Abstract
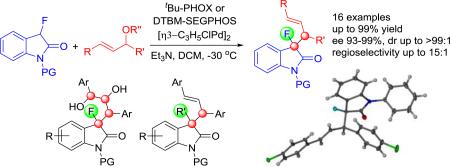
Synthetically versatile 3,3-disubstituted fluorooxindoles exhibiting vicinal chirality centers were obtained in high yields and with excellent enantio-, diastereo- and regioselectivity by catalytic asymmetric fluoroenolate alkylation with allylic acetates. The reaction proceeds under mild conditions and can be upscaled without compromising the asymmetric induction. The unique synthetic usefulness of the products is highlighted with the incorporation of additional functionalities and the formation of 3-fluorinated oxindoles exhibiting an array of four adjacent chirality centers. A new C-F bond functionalization path that provides unprecedented means for stereoselective generation of a chiral quaternary carbon center in the alkaloid scaffold is introduced.
Keywords: synthetic methods, homogeneous catalysis, quaternary chiral center, alkaloids, organofluorines
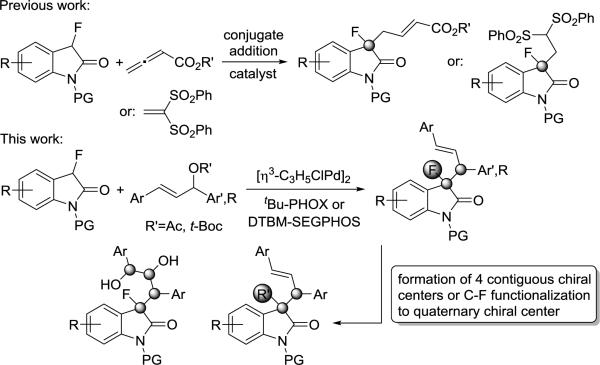
The widespread popularity of fluorinated drugs has been attributed to advantageous bioavailability, metabolic stability and other pharmacological properties that often compare favorably to those of the nonfluorinated parent compounds.[1] The high impact of chiral organofluorines on the health sector continuous to stimulate the advance of methods that exploit fluoroenolates for asymmetric C-C bond formation.[2] However, the presence of a fluorine atom is known to alter the reactivity and stability of enolates which may ultimately compromise yields and stereoselectivities, in particular when challenging structures are targeted. The importance of the 3,3-disubstituted oxindole ring, a common motif in many natural compounds and drugs, has fueled the development of intriguing synthetic strategies.[3] Following reports on the medicinal potential of 3-substituted 3-fluorooxindoles,[4] several groups have developed effective enantioselective fluorination methods for 3-alkyl and 3-aryloxindoles.[5] By contrast, catalytic asymmetric C-C bond formation with 3-fluorooxindoles has remained difficult and only three reports on conjugate additions have appeared in the literature.[6] The synthesis of C3-substituted oxindoles carrying two or more contiguous stereogenic centers is equally demanding albeit a few impressive examples are known.[7] A catalytic method that affords 3-fluorooxindoles exhibiting vicinal chirality centers with one being fluorinated would achieve both tasks at once and significantly widen the scope and availability of this important class of compounds.[8] In this regard, Trost's asymmetric allylic alkylation (AAA) would be a preferable reaction due to its outstanding value in natural product synthesis.[9] Although many examples of allylic alkylations producing a single chiral center with high asymmetric induction are known, the stereocontrolled formation of two adjacent chirality centers continues to be a challenge.[10]
We now present a catalytic method that addresses the challenges mentioned above by providing practical access to multifunctional 3-fluoroxoindoles with contiguous chirality centers using readily available allylic acetates and carbonates (Scheme 1). The products are obtained in high yields and with excellent enantio-, diastereo- and regioselectivity under mild conditions. The stereoselective introduction of additional functionalities, formation of fluorinated oxindoles having 4 adjacent chiral centers and the demonstration of stereoselective C-F bond functionalization underscore the general synthetic utility and prospect of this approach.
Scheme 1.
Asymmetric synthesis with fluorooxindoles.
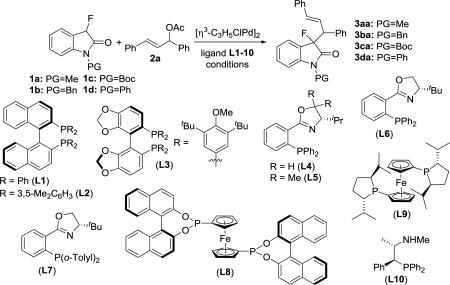
At the onset of this study, we tested the asymmetric allylic alkylation of N-methyl 3-fluorooxindole, 1a, with (E)-1,3-diphenylallyl acetate, 2a, using a variety of transition metal catalysts. After some initial screening, we were pleased to find that the desired C3-substituted fluorooxindole 3aa with two contiguous chiral centers can be obtained in 85-89% yield and up to 96% ee in the presence of 10 mol% of a palladium(II) BINAP or DTBM -Segphos complex, stoichiometric amounts of sodium acetate and 3 equivalents of bis(trimethylsilyl)acetamide (BSA) at room temperature. The results of the Pd(II) catalysis with ligands L1-3 were promising albeit the diastereoselectivity of this reaction was low (Table 1, entries 1-3). We then employed phosphine ligands L4-10 in the same protocol (entries 4-10). The introduction of the phosphinooxazoline L6 further improved both yield and asymmetric induction. Under these conditions, nearly quantitative amounts of 3aa were produced with excellent ee's (entry 6). The diastereomeric ratio, however, increased only slightly to 3:1. Extensive screening of inorganic bases revealed only small effects on the diastereoselectivity and the testing of various solvents did also not give superior results (see entries 11 and 12 and SI).
Table 1.
Optimization of the asymmetric allylic alkylation of fluorooxindoles.[a]
| ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | 1 | ligand | conditions | 3 | yield (%) | dr | ee (%) | |
| major | minor | |||||||
| 1 | 1a | L1 | BSA/NaOAc (3:1), 25 °C | 3aa | 89 | 2.2:1 | 92 | 92 |
| 2 | 1a | L2 | BSA/NaOAc (3:1), 25 °C | 3aa | 88 | 1.5:1 | 86 | 94 |
| 3 | 1a | L3 | BSA/NaOAc (3:1), 25 °C | 3aa | 85 | 1.1:1 | 96 | 93 |
| 4 | 1a | L4 | BSA/NaOAc (3:1), 25 °C | 3aa | 98 | 2.7:1 | 98 | 96 |
| 5 | 1a | L5 | BSA/NaOAc (3:1), 25 °C | 3aa | 99 | 2.7:1 | 98 | 96 |
| 6 | 1a | L6 | BSA/NaOAc (3:1), 25 °C | 3aa | 98 | 3.0:1 | 99 | 98 |
| 7 | 1a | L7 | BSA/NaOAc (3:1), 25 °C | 3aa | 93 | 2.5:1 | 30 | 28 |
| 8 | 1a | L8 | BSA/NaOAc (3:1), 25 °C | 3aa | 98 | 1.1:1 | 68 | 60 |
| 9 | 1a | L9 | BSA/NaOAc (3:1), 25 °C | 3aa | 96 | 1.9:1 | 64 | 72 |
| 10 | 1a | L10 | BSA/NaOAc (3:1), 25 °C | 3aa | 91 | 2.3:1 | 38 | 28 |
| 11 | 1a | L6 | BSA/KOAc (3:1), 25 °C | 3aa | 98 | 3.0:1 | 99 | 99 |
| 12 | 1a | L6 | BSA/Cs2CO3 (3:1), 25 °C | 3aa | 88 | 3.1:1 | 99 | 99 |
| 13 | 1b | L6 | BSA/KOAc (3:1), 25 °C | 3ba | 97 | 3.3:1 | 99 | >99 |
| 14 | 1c | L6 | BSA/KOAc (3:1), 25 °C | 3ca | 96 | 4.1:1 | 99 | 99 |
| 15 | 1d | L6 | BSA/KOAc (3:1), 25 °C | 3da | 93 | 4.7:1 | 99 | >99 |
| 16[b] | 1a | L6 | Et3N, 25 °C | 3aa | 99 | 3.1:1 | >99 | 99 |
| 17[b] | 1a | L6 | iPr2EtN, 25 °C | 3aa | 91 | 2.9:1 | >99 | 99 |
| 18[b] | 1a | L6 | DABCO, 25 °C | 3aa | 64 | 2.4:1 | >99 | 99 |
| 19[b] | 1a | L6 | DBU, 25 °C | 3aa | 95 | 2.5:1 | >99 | 99 |
| 20[b] | 1d | L6 | Et3N, 25 °C | 3da | 99 | 4.7:1 | >99 | 99 |
| 21[b,d] | 1d | L6 | Et3N, 0 °C | 3da | 99 | 7.0:1 | >99 | >99 |
| 22[c,e] | 1d | L6 | Et3N, −10 °C | 3da | 98 | 9.8:1 | >99 | >99 |
| 23[c,f] | 1d | L6 | Et3N, −30 °C | 3da | 96 | >19.0:1 | >99 | >99 |
All reactions were carried out using equimolar amounts of 1 and 2, 5 mol% of the Pd complex and 12 mol% of L1-10 in dichloromethane for 14-18 hours.
2 Equivalents of base.
3 Equivalents of base.
24 h.
36 h.
72 h.
The ee's were determined by chiral chromatography on Chiralpak IA, Amylose I and Cellulose 3. The diastereomeric ratio was obtained from 19F NMR analysis. Boc = tert-butoxycarbonyl, BSA = bis(trimethylsilyl)acetamide, DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene, DABCO = 1,4-diazabicyclo[2.2.2]octane.
At this point, we considered other options. A literature survey pointed us to asymmetric allylic substitution reactions that utilize chiral iridium,[11] copper,[12] ruthenium,[13] rhodium[14] and tungsten[15] complexes. Unfortunately, all of these catalytic systems gave 3aa in lower yields and decreased stereoselectivities. The reverse approach, i.e. introduction of the allyl group at C3 in oxindoles prior to asymmetric formation of the C-F bond gave unsatisfactory results.[16] Variation of the protecting group revealed that the placement of a Boc or a phenyl group at the oxindole nitrogen increases the dr's to 4.7:1 (entries 11 and 13-15). We then found that the reaction can also be conducted using organic bases (entries 16-19). The screening of several amines showed that the allylation of 1a and 1d gives 3aa and 3da, respectively, with very similar results when BSA and potassium acetate are replaced with triethylamine (compare entries 11 and 16 or 15 and 20). The use of expensive and moisture-sensitive bases such as BSA in asymmetric allylic alkylations is common and we were delighted to find that the introduction of triethylamine furnished a venue for improving the diastereoselectivity in combination with economical and operational advantages. Optimization of the temperature finally allowed us to produce 3da in excellent yield, ee's and more than 19:1 dr at −30 °C (entries 21-23). It is noteworthy that this protocol is equally successful with fluorooxindoles that carry removable protecting groups at the lactam nitrogen atom, such as benzyl, para-methoxyphenyl (PMP) and para-benzyloxyphenyl moieties, vide infra.
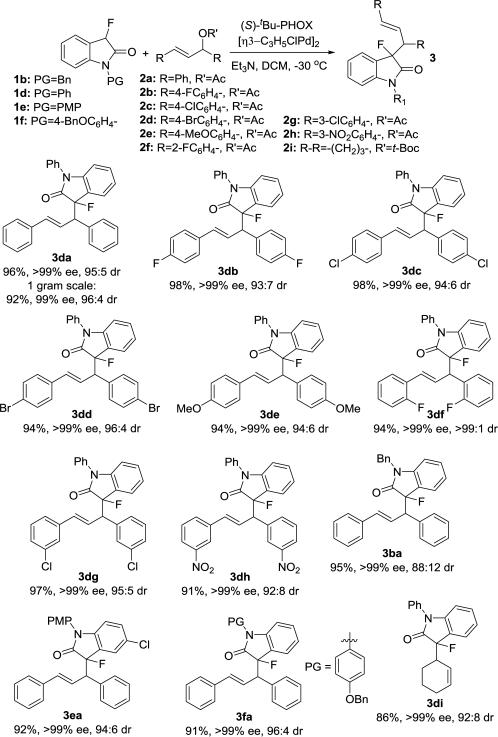
With an optimized procedure in hand, we continued with the evaluation of the reaction scope. We first used the N-phenyl fluorooxindole, 1d, in a variety of combinations with allylic acetates and carbonates 2a-i (Scheme 2). The corresponding products 3 were isolated in 86-98% yield, >99% ee and high dr's. Interestingly, the reaction with the ortho-substituted allylic acetate 2f gave oxindole 3df in 94% yield, >99% ee and more than 99:1 dr. When we employed N-benzyl-, N-4-methoxyphenyl- and N-4-benzyloxyphenyl-3-fluorooxindole, 1b, 1e and 1f, in our procedure we obtained 3ba, 3ea and 3fa in 91-95% yield, >99% ee and up to 96:4 dr. Importantly, the reaction is amenable to upscaling and we were able to prepare 1.15 g of 3da in 92% yield, >99% ee and 96:4 dr.
Scheme 2.
Reaction scope of the asymmetric catalytic allylation of fluorooxindoles.
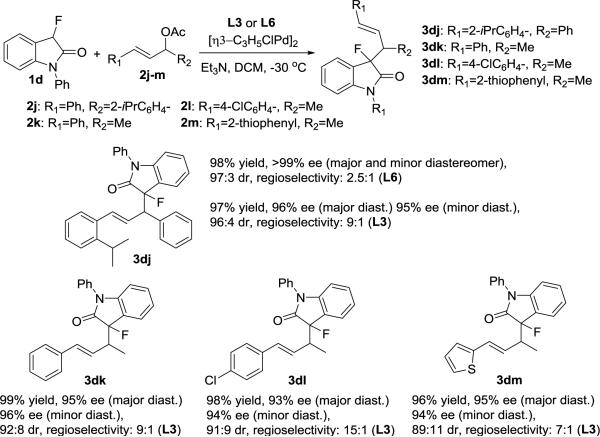
In addition to the highly enantio- and diastereoselective formation of the 3,3-disubstituted fluorooxindoles shown in Scheme 2, the possibility of regioselective asymmetric alkylation with nonsymmetrically substituted allylic acetates 2j-m was investigated.[17] The reaction between the fluorooxindole 1d and (E)-1-(2-isopropylphenyl)-3-phenylallyl acetate, 2j, gave 3dj as the major regioisomer in 98% yield and with excellent enantio- and diastereoselectivity (Scheme 3). Ligand screening revealed that the regioselectivity increases from 2.5:1 to 9:1 and without compromising yield and asymmetric induction when L6 is replaced with L3. We were pleased to find that this protocol is also successful with allylic acetates 2k-m carrying only one aryl terminus. The corresponding 3,3-disubstituted fluorooxindoles 3dk-3dm were obtained in 96-99% yield and with high stereo- and regioselectivity. The C-C bond formation occurs in all cases at the less hindered site of 2j-m irrespective of the original position of the acetyl group.
Scheme 3.
Regioselective asymmetric allylation with acetates 2j-m.
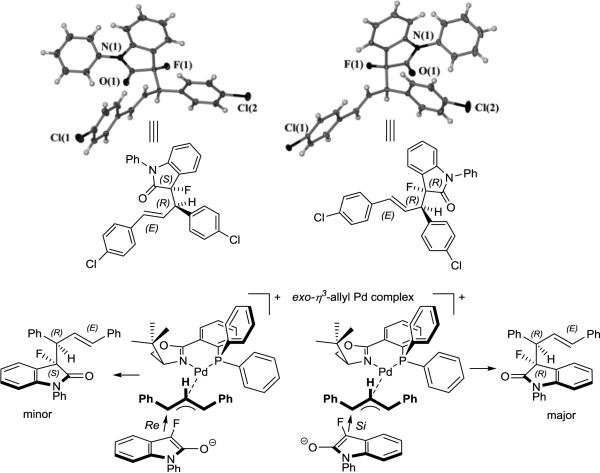
We then attempted to examine the sense of the asymmetric induction of the [Pd (S)-tBuPHOX] catalyzed reaction. Fortunately, slow evaporation of solutions of the dichlorosubstituted product 3dc in hexanes/isopropyl alcohol and hexanes/dichloromethane solutions gave single crystals of the major and minor diastereomers, respectively, which were identified by chiral HPLC. Crystallographic analysis showed that (R)-3-(R,E)-3-(1,3-bis(4-chlorophenyl)allyl)-3-fluoro-1-phenylindolin-2-one is the major diastereomer while the minor diastereomer has (S)-3-(R,E)-configuration (Figure 1).[18] Importantly, the only observed enantiomers of the major and minor diastereomers have opposite configuration at the C3 oxindole carbon, but the configuration at the sidechain stereocenter is the same in both cases. In accordance with literature reports,[19] the stereochemical outcome can be explained with a preferred attack from the Si-face of the fluoroenolate at the allylic position trans to the phosphorus atom of the PHOX ligand in a favored exo-η3-allyl palladium complex. This pathway yields the major (R,R,E)-isomer, while the attack from the Re-face of the fluoroenolate affords the minor (S,R,E)-diastereomer.[20]
Figure 1.
Top: Crystal structures of the minor (left) and the major (right) diastereomer of 3dc. Bottom: Proposed formation of the only observed two stereoisomers.
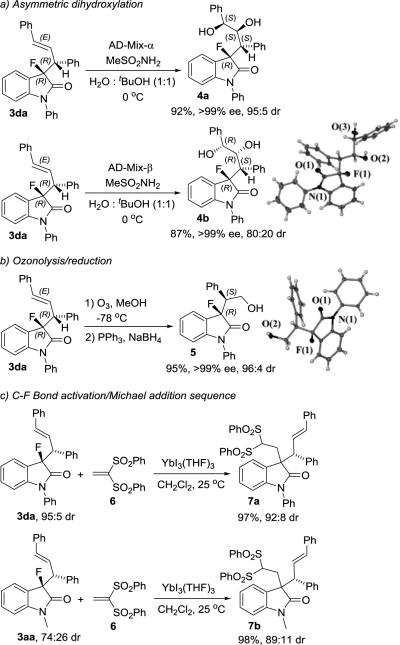
In addition to the medicinal potential, the chiral 3-fluorooxindoles prepared herein have unique synthetic value and offer unprecedented venues for further modifications. As mentioned above, the synthesis of C3-substituted oxindoles carrying two or more contiguous stereocenters remains a major challenge, in particular if this includes a fluorinated tertiary chiral carbon center. We anticipated that the incorporation of an allylic group into the fluorooxindole scaffold would facilitate introduction of new functionalities and formation of an array of 4 chirality centers. With that in mind we subjected 3da to a variety of double bond manipulations (Scheme 4). We were pleased to find that the Sharpless asymmetric dihydroxylation occurs with excellent stereocontrol.[21] The AD-mix-α catalyzed reaction gave 4a in high enantiomeric and diastereomeric purity with 92% yield, and it establishes 4 adjacent chiral centers along the alkyl sidechain. As expected, the dihydroxylation of 3da with AD-mix-β constitutes a mismatched pair and we obtained 4b in 87% yield, >99% ee and 4:1 dr. The sense of asymmetric induction was determined by crystallographic analysis. Alternatively, the double bond can be cleaved via ozonolysis and subsequent reduction to give the alcohol 5 in 95% yield and without any sign of erosion of the stereochemical purity.
Scheme 4.
Selective transformations of 3-fluorooxindoles. The absolute and relative stereochemistry of 3da was assigned in accordance with the crystallographic data obtained with 3dc.
The steadily increasing availability of fluorinated compounds has raised recent interest in C-F bond activation strategies.[22] To this end, progress with aliphatic substrates has been largely limited by the chemical inertness and stability of the Csp3-F bond. Intriguing reports on fluoride/halide exchange[23] and aliphatic C-F bond cleavage followed by carbon-heteroatom[24] or carbon-carbon coupling[25] have emerged in the last few years. Selective transformations that proceed under mild conditions and in the presence of other functional groups, however, are rare and particularly difficult. We have identified a unique defluorination pathway that provides a new entry to selective C-C bond formation. The treatment of 3da with ytterbium triiodide in the presence of equimolar amounts of 1,1-(diphenylsulfonyl)ethylene, 6, gave the Michael addition product 7a in quantitative yields and high dr at 25 °C (Scheme 4). Similar results were obtained when we employed 3aa, which was available in relatively low diastereomeric purity from the AAA optimization study, in the same reaction to produce 7b in 98% yield and with 89:11 dr. To the best of our knowledge this is the first example showing formation of a quaternary chiral carbon center from a tertiary alkyl fluoride and it further highlights the general synthetic usefulness of our 3-fluorooxindoles.[26]
In conclusion, we have developed a catalytic asymmetric alkylation method that affords practical and high-yielding access to a series of 3,3-disubstituted fluorooxindole alkaloids exhibiting vicinal chirality centers. The reaction proceeds with excellent enantio-, diastereo- and regioselectivity and it can be upscaled without compromising the stereochemical outcome. The unique synthetic utility of the products is demonstrated with the introduction of additional functionalities and the formation of fluorinated oxindoles exhibiting four contiguous chiral centers. Furthermore, a new C-F bond functionalization path that provides unprecedented means for stereoselective incorporation of a chiral quaternary carbon center into the alkaloid scaffold is presented. The synthetic prospect and mechanism of the C-F bond activation/C-C bond formation sequence is currently under investigation in our laboratory and will be reported in due course.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support from NIH (R15GM106260) and the donors of the American Chemical Society Petroleum Research Fund (PRF52737ND).
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Zhou Y, Wang J, Gu Z, Wang S, Zhu W, Acena JL, Soloshonok VA, Izawa K, Liu H. Chem. Rev. 2016;116:422. doi: 10.1021/acs.chemrev.5b00392. [DOI] [PubMed] [Google Scholar]
- 2.a Zhang P, Wolf C. J. Org. Chem. 2012;77:8840. doi: 10.1021/jo3017583. [DOI] [PubMed] [Google Scholar]; b Saidalimu I, Fang X, He XP, Liang J, Yang XY, Wu FH. Angew. Chem. Int. Ed. 2013;52:5566. doi: 10.1002/anie.201301443. [DOI] [PubMed] [Google Scholar]; c Saidalimu I, Fang X, Lv W, Yang X, He X, Zhang J, Wu FH. Adv. Synth. Catal. 2013;355:857. [Google Scholar]; d Zhang P, Wolf C. Angew. Chem. Int. Ed. 2013;52:7869. doi: 10.1002/anie.201303551. [DOI] [PubMed] [Google Scholar]; e Xie C, Wu L, Mei H, Soloshonok VA, Han J, Pan Y. Org. Biomol. Chem. 2014;12:7836. doi: 10.1039/c4ob01575d. [DOI] [PubMed] [Google Scholar]; f Wu C, Li G, Sun W, Zhang M, Hong L, Wang R. Org. Lett. 2014;16:1960. doi: 10.1021/ol500517d. [DOI] [PubMed] [Google Scholar]; g Xie C, Wu L, Han J, Soloshonok VA, Pan Y. Angew. Chem. Int. Ed. 2015;54:6019. doi: 10.1002/anie.201500908. [DOI] [PubMed] [Google Scholar]; h Xie C, Dai Y, Mei H, Han J, Soloshonok VA, Pan Y. Chem. Commun. 2015;51:9149. doi: 10.1039/c5cc02256h. [DOI] [PubMed] [Google Scholar]; i Saadi J, Wennemers H. Nat. Chem. 2016;8:276. doi: 10.1038/nchem.2437. [DOI] [PubMed] [Google Scholar]; j Balaraman K, Moskowitz M, Liu Y, Wolf C. Synthesis. 2016;48:2376. doi: 10.1055/s-0035-1561433. [DOI] [PMC free article] [PubMed] [Google Scholar]; For a general review. [Google Scholar]; k Mei H, Acena JL, Soloshonok VA, Roeschenthaler G-V, Han J. Eur. J. Org. Chem. 2015:6401. [Google Scholar]
- 3.a Ma S, Han X, Krishnan S, Virgil SC, Stoltz BM. Angew. Chem. Int. Ed. 2009;48:8037. doi: 10.1002/anie.200902943. [DOI] [PubMed] [Google Scholar]; b Antonchick AP, Gerding-Reimers C, Catarinella M, Schürmann M, Preut H, Ziegler S, Rauh D, Waldmann H. Nat. Chem. 2010;2:735. doi: 10.1038/nchem.730. [DOI] [PubMed] [Google Scholar]; c Guo C, Song J, Huang J-Z, Chen P-H, Luo S-W, Gong L-Z. Angew. Chem. Int. Ed. 2012;51:1046. doi: 10.1002/anie.201107079. [DOI] [PubMed] [Google Scholar]; d Zhu B, Zhang W, Lee R, Han Z, Yang W, Tan D, Huang K-W, Jiang Z. Angew. Chem. Int. Ed. 2013;52:6666. doi: 10.1002/anie.201302274. [DOI] [PubMed] [Google Scholar]; e Xie W, Jiang G, Liu H, Hu J, Pan X, Zhang H, Wan X, Lai Yi., Mae D. Angew. Chem. Int. Ed. 2013;52:12924. doi: 10.1002/anie.201306774. [DOI] [PubMed] [Google Scholar]; f Mitsunuma H, Shibasaki M, Kanai M, Matsunaga S. Angew. Chem. Int. Ed. 2012;51:5217. doi: 10.1002/anie.201201132. [DOI] [PubMed] [Google Scholar]; g Zong L, Du S, Chin KF, Wang C, Tan C-H. Angew. Chem. Int. Ed. 2015;54:9390. doi: 10.1002/anie.201503844. [DOI] [PubMed] [Google Scholar]; h Biswas P, Paul S, Guin J. Angew. Chem. Int. Ed. 2016;55:7756. doi: 10.1002/anie.201603809. [DOI] [PubMed] [Google Scholar]; i Sankar MG, Garcia-Castro M, Golz C, Strohmann C, Kumar K. Angew. Chem. Int. Ed. 2016;55:9709. doi: 10.1002/anie.201603936. [DOI] [PubMed] [Google Scholar]; j Kong W, Wang Q, Zhu J. Angew. Chem. Int. Ed. 2016;55:9714. doi: 10.1002/anie.201603950. [DOI] [PubMed] [Google Scholar]
- 4.a Gribkoff VK, Starrett JE, Jr., Dworetzky SL, Hewawasam P, Boissard CG, Cook DA, Frantz SW, Heman K, Hibbard JR, Huston K, Johnson G, Krishnan BS, Kinney GG, Lombardo LA, Meanwell NA, Molinoff PB, Myers RA, Moon SL, Ortiz A, Pajor L, Pieschl RL, Post-Munson DJ, Signor LJ, Srinivas N, Taber MT, Thalody G, Trojnacki JT, Wiener H, Yeleswaram K, Yeola SW. Nat. Med. 2001;7:471. doi: 10.1038/86546. [DOI] [PubMed] [Google Scholar]; b Hewawasam P, Gribkoff VK, Pendri Y, Dworetzky SI, Meanwell NA, Martinez E, Boissard CG, Post-Munson DJ, Trojnacki JT, Yeleswaram K, Pajor LM, Knipe J, Gao Q, Perrone R, Starrett JE., Jr. Bioorg. Med. Chem. Lett. 2002;12:1023. doi: 10.1016/s0960-894x(02)00101-4. [DOI] [PubMed] [Google Scholar]
- 5. Selected examples. [Google Scholar]; a Shibata N, Suzuki E, Asahi T, Shiro M. J. Am. Chem. Soc. 2001;123:7001. doi: 10.1021/ja010789t. [DOI] [PubMed] [Google Scholar]; b Hamashima Y, Suzuki T, Takano H, Shimura Y, Sodeoka M. J. Am. Chem. Soc. 2005;127:10164. doi: 10.1021/ja0513077. [DOI] [PubMed] [Google Scholar]; c Shibata N, Kohno J, Takai K, Ishimaru T, Nakamura S, Toru T, Kanemasa S. Angew. Chem., Int. Ed. 2005;44:4204. doi: 10.1002/anie.200501041. [DOI] [PubMed] [Google Scholar]; d Ishimaru T, Shibata N, Horikawa T, Yasuda N, Nakamura S, Toru T, Shiro M. Angew. Chem., Int. Ed. 2008;47:4157. doi: 10.1002/anie.200800717. [DOI] [PubMed] [Google Scholar]; e Deng Q-H, Wadepohl H, Gade LH. Chem. Eur. J. 2011;17:14922. doi: 10.1002/chem.201102375. [DOI] [PubMed] [Google Scholar]; f Shen K, Liu XH, Lin LL, Feng XM. Chem. Sci. 2012;3:327. [Google Scholar]; g Li J, Cai Y, Chen W, Liu X, Lin L, Feng X. J. Org. Chem. 2012;77:9148. doi: 10.1021/jo301705t. [DOI] [PubMed] [Google Scholar]; h Gu X, Zhang Y, Xu Z-J, Che C-M. Chem. Commun. 2014;50:7870. doi: 10.1039/c4cc01631a. [DOI] [PubMed] [Google Scholar]; For an orthogonal approach based on asymmetric ring construction, see. [Google Scholar]; i Wu L, Falivene L, Drinkel E, Grant S, Linden A, Cavallo L, Dorta R. Angew. Chem. Int. Ed. 2012;51:2870. doi: 10.1002/anie.201200206. [DOI] [PubMed] [Google Scholar]
- 6.a Dou X, Lu Y. Org. Biomol. Chem. 2013;11:5217. doi: 10.1039/c3ob41267a. [DOI] [PubMed] [Google Scholar]; b Wang T, Hoon DL, Lu Y. Chem. Commun. 2015;51:10186. doi: 10.1039/c5cc03289j. [DOI] [PubMed] [Google Scholar]; c Kim YS, Kwon SJ, Kim DY. Bull. Korean Chem. Soc. 2015;36:1512. [Google Scholar]
- 7.a Ogawa S, Shibata N, Inagaki J, Nakamura S, Toru T, Shiro M. Angew. Chem. Int. Ed. 2007;46:8666. doi: 10.1002/anie.200703317. [DOI] [PubMed] [Google Scholar]; b Bui T, Syed S, Barbas CF., III J. Am. Chem. Soc. 2009;131:8758. doi: 10.1021/ja903520c. [DOI] [PubMed] [Google Scholar]; c Wu M-Y, He W-W, Liu X-Y, Tan B. Angew. Chem. Int. Ed. 2015;54:9409. doi: 10.1002/anie.201504640. [DOI] [PubMed] [Google Scholar]; d Ohmatsu K, Ando Y, Ooi T. J. Am. Chem. Soc. 2013;135:18706. doi: 10.1021/ja411647x. [DOI] [PubMed] [Google Scholar]; e Tan B, Candeias NR, Barbas CF. Nat. Chem. 2011;3:473. doi: 10.1038/nchem.1039. [DOI] [PubMed] [Google Scholar]; f Guo Q-X, Liu Y-W, Li X-C, Zhong L-Z, Peng Y-G. J. Org. Chem. 2012;77:3589. doi: 10.1021/jo202585w. [DOI] [PubMed] [Google Scholar]; g Lv H, Tiwari B, Mo J, Xing C, Chi YR. Org. Lett. 2012;14:5412. doi: 10.1021/ol302475g. [DOI] [PubMed] [Google Scholar]; h Zhao J, Fang B, Luo W, Hao X, Liu X, Lin L, Feng X. Angew. Chem. Int. Ed. 2015;54:241. doi: 10.1002/anie.201408730. [DOI] [PubMed] [Google Scholar]; i Engl OD, Fritz SP, Wennemers H. Angew. Chem. Int. Ed. 2015;54:8193. doi: 10.1002/anie.201502976. [DOI] [PubMed] [Google Scholar]; j Shan J, Cui B, Wang Y, Yang C, Zhou X, Han W, Chen Y. J. Org. Chem. 2016;81:5270. doi: 10.1021/acs.joc.6b00278. [DOI] [PubMed] [Google Scholar]; k You Y, Wu Z-J, Chen J-F, Wang Z-H, Xu X-Y, Zhang X-M, Yuan W-C. J. Org. Chem. 2016;81:5759. doi: 10.1021/acs.joc.6b00896. [DOI] [PubMed] [Google Scholar]
- 8.Xie C, Zhang L, Sha W, Soloshonok VA, Han J, Pan Y. Org. Lett. 2016;18:3270. doi: 10.1021/acs.orglett.6b01516. For a diastereoselective Mannich reaction with fluorooxindoles, see. [DOI] [PubMed] [Google Scholar]
- 9.a Trost BM, Krische MJ, Radinov R, Zanoni G. J. Am. Chem. Soc. 1996;118:6297. [Google Scholar]; b Trost BM, Toste FD. J. Am. Chem. Soc. 1999;121:3543. [Google Scholar]; c Trost BM, Patterson DE, Hembre EJ. J. Am. Chem. Soc. 1999;121:10834. [Google Scholar]; d Trost BM, Bunt RC, Lemoine RC, Calkins TL. J. Am. Chem. Soc. 2000;122:5968. [Google Scholar]; e Trost BM, Fandrick DR. Org. Lett. 2005;7:823. doi: 10.1021/ol047513l. [DOI] [PubMed] [Google Scholar]; f Trost BM, Machacek MR, Tsui H-C. J. Am. Chem. Soc. 2005;127:7014. doi: 10.1021/ja050340q. [DOI] [PubMed] [Google Scholar]; g Trost BM, Machacek MR, Aponick A. Acc. Chem. Res. 2006;39:747. doi: 10.1021/ar040063c. [DOI] [PubMed] [Google Scholar]; For examples with oxindoles [Google Scholar]; h Trost BM, Zhang Y. J. Am. Chem. Soc. 2006;128:4590. doi: 10.1021/ja060560j. [DOI] [PubMed] [Google Scholar]; i Jiang K, Peng J, Cui H-L, Chen Y-C. Chem. Commun. 2009:3955. doi: 10.1039/b905177e. [DOI] [PubMed] [Google Scholar]; j Trost BM, Malhotra S, Chan WH. J. Am. Chem. Soc. 2011;133:7328. doi: 10.1021/ja2020873. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Trost BM, Xie J, Sieber JD. J. Am. Chem. Soc. 2011;133:20611. doi: 10.1021/ja209244m. [DOI] [PMC free article] [PubMed] [Google Scholar]; l Franckevičius V, Cuthbertson JD, Pickworth M, Pugh DS, Taylor RJK. Org. Lett. 2011;13:4264. doi: 10.1021/ol201613a. [DOI] [PubMed] [Google Scholar]; m Trost BM, Masters JT, Burns AC. Angew. Chem., Int. Ed. 2013;52:2260. doi: 10.1002/anie.201209783. [DOI] [PMC free article] [PubMed] [Google Scholar]; n Trost BM, Osipov M. Angew. Chem., Int. Ed. 2013;52:9176. doi: 10.1002/anie.201302805. [DOI] [PMC free article] [PubMed] [Google Scholar]; o Jayakumar S, Kumarswamyreddy N, Prakash M, Kesavan V. Org. Lett. 2015;17:1066. doi: 10.1021/acs.orglett.5b00034. [DOI] [PubMed] [Google Scholar]; p Zhou H, Zhang L, Xu C, Luo S. Angew. Chem., Int. Ed. 2015;54:12645. doi: 10.1002/anie.201505946. [DOI] [PubMed] [Google Scholar]; q Kita Y, Kavthe RD, Oda H, Mashim K. Angew. Chem., Int. Ed. 2016;55:1098. doi: 10.1002/anie.201508757. [DOI] [PubMed] [Google Scholar]; r Pritchett BP, Kikuchi J, Numajiri Y, Stoltz BM. Angew. Chem. Int. Ed. 2016;55:13529. doi: 10.1002/anie.201608138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a Nakamura M, Hajra A, Endo K, Nakamura E. Angew. Chem. Int. Ed. 2005;44:7248. doi: 10.1002/anie.200502703. [DOI] [PubMed] [Google Scholar]; b Belanger E, Houze C, Guimond N, Cantin K, Paquin J-F. Chem. Commun. 2008:3251. doi: 10.1039/b803097a. [DOI] [PubMed] [Google Scholar]; c Huang Y, Zhang Q-S, Fang P, Chen T-G, Zhu J, Hou X-L. Chem. Commun. 2014;50:6751. doi: 10.1039/c4cc02158d. [DOI] [PubMed] [Google Scholar]; d Jiang X, Chen W, Hartwig JF. Angew. Chem. Int. Ed. 2016;55:5819. doi: 10.1002/anie.201600235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a Bartels B, Helmchen G. Chem. Commun. 1999:741. [Google Scholar]; b Takeuchi R, Ue N, Tanabe K, Yamashita K, Shiga N. J. Am. Chem. Soc. 2001;123:9525. doi: 10.1021/ja0112036. [DOI] [PubMed] [Google Scholar]
- 12.van Zijl AW, Arnold LA, Minnaard AJ, Feringa BL. Adv. Synth. Catal. 2004;346:413. [Google Scholar]
- 13.a Matsushima Y, Onitsuka K, Kondo T, Mitsudo T, Takahashi S. J. Am. Chem. Soc. 2001;123:10405. doi: 10.1021/ja016334l. [DOI] [PubMed] [Google Scholar]; b Trost BM, Fraisse PL, Ball ZT. Angew. Chem. Int. Ed. 2002;41:1059. doi: 10.1002/1521-3773(20020315)41:6<1059::aid-anie1059>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Evans PA, Leahy DK. Chemtracts. 2003;16:567. [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd-Jones GC, Pfaltz A. Angew. Chem. Int. Ed. 1995;34:462. [Google Scholar]
- 16.a Liang T, Neumann CN, Ritter T. Angew. Chem. Int. Ed. 2013;52:8214. doi: 10.1002/anie.201206566. [DOI] [PubMed] [Google Scholar]; b Yang X, Wu T, Phipps RJ, Toste FD. Chem. Rev. 2015;115:826. doi: 10.1021/cr500277b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. For nonstereoselective regiodivergent decarboxylative allylations with difluoroenolate synthons, see. [Google Scholar]; a Yang M-H, Orsi DL, Altman RA. Angew. Chem. Int. Ed. 2015;54:2361. doi: 10.1002/anie.201410039. [DOI] [PMC free article] [PubMed] [Google Scholar]; Examples of regioselective AAA with nonfluorinated substrates [Google Scholar]; b Trost BM, Toste FD. J. Am. Chem. Soc. 1999;121:4545. [Google Scholar]; c Kazmaier U, Stolz D. Angew. Chem. Int. Ed. 2006;45:3072. doi: 10.1002/anie.200600100. [DOI] [PubMed] [Google Scholar]; d Grassi D, Alexakis A. Angew. Chem. Int. Ed. 2013;52:13642. doi: 10.1002/anie.201307591. [DOI] [PubMed] [Google Scholar]; e Liu W-B, Reeves CM, Stoltz BM. J. Am. Chem. Soc. 2013;135:17298. doi: 10.1021/ja4097829. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Huwig K, Schultz K, Kazmaier U. Angew. Chem. Int. Ed. 2015;54:9120. doi: 10.1002/anie.201502975. [DOI] [PubMed] [Google Scholar]
- 18. CCDC numbers 1494553 [(R,R,E)-3dc], 1494554 [[(S,R,E)-3dc], 1494555 [1d], 1502269 [4b] and 1502270 [5] contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre.
- 19.a von Matt P, Pfaltz A. Angew. Chem. Int. Ed. 1993;32:566. [Google Scholar]; b Sprinz J, Helmchen G. Tetrahedron Lett. 1993;34:1769. [Google Scholar]; c Dawson GJ, Frost CG, Williams JMJ, Coote SJ. Tetrahedron Lett. 1993;34:3149. [Google Scholar]; d Helmchen G, Pfaltz A. Acc. Chem. Res. 2000;33:336. doi: 10.1021/ar9900865. [DOI] [PubMed] [Google Scholar]
- 20. The isomerization between exo- and endo-η3-allyl palladium complexes is expected to be fast. However, the exo-isomer is typically preferred and given the generally accepted nucleophilic attack at the allylic position trans to the phosphorus atom the experimental results are in agreement with a pathway involving the exo-allyl Pd complex. Further reading. [Google Scholar]; a Kollmar M, Goldfuss B, Reggelin M, Rominger F, Helmchen G. Chem. Eur. J. 2001;7:4913. doi: 10.1002/1521-3765(20011119)7:22<4913::aid-chem4913>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]; b Kollmar M, Steinhagen H, Janssen JP, Goldfuss B, Malinovskaya SA, Vazquez J, Rominger F, Helmchen G. Chem. Eur. J. 2002;8:3103. doi: 10.1002/1521-3765(20020715)8:14<3103::AID-CHEM3103>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 21.Sharpless KB, Amberg W, Bennani YL, Crispino GA, Hartung J, Jeong K-S, Kwong H-L, Morikawa K, Wang Z-M, Xu D, Zhang X-L. J. Org. Chem. 1992;57:2768. [Google Scholar]
- 22.a Douvris C, Ozerov OV; Science. 2008;321:1188. doi: 10.1126/science.1159979. [DOI] [PubMed] [Google Scholar]; b Choi J, Wang DY, Kundu S, Choliy Y, Emge TJ, Krogh-Jespersen K, Goldman AS. Science. 2011;332:1545. doi: 10.1126/science.1200514. [DOI] [PubMed] [Google Scholar]; c Caputo CB, Hounjet LJ, Dobrovetsky R, Stephan DW. Science. 2013;341:1374. doi: 10.1126/science.1241764. [DOI] [PubMed] [Google Scholar]; d Stahl HF, Klare T, Oestreich M. ACS Catal. 2013;3:1578. [Google Scholar]; e Kuehnel MF, Lentz D, Braun T. Angew. Chem. Int. Ed. 2013;52:3328. doi: 10.1002/anie.201205260. [DOI] [PubMed] [Google Scholar]; f Stahl T, Klare HFT, Oestreich M. J. Am. Chem. Soc. 2013;135:1248. doi: 10.1021/ja311398j. [DOI] [PubMed] [Google Scholar]; g Ahrens M, Scholz G, Braun T, Kemnitz E. Angew. Chem. Int. Ed. 2013;52:5328. doi: 10.1002/anie.201300608. [DOI] [PubMed] [Google Scholar]; h Ohashi M, Shibata M, Ogoshi S. Angew. Chem. Int. Ed. 2014;53:13578. doi: 10.1002/anie.201408467. [DOI] [PubMed] [Google Scholar]; i Liu X-W, Echavarren J, Zarate C, Martin R. J. Am. Chem. Soc. 2015;137:12470. doi: 10.1021/jacs.5b08103. [DOI] [PubMed] [Google Scholar]
- 23.a Traff AM, Janjetovic M, Ta L, Hilmersson G. Angew. Chem. Int. Ed. 2013;52:12073. doi: 10.1002/anie.201306104. [DOI] [PubMed] [Google Scholar]; b Janjetovic M, Ekebergh A, Traff AM, Hilmersson G. Org. Lett. 2016;18:2804. doi: 10.1021/acs.orglett.6b01022. [DOI] [PubMed] [Google Scholar]
- 24.Traff AM, Janjetovic M, Hilmersson G. Chem. Commun. 2015;51:13260. doi: 10.1039/c5cc04723d. [DOI] [PubMed] [Google Scholar]
- 25.a Gu W, Haneline MR, Douvris C, Ozerov OV. J. Am. Chem. Soc. 2009;131:11203. doi: 10.1021/ja903927c. [DOI] [PubMed] [Google Scholar]; b Champagne PA, Benhassine Y, Desroches J, Paquin J-F. Angew. Chem. Int. Ed. 2014;53:13835. doi: 10.1002/anie.201406088. [DOI] [PubMed] [Google Scholar]; c Zhu J, Perez M, Caputo CB, Stephan DW. Angew. Chem. Int. Ed. 2016;55:1417. doi: 10.1002/anie.201510494. [DOI] [PubMed] [Google Scholar]; d Dryzhakov M, Moran J. ACS Catal. 2016;6:3670. [Google Scholar]; e Yoshida S, Shimomori K, Kim Y, Hosoya T. Angew. Chem. Int. Ed. 2016;55:10406. doi: 10.1002/anie.201604776. [DOI] [PubMed] [Google Scholar]
- 26.Kato Y, Furutachi M, Chen Z, Mitsunuma H, Matsunaga S, Shibasaki M. J. Am. Chem. Soc. 2009;131:9168. doi: 10.1021/ja903566u. For an alternative asymmetric route toward analogues of 7 having two contiguous chiral centers, see. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.