Abstract
We attempted the isolation of variant infectious bursal disease (IBD) viruses by using sentinel chickens immunized with inactivated classical-type IBD vaccine. Immunized sentinel chickens with high levels of neutralizing antibodies and non-immunized sentinel chickens were raised together with broiler chickens in a commercial farm. Severe atrophy of the bursa of Fabricius was observed from the second week after cohabitation in non-immunized sentinel chickens. However, in immunized sentinel chickens and broiler chickens, atrophy was observed from the third week after cohabitation. The IBD virus (IBDV) isolated from the bursa of Fabricius of immunized sentinel chickens, designated as strain IBDV TY2, showed severe atrophy of the bursa in infected SPF chickens. Antiserum to the IBDV TY2 strain showed higher neutralizing activity to heterologous IBDV strains than did antiserum to the K strain vaccine virus. Phylogenetic analysis revealed that the nucleotide sequences encoding the hypervariable region of virus protein 2 of the IBDV TY2 strain did not cluster with the classical, variant or very virulent IBDV groups. Based on these results, we suggest that the IBDV TY2 strain may constitute a novel variant type of IBDV.
Keywords: bursa of Fabricius, IBD, sentinel bird, variant IBDV
Infectious bursal disease (IBD) is a highly contagious immunosuppressive disease that affects young chickens; IBD causes extensive economic losses in the poultry industry [4, 12]. The IBD virus (IBDV), which replicates in the bursa of Fabricius and other lymphoid tissues, destroys B lymphocytes, resulting in immunosuppression [4, 20]. There are two IBDV serotypes, serotypes 1 and 2. Both serotypes infect chickens; however, disease is only observed in serotype-1 infections [9, 11]. Classical serotype-1 IBDVs have two different levels of virulence: virulent and very virulent [4, 6, 16]. IBDVs also have been characterized by antigenic type into classical and variant types. In the United States of America, Australia and other countries, variant types of IBDV that induce severe atrophy of the bursa, but against which the classical type of IBD vaccine does not protect, have been isolated [8, 10, 18, 21]. These variant IBDV types have not (to our knowledge) been isolated in Japan. Generally, IBD is effectively controlled by vaccination worldwide [16]. Newly hatched chickens are protected from IBDV for a few weeks by maternal antibodies and thereafter by antibodies produced by vaccination with live attenuated IBD vaccines [17, 28]. In Japan, despite the routine use of mild type live attenuated IBD vaccines, severe atrophy of the bursa of Fabricius in young broilers has been observed in the field [13]. Bursal atrophy in young broilers can be caused by various factors. While the factors have not yet been identified, one of the factors may be an IBDV of distinct antigenic type.
In this study, in order to investigate whether a variant type of IBDV is a causal agent of bursal atrophy, we attempted the isolation of an IBDV variant at a commercial broiler farm by using sentinel chickens immunized with a classical-type inactivated IBD vaccine. In addition, the pathogenicity of the isolated IBDV was examined in specific-pathogen-free (SPF) chickens, and serological and genetic characteristics were analyzed.
MATERIALS AND METHODS
Preparation of sentinel chickens
Forty-nine one-week-old SPF chickens (White Leghorn, maintained at the Chemo-Sero-Therapeutic Research Institute, Kumamoto, Japan) were used. The animals were injected subcutaneously with a commercial inactivated IBD vaccine (Nisseiken Inactivated IBD Vaccine, composed with the I • Q strain of classical serotype 1; Nisseiken LTD., Tokyo, Japan) at 1 and 3 weeks of age; these animals were used as the immunized group starting at 4 weeks of age. The average virus-neutralizing (VN) antibody titer of the immunized group against the IBDV K strain was 1:1,040. Forty-nine four-week-old SPF chickens also were used for the non-immunized group. These two groups were transferred to a broiler farm at 4 weeks of age for use as sentinel birds.
Broiler farm and broiler chickens used in this study
The broiler farm used for this study was located in the south of Kyushu, Japan. The broiler house was windowless, with a floor area of 9 m by 90 m. In this broiler house, 20,000 commercial broiler chickens were housed until approximately 52 days of age. Of these 20,000 broiler chickens, 49 two-week-old chickens were used in the present study.
Vaccines
We used two commercial IBD vaccines: the above-mentioned Nisseiken Inactivated IBD Vaccine for sentinel SPF chickens and Live IBD Vaccine L “Kaketsuken” (composed with the attenuated K strain of classical serotype 1, classified as “mild type”; Kaketsuken, Kumamoto, Japan) for the broiler chickens.
Experimental design
In order to ensure the same conditions (feed, temperature and the influence of etiological agents) among the test groups, the group of 49 immunized sentinel chickens and the group of 49 non-immunized sentinel chickens were caged separately and placed in the center of the broiler house. Additionally, 49 broiler chickens also were enclosed in a cage of the same size and placed between these two groups. For all three groups, the same feed and drinking lines were used.
The sentinel chickens were raised with 2-week-old broiler chickens on the farm for 5 weeks, to 9 weeks and 7 weeks of age, respectively. On this farm, the live attenuated IBD vaccine was routinely administered to broilers at 2, 3 and 4 weeks of age. For the present study, all chickens, including immunized and non-immunized sentinel chickens, were vaccinated with the live vaccine 3 times (sentinel chickens: 4, 5 and 6 weeks of age) by administration via the drinking water. Chickens were observed daily for clinical symptoms, and 7 chickens from each group were randomly selected every week after cohabitation. For the weekly selection of animals, the chickens were bled, euthanized and subjected to necropsy and examination for pathological changes; the bursa of Fabricius was collected from each animal. The bursa weight and body weight of each chicken were recorded, and the ratio of bursa to body weight was calculated as bursa weight (grams)/body weight (grams) ×100. The bursas of each group then were pooled and used as a substrate for detection of IBDV genetic material. The pooled bursas of the immunized sentinel group collected at 2 weeks after cohabitation were used for virus isolation.
IBDV-neutralizing antibody titers
VN antibody titer against the IBDV in serum was measured using the attenuated IBDV K strain of the vaccine virus. Serum was subjected to two-fold serial dilution in 96-well microplates; diluted serum then was mixed with an equal volume of the IBDV K strain adjusted to 200 TCID50/well and maintained at 37°C for one hr. The virus-serum mixtures then were combined with an equal volume of chicken embryo cells and incubated in a 5% CO2 atmosphere at 37°C. Microplates were observed for cytopathogenic effects after 7 days of incubation. The value of the highest serum dilution that did not show cytopathogenic effects was taken as the antibody titer.
Reverse transcription-polymerase chain reaction (RT-PCR)
Viral RNA was extracted from the bursas using a QIAamp Viral RNA Kit according to the manufacturer’s instructions (Qiagen Inc., Valencia, CA, U.S.A.). Amplification of the VP2 genes was performed by RT-PCR using a One Step RT-PCR Kit (Qiagen Inc.). Reverse transcription was performed at 50°C for 30 min, early PCR activation was performed at 94°C for 4 min, and then, a cycle consisting of denaturing at 94°C for 1 min, annealing at 52°C for 1 min and extension at 72°C for 2 min was repeated 40 times, with a subsequent further extension at 72°C for 10 min. The RT-PCR oligonucleotide primers (P2.3: 5′-CCCAGAGTCTACACCATA-3′ and RP5.3: 5′-TCCTGTTGCCACTCTTTC-3′) corresponded to the sequences encoding the hypervariable region (HVR) of the IBDV virus protein 2 (VP2) [15].
Restriction fragment length polymorphism analysis (RFLP)
RFLP analysis was conducted to simplify distinguishing the isolated IBDV TY2 strain from the live vaccine viruses (K strain; used on the present farm, the Luckert BP and 2512 G-61 strains), a very virulent strain (F539 strain) and a field isolate (TA-03 strain; unpublished). RT-PCR products were digested via the restriction enzymes TaqI, SspI and MvaI. The restriction fragments were electrophoretically separated in 2.0% agarose gels submerged in Tris-phosphate buffer. Gels were stained with ethidium bromide, and the DNA fragments were visualized under ultraviolet light.
Nucleotide sequence analysis
Sequencing of the RT-PCR fragments of the VP2 genes was outsourced to Takara Bio (Tokyo, Japan). Phylogenetic tree analysis of the 435-bp fragment encoding the HVR of the IBDV VP2 protein was conducted by neighbor-joining method with the Kimura’s 2-parameter model using MEGA software version 6.06. The sequences of the amplified fragments were compared to the corresponding sequences of the 228E strain (accession No. AF457104), 2512 strain (accession No. AF457105), D78 strain (accession No. Y14962), K strain (accession No. D16678), Cu-1 strain (accession No. D00867), 52/70 strain (accession No. D00869), GBF-1 strain (accession No. D16828), STC strain (accession No. D00499), Variant A strain (accession No. M64285), Variant E strain (accession No. D10065), GLS strain (accession No. AY368653), K357/88 strain (accession No. AF159216), OKYM strain (accession No. D49706), UK661 strain (accession No. X92760), F539 strain (accession No. LC136908), 08/95 strain (accession No. AF148081) and OH strain (accession No. U30818).
Virus isolation
We isolated IBDV from the immunized sentinel chickens based on the results of RT-PCR and RFLP. Bursas collected from 7 immunized sentinel birds at 2 weeks after cohabitation were pooled and homogenized with 10% phosphate buffered saline supplemented with penicillin and streptomycin (100 µg/ml each). The suspension of 10% homogenized bursas was centrifuged at 3,000 × g for 5 min at 4°C, and 5 four-week-old SPF chickens were orally inoculated with 1 ml of the resulting supernatant. Four days post-inoculation, the supernatant-immunized SPF chickens were euthanized, and their bursas were collected, processed as above and used for another round of inoculation of naïve animals. We conducted this procedure a total of 3 times. The 10% homogenate of bursa obtained in the final round was subjected to RT-PCR as above to confirm the presence of the IBDV genetic material.
Pathogenicity of the isolated IBDV in SPF chickens
The resulting IBDV isolate, designated as the IBDV TY2 strain, was administered orally at 104.1 50% mean egg infectious doses (EID) per bird to 25 four-week-old SPF chickens, and the chickens were observed daily over the following 14 days for clinical symptoms. The same number of 4-week-old SPF chickens was prepared as control animals. Five chickens from each group were euthanized and necropsied at 3, 5, 7, 10 and 14 days post-inoculation, and the bursal appearance was observed. For each animal, the bursa of Fabricius was collected, and the bursa to body weight ratio was calculated as above. The collected bursas were fixed in 10% neutral buffered formalin. After routine processing, the tissues were embedded in paraffin, cut into approximately 3-µm sections and prepared with hematoxylin and eosin staining for histopathological evaluation.
Cross VN test in embryonated eggs
The VN cross-reactivity test was performed by α neutralization procedure (constant-serum diluted-virus method) using 11-day-old SPF embryonated eggs with the isolated IBDV TY2 strain, IBDV K strain, very virulent IBDV F539 strain [23, 24] and antisera prepared from convalescent SPF chickens infected with either the IBDV TY2 or IBDV K strain. Serial ten-fold dilutions (10−1–10−6) of each IBDV were prepared, and each dilution was mixed with the same volume of either antiserum (diluted 5 times) and maintained at 37°C for one hr. The chorioallantoic membrane of 5 eggs was inoculated with 0.2 ml of the virus-serum mixtures at each serial dilution, and the eggs were incubated at 37°C. For the virus positive control, the virus and SPF chicken serum (negative serum) mixtures were inoculated in the same manner as described above. Seven days after inoculation, pathological changes in the chorioallantoic membrane and embryos were observed, and the EID50/0.2 ml was determined. The neutralization index (NI) of the serum was calculated as the difference between the log titer of the negative serum-virus mixture and the log titer of the positive serum-virus mixture [25].
RESULTS
Clinical signs and necropsy findings in sentinel birds
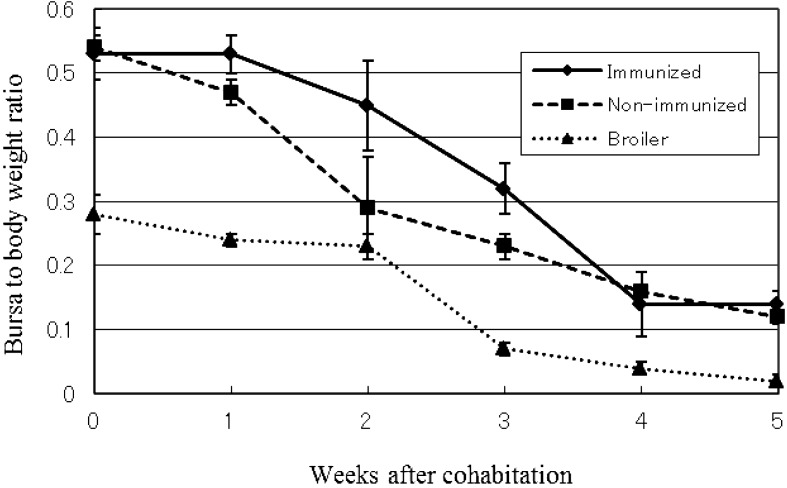
No clinical symptoms were observed in immunized and non-immunized sentinel chicken groups after cohabitation with commercial broilers. However, necropsy findings of acute inflammation of the bursa of Fabricius were observed in the non-immunized group at one week after cohabitation (5 weeks of age) and in the immunized group at two weeks after cohabitation (6 weeks of age). Atrophy of the bursa of Fabricius was observed one week after inflammatory reactions were observed. Although no clinical symptoms were observed in the broiler group, the same necropsy findings were observed, including significant atrophy of the bursa of Fabricius at 2 weeks after cohabitation (4 weeks of age). The average bursa to body weight ratio is shown in Fig. 1. No other necropsy changes were observed.
Fig. 1.
Average of bursa to body weight ratio of immunized, non-immunized and broiler groups. The data are presented as the mean ± SD.
VN antibody titer
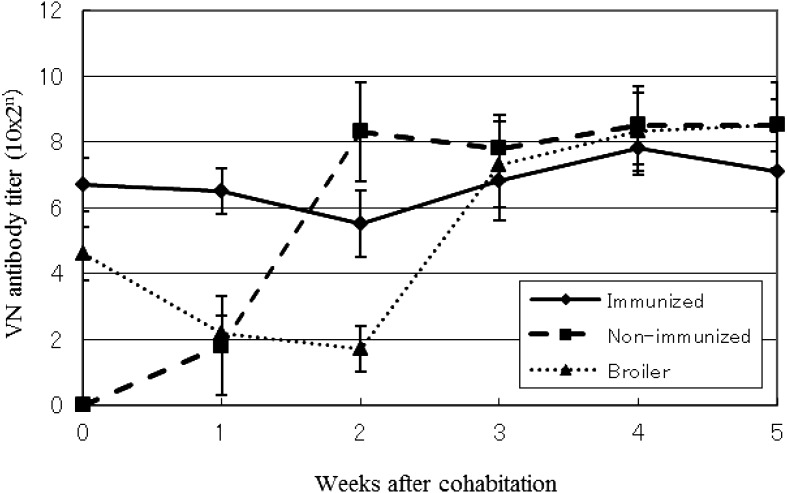
A time course of the average VN antibody titer against the IBDV K strain in each group is shown in Fig. 2. In immunized sentinel chickens, the VN antibody titer at the beginning of cohabitation was 1:1,024, a value that gradually decreased until 2 weeks after cohabitation. However, after two weeks, the titer rose to levels higher than those observed during cohabitation. The VN antibody titer of non-immunized sentinel chickens increased rapidly immediately after the start of cohabitation. In broiler chickens, the VN antibody titer decreased until 2 weeks after cohabitation (4 weeks of age), subsequently increasing to levels similar to those seen in the non-immunized sentinel chickens.
Fig. 2.
VN antibody titer after cohabitation of immunized, non-immunized and broiler groups. The data are presented as the mean ± SD.
RT-PCR and RFLP
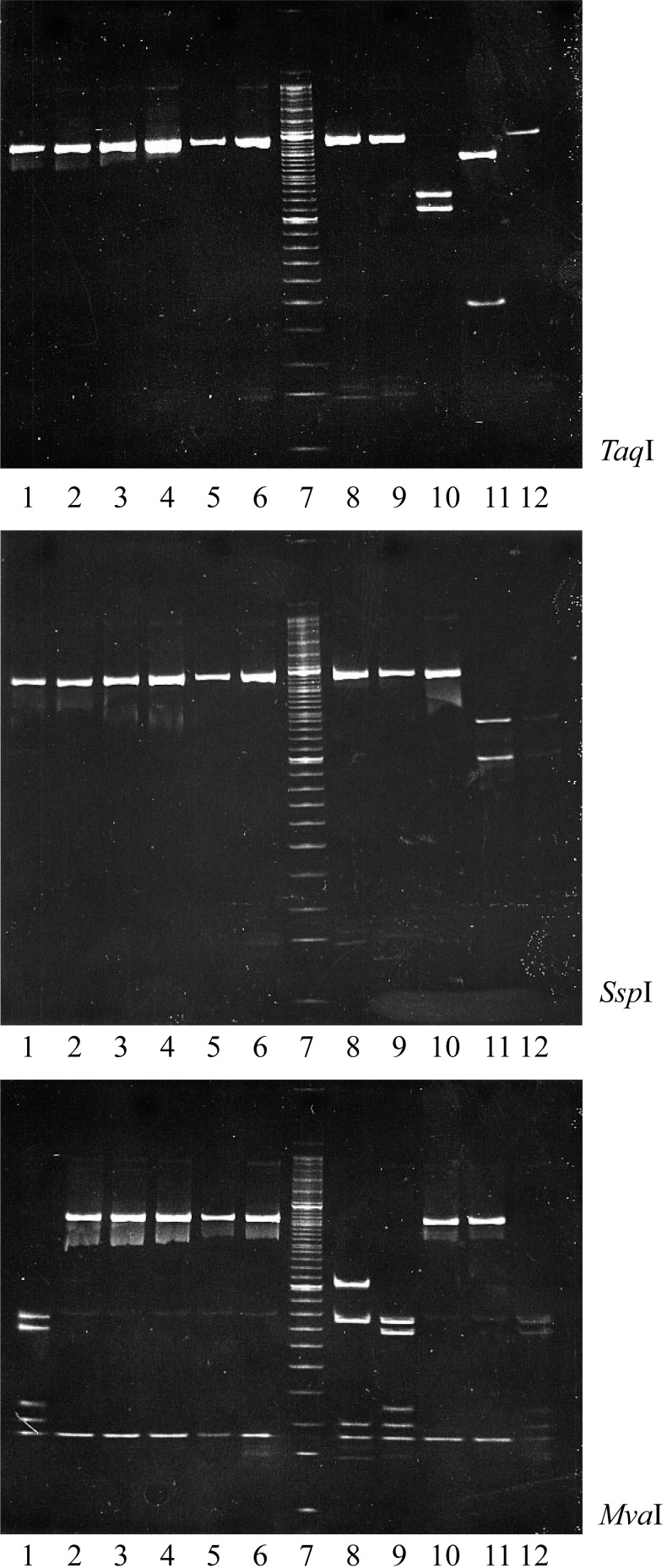
The RT-PCR fragments amplified from all samples were similar in size. The RT-PCR fragments amplified from the tested animals (lanes 1–6) were not cut by digestion with TaqI or SspI, but were cleaved by MvaI. However, only the non-immunized sentinel chickens collected 1 week after cohabitation (lane 1) showed the same restriction fragment pattern (for all three restriction enzymes) as that of the live attenuated IBD vaccine (lane 9) that was used on this study’s farm (Fig. 3). The RFLP analysis of their cutting patterns is summarized in Table 1. The samples from the immunized sentinel, non-immunized sentinel and broiler groups from the second week after cohabitation showed a unique restriction fragment pattern, distinct from those of well-known IBDV strains, including those of commercial vaccines, very virulent strains and other farm strains (marked V in Table 1).
Fig. 3.
RFLP analysis (via agarose gel electrophoresis) of IBDV VP2-encoding fragments amplified from the bursas of immunized, non-immunized, broiler chickens and other IBDVs. RT-PCR products were digested with the restriction enzymes TaqI, SspI or MvaI. 1: Non immunized. (1w: after cohabitation), 2: Non immunized. (2w), 3: Immunized. (2w), 4: Broiler (2w), 5: Non immunized. (3w), 6: Immunized. (3w), 7: Size marker, 8: Field isolate (TA-03 strain; unpublished), 9: Vaccine strain (K), 10: Vaccine strain (Luckert BP), 11: Very virulent strain (F539), 12: Vaccine strain (2512 G-61).
Table 1. Detection of IBDV genes from sentinel chickens and their types.
| Groups of sentinel chickens | Weeks after cohabitation / Identity of gene types |
|||||
|---|---|---|---|---|---|---|
| 0* | 1* | 2* | 3 | 4 | 5 | |
| Immunized | – | – | V a) | V | V | – |
| Non-immunized | – | A | V | V | – | – |
| Broiler | – | – | V | V | V | – |
a) V: Unique gene type that was different from that of known IBDVs. A: Gene type of the live vaccine virus (K strain) used on the study farm. * Time point at which live vaccine was administered.
Virus isolation
The bursas of all SPF chickens inoculated orally with bursal homogenate from immunized sentinel birds exhibited findings similar to those of the immunized sentinel chickens. Passage of the affected bursas to new SPF chickens was performed three times, and the same bursal findings were observed each time. The specific IBDV gene was confirmed using RT-PCR in the 10% homogenate of bursas collected following the 3rd round of passage through SPF chickens. Therefore, we considered the IBDV to be successfully isolated and designated the virus as strain IBDV TY2.
Pathogenicity of the IBDV TY2 strain
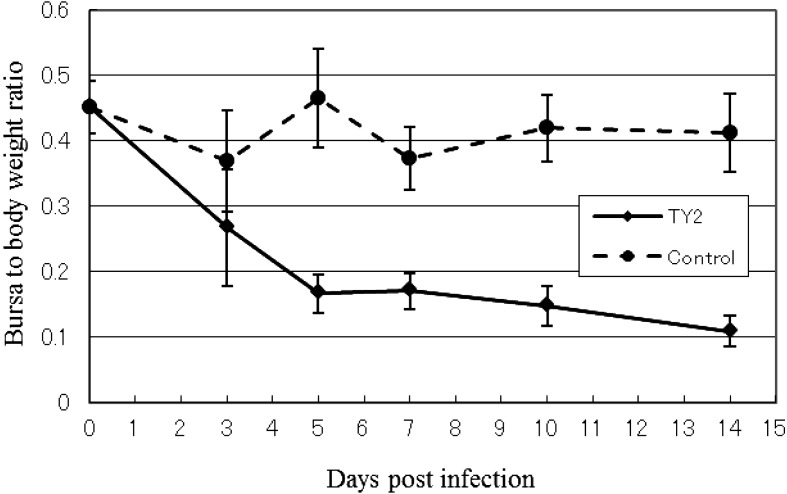
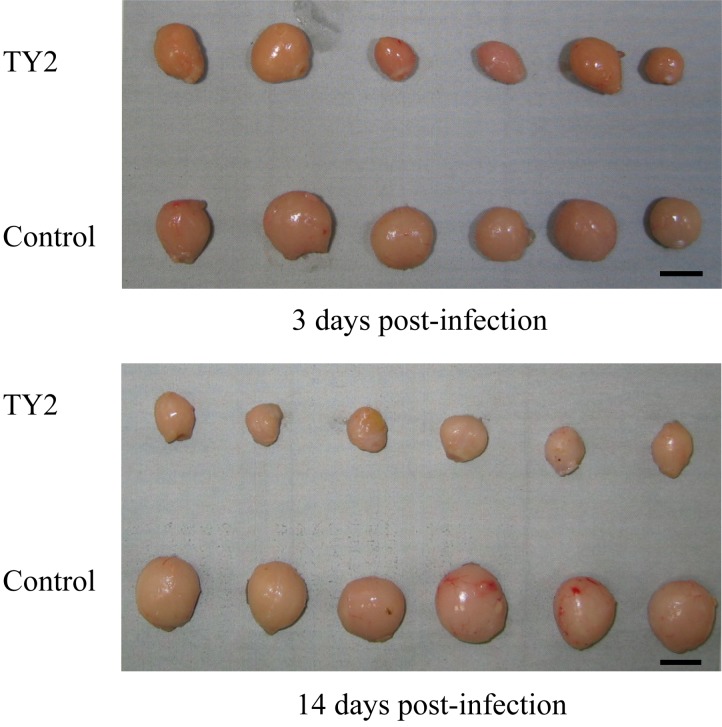
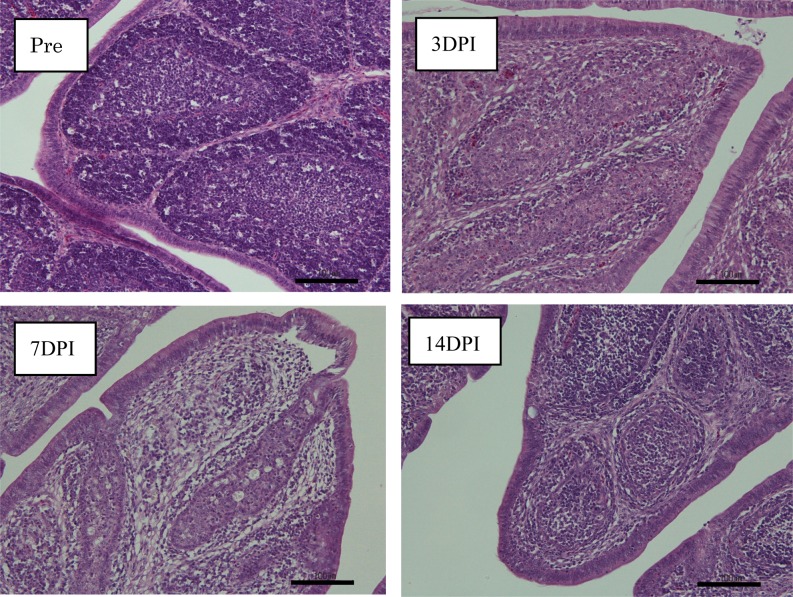
The bursal lesions typical of IBDV, such as atrophy and change to a gelatin-like substance, were observed at 3 days post-inoculation in SPF chickens inoculated orally with homogenate containing the IBDV TY2 strain, but no clinical signs were observed. The degree of bursal atrophy was demonstrated by the decreased bursa to body weight ratio. Severe atrophy of the bursa was observed from 3 days post-inoculation (Figs. 4 and 5). Histopathological evaluation of the bursas revealed degeneration and necrosis of lymphocytes in the medullary area of bursal follicles from 3 to 7 days post-infection. Inflammatory reactions also occurred, but were not severe. Hemorrhage was not observed. Phagocytosis of histiocytes and fibroplasia in the interfollicular connective tissue was observed at 7 days post-infection. However, regeneration of follicles, including reconstruction and activation of follicle structures, was also observed at 14 days post-infection (Fig. 6).
Fig. 4.
Average of bursa to body weight ratio of SPF chickens inoculated with IBDV TY2 strain. The data are presented as the mean ± SD.
Fig. 5.
Bursas of SPF chickens inoculated with IBDV TY2 strain or control. Bar=1 cm.
Fig. 6.
Histopathological observations in the bursas of SPF chickens inoculated with IBDV TY2 strain. Pre: pre-inoculation, 3DPI: 3 days post-infection, 7DPI: 7 days post-infection, 14DPI: 14 days post-infection. Bar=100 µm.
Cross VN test in eggs
NI is shown in Table 2. The NIs of IBDV TY2 and IBDV K strains against the homologous antiserum were 3.15 and 3.21, respectively. Against IBDV K and F539 strains, the anti-IBDV TY2 strain serum showed an NI of similar value, with a range of 2.46 to 3.05, even though the anti-IBDV K strain serum against heterologous IBDVs showed a range of 2.00 to 2.84.
Table 2. Neutralizing cross-reactivity test.
| IBDV strain | Antiserum |
|
|---|---|---|
| TY2 | K | |
| TY2 | 3.15a) | 2.00 |
| K | 2.46 | 3.21 |
| F539 | 3.05 | 2.84 |
a) Neutralizing index.
Nucleotide sequence analysis of IBDV TY2 strain
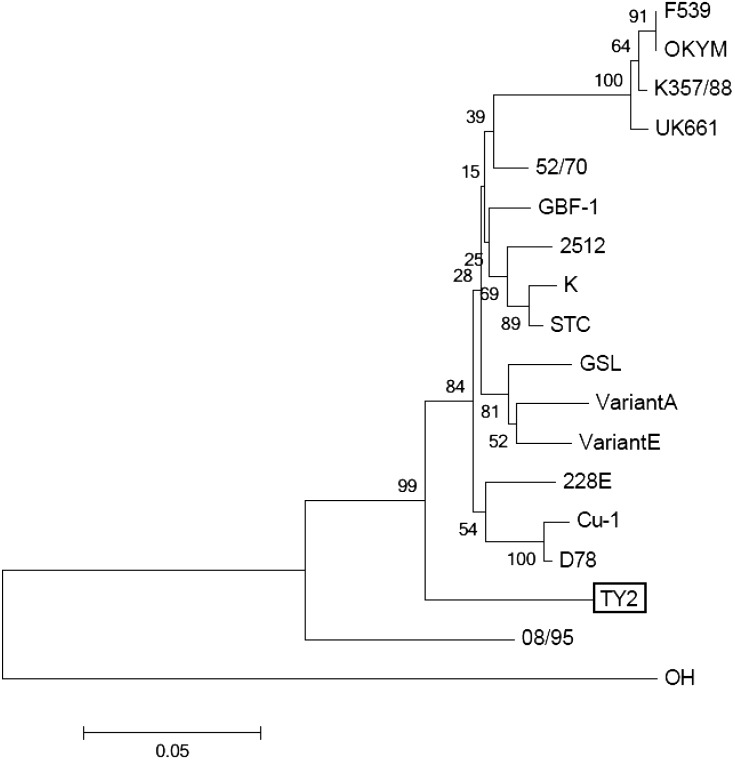
Based on the nucleotide sequences encoding the HVR of VP2, the IBDV TY2 strain did not cluster with any classical, variant or very virulent IBDV (vv-IBDV) groups in a phylogenetic tree (Fig. 7).
Fig. 7.
Phylogenetic tree based on nucleotide sequences encoding the hypervariable region of the VP2 protein. The tree was constructed using the neighbor-joining method. Bootstrap values, based on 1,000 replications. The scale bar represents 0.05 substitutions per site.
DISCUSSION
In this study, atrophy of the bursa of Fabricius was observed in both immunized and non-immunized sentinel chickens, as well as in broiler chickens. However, the starting time of atrophy in immunized sentinel chickens was one week later than that in non-immunized sentinel chickens. In the present study, mild-type live attenuated IBD vaccine was administered at 2, 3 and 4 weeks of age in the broilers (4, 5 and 6 weeks of age in the sentinel chickens), with the first vaccination administered just after the start of cohabitation. As such, it was possible to consider vaccination as one potential contributing factor in atrophy of the bursa and elevated VN antibody titers in the non-immunized sentinel chickens. However, titers were too high to suggest that atrophy reflected this effect alone. Notably, the VN antibody titer in immunized sentinel chickens re-ascended from 3 weeks after cohabitation. On the other hand, we successfully isolated the novel IBDV TY2 strain from 6-week-old immunized sentinel chickens at 2 weeks after cohabitation, even though the neutralizing antibody titer was as high as 1:1,040. Our previous study experimented in an isolated room showed that the chickens with a neutralizing antibody titer of 1:691 exhibited a high rate of protection of clinical signs (96.2%) against challenge by vv-IBDVs (unpublished data). From these points of view, the immunized sentinel chickens were expected to have possessed sufficient protection against infection by the classical type of IBDV, including vv-IBDV. Therefore, we inferred that the IBDV TY2 strain might have a different antigenicity than that of the classical type of IBDV.
While the IBDV TY2 strain exhibited very low pathogenicity in SPF chickens, histopathological examination revealed severe damage to the bursa of Fabricius in these animals. The bursa of birds infected with the IBDV TY2 strain showed typical IBD lesions, characterized by the degeneration and necrosis of lymphocytes and inflammatory reactions. These findings are distinct from findings reported in animals infected with the common IBDV variant strain in the United States of America, in which inflammatory reactions are mostly absent [19].
In a test of the neutralizing cross-reactivity of the IBDV TY2 strain against the classical IBDV, the IBDV TY2 antiserum fully covered the classical type, but IBDV TY2 strain was not fully covered by the classical IBDV K antiserum. This cross-reactivity test was performed using embryonated eggs, because TY2 strain had not yet been adapted for growth in cultured cells. If the test could be done in cultured cells using adapted strains, the serological features of IBDV TY2 strain might be shown more clearly.
As assessed by RFLP analysis, the IBDV TY2 strain was different from the classical IBDV strains, such as live vaccines or vv-IBDV. To our knowledge, the RFLP of the IBDV TY2 strain does not match that reported for any other IBDV strain.
Analysis of the HVR of the IBDV VP2-encoding gene is commonly used to differentiate IBDVs and to identify antigenic variations [2, 5, 27]. This technique is employed, because VP2 has been identified as the major source of protective antigen for IBDV, with the HVR representing the antigenic region responsible for recognition by neutralizing antibodies [1, 3, 7]. Moreover, the antigenic hydrophilic regions A (aa 212 to 224) and B (aa 314 to 325) in HVR also are important for identifying antigenic variation, especially for US variants, such as the Variant E strain or the GLS strain [4, 8, 14]. However, the nucleotide sequences encoding the antigenic hydrophilic regions A and B of the IBDV TY2 strain differed from those of the US variant strains. Furthermore, a phylogenetic tree, based on the nucleotide sequences encoding the HVR of VP2, confirmed that the IBDV TY2 strain did not cluster with any of the classical, variant or vv-IBDV groups. Thus, we propose that the IBDV TY2 strain may need to be classified as a new type of variant IBDV. Our concern regarding the IBDV TY2 strain is its origin as well as its lack of susceptibility to cross-neutralization by US or other variant types of IBDV. Therefore, it will be necessary to examine the neutralizing relation of the IBDV TY2 strain to variant strains, such as Del-A, Del-E, GLS or the 08/95 Australian variant strain, previously detected in other countries [18, 22, 26]. Further experiments will be necessary to better characterize the antigenicity of the IBDV TY2 strain.
In conclusion, we report the isolation, from a Japanese commercial broiler farm, of the novel IBDV TY2 strain, which has interesting characteristics in term of antigenicity and genetics. The use of sentinel chickens immunized with inactivated vaccine may be useful in the isolation of antigenically divergent viruses. Further, pathogenicity tests suggested that the IBDV TY2 strain is a contributing factor to the atrophy of the bursa in broiler farm animals. Development of a new vaccine using the IBDV TY2 strain may prove beneficial to prevent bursal atrophy in broiler farms.
REFERENCES
- 1.Azad A. A., Jagadish M. N., Brown M. A., Hudson P. J.1987. Deletion mapping and expression in Escherichia coli of the large genomic segment of a birnavirus. Virology 161: 145–152. doi: 10.1016/0042-6822(87)90180-2 [DOI] [PubMed] [Google Scholar]
- 2.Bayliss C. D., Spies U., Shaw K., Peters R. W., Papageorgiou A., Müller H., Boursnell M. E.1990. A comparison of the sequences of segment A of four infectious bursal disease virus strains and identification of a variable region in VP2. J. Gen. Virol. 71: 1303–1312. doi: 10.1099/0022-1317-71-6-1303 [DOI] [PubMed] [Google Scholar]
- 3.Becht H., Müller H., Müller H. K.1988. Comparative studies on structural and antigenic properties of two serotypes of infectious bursal disease virus. J. Gen. Virol. 69: 631–640. doi: 10.1099/0022-1317-69-3-631 [DOI] [PubMed] [Google Scholar]
- 4.Berg T. P.2000. Acute infectious bursal disease in poultry: a review. Avian Pathol. 29: 175–194. doi: 10.1080/03079450050045431 [DOI] [PubMed] [Google Scholar]
- 5.Brown M. D., Green P., Skinner M. A.1994. VP2 sequences of recent European ‘very virulent’ isolates of infectious bursal disease virus are closely related to each other but are distinct from those of ‘classical’ strains. J. Gen. Virol. 75: 675–680. doi: 10.1099/0022-1317-75-3-675 [DOI] [PubMed] [Google Scholar]
- 6.Chettle N., Stuart J. C., Wyeth P. J.1989. Outbreak of virulent infectious bursal disease in East Anglia. Vet. Rec. 125: 271–272. doi: 10.1136/vr.125.10.271 [DOI] [PubMed] [Google Scholar]
- 7.Fahey K. J., Erny K., Crooks J.1989. A conformational immunogen on VP-2 of infectious bursal disease virus that induces virus-neutralizing antibodies that passively protect chickens. J. Gen. Virol. 70: 1473–1481. doi: 10.1099/0022-1317-70-6-1473 [DOI] [PubMed] [Google Scholar]
- 8.Heine H. G., Haritou M., Failla P., Fahey K., Azad A.1991. Sequence analysis and expression of the host-protective immunogen VP2 of a variant strain of infectious bursal disease virus which can circumvent vaccination with standard type I strains. J. Gen. Virol. 72: 1835–1843. doi: 10.1099/0022-1317-72-8-1835 [DOI] [PubMed] [Google Scholar]
- 9.Ismail N. M., Saif Y. M., Wigle W. L., Havenstein G. B., Jackson C.1990. Infectious bursal disease virus variant from commercial Leghorn pullets. Avian Dis. 34: 141–145. doi: 10.2307/1591345 [DOI] [PubMed] [Google Scholar]
- 10.Jackwood D. H., Saif Y. M.1987. Antigenic diversity of infectious bursal disease viruses. Avian Dis. 31: 766–770. doi: 10.2307/1591028 [DOI] [PubMed] [Google Scholar]
- 11.Jackwood D. J., Saif Y. M., Hughes J. H.1982. Characteristics and serologic studies of two serotypes of infectious bursal disease virus in turkeys. Avian Dis. 26: 871–882. doi: 10.2307/1589875 [DOI] [PubMed] [Google Scholar]
- 12.Kibenge F. S., Dhillon A. S., Russell R. G.1988. Biochemistry and immunology of infectious bursal disease virus. J. Gen. Virol. 69: 1757–1775. doi: 10.1099/0022-1317-69-8-1757 [DOI] [PubMed] [Google Scholar]
- 13.Konishi Y., Takase K., Yamazaki K., Taika K., Takase Y.2004. Atrophy of bursa of Fabricius at early ages of broiler chickens. Bulletin of the Faculty of Agriculture. Kagoshima University 54: 9–14. [Google Scholar]
- 14.Letzel T., Coulibaly F., Rey F. A., Delmas B., Jagt E., van Loon A. A., Mundt E.2007. Molecular and structural bases for the antigenicity of VP2 of infectious bursal disease virus. J. Virol. 81: 12827–12835. doi: 10.1128/JVI.01501-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Z., Kato A., Otaki Y., Nakamura T., Sasmaz E., Ueda S.1993. Sequence comparisons of a highly virulent infectious bursal disease virus prevalent in Japan. Avian Dis. 37: 315–323. doi: 10.2307/1591655 [DOI] [PubMed] [Google Scholar]
- 16.Müller H., Islam M. R., Raue R.2003. Research on infectious bursal disease--the past, the present and the future. Vet. Microbiol. 97: 153–165. doi: 10.1016/j.vetmic.2003.08.005 [DOI] [PubMed] [Google Scholar]
- 17.Muskett J. C., Hopkins I. G., Edwards K. R., Thornton D. H.1979. Comparison of two infectious bursal disease vaccine strains: efficacy and potential hazards in susceptible and maternally immune birds. Vet. Rec. 104: 332–334. doi: 10.1136/vr.104.15.332 [DOI] [PubMed] [Google Scholar]
- 18.Sapats S. I., Ignjatovic J.2000. Antigenic and sequence heterogeneity of infectious bursal disease virus strains isolated in Australia. Arch. Virol. 145: 773–785. doi: 10.1007/s007050050670 [DOI] [PubMed] [Google Scholar]
- 19.Sharma J. M., Dohms J. E., Metz A. L.1989. Comparative pathogenesis of serotype 1 and variant serotype 1 isolates of infectious bursal disease virus and their effect on humoral and cellular immune competence of specific-pathogen-free chickens. Avian Dis. 33: 112–124. doi: 10.2307/1591076 [DOI] [PubMed] [Google Scholar]
- 20.Sharma J. M., Kim I. J., Rautenschlein S., Yeh H. Y.2000. Infectious bursal disease virus of chickens: pathogenesis and immunosuppression. Dev. Comp. Immunol. 24: 223–235. doi: 10.1016/S0145-305X(99)00074-9 [DOI] [PubMed] [Google Scholar]
- 21.Snyder D. B., Lana D. P., Savage P. K., Yancey F. S., Mengel S. A., Marquardt W. W.1988. Differentiation of infectious bursal disease viruses directly from infected tissues with neutralizing monoclonal antibodies: evidence of a major antigenic shift in recent field isolates. Avian Dis. 32: 535–539. doi: 10.2307/1590924 [DOI] [PubMed] [Google Scholar]
- 22.Snyder D. B., Vakharia V. N., Savage P. K.1992. Naturally occurring-neutralizing monoclonal antibody escape variants define the epidemiology of infectious bursal disease viruses in the United States. Arch. Virol. 127: 89–101. doi: 10.1007/BF01309577 [DOI] [PubMed] [Google Scholar]
- 23.Takase K., Baba G. M., Ariyoshi R., Fujikawa H.1996. Susceptibility of chicken embryos to highly virulent infectious bursal disease virus. J. Vet. Med. Sci. 58: 1129–1131. doi: 10.1292/jvms.58.11_1129 [DOI] [PubMed] [Google Scholar]
- 24.Takase K., Uchimura T., Katsuki N., Yamamoto M.1993. Agar gel precipitin line patterns and pathogenicity of infectious bursal disease viruses. J. Vet. Med. Sci. 55: 137–139. doi: 10.1292/jvms.55.137 [DOI] [PubMed] [Google Scholar]
- 25.Thayer S. G., Beard C. W.1998. Serologic procedures. pp. 235–266. In: A Laboratory Manual for the Isolation and Identification of Avian Pathogens, 4th ed. (Swayne, D.E., Glisson, J.R., Jackwood, M.W. and Reed, W.R. eds.), The American Association of Avian Pathologists, Kennett Square. [Google Scholar]
- 26.Vakharia V. N., He J., Ahamed B., Snyder D. B.1994. Molecular basis of antigenic variation in infectious bursal disease virus. Virus Res. 31: 265–273. doi: 10.1016/0168-1702(94)90009-4 [DOI] [PubMed] [Google Scholar]
- 27.Wu C. C., Rubinelli P., Lin T. L.2007. Molecular detection and differentiation of infectious bursal disease virus. Avian Dis. 51: 515–526. doi: 10.1637/0005-2086(2007)51[515:MDADOI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 28.Wyeth P. J., O’Brien J. D. P., Cullen G. A.1981. Improved performance of progeny of broiler parent chickens vaccinated with infectious bursal disease oil-emulsion vaccine. Avian Dis. 25: 228–241. doi: 10.2307/1589849 [DOI] [PubMed] [Google Scholar]