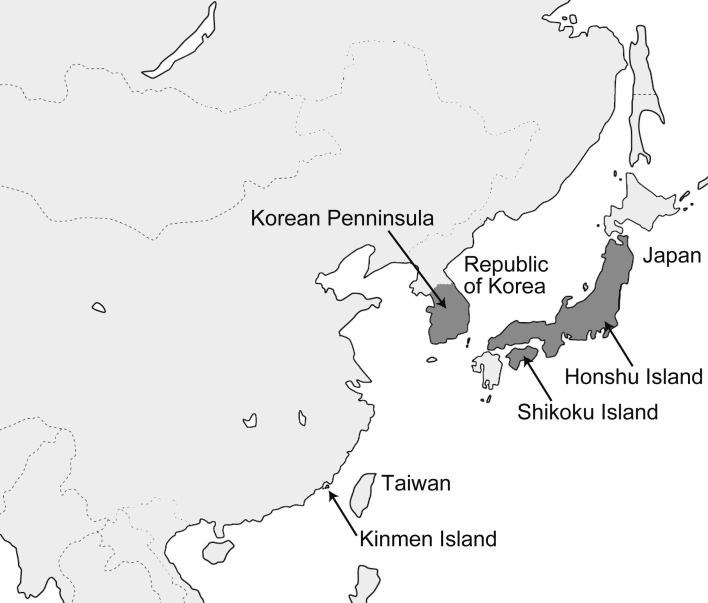
Fig. 1.
Collected locations of the specimens used in this study indicated by dark-gray area and arrowed lines: Japanese islands (Honshu and Shikoku islands), Korean peninsula and Kinmen Island, Taiwan.
We undertook a comparison of the skulls of the Eurasian otters from East Asian regions (the Japanese islands, Korean Peninsula and Kinmen Island) (Fig. 1), because of the critical status and the limited morphological data on this species. Our aim was to identify the differences between the subspecies or species in terms of size and shape. Besides the molecular phylogenetic clarification [9, 10, 12, 21, 24], the morphological definition might also contribute to better understanding of Lutra in East Asia. We used the same methods as in our previous sexual dimorphism study, geometric morphometrics [3, 25]. The method allows independent comparisons of shape and size [3, 14, 25].
MATERIALS AND METHODS
We examined the specimens collected in the Japanese islands (Honshu and Shikoku), Korean Peninsula and Kinmen Island, Taiwan (Fig. 1). One specimen from Hokkaido, deposited in the Kyoto University Museum, was also examined. As its taxonomic position is controversial [10], we did not include this specimen in the Japanese island group in our further analysis. The number of the specimens and their sex are shown in Table 1. The details of the museums where we obtained the specimens are shown in Table 2. We selected only the adult skulls with complete suture closure and fully erupted molar teeth. Even though sexual dimorphism is a distinct characteristic in mustelids [13], in this study, we analyzed the skulls without separating the males and females. The results of our previous study of sexual dimorphism in Korean otter population have shown a low level of sexual dimorphism in terms of shape [14]. Furthermore, in the current study, we had a similar number of male and female specimens of each species or subspecies.
Table 1. Number of specimens for each sex of each country that were used in this study for both dorsal and ventral views of cranium and mandible.
| Country | Sex |
Number of specimens |
|||
|---|---|---|---|---|---|
| Male | Female | Unknown | Total | ||
| Cranium: Dorsal view | Japan | 2 | 2 | 1 | 5 |
| Korea | 16 | 13 | 29 | ||
| Taiwan | 3 | 2 | 5 | ||
| Total | 21 | 18 | 1 | 40 | |
| Cranium: Ventral view | Japan | 2 | 2 | 1 | 5 |
| Korea | 16 | 13 | 29 | ||
| Taiwan | 1 | 1 | 1 | 3 | |
| Total | 19 | 17 | 2 | 38 | |
| Mandible | Japan | 2 | 2 | 1 | 5 |
| Korea | 15 | 13 | 28 | ||
| Taiwan | 2 | 1 | 1 | 4 | |
| Total | 19 | 17 | 2 | 38 | |
Table 2. Number of specimens and specimen ID based on the country and institution.
| Country and institution | Number of specimens and specimen ID | |
|---|---|---|
| Japan | National Museum of Nature and Science, Tokyo | 4 (M5761, M10426, M16201, M33858) |
| Ehime Tobe Zoo | 1 (NA) | |
| Total: 5 | ||
| Hokkaido, Japana) | The Kyoto University Museum | Total: 1 (3568) |
| Korea | Laboratory of Anatomy and Cell Biology in Seoul National University | 18 (KJ1150, KJ1152, KJ1157, KJ1158, KJ1160, KJ1162, KJ1168, KJ1189, KJ1190, KJ1203, KJ1204, KJ1206, KJ1209, KJ1210, KJ1212, KJ1214, KJ1215, KJ9025) |
| Korean Otter Research Centre | 11 (M3, M4, M5, M7, M8, M9, F1, F2, F4, F5, F6) | |
| Total: 29 | ||
| Taiwan | National Taiwan University | 1 (057) |
| Taipei Zoo | 1 (556) | |
| National Museum of Natural Science, Taichung | 1 (17105) | |
| Kinmen National Park and Kinmen County Government, Kinmen Island | 3 (NA) | |
| Total: 6 |
a) A specimen from Hokkaido is not used for the statistical analyses.
For all the specimens, we obtained two-dimensional images of the dorsal and ventral views of the cranium, as well as the right lateral views of the mandible, with a scale included. We then digitized all the images using the tpsDig2 program [18], assigning landmarks to the structures to be compared. Landmarks are homologous points that can be found easily in all the images [3, 25]. We used the same set of landmarks as in our previous study of sexual dimorphism in the Korean otter [14]. We compared only the right sides of our specimens, assuming that they were symmetrical. Centroid size is the measure of size used in geometric morphometrics, independent from the shape [3, 25]. The landmark coordinates created with tpsDig2 program [18] were then used for the RWs analysis using tpsRelw program [19]. In geometric morphometrics, the RW analysis explains shape variations independently from the centroid size [25]. The method is similar to the principal component analysis, but instead of principal component scores, it generates the RW scores [3, 25]. The RW scores were used in the statistical analysis. Shape deformations along the significant RW axis were visualized using tpsRelw program [19] and presented using thin-plate splines [3, 25].
The data obtained from the RW analysis were entered into the PAST program [7] for statistical analysis. Mann-Whitney U-test, Pearson’s correlation test (Pearson’s r/two-tailed t-test of significance) and MANOVA (multivariate analysis of variance) were performed. The Mann-Whitney U-test was conducted to compare the centroid sizes within the populations. Then, we compared the RW scores in different populations to identify the shape variations. We used the Pearson’s test to analyze the correlation between RW scores and centroid size: first, using all specimens and then for each population separately. MANOVA was conducted with 90% cumulative of RW scores. All the significant data were further adjusted using the Holm-Bonferroni correction. Box plots were used to visualize the centroid size differences between the populations.
RESULTS
The results of the Mann-Whitney U-test and Pearson’s correlation test are shown in Tables 3 and 4. Table 5 shows the MANOVA results. Figure 3 shows the box plots for the three populations with their centroid sizes. RW plots are shown in Fig. 4. Shape deformations along the significant RW axis of the dorsal and ventral views of the skull, as well as the right lateral mandible view, are shown in Fig. 5.
Table 3. Results for Mann-Whitney U test with the centroid size between the specimens (dorsal view of the skull) from Japanese islands, Korean peninsula and Kinmen Island, Taiwan, with Holm Bonferroni correction.
| Japanese islands | Korean peninsula | Kinmen Island, Taiwan | |
|---|---|---|---|
| Japanese islands | 0.381 | 0.293 | |
| Korean peninsula | 0.381 | 0.024 | |
| Kinmen Island, Taiwan | 0.293 | 0.024 |
Significance with P<0.05, P<0.025 for the second smallest P value, P<0.017 for the smallest P value. Numbers in bold and italic indicate significance.
Table 4. Results for Mann-Whitney U test and Pearson correlation test for specimens from Japanese islands, Korean peninsula and Kinmen Island, Taiwan (Indicated in short as Japan, Korea and Taiwan in the table). Results with Holm Bonferroni correction.
| Relative warps (RW) | Result of U-test (P=) | Singular values (%) | Linear r/P value (RW/centroid size) | Linear r/P value (Country/ centroid size) | ||||
|---|---|---|---|---|---|---|---|---|
| Japan | Korea | Taiwan | ||||||
| Cranium: Dorsal view | RW1 | Japan | 0.015 | 0.249 | 33.18 | 0.403 | 0.383 | |
| Korea | 0.015 | 0.496 | 0.307 | |||||
| Taiwan | 0.249 | 0.496 | 0.427 | |||||
| RW2 | Japan | 0.025 | 0.835 | 15.62 | 0.000 | 0.183 | ||
| Korea | 0.025 | 0.011 | 0.000 | |||||
| Taiwan | 0.835 | 0.011 | 0.952 | |||||
| RW3 | Japan | 0.466 | 0.676 | 14.27 | 0.003 | 0.486 | ||
| Korea | 0.466 | 0.734 | 0.375 | |||||
| Taiwan | 0.676 | 0.734 | 0.508 | |||||
| RW4 | Japan | 0.005 | 0.296 | 9.37 | 0.498 | 0.834 | ||
| Korea | 0.005 | 0.080 | 0.416 | |||||
| Taiwan | 0.296 | 0.080 | 0.410 | |||||
| Cranium: Ventral view | RW1 | Japan | 0.466 | 0.766 | 21.30 | 0.733 | 0.340 | |
| Korea | 0.466 | 0.245 | 0.824 | |||||
| Taiwan | 0.766 | 0.245 | 0.688 | |||||
| RW2 | Japan | 0.007 | 0.037 | 14.42 | 0.403 | 0.922 | ||
| Korea | 0.007 | 0.245 | 0.522 | |||||
| Taiwan | 0.037 | 0.245 | 0.232 | |||||
| RW3 | Japan | 0.072 | 0.037 | 12.11 | 0.071 | 0.181 | ||
| Korea | 0.072 | 0.039 | 0.390 | |||||
| Taiwan | 0.037 | 0.039 | 0.771 | |||||
| RW4 | Japan | 0.065 | 0.371 | 8.91 | 0.114 | 0.638 | ||
| Korea | 0.065 | 0.796 | 0.141 | |||||
| Taiwan | 0.371 | 0.796 | 0.264 | |||||
| Mandible | RW1 | Japan | 0.940 | 0.178 | 26.62 | 0.193 | 0.045 | |
| Korea | 0.940 | 0.073 | 0.051 | |||||
| Taiwan | 0.178 | 0.073 | 0.995 | |||||
| RW2 | Japan | 0.269 | 0.623 | 20.02 | 0.257 | 0.694 | ||
| Korea | 0.269 | 0.628 | 0.288 | |||||
| Taiwan | 0.623 | 0.628 | 0.677 | |||||
| RW3 | Japan | 0.042 | 0.111 | 17.19 | 0.001 | 0.214 | ||
| Korea | 0.042 | 0.104 | 0.024 | |||||
| Taiwan | 0.111 | 0.104 | 0.069 | |||||
| RW4 | Japan | 0.219 | 0.178 | 9.34 | 0.708 | 0.480 | ||
| Korea | 0.219 | 0.442 | 0.312 | |||||
| Taiwan | 0.178 | 0.442 | 0.705 | |||||
Significance with P<0.05, P<0.025 for the second smallest P value, P<0.017 for the smallest P value. Numbers in bold and italic indicate significance.
Table 5. MANOVA results with 90% cumulative relative warps for all the three views with Holm Bonferroni correction.
| Country | Japan | Korea | Taiwan | |
|---|---|---|---|---|
| Cranium: Dorsal view | Japan | 0.000 | ||
| Korea | 0.014 | |||
| Taiwan | ||||
| Cranium:Ventral view | Japan | 0.057 | ||
| Korea | 0.043 | |||
| Taiwan | ||||
| Mandible | Japan | 0.034 | 0.757 | |
| Korea | 0.252 | |||
| Taiwan |
Significance with P<0.05, P<0.025 for the second smallest P value, P<0.017 for the smallest P value). Numbers in bold and italic indicate significance.
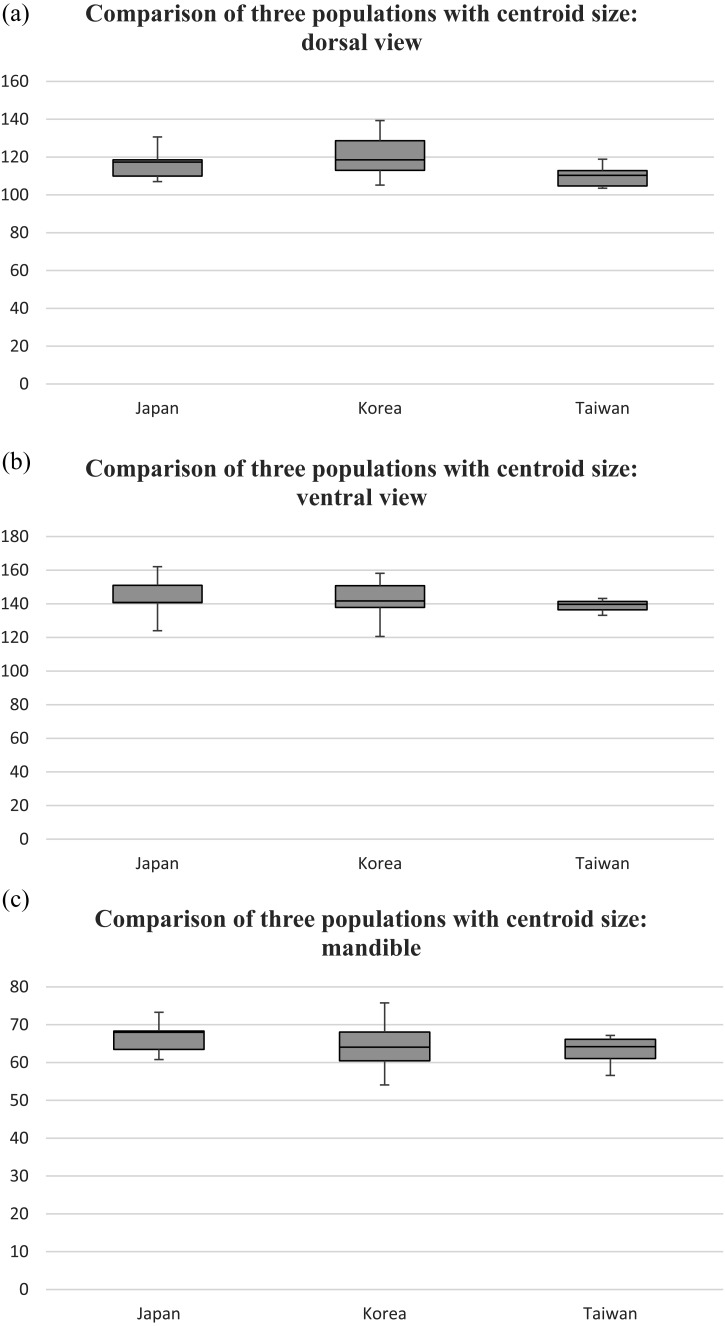
Fig. 3.
Box plots of each population (X axis) from Japanese islands, Korean peninsula and Kinmen Island, Taiwan (termed as Japan, Korea and Taiwan in chart) were compared for (a) dorsal view; (b) ventral view of the cranium and (c) mandible with the centroid size (Y axis). The third quartile is indicated by the first horizontal line, following by the median and the first quartile. The whiskers extend to the maximum value (on the top) and minimum value (on the bottom).
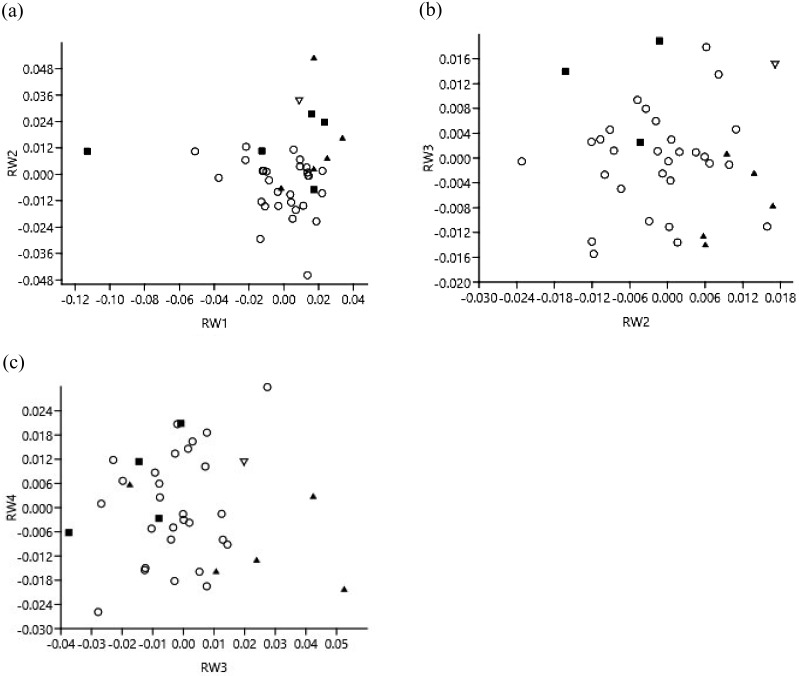
Fig. 4.
Relative warp (RW) plots of RWs (X axis and Y axis) to show the differences between the populations at (a) RW1 and RW2 of dorsal view, (b) RW2 and RW3 of ventral view of skull and (c) RW3 and RW4 of right lateral mandible. Triangles indicate the specimens from Japanese islands (filled triangles indicate Shikoku and Honshu, and inverted open triangle indicates Hokkaido); open circles indicate Korean peninsula; filled squares indicate Kinmen Island, Taiwan.
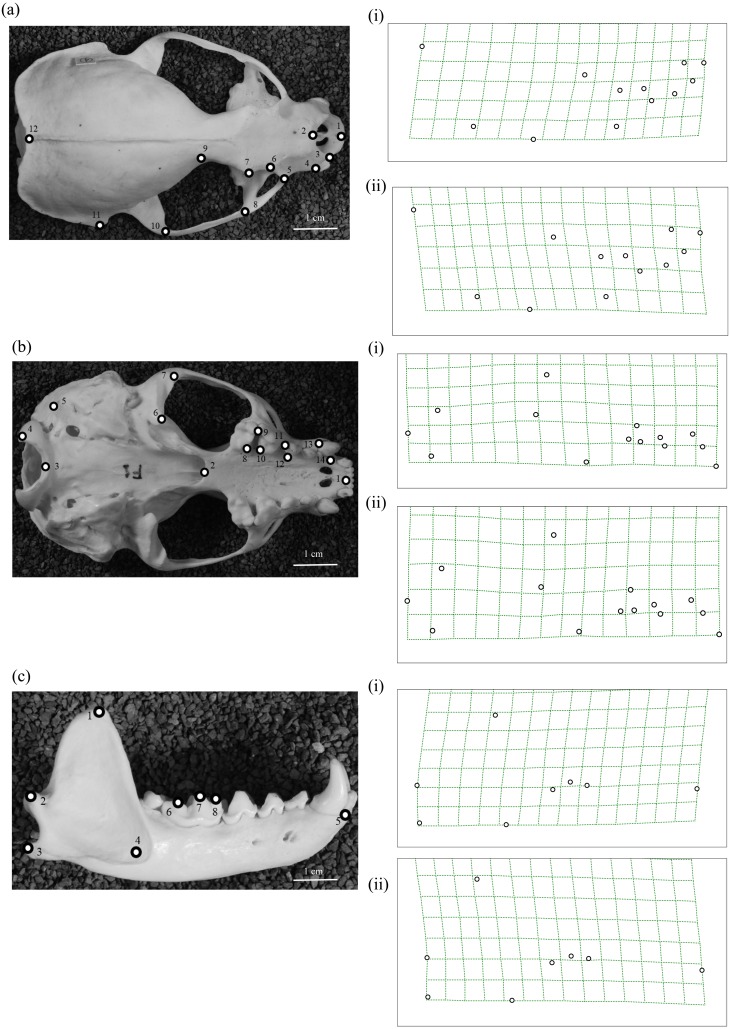
Fig. 5.
Thin-plate spline showing shape deformation for (a) dorsal view with RW1, (b) ventral view with RW2 of the skull and (c) right lateral mandible with RW1 on deformation grid with (a) (i) from 0.00 to 0.05 and (ii) from 0.00 to −0.05; (b) (i) from 0.00 to 0.03 and (ii) from 0.00 to −0.03 and (c) (i) from 0.00 to 0.05 and (ii) from 0.00 to −0.05.
The comparison of centroid sizes of the populations using the Mann-Whitney U-test and Holm-Bonferroni correction revealed that they were not significantly different (Table 3). However, the box plots showed that specimens from the Korean Peninsula were larger than those from the Japanese islands and Kinmen Island, Taiwan (Fig. 3). Specimens from Kinmen Island were also smaller than those in the Japanese and Korean populations (Fig. 3).
Results of the Mann-Whitney U-test showed that RW1 analysis (P=0.015 for Korean and Japanese pair; U-test), RW2 (P=0.011 for Korean and Taiwanese pair; U-test) and RW4 (P=0.005 for Korean and Japanese pair; U-test) (Table 4) for the dorsal view of the skull detected significant differences between the populations. RW1 to RW4 explained 72.98% of the total variations in the dorsal views of the skull. Among the RW plots (Fig. 4a), the first axis (RW1) best explained the variations between these three populations. The shape variation was observed at the snout region and the parietal bone (Fig. 5a). We noted that as the RW1 increased, the nasal aperture narrowed, as shown in the deformation grid (Fig. 5a and 5i). The distance between the anterior end and mesial end of the orbital region appeared to be smaller, too (Fig. 5a and 5i). Furthermore, the distance between the postorbital constriction and the zygomatic process of frontal bone increased, as did the maximum breadth of the cranial vault and the external occipital protuberance (Fig. 5a and 5ii). In the RW plot, the specimens from Kinmen Island overlapped with the specimens from the Korean peninsula (Fig. 4a). Overall, the individuals from the Japanese islands had a broader nasal aperture than the specimens from the Korean Peninsula. The Korean specimens had much narrower postorbital constriction and smaller nasal aperture and orbit. However, the Korean group showed the larger distance between the maximum width of the cranial vault and the external occipital protuberance, which indicated that the parietal bone in the Korean group was larger than in the other two populations. The specimens from the Japanese islands and Kinmen Island had a smaller distance between these two landmarks than the Korean individuals (Fig. 5a).
The ventral view of the skull was significantly different in the Japanese and Korean populations in the RW2 (P=0.007; U-test) (Table 4). RW2 explained 14.08% of the total deformation. The RW plots (Fig. 4b) showed the differences for the mandibular fossa, basion, occipital condyle and the mastoid process. Changes were also observed at the premolar and molar junctures (Fig. 5b). There was no correlation between the centroid size and the shape variations (Table 4) either in the comparisons of all the specimens or in each population. Thus, the variations observed in the RWs were not a result of size differences.
MANOVA results revealed that, in the dorsal view of the skull, the skull shape was significantly different in the specimens from the Korean peninsula in comparison with the Japanese (P=0.000; MANOVA) and Kinmen Island specimens (P=0.014; MANOVA) (Table 5). Other pair comparisons (such as between the Japanese islands and Kinmen Island) did not show significant differences for either the dorsal or the ventral view of the skull. For the mandible, no significant differences were shown in either the Mann-Whitney U-test (Table 4) or MANOVA results (Table 5) for any of the pairs.
In the RW plots (Fig. 4a–4c), all populations overlap for dorsal and ventral views of the skull, as they do for the right lateral mandible view. One specimen from Hokkaido that had been included in the RW plots plotted closer to the specimens from the Japanese islands.
DISCUSSION
A recent molecular phylogeographic study of the Japanese river otter by Waku et al. [24] has concluded that their specimen from Kochi (Shikoku) has a distinct lineage in the Lutra clade, while a specimen from Kanagawa (Honshu) might be a subspecies of L. lutra. An earlier study by Suzuki et al. [21] has also used the specimen from Kochi and a specimen from Ehime. The study conducted a cytochrome b gene analysis and detected a difference between the nucleotide sequence from the Japanese river otter and the Eurasian otters from Latvia and China. The authors have concluded that the Japanese river otter could be a distinct species rather than a subspecies of L. lutra. Imaizumi and Yoshiyuki [10] have described the Japanese river otters from Honshu, Shikoku and Kyushu as L. nippon, a distinct species. Our findings are compatible with their taxonomic studies as we could see significant differences between the specimens from the Japanese islands and Korean peninsula (Mann-Whitney U-test and MANOVA; Table 4 and Table 5). Furthermore, the deformation grids (Fig. 5a and 5b) also revealed craniodental differences. These were mainly the different width of the nasal aperture, broader or narrower snout, and parietal bone size in the dorsal view of the skull. There were also differences at the mandibular fossa and premolar and molar juncture in the ventral view. Imaizumi and Yoshiyuki [10] have reported that the Japanese river otter has a different shape of rhinarium and a larger nostril pad but smaller ala nasi. These findings are consistent with the craniodental changes that were observed at the nasal aperture and snout region of the skulls in our study. The divergence of the Japanese otters may be explained by a migration via the land bridge around 1.27 Ma and the subsequent isolation of the species [24].
As mentioned in the study of Koh et al. [12], the subspecies found in the Korean peninsula is probably closer to the subspecies in the Amur and Northeast China regions than to the European otter. The subspecies in Kinmen Island and Taiwan is L. l. chinensis [22]. L. l. chinensis is considered separate from L. lutra in Korea and Europe according to the molecular phylogenetic tree of Park et al. (personal communication). In our study, the sample from Taiwan was small, making the comparisons harder. However, we found significant differences between the dorsal views of the skull in the Korean and Taiwan specimens (Table 4). We observed that the distance between the external occipital protuberance and other landmarks became shorter, which indicated a smaller parietal bone. Variations were also observed for the landmarks around the nasal region and orbital region. In comparison with the Korean population, specimens from Kinmen Island had a shorter snout region (Fig. 5a). The craniodental differences between the animals from Kinmen Island and the Korean peninsula could be due to their different divergence. However, there was no significant difference between skulls from the Japanese islands and from Taiwan, which could be due to the paucity of available Taiwan specimens.
Hernadez-Romero et al. [8] have analyzed geographical variation in Lontra longicaudis (neotropical otter) using geometric morphometrics. The study has included species distributed across South America and found craniodental changes that could be partly due to geographical separation and distance. The authors have shown that the sizes of the mandibles in the northern (Mexico) and southern (La Plata) populations were significantly different, and the differences followed Bergmann’s rule. We did not perform a regression analysis of the correlation between environmental factors (such as climate and temperature) and the skull size. However, the temperatures are lower in Korea than in Japan and lower in Japan than in Taiwan. This factor might contribute to the size difference shown in the box plots (Fig. 3). We observed that the specimens from the Korean Peninsula were generally the biggest, followed by the specimens from the Japanese islands and Kinmen Island. The differences were not significant (Mann-Whitney U-test) (Table 3) after Holm-Bonferroni correction. This might be due to the small sample sizes of the Japanese and Taiwanese populations.
Other factors, such as adaptation to different environments, could also have contributed to the significant changes in craniodental morphology. The diets and different feeding habits are two of the crucial factors that affect craniodental morphology and feeding performance. A recent study by Timm-Davis et al. [23] has analyzed otter skull morphology, taking into account two different feeding habits. The results suggest that the divergences in skull morphology correspond to divergences in feeding modes. The authors have also concluded that the otters have divergent feeding habits. Similarly, Lynch and O’ Sullivan [16] have reported that the cranial morphology of Irish otters might be related to their diet and feeding habits. Brain size and masticatory muscles, particularly the temporalis muscle, might affect the shape of the skull. In our study, we observed variations in the size of the parietal bone (Fig. 5a), which forms part of the brain case. These bones were smaller in the Japanese and Kinmen Island specimens than in the Korean animals. Furthermore, the width of the postorbital constriction is associated with the size of the temporalis muscle [16]. Our Korean specimens had narrower postorbital constriction (Fig. 5a) and thus a larger temporalis muscle, which allows more powerful bites. Our previous study of sexual dimorphism in Korean otters discussed the relationship between the temporalis muscle and biting force, which affects hunting [14]. We have also concluded that differences in biting force might be an evolutionary factor in Carnivora, associated with adaptation to different habitats [14].
In summary, we observed the geographic variations in the size and shape of the craniodental morphology elements in specimens from the Japanese islands, Korean Peninsula and Kinmen Island. The shape variations observed in our study might be a result of divergences caused by geographical separation and adaptation to different environments. Some difficulties encountered in our study included a paucity of specimens in Japan and Taiwan and a lack of phylogenetic studies of the Korean and Taiwanese populations. Further integrative research, employing both molecular and morphological methods, should include specimens from mainland China and Taiwan.
Acknowledgments
We are grateful to: the National Museum of Nature and Science, Tokyo; The Kyoto University Museum; and the Ehime Tobe Zoo in Japan; the Laboratory of Anatomy and Cell Biology at Seoul National University; and the Korean Otter Research Centre in Korea; National Taiwan University; the National Museum of Natural Science (Taichung); Kinmen National Park; and the Kinmen County Government of Kinmen Island in Taiwan, for allowing us to examine their collected specimens. We thank all the staff members from the institutions mentioned above for the assistance given to us during our visits. We would also like to express our gratitude to Dr. Young-Jun Kim, for offering us one of his great photograph on the Eurasian otter.
REFERENCES
- 1.Ando M., Son S. W., Shiraishi S.1985. The common otter, Lutra lutra, in Southern Korea. Sci. Bull. Fac. Agr. Kyushu Univ 40: 1–5. [Google Scholar]
- 2.Ando M.2008. The Japanese otter −lessons from its extinction. University of Tokyo Press, Tokyo. [Google Scholar]
- 3.Bookstein F. L.1991. Morphometric tools for landmark data: geometry and biology. Cambridge University Press, Cambridge. [Google Scholar]
- 4.Conroy J., Melisch R., Chanin P.1998. The distribution and status of the Eurasian otter (Lutra lutra) in Asia—A preliminary review. IUCN Otter Spec. Group Bull. 15: 1–67. [Google Scholar]
- 5.Endo H., Ye X. D., Kogiku H.2000. Osteometrical study of Japanese otter (Lutra nippon) from Ehime and Koichi prefectures. Mem. Natn. Sci. Mus., Tokyo 33. [Google Scholar]
- 6.Foster-Turley P.1991. The status of otters in Asia. Reuther, C.; Rochert, R. (eds): Proceedings of the V. International Otter Colloquium. Habitat 6.
- 7.Hammer D. A., Harper T., Ryan P. D.2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electronica 4: 9. [Google Scholar]
- 8.Hernández-Romero P. C., Guerrero J. A., Valdespino C.2015. Morphological variability of the cranium of Lontra longicaudis (Carnivora: Mustelidae): a morphometric and geographic analysis. Zool. Stud. 54: 50. doi: 10.1186/s40555-015-0127-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hung C. F., Li S. H., Lee L. L.2004. Fecal DNA typing to determine the abundance and spatial organization of otters (Lutra lutra) along two stream systems in Kinmen. Anim. Conserv. 7: 301–311. doi: 10.1017/S1367943004001453 [DOI] [Google Scholar]
- 10.Imaizumi Y., Yoshiyuki M.1989. Taxonomic status of the Japanese otter (Carnivora, Mustelidae), with a description of a new species. Bull. Nat. Sci. Mus. Tokyo series A. 15: 177–188. [Google Scholar]
- 11.Kim H. J., Ando M., Han S. Y., Sasaki H., Ogawa H.2011. Recovery of the Eurasian Otter Lutra lutra in Korea and change in public attitude. Proceedings of XI International Otter Colloquium, IUCN Otter Spec. Group Bull. Vol. 28: 85–90.
- 12.Koh H. S., Yoo M. H., Lee B. G., Park J. G.2004. Molecular DNA systematic analyses of East Asian mammals: sequence variation of cytochrome b gene and control region of mitochondrial DNA of common otter, Lutra lutra lutra L. (Mammalia, Carnivora) from Korea. Korean J. Biol. Sci. 8: 231–233. doi: 10.1080/12265071.2004.9647755 [DOI] [Google Scholar]
- 13.Kruuk H.2006. Otters: ecology, behaviour and conservation. Oxford University Press, Oxford. [Google Scholar]
- 14.Lau A. C. C., Asahara M., Han S. Y., Kimura J.2016. Sexual dimorphism of the Eurasian otter (Lutra lutra) in South Korea: Craniodental geometric morphometry. J. Vet. Med. Sci. 78: 1007–1011. doi: 10.1292/jvms.16-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee L. L., Lee P.2015. Distribution and conservation status of Eurasian otter in Kinmen Islands. The international conference for the conservation breeding and reintroduction of endangered small carnivores. Taipei Zoo, Taiwan. Available at reg.zoo.gov.tw/public/attachment/562611265054.pdf.
- 16.Lynch J. M., O’Sullivan W. M.1993. Cranial form and sexual dimorphism in the Irish Otter Lutra lutra. Biol. Environ. Proc. R. Irish Acad 93: 97–105. [Google Scholar]
- 17.Ohdachi S. D., Ishibashi Y., Iwasa M. A., Fukui D., Saitoh T.2015. The wild mammals of Japan. 2nd ed. Shoukadoh, Kyoto. [Google Scholar]
- 18.Rohlf F. J.2010a. TpsDig, version 2.16, Department of Ecology and Evolution, State University of New York at Stony Brook. Available at http://life.bio.sunysb.edu/morph/.
- 19.Rohlf F. J.2010b. TpsRelw, version 2.16, Department of Ecology and Evolution, State University of New York at Stony Brook. Available at http://life.bio.sunysb.edu/morph/.
- 20.Smith A. T., Xie Y.2009. A guide to the mammals in China. J. Mammal. 90: 520–521. [Google Scholar]
- 21.Suzuki T., Yuasa H., Machida Y.1996. Phylogenetic position of the Japanese river otter Lutra nippon inferred from the nucleotide sequence of 224 bp of the mitochondrial cytochrome b gene. Zoolog. Sci. 13: 621–626. doi: 10.2108/zsj.13.621 [DOI] [PubMed] [Google Scholar]
- 22.The IUCN Red List of Threatened Species Version 2015–4. www.iucnredlist.org. Downloaded on 12 April 2016.
- 23.Timm-Davis L. L., DeWitt T. J., Marshall C. D.2015. Divergent skull morphology supports two trophic specializations in otters (Lutrinae). PLOS ONE 10: e0143236. doi: 10.1371/journal.pone.0143236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waku D., Segawa T., Yonezawa T., Akiyoshi A., Ishige T., Ueda M., Ogawa H., Sasaki H., Ando M., Kohno N., Sasaki T.2016. Evaluating the phylogenetic status of the extinct Japanese otter in the basis of mitochondrial genom analysis. PLOS ONE 11: e0149341. doi: 10.1371/journal.pone.0149341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zelditch M. L., Swiderski D. L., Sheets H. D.2012. Geometric Morphometrics for Biologists: A Primer. 2nd ed. Elsevier Inc., New York and London. [Google Scholar]