Abstract
Equine herpesvirus type 4 (EHV-4) is one of the most important pathogens in horses. To clarify the key genes of the EHV-4 genome that cause abortion in female horses, we determined the whole genome sequences of a laboratory strain and 7 Japanese EHV-4 isolates that were isolated from 2 aborted fetuses and nasal swabs of 5 horses with respiratory disease. The full genome sequences and predicted amino acid sequences of each gene of these isolates were compared with of the reference EHV-4 strain NS80567 and Australian isolates that were reported in 2015. The EHV-4 isolates clustered in 2 groups which did not reflect their pathogenicity. A comparison of the predicted amino acid sequences of the genes did not reveal any genes that were associated with EHV-4-induced abortion.
Keywords: abortion, genome sequence, Japanese equine herpesvirus type 4
Equine herpesviruses type 1 (EHV-1) and type 4 (EHV-4) cause equine respiratory disease (rhinopneumonia) in horses. EHV-1 and EHV-4 are classified as members of the genus Varicellovirus of Alphaherpesvirinae. The EHV-1 and EHV-4 genomes are linear, double-stranded DNA molecules of 150 kbp and 146 kbp in length, respectively [21, 22]. The genome structure of both EHV-1 and EHV-4 consists of a long unique region (UL) flanked by a short inverted repeat (TRL/IRL) linked to a short unique region (US) flanked by a substantial inverted repeat (TRS/IRS) [21, 22]. The genome of EHV-1 encodes 80 open reading frames (ORFs), while EHV-4 encodes 79 ORFs. EHV-1 and EHV-4 are genetically closely related viruses. The predicted amino acid sequences of their genes have identities in the range of 54.9–96.4% [22], and EHV-4 and EHV-1 are serologically similar. However, the symptoms of EHV-1 and EHV-4 are different: EHV-1 causes respiratory diseases in mostly 3-year-old horses and abortion in mares at the late stage of gestation [1, 15], while EHV-4 causes respiratory diseases in 2-year-old and under foals and rarely causes abortion [15, 22]. Other studies have shown that these differences are derived from the cellular tropisms of EHV-1 and EHV-4 [13]. However, it is unknown how these similar viruses have different pathogenicities in horses.
The full genome sequence of EHV-4 was first reported for an Irish isolate in 1998 [22]. Additional genome sequences of Australian isolates were reported in 2015 [24]. Maeda et al. reported the genomic variability among Japanese EHV-4 field isolates by restriction endonuclease digestion analyses and PCR to identify unique markers for the isolates [14]. Although EHV-4 is rarely associated with abortion in horses, at least 2 isolates from aborted fetuses were reported in Japan [14]. The incidence of abortion due to EHV-4 in the field is not well known, but it was reported to be less than 1% in Kentucky, U.S.A. between 1983 and 1992 and up to 16% in England between 1987 and 1993 [18, 26]. EHV-4 was also detected in a nasal swab of an aborted fetus by real-time PCR in Turkey [23].
In the present study, we determined the full genome sequences of EHV-4 isolates from 8 horses in Japan. To identify EHV-4 genes associated with abortion and to establish diagnostic methods for predicting and preventing abortion, we compared the full genome sequences of isolates of respiratory associated isolates and abortion associated isolates from Japan, Ireland and Australia.
MATERIALS AND METHODS
Cells and viruses
Fetal equine kidney (FEK) cells were used to propagate viruses. The FEK cells were maintained in Dulbecco’s Modified Eagle’s Medium (D-MEM) (Wako Pure Chemical Industries, Ltd., Tokyo, Japan) with 10% fetal bovine serum (FBS) (Invitrogen, Life Technologies, Tokyo, Japan).
Seven EHV-4s were isolated from aborted fetus in 1991 and 2012 (isolates 91c1 and 12-I-203, respectively) and nasal swabs of racehorses in 1983, 2001, 2003, 2005 and 2011 (isolates 83-MB, 01-10-1, 03-VR, 05-I-202 and 11-10, respectively). The isolate of 91c1 was kindly provided by Chubu Livestock Hygiene Service Center, Saga, Japan. A Japanese strain, TH20p, was plaque purified from TH20 which was isolated from a colt with respiratory disease in the first rhinopneumonitis outbreak in Japan was also used [14]. The other isolates and TH20p were kindly provided by Dr. Matsumura and Dr. Tsujimura (Japan Racing Association, Shimotsuke, Japan). The viruses were propagated in FEK cells for 2 passages, and supernatant was harvested when 80% CPE were observed.
Genome DNA extraction
The supernatant of a virus-infected cell culture was centrifuged at 3,000 rpm for 15 min at 4°C to remove cell debris and was ultracentrifuged at 25,000 rpm for 1 hr at 4°C to collect virions as a pellet. Viral genomic DNA was extracted from the pellet as described previously [25].
Sequencing and analysis of the genomes
Genome sequences of TH20p, 05-I-202 and 12-I-203 were read by the next generation sequencer (NGS) GS Junior (Roche Diagnostics K. K., Tokyo, Japan), and those of 83-MB, 91c1, 01-10-1 03-VR and 11-10 were read by the NGS MiSeq (Illumina lnc., Tokyo, Japan) according to the manufacturer’s protocol. The complete genome sequences were assembled by reference sequence mapping with Bowtie 2 [10] using EHV-4 strain NS80567 sequence (GenBank accession number AF030027) or TH20p sequence as the reference and editing with Consed [4] and SnapGene software (GSL Biotech; available at snapgene.com). The full genome sequence of TH20p was determined by mapping against the NS80567 sequence. The genome sequences of the other 7 Japanese isolates were determined by mapping against TH20p as a reference sequence. Because ORFs 24 and 71 included large regions of direct repeat sequences, these regions were amplified by PCR and further analyzed by Sanger sequencing. The primers used to amplify the ORFs 24 and 71 were ORF24-F (5′-gctttccaaaccttggcgtccatcgatacg-3′), ORF24-R (5′-ccgcgcggtttatctgtagatcatattcaagttc-3′), ORF71-F (5′-ctacatcaacctcggtgtc-3′) and ORF71-R (5′-cctgtttgatccaaccgacc-3′). Direct repeat sequences were analyzed by Tandem Repeat Finder [3] and XSTREAM [16] and removed from sequences before multiple alignments. The locations of removal are indicated in Table 1. The whole genome, UL, US and IR of EHV-4s including sequences determined in the present study, and EHV-4 reference strain NS80567 and 14 sequences of Australian isolates [24] were multiple aligned by MAFFT version7 [7] and AliView 1.18 [11]. Phylogenetic trees were constructed by neighbor-joining method using the Molecular Evolutionary Genetic Analysis (MEGA7) software [9] based on the nucleotide sequences excluding direct repeat sequences. Each ORF’s amino acid (AA) sequence was analyzed as the same as nucleic acid sequences. The AA differences were counted using AliView 1.18 manually.
Table 1. The deleted repeats location and size of NS80567 for alignment.
| Location | Size (bp) | Location | Size (bp) |
|---|---|---|---|
| 100..507 | 408 | 122,168..122,311 | 144 |
| 44,157..44,696 | 540 | 127,846..127,905 | 60 |
| 44,763..44,906 | 144 | 127,906..128,205 | 300 |
| 45,048..45,095 | 48 | 128,261..128,344 | 84 |
| 73,525..73,775 | 251 | 135,725..135,868 | 144 |
| 108,448..108,735 | 288 | 138,102..138,281 | 180 |
| 112,550..112,564 | 15 | 145,135..145,446 | 312 |
| 112,583..112,894 | 312 | 145,468..145,482 | 15 |
| 119,751..119,930 | 180 |
Nucleotide sequence accession numbers
The 8 EHV-4 genome sequences were submitted to DNA Data Bank of Japan with the following accession numbers: LC063142 (TH20p), LC075582 (83-MB), LC075583 (91c1), LC075584 (01-10-1), LC075585 (03-VR), LC075586 (05-I-202), LC075587 (11-10) and LC075588 (12-I-203).
RESULTS
The EHV-4 isolates consisted of 2 genetic groups
We determined full genome sequences of 8 Japanese EHV-4 isolates. The sizes of the EHV-4 full-length genomes ranged from 143,645 bp (91c1) to 144,802 bp (TH20p) (Table 2). Each virus genome encoded 79 of open reading frames (ORFs) as well as EHV-4 strain NS80567.
Table 2. EHV-4 field isolates sequenced in this study.
| Field isolates | Isolation site | Disease | Year of isolation | Accession number | Genome size (bp) |
|---|---|---|---|---|---|
| TH20p | Nasal swab | Respiratory disease | 1962 | LC063142 | 144,802 |
| 83-MB | Nasal swab | Respiratory disease | 1983 | LC075582 | 144,292 |
| 91c1 | Aborted fetus | Abortion | 1991 | LC075583 | 143,645 |
| 01-10-1 | Nasal swab | Respiratory disease | 2001 | LC075584 | 144,636 |
| 03-VR | Nasal swab | Respiratory disease | 2003 | LC075585 | 144,108 |
| 05-I-202 | Nasal swab | Respiratory disease | 2005 | LC075586 | 144,655 |
| 11-10 | Nasal swab | Respiratory disease | 2011 | LC075587 | 144,670 |
| 12-I-203 | Aborted fetus | Abortion | 2012 | LC075588 | 143,996 |
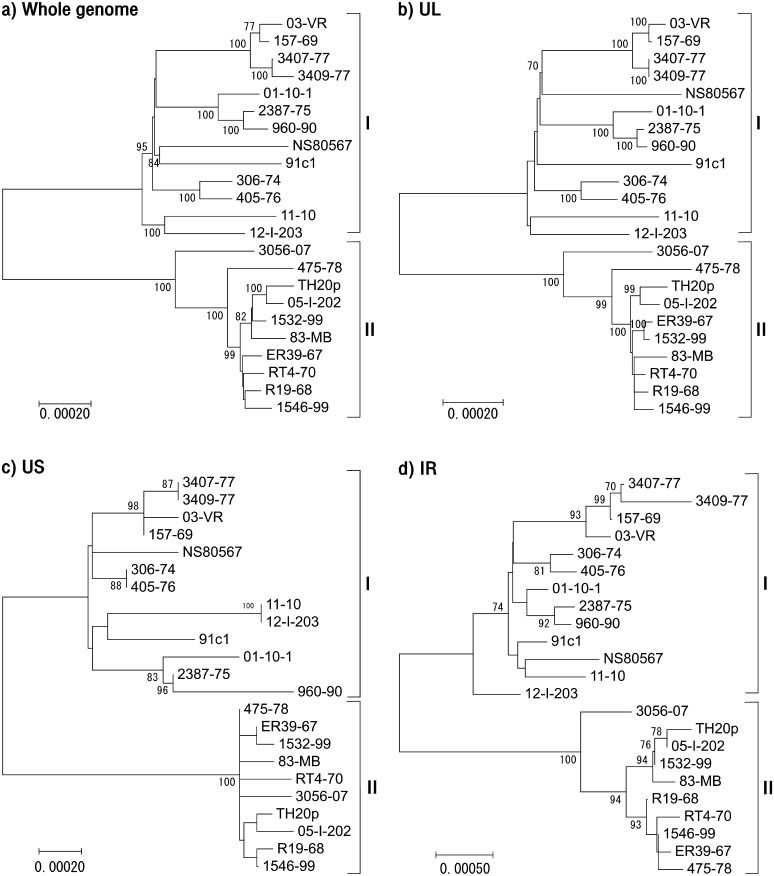
Next, we compared the genome of 8 Japanese isolates with other 15 EHV-4 isolates from Ireland and Australia. The phylogenetic trees were constructed by MEGA7 based on the nucleotide sequences excluding direct repeat sequences (Fig. 1a–1d). In the phylogenetic trees, the EHV-4 isolates fell into 2 genetic groups, groups I and II. Group I consisted of 13 isolates, and group II consisted of 10 isolates. All but one of the abortion isolates belonged to group I, the exception being 475-78 in group II.
Fig. 1.
Phylogenetic trees indicating evolutionary relationships generated from Japanese, Irish and Australian EHV-4 nucleotide alignments (excluding sequence repeats) using MEGA7. (a) Whole genome sequence, (b) UL region, (c) US region and (d) IR region. In all region, isolates and strains were clustered into group I or II. These groups were not associated with viral pathogenicity.
Group II strains had a higher sequence similarity than group I strains. The structure of the UL tree (Fig. 1b) was very similar to that of the whole genome tree (Fig. 1a). Although the grouping in the US tree (Fig. 1c) was the same as that in the whole genome tree, the structures of the 2 trees were different. The variation was greatest in the IR region (Fig. 1d) which has higher GC mol% than in UL and US. GC mol% of ORFs and non-coding regions in IR are 66.7% and 53%, respectively.
The structures of ORFs 24 and 71 containing direct repeat sequences
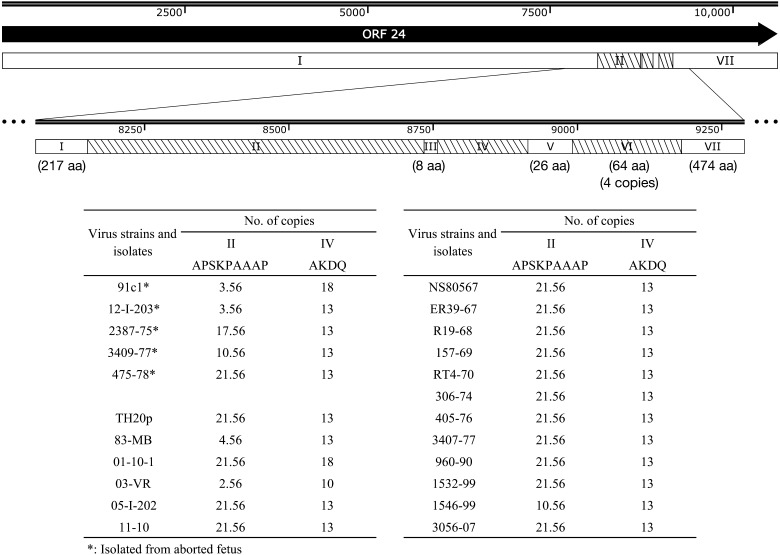
To see if differences in the direct repeat sequences were related to viral pathogenicity, we analyzed the predicted AA sequences of ORF 24 in UL and ORF 71 in US. We searched direct repeat regions of AA sequence by XSTREAM, and other regions were recognized as unique sequence regions.
ORF 24, which is the largest tegument protein of EHV-4, has 3 direct repeat regions (regions II, IV and VI) and 4 unique sequence regions (regions I, III, V and VII) (Fig. 2). The lengths of regions I, III, V, VI and VII were the same among the isolates analyzed in this study. All the isolates had the same number of copies in region VI, while they had different number of copies in regions II and IV (Fig. 2). In region II, 7 isolates had less copy numbers and tended to include abortion isolates. Japanese isolates had more variations in region IV, one Japanese isolate had less than 13 copies, and 2 Japanese isolates had more than 13 copies.
Fig. 2.
Amino acid sequence structure of ORF 24. ORF 24 was divided into 7 regions indicated in the schematic diagram above, and the details of numbers of copied sequences and amino acid sequences were shown in the table below. Regions I, III, V and VII were not repeat sequences. Region II, IV and VI were direct repeat sequences. The ORF 24 sequence was highly conserved, except for 2 regions, Regions II and IV, and those were repeat sequences.
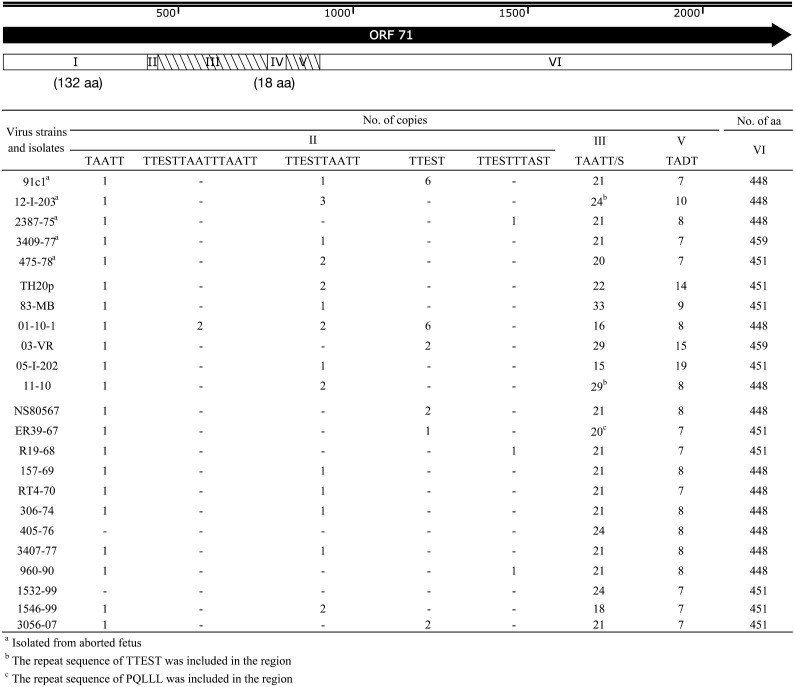
ORF 71, which is a large glycoprotein, has 6 regions which contains 2 direct repeat regions (regions III and V) and 4 unique sequence regions (regions I, II, IV and VI) (Fig. 3). All the isolates analyzed in the study had the same number of AAs in regions I and IV, while they had significantly different numbers of copies in regions III and V and significantly different patterns of direct repeat unit sequences (Fig. 3). The length and sequence pattern of region II were different among the isolates. We focused on direct repeat regions to find variation of AA sequence of ORF 71, but the sequence variation was seen in region II.
Fig. 3.
Amino acid sequence structure of ORF 71. ORF 71 was divided into 6 regions indicated in the schematic diagram above, and the details of numbers of copied sequences and amino acid sequences were shown in the table below. Regions I, II, IV and VI were not repeat sequences, but region II partially included repeat sequence. Regions III and V were direct repeat sequences which determined the length of ORF 71 amino acid sequence. Regions II and III were the most variable region.
The predicted AA sequences for both ORFs 24 and 71 varied among the isolates, however, abortion isolates did not have common feature of sequence patterns. These results indicate that neither of ORF was related to the pathogenicity of EHV-4.
Comparison of AA sequences of individual viral genes
To elucidate the genes that might be related to the pathogenicity of EHV-4, the predicted AA sequences of all 76 ORFs were compared among 23 EHV-4 isolates and strains (Table S1). Nine ORFs, including ORFs 4, 6, 8, 11, 25, 26, 44, 51 and 58, were completely conserved among the sequences examined. The predicted AA sequences for other ORFs varied among the isolates, as well as ORFs 24 and 71, and none of the ORFs seemed to correlate with pathogenicity of EHV-4 in horses.
DISCUSSION
In the present study, we determined the full genome sequence of 1 laboratory strain, TH20p and 7 Japanese isolates. Phylogenetic analyses were performed on a total of 23 isolates and strains of EHV-4 including the 8 Japanese strain and isolates, NS80567 (the Irish reference strain) and 14 Australian isolates. They clustered into 2 groups, I and II. The genome sequences of the 23 isolates and strains appeared to be very similar, especially in group II. TH20p is the oldest EHV-4 in this study, which was isolated in 1962 and maintained in laboratories in Japan. The newest Japanese isolate in group II, 05-I-202, was isolated in 2005. TH20p and 05-I-202 showed the highest similarity among Japanese isolates. This high similarity suggests that these 2 viruses have the same ancestor and have been conserved in horses in Japan. In phylogenetic tree analysis of EHV-4 genome, we found that IR region had the most variation among isolates. Natural recombination of viral genome is being recognized as important mechanism in alphaherpesviruses including EHV-4 [12, 24]. We did not examine recombination frequency of the EHV-4 genome in this study, however, a higher GC content in the repeats may increase their recombination frequency [12]. This indicates that higher mutation rate in high GC mol% of EHV-4 IR may contribute to recombination of viral genome in hosts to adapt them.
When we completed sequencing of EHV-4 isolates and the laboratory strain isolated in Japan, Australian group reported the genome sequences of Australian EHV-4 isolates [24]. Therefore, we analyzed the sequences of Japanese EHV-4 adding with those of Australian EHV-4 in the present study. We newly found that Japanese, Irish and Australian isolates were clustered into either the cluster I or II, indicating that genomic diversity of EHV-4 did not depend on geographical origins. This result suggested that globally distributed EHV-4 is clustered into the cluster I or II. To confirm this hypothesis, more EHV-4 sequences from other regions should be analyzed.
It is reported that repeat sequences of ORFs 24 and 71 were variable among EHV-4 field isolates [5, 14]. A large number of alterations were expected in ORFs 24 and 71, because these genes contain repeat regions. Although ORF 24 sequence is longer than ORF 71 sequence, it was revealed that ORF 24 had less repeat patterns and mutations compared to those of ORF 71. EHV-1 ORF 71, which has 61.1% identity with EHV-4 ORF 71 [22], is reported to be sufficient to restore respiratory virulence to the attenuated KyA strain [19].
The economic loss due to abortion caused by EHV-1 and 4 is a severe problem in the horse industry. It is believed that the mechanism of abortion by EHV-1 and 4 is similar but the pathogenesis of EHV-4 has not been well studied [18]. The neuropathogenicity of EHV-1 is determined by an AA change of ORF 30 (D752N) [17]. We hypothesized that the molecular marker related to abortion would be clear in EHV-4 because abortion rarely happens in EHV-4. However, we could not find the key gene (s) of abortion in the present study.
We consider the reasons of failure to identify the key gene (s) associated with abortion by EHV-4 as follows. First, the mechanism of abortion caused by EHV-1 or EHV-4 is complicated, for instance, not only one gene or SNP might determine the viral pathogenicity. Second, not only the viral factors but also host factors are critical to induce abortion. Third, the viral passages in culture cells derived from unnatural species induce mutations in the EHV-4, because mutations associated with viral passages in culture cells were observed in EHV-1 genomes [2, 6, 8, 20]. In conclusion, to solve these problems, we need to collect more viral and host samples to compare the genomic diversity statistically between respiratory associated isolates and abortion associated isolates. Furthermore, DNA sample preparation for next generation sequence should be done directly from specimen in a future study to avoid detecting mutations derived from viral passages in culture cells.
Supplementary
Acknowledgments
We thank Japan Racing Association and Chubu Livestock Hygiene Service Center in Saga for giving us EHV-4 isolates.
REFERENCES
- 1.Allen G. P., Kydd J. H., Slater J. D., Smith K. C.2004. Equid herpesvirus 1 and equid herpesvirus 4 infections. pp. 829–859. In: Infectious Diseases of Livestock (Coetzer, J. A. W. and Tustin, R. C. eds.), Oxford University Press, Cape Town. [Google Scholar]
- 2.Allen G. P., Yeargan M. R., Bryans J. T.1983. Alterations in the equine herpesvirus 1 genome after in vitro and in vivo virus passage. Infect. Immun. 40: 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson G.1999. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27: 573–580. doi: 10.1093/nar/27.2.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon D., Green P.2013. Consed: a graphical editor for next-generation sequencing. Bioinformatics 29: 2936–2937. doi: 10.1093/bioinformatics/btt515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Ja J. A., Ficorilli N., Hartley C. A., Allen G. P., Studdert M. J.2002. Polymorphism of open reading frame 71 of equine herpesvirus-4 (EHV-4) and EHV-1. J. Gen. Virol. 83: 525–531. doi: 10.1099/0022-1317-83-3-525 [DOI] [PubMed] [Google Scholar]
- 6.Hübert P. H., Birkenmaier S., Rziha H. J., Osterrieder N.1996. Alterations in the equine herpesvirus type-1 (EHV-1) strain RacH during attenuation. Zentralbl. Veterinarmed. B. 43: 1–14. [DOI] [PubMed] [Google Scholar]
- 7.Katoh K., Standley D. M.2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30: 772–780. doi: 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirisawa R., Ui S., Takahashi A., Kawakami Y., Iwai H.1994. Comparison of the genomes of attenuated equine herpesvirus-1 strains with their parent virulent strain. Virology 200: 651–660. doi: 10.1006/viro.1994.1228 [DOI] [PubMed] [Google Scholar]
- 9.Kumar S., Stecher G., Tamura K.2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33: 1870–1874. doi: 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langmead B., Salzberg S. L.2012. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9: 357–359. doi: 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson A.2014. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30: 3276–3278. doi: 10.1093/bioinformatics/btu531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee K., Kolb A. W., Sverchkov Y., Cuellar J. A., Craven M., Brandt C. R.2015. Recombination analysis of herpes simplex virus 1 reveals a bias toward GC content and the inverted repeat regions. J. Virol. 89: 7214–7223. doi: 10.1128/JVI.00880-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma G., Azab W., Osterrieder N.2013. Equine herpesviruses type 1 (EHV-1) and 4 (EHV-4)--masters of co-evolution and a constant threat to equids and beyond. Vet. Microbiol. 167: 123–134. doi: 10.1016/j.vetmic.2013.06.018 [DOI] [PubMed] [Google Scholar]
- 14.Maeda K., Kai K., Matsumura T.2005. Genomic diversity among equine herpesvirus-4 field isolates. J. Vet. Med. Sci. 67: 555–561. doi: 10.1292/jvms.67.555 [DOI] [PubMed] [Google Scholar]
- 15.Matsumura T., Sugiura T., Imagawa H., Fukunaga Y., Kamada M.1992. Epizootiological aspects of type 1 and type 4 equine herpesvirus infections among horse populations. J. Vet. Med. Sci. 54: 207–211. doi: 10.1292/jvms.54.207 [DOI] [PubMed] [Google Scholar]
- 16.Newman A. M., Cooper J. B.2007. XSTREAM: a practical algorithm for identification and architecture modeling of tandem repeats in protein sequences. BMC Bioinformatics 8: 382. doi: 10.1186/1471-2105-8-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nugent J., Birch-Machin I., Smith K. C., Mumford J. A., Swann Z., Newton J. R., Bowden R. J., Allen G. P., Davis-Poynter N.2006. Analysis of equid herpesvirus 1 strain variation reveals a point mutation of the DNA polymerase strongly associated with neuropathogenic versus nonneuropathogenic disease outbreaks. J. Virol. 80: 4047–4060. doi: 10.1128/JVI.80.8.4047-4060.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel J. R., Heldens J.2005. Equine herpesviruses 1 (EHV-1) and 4 (EHV-4)--epidemiology, disease and immunoprophylaxis: a brief review. Vet. J. 170: 14–23. doi: 10.1016/j.tvjl.2004.04.018 [DOI] [PubMed] [Google Scholar]
- 19.Smith P. M., Kahan S. M., Rorex C. B., von Einem J., Osterrieder N., O’Callaghan D. J.2005. Expression of the full-length form of gp2 of equine herpesvirus 1 (EHV-1) completely restores respiratory virulence to the attenuated EHV-1 strain KyA in CBA mice. J. Virol. 79: 5105–5115. doi: 10.1128/JVI.79.8.5105-5115.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Studdert M. J., Fitzpatrick D. R., Browning G. F., Cullinane A. A., Whalley J. M.1986. Equine herpesvirus genomes: heterogeneity of naturally occurring type 4 isolates and of a type 1 isolate after heterologous cell passage. Arch. Virol. 91: 375–381. doi: 10.1007/BF01314296 [DOI] [PubMed] [Google Scholar]
- 21.Telford E. A. R., Watson M. S., McBride K., Davison A. J.1992. The DNA sequence of equine herpesvirus-1. Virology 189: 304–316. doi: 10.1016/0042-6822(92)90706-U [DOI] [PubMed] [Google Scholar]
- 22.Telford E. A. R., Watson M. S., Perry J., Cullinane A. A., Davison A. J.1998. The DNA sequence of equine herpesvirus-4. J. Gen. Virol. 79: 1197–1203. doi: 10.1099/0022-1317-79-5-1197 [DOI] [PubMed] [Google Scholar]
- 23.Turan N., Yildirim F., Altan E., Sennazli G., Gurel A., Diallo I., Yilmaz H.2012. Molecular and pathological investigations of EHV-1 and EHV-4 infections in horses in Turkey. Res. Vet. Sci. 93: 1504–1507. doi: 10.1016/j.rvsc.2012.01.019 [DOI] [PubMed] [Google Scholar]
- 24.Vaz P. K., Horsington J., Hartley C. A., Browning G. F., Ficorilli N. P., Studdert M. J., Gilkerson J. R., Devlin J. M.2016. Evidence of widespread natural recombination among field isolates of equine herpesvirus 4 but not among field isolates of equine herpesvirus 1. J. Gen. Virol. 97: 747–755. doi: 10.1099/jgv.0.000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkening J. D., Spatz S. J.2009. Purification of DNA from the cell-associated herpesvirus Marek’s disease virus for 454 pyrosequencing using micrococcal nuclease digestion and polyethylene glycol precipitation. J. Virol. Methods 157: 55–61. doi: 10.1016/j.jviromet.2008.11.017 [DOI] [PubMed] [Google Scholar]
- 26.Whitwell K., Smith K., Sinclair R., Mumford J.1994. Fetal lesions in spontaneous EHV-4 abortions in mares. p. 354. In: Proceedings of the seventh International Conference on Equine Infection Diseases, (Nakajima, H. and Plowright, W. eds.), R & W Publications (Newmarket) Limited, Tokyo.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.