Abstract
The role of optic nerve sheath fenestration (ONSF) in the management of idiopathic intracranial hypertension remains controversial, with indications, risks, and benefits compared to cerebro-spinal fluid diversion procedures not fully elucidated. We report a retrospective record review of 37 patients (50 eyes) which had undergone ONSF by a single surgeon. Visual acuity (VA) improved in 22% of operated eyes and 17% of fellow eyes; stabilized in 54% of operated and 74% of fellow eyes; and deteriorated in 24% of operated and 9% of fellow eyes. Better pre-operative VA (p = 0.01), colour vision (p = 0.002), and earlier intervention (p = 0.04) were associated with stabilization. We conclude that ONSF often stabilizes vision and visual fields. Our results were best in patients with better pre-operative vision and in those with earlier intervention.
Keywords: Idiopathic intracranial hypertension, optic nerve sheath fenestration, optic neuropathy
Introduction
Idiopathic intracranial hypertension (IIH) is a relatively common disorder predominately affecting young obese women. The mainstays of treatment remain weight loss and medical management with diuretics; however in certain cases of severe acute IIH or in those patients who are refractory to medical treatments, surgical intervention is occasionally required. Surgical options include optic nerve sheath fenestration (ONSF) or cerebrospinal fluid (CSF) diversion procedures, such as lumbar-peritoneal or ventricular-peritoneal shunts. Although there are no evidence-based recommendations supported by randomized clinical trials, all three procedures are accepted as effective treatments to halt vision loss in these patients.1,2 In recent years, shunt procedures have been utilized with increasing frequency,3 however these procedures are known to carry a high risk of systemic complications and shunt failure.1 In order to identify pre-operative characteristics associated with better outcomes and hoping to refine treatment guidelines and decision making, we examined the results of our experience using ONSF as a primary surgical treatment for IIH-associated vision loss, focusing specifically on visual acuity (VA) and visual field (VF) outcomes and evaluating for pre-operative factors associated with better outcomes.
Methods
This study was approved by the University of Pennsylvania Institutional Review Board and conformed to the requirements of the US Health Insurance Portability and Accountability act. The clinical records of all patients at the Scheie Eye Institute at the University of Pennsylvania with IIH who underwent ONSF by a single surgeon (NJV) were retrospectively reviewed. Those subjects who had at least four weeks of post-operative follow-up were included in the analysis. Patients were excluded if there was any history of prior ONSF or CSF diversion procedure. IIH was defined by the modified Dandy criteria.4
The following pre-operative characteristics were recorded from the patients’ charts: age at onset, duration of signs and symptoms, age at surgery, gender, best corrected VA, colour vision tested with a standard Ishihara colour plate book, pre-operative medical treatments, risk factors for IIH, symptoms of diplopia, pulsatile tinnitus, headache, and transient visual obscurations, and results of serial VF examinations and optic nerve evaluations. In addition, all subsequent surgical procedures and complications were noted. For fellow eyes that eventually underwent subsequent ONSF, data were taken from the final post-operative visit after the first eye but before the second eye. If the second eye surgery was performed within four weeks of the first eye, then the fellow eye from the patient was excluded. The procedure used for all ONSF procedures is published elsewhere.5 In general, patients were selected to undergo ONSF if they presented with profound or severe vision loss, were refractory to medical treatment, or if they were unable to comply with prescribed medication or follow-up.
VA was tested for all patients using projected linear ETDRS optotypes tested monocularly. Attempt was made to perform automated VFs for all patients diagnosed with IIH. However, if patients were unable to perform automated VF tests with adequate reliability (<50% fixation losses) they were instead followed with manual kinetic perimetry. Failure to perform reliable automated VF could be secondary to poor central VA or lack of patient cooperation. Manual kinetic perimetry was performed by a trained technician and utilized a test object speed of approximately 3°/s as objects were moved from peripheral non-seeing areas inward. To delineate scotomata and the blind spot, stimuli were moved from inside the scotoma outward. Manual perimetry was considered unreliable if the examiner assessed patient fixation to be poor or if the blind spot could not be mapped. We considered as a significant change in manual perimetry results a change in the extent of VF loss from baseline by at least 10° in two isopters or resolution of the defect. Colour vision was tested monocularly using standard Ishihara colour plates. The percentage of plates read correctly defined the outcome for each eye.
For subjects diagnosed with functional visual loss during the treatment course, objective features of the examination were used to assess improvement. Functional visual loss was typically defined by standard testing methods such as tubular VFs, prism dissociation testing, stereoacuity, and reverse Snellen acuity testing, or any other inconsistencies during the examination when testing VA, VF, or colour vision. If the diagnosis of functional visual loss was mentioned in the record with regard to any metric of visual performance (VA, VF, and colour vision), those data were excluded. However, for objective outcomes such as pupil examination and resolution of optic disc oedema, these records were included. Patients with functional visual loss were operated on when there was a definite true component of vision loss represented by examination features such as atrophic papilloedema or disc pallor at presentation.
Outcome Groups
Patients were divided into a final outcome group based upon their post-operative VA at the last visit. An eye was considered to be “improved” if the VA improved by at least −0.2 logmar from the pre-operative value; “stable” if the VA was within 0.2 logmar, and “deteriorated” if the final VA was ≥0.2 logmar from the pre-operative value. Similarly, those patients who were followed serially with automated perimetry were divided into a final outcome group based upon their final mean deviation (MD). Eyes in which the MD improved by at least 5 dB were considered “improved”, within 5 dB were considered “stable”, and a worsening of MD by at least 5 dB were considered “deteriorated”. All automated and manual VFs were then analysed blindly by a masked grader (SLP) who determined the pattern and extent of VF loss using similar criteria to the Ocular Hypertension Treatment Study scheme which defines multiple patterns of VF loss from which to classify VFs and then an assessment of the extent of VF loss (measured in degrees).6 The VF pattern configurations of generalized constriction and enlarged blind spot were added to the choices of patterns due to their unique appearance and high prevalence in patients with IIH. Optic nerve photographs were also analysed blindly by a masked grader (SLP) and were assigned a Frisen7 score.
Statistical Analysis
Statistical analyses were performed using statistical software, STATA version 10.0 and Microsoft Excel. Pre-operative and final post-operative values were compared using a two-tailed Student’s t-test. A p value of <0.05 was considered statistically significant. Univariate analyses were done using logistic regression models to evaluate for associations between pre-operative factors (gender, VA, colour vision, MD, presence of a relative afferent pupillary defect, optic nerve pallor, and time to surgery) and VA stabilization/improvement or MD stabilization/improvement.
Results
Patient Population
General patient demographic information is presented in Table 1. Thirty-seven patients who underwent 50 ONSF procedures met the study inclusion criteria (13 subjects had bilateral ONSF). The post-operative follow-up ranged from 4 to 700 weeks, with 3 patients having less than 3 months follow-up and 26 patients having greater than 1 year (post-operative follow-up 2–5 years in 7 patients and >5 years in 14 patients). Age at surgery ranged from 19–74 years; the median age was 31 years and all but 3 patients were under the age of 50 years.
TABLE 1 .
Demographic and pre-operative information for patients undergoing ONSF for IIH (n = 37).
| Mean ± standard deviation | Range | |
|---|---|---|
| Age (years) | 33 ± 11 | 19–74 |
| Gender | 5/37 (13%) male | |
| Post-operative follow-up (weeks) | 210 ± 200 | 4–700 |
| Unilateral/bilateral | 24 unilateral, 13 bilateral | |
| Subsequent CSF diversion procedures | 8 (4 after second eye ONSF) | |
| Time to surgery (weeks) | 36 ± 54 | 0.5–226 |
| Time to second procedure (weeks) | 6 ± 7 | |
| Clinical recurrence | 12/37 (32%) | |
| Time to clinical recurrence (weeks) | 55 ± 56 | 5–375 |
| Pre-operative steroids | 10/37 (27%) | |
| Presenting symptoms | Headache: 35/37 (95%) | |
| Pulsatile tinnitus 18/37 (49%) | ||
| Transient visual obscuration 17/37 (46%) | ||
| Diplopia 2/37 (5%) | ||
| Pre-operative risk factors for IIH | Obesity 36/37 (97%) | |
| Tetracycline 3/37 (8%) | ||
| Obstructive sleep apnea 2/37 (5%) | ||
| Oral contraceptive use 2/37 (5%) | ||
Examination Findings
The overall mean pre-operative VA was 0.38 ± 0.5 logmar in the surgical eye and 0.33 ± 0.5 logmar in the fellow eye. Post-operatively, the overall mean VA was 0.7 ± 0.8 logmar and 0.16 ± 0.36 logmar in the surgical and fellow eyes, respectively. There was no significant difference in the pre-operative and post-operative VA when the means for the surgical and fellow eyes were compared (p = 0.07 for surgical and fellow eye comparisons). In the 17 patients in whom serial HVFs (>2 HVFs) were performed, the mean pre-operative MD was −17 ± 7.5 dB in the surgical eye and −12 ± 8 dB in the fellow eye. The MD improved overall for each group, with a mean post-operative MD of −13 ± 11 dB and −7 ± 6 dB in the surgical and fellow eyes, respectively. The trend towards improvement in MD was not statistically significant (p = 0.2 and 0.09 for surgical and fellow eyes, respectively). The mean number of colour plates correctly identified was 59 ± 46% in the surgical eye and 69 ± 40% in the fellow eye. Post-operatively, the mean percentage of colour plates identified correctly was 58 ± 49% and 62 ± 46% in the surgical and fellow eye groups, respectively. Pre-operatively, 19 patients demonstrated a relative afferent pupillary defect; of these 19 patients, 5 had a resolution of the afferent pupillary defect post-operatively. Seven additional patients developed relative afferent pupillary defects during the post-operative course.
VA Outcomes
VA outcomes are summarized in Table 2. The results of risk factor analysis for VA deterioration are summarized in Table 3. Overall, better pre-operative VA (p = 0.01), better pre-operative colour vision (p = 0.002), and shorter time to surgery (p = 0.04) were associated with VA improvement or stabilization. In addition, the overall final VA results were calculated using the final VA at the very last follow-up visit regardless of subsequent ONSF (results in Table 2 are the final VA prior to any additional ONSF procedures). There were 6/37 (16%) total eyes that deteriorated, there were 26 (68%) eyes that were stable, and there were 5 (14%) eyes that showed overall improvement – these numbers are similar to the fellow eye results presented in Table 2, which demonstrate the values prior to subsequent procedures.
TABLE 2 .
Results of optic nerve sheath in patients with IIHa.
| Measure | Eye | Improved | Stabilized | Deteriorated |
|---|---|---|---|---|
| VA | Operated (n = 37) | 8 (22%) | 20 (54%) | 9 (24%) |
| Fellow (n = 31) | 5 (17%) | 23 (74%) | 3 (9%) | |
| MDb | Operated (n = 17) | 8 (47%) | 6 (35%) | 3 (18%) |
| Fellow (n = 13) | 6 (46%) | 6 (46%) | 1 (8%) |
aAn eye was considered to be “improved” if the VA improved by at least −0.2 logmar from the pre-operative value; “stable” if the VA was within 0.2 logmar, and “deteriorated” if the final VA was ≥0.2 logmar from the pre-operative value.
bMD is only reported in eyes of patients who were reliably able to undergo automated perimetry (n = 17).
TABLE 3 .
The association of VA improvement or stabilization (within 0.2 logmar) in the surgical eye (n = 37) and pre-operative risk factors in patients undergoing ONSF for IIHa.
| Measure | Coefficient from logistic regression model | Odds ratio | 95% CI | p Value |
|---|---|---|---|---|
| Pre-operative VA (logmar) | −2.3 | 0.1 | 0.02, 0.7 | 0.01* |
| Pre-operative MD | −0.01 | 0.99 | 0.8, 1.2 | 0.9 |
| Pre-operative colour vision | 3.1 | 21.6 | 3.2, 144 | 0.002* |
| Pre-operative optic nerve pallor | 0.6 | 1.8 | 0.3, 10 | 0.5 |
| Gender | 0.02 | 1.0 | 0.2, 7 | 0.9 |
| Age | 0.008 | 1 | 0.9, 1.1 | 0.78 |
| Time to operation | −0.02 | 0.98 | 0.96, 0.99 | 0.04* |
| Afferent pupillary defect | −0.6 | 0.56 | 0.2, 2.1 | 0.39 |
| Follow-up length | 0.002 | 0.99 | 0.99, 1.0 | 0.28 |
aEyes in which the MD improved by at least 5 dB were considered “improved”, within 5 dB were considered “stable”, and a worsening of MD by at least 5 dB were considered “deteriorated”.
*p Value < 0.05 considered statistically significant.
Visual Fields
VFs were followed serially by automated perimetry in 17 patients (the remainder of subjects were followed serially by manual kinetic perimetry). VF MD data are summarized in Table 2. The results of risk factor analysis for VF deterioration are summarized in Table 4. Overall, better pre-operative VA (p = 0.04) and pre-operative colour vision (p = 0.02) were associated with VF MD improvement or stabilization. For patients who were followed serially by manual kinetic perimetry, VF loss improved in 6/16 patients, stayed stable in 2/16 patients, and worsened by the last follow-up visit in 8/16 patients.
TABLE 4 .
The association of VF MD improvement or stabilization (within 5 dB) in the surgical eye (n = 17) and pre-operative factors in patients undergoing ONSF for IIHa.
| Measure | Coefficient from logistic regression model | Odds ratio | 95% CI | p Value |
|---|---|---|---|---|
| Pre-operative VA (logmar) | −3.5 | 0.03 | 8e − 4, 0.95 | 0.04* |
| Pre-operative MD | 0.05 | 0.95 | 0.8, 1.1 | 0.6 |
| Pre-operative colour vision | 2.1 | 7.9 | 1.3, 48 | 0.02* |
| Pre-operative optic nerve pallor | −0.2 | 0.8 | 0.1, 9.8 | 0.8 |
| Gender | −0.4 | 0.7 | 0.1, 4.8 | 0.7 |
| Age | −0.01 | 0.99 | 0.9, 1.1 | 0.7 |
| Time to operation | −0.01 | 0.99 | 0.98, 1.0 | 0.4 |
| Afferent pupillary defect | −0.55 | 0.58 | 0.1, 2.2 | 0.4 |
| Follow-up length | −0.05 | 0.99 | 0.98, 1.0 | 0.09 |
aThis regression model was constructed using only those patients undergoing automated perimetry (n = 17).
*p Value < 0.05 considered statistically significant.
Pattern of VF loss was also assessed in all VFs (automated and manual kinetic). In those subjects in whom a clear pattern of VF loss could be distinguished pre-operatively on either automated or manual perimetry, the incidence of various patterns can be found in Table 5. VF defects were most likely to resolve completely by the last post-operative examination if they were “enlarged blind spot” patterns (60%); they were least likely to resolve if they were arcuate or central scotomas (0% resolved).
TABLE 5 .
VF loss patterns and resolution from automated (n = 17) or kinetic perimetry (n = 20) in operated and fellow eyes of patients undergoing ONSF for IIH.
| VF pattern | Eye | Number of subjects with defect | Percent resolved |
|---|---|---|---|
| Generalized constriction | Operateda | 12 | 25% |
| Fellowb | 8 | 13% | |
| Arcuate defect | Operated | 4 | 0 |
| Fellow | 7 | 0 | |
| Nasal step | Operated | 10 | 20% |
| Fellow | 13 | 8% | |
| Enlarged blind spot | Operated | 5 | 60% |
| Fellow | 10 | 50% | |
| Central scotoma | Operated | 8 | 0 |
| Fellow | 1 | 0 |
aTotal number of operated eyes followed by automated or kinetic perimetry = 37.
bTotal number of fellow eyes followed by automated or kinetic perimetry = 31.
Serial Optic Nerve Examinations
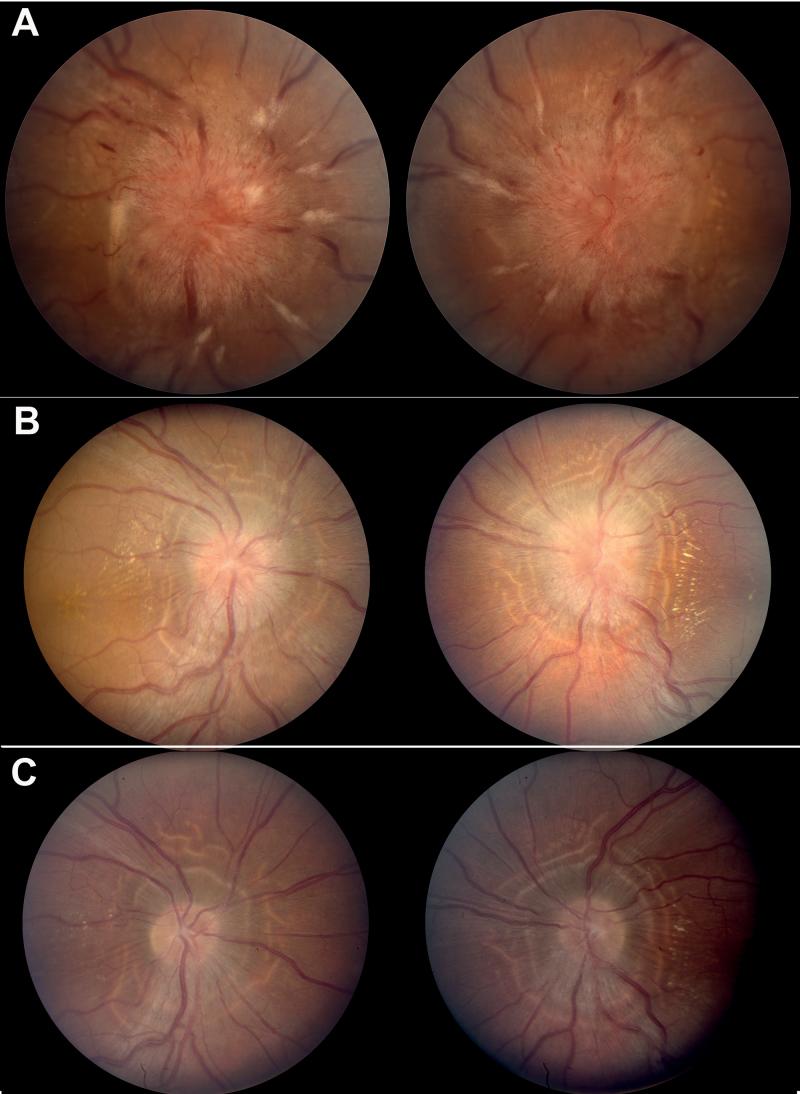
Overall, optic nerve oedema resolved completely first temporally and lastly in the nasal quadrant. The shortest duration to complete resolution of optic nerve oedema to a Frisen scale score of 0 was 2 weeks. Several examples of fairly dramatic change in disc appearance within days of surgery were seen (Figure 1).
FIGURE 1.
Example of dramatic improvement in optic nerve oedema after optic nerve sheath decompression: (A) pre-operative photographs reveal 4+ optic nerve oedema with associated haemorrhages, cotton wool spots, and exudates; (B) post-operative week 2 photographs reveal resolution of hyperaemia, haemorrhages, and cotton wool spots. In addition, the nerve fibre layer oedema and opacification has diminished; and (C) post-operative week 8 photographs reveal resolution of oedema, hyperaemia, haemorrhages, and cotton wool spots. “High water marks” delineate the region of previous nerve fibre layer oedema. Note: Figure 1 of this article is available in colour online at www.informahealthcare.com/oph.
Surgical Complications and Failures
The most common complication was a post-operative tonic pupil with resultant anisocoria in two patients. One patient had a conjunctival abscess and one had persistent post-operative diplopia. In the five subjects diagnosed with functional visual loss, there were no surgical complications, thereby showing that ONSF is can be considered for those high-risk patients who cannot be followed by objective measures.
Twelve patients (32%) re-presented with a clinical recurrence post-operatively. Recurrence time ranged from 5 to 375 weeks and was manifest principally by headache (n = 8) or recurrent vision loss in the operated eye (n = 4). Of the patients presenting with recurrences, six (50%) required additional proceudres as the recurrence could not be managed medically: four underwent CSF diversion and two underwent fellow eye ONSF. Seventeen subjects total (including recurrences mentioned above) underwent subsequent procedures. Thirteen subjects underwent ONSF in the fellow eye, with five planned pre-operatively due to bilateral severe disease. In the remaining eight, the decision was made due to further deterioration of the fellow eye VA or VF. Eight subjects underwent subsequent CSF diversion procedures, four of which followed a fellow eye OSNF. The indication was either was intractable headaches (n = 5) or continued loss of VA or VF (n = 3).
There were 14 subjects overall who either went on to require CSF diversion or had a deterioration of VA < −0.2 logmar. These subjects were more likely to have undergone their first ONSF more than two months after symptom onset (72% versus 30% of the remaining subjects) and were more likely to present with VA worse than 20/200 (29% versus 4% of the remaining subjects).
Discussion
The treatment of IIH is controversial because there are no definitive guidelines for medical or surgical management. Although a randomized clinical trial for medical treatment and weight loss management is underway, there is currently no consensus by which to base decisions related to surgical intervention in those patients with severe acute vision loss or in those with progressive vision loss unresponsive to maximal medical treatment. In our practice of using ONSF for patients with profound or severe vision loss, in those cases that are refractory to medical treatment, or for those patients who are unable to comply with medications or follow-up, our long-term results reveal that most demonstrated stabilization or improvement of VA and VF MD. In addition, patients were more likely to obtain better results if they had better pre-operative visual function and a shorter time to surgical intervention. This finding is not surprising since VA and colour vision are metrics that provide functional assessments of optic nerve function. The more favourable prognosis for those patients with relatively early intervention makes surgical decision-making difficult when the risks of surgical intervention have to be weighed against the chance for early and more complete reversal of vision loss with earlier surgery. Decreased pre-operative optic nerve function may indicate a level of permanent damage that cannot be re-gained after optic nerve sheath decompression. The association of a shorter time to surgery with better results is further supported by one of the earliest case series describing ONSF in Spoor et al.8 in which ONSF produced improved visual function in all 69 eyes undergoing ONSF with acute papilloedema, but only 10 of 32 eyes with chronic oedema.
These results corroborate previous findings in other case series of ONSF, many of which have been combined in a meta-analysis reported by Feldon9 in 2007. In this series, seven of the largest retrospective case series were merged to report the results of a total of 252 patients (423 eyes). VA or VF worsened in 11%, which is lower than in our series but may be biased in that there was no single standard by which to categorize improvement or stabilization. In the same series, second eye surgeries were performed in 59% of cases and CSF diversion procedures were performed in 4% of cases compared with 35% and 22% in our series, respectively. This difference most likely represents our practice to recommend shunting after ONSF if headaches are the remaining persistent complaint. In the largest single centre case series, Banta and Farris10 found that VA was stabilized in 94% and VFs were stabilized in 88% of eyes. In this study, there was a clinical relapse in 11 of 86 patients (13%), which occurred after an unpredictable time course up to five years post-operatively (similarly to our population). Although more of the ONSF cases underwent bilateral procedures in this series (84% versus 35%), recent data from Alsuhaibani et al.11 suggests that fellow eye response to ONSF may be greater than previously thought, and that both optic nerve oedema and VF stability are improved in fellow eyes of patients undergoing unilateral ONSF.
Aside from ONSF, several groups have evaluated CSF diversion procedures, such as ventricular-peritoneal and lumbar-peritoneal shunts, as a primary surgical intervention for IIH. These authors have argued that shunt procedures are more effective for headache control and are subject to less late failures than ONSF.1 However, results of these procedures are highly variable in terms of success, shunt failure, and complications rates.12–18 In three of the largest studies focusing on patients with vision loss, Rosenberg et al.,15 Eggenberger et al.,13 and Bynke et al.18 reviewed the records of 37, 27, and 17 patients, respectively. Rosenberg et al.15 found that VA improved in 35% of patients and stabilized in 35% of patients who underwent either lumbar-peritoneal or ventricular-peritoneal shunts. Shunt failure occurred in 55% of cases, and occurred as late as seven years after insertion. Complications included low-pressure headaches in 21% of patients, as well as infection, pain, and CSF leak. However, in their series of 27 patients treated with lumbar-peritoneal shunts, Eggenberger et al.13 found that there were no patients with low-pressure headache or pain, and reported that the only complication of shunt was shunt failure requiring revision (56%). In a study made up of patients treated only with ventricular-peritoneal shunts, Bynke et al.18 reported a lower shunt revision rate (40%) and improved VA results from those previous studies including patients with lumbar-peritoneal shunts, with VA improvement in 3 eyes (9%) and stability in 31 eyes (91%). Although newer techniques for ventricular-peritoneal shunts have greatly improved them compared to lumbo-peritoneal shunts, allowing them to require less revisions and better symptom control,17 they are subject to a higher level of risk due to the required craniotomy and are more expensive.19 Both CSF diversion procedures usually require inpatient hospital admission and lengthy observation, whereas ONSF can often be performed on an outpatient basis or with a minimal in-hospital post-operative observation period. Furthermore, the possibility of subarachnoid space compartmentalization with accumulation of toxic substances to the optic nerve is a theoretical risk for shunt failure that is alleviated by ONSF.20
When compared to our results, the results of these and other studies of lumbar-peritoneal and ventricular-peritoneal shunts suggest that ONSF provides similar improvement or stability in visual function with less systemic complications. In addition, the need for a second procedure was lower in our series than in most other reports. However, given that clinical recurrences occurred in our series as late as seven years, it is possible that ONSF may be less reliable long-term. Citing this possibility of long-term failure, some authors have argued that CSF diversion procedures are, therefore, the procedure of choice for IIH. However, in our series, the number of subjects requiring shunting procedures (n = 8, 22%) is comparable or better than reported shunt revision rates, which range between 38% and 64%.21 Our rates for subsequent procedures and clinical recurrences are similar to that described in long-term follow-up data from Spoor and McHenry22 who found that 32% of their ONSF subjects experienced visual function deterioration during a post-operative follow-up of at least six months.
The results of our study, which indicate that ONSF is a useful procedure to stabilize vision loss in IIH, should be understood within the context of its limitations. First, this was a retrospective chart review, and therefore is subject to selection bias as well as that which is introduced by differing post-operative follow-up lengths. Although our series of patients is amongst the largest reported, it is still a relatively small group, and therefore may not be generalizable to all practices. Given the retrospective nature of the study, there was not standardization as to how individual patient’s visual function was tracked. Approximately half of the patients were not followed over time with automated perimetry, thereby limiting our VF analysis of MD to those with enough visual function to perform automated perimetry, most likely creating further bias. In addition, in order to fully assess the impact of the initial ONSF, we performed our primary VA outcome analysis prior to any subsequent ONSF procedures which may have led to a positive selection bias by excluding patients with presumably more aggressive disease; however, our analysis of final VA outcomes despite subsequent procedures was fairly similar to the results reported prior to fellow eye ONSF. Finally, due to the retrospective nature of our study, we do not have a control group in whom we can compare the improvement and stabilization experienced by our patients.
In spite of its limitations, our study suggests that ONSF is a useful procedure for patients with severe optic neuropathy secondary to IIH. Our findings suggest that earlier intervention combined with better pre-operative visual function is associated with improved outcomes. Therefore, clinicians should consider ONSF earlier in the course for those patients with progressive vision loss.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Research supported in part by an unrestricted grant (NJV) from Research to Prevent Blindness.
References
- 1.Brazis PW. Clinical review: the surgical treatment of idiopathic pseudotumour cerebri (idiopathic intracranial hypertension). Cephalalgia 2008;28:1361–1373 [DOI] [PubMed] [Google Scholar]
- 2.Lueck C, McIlwaine G. Interventions for idiopathic intracranial hypertension. Cochrane Database Syst Rev 2005;Jul 20;(3):CD003434. Review. [DOI] [PubMed] [Google Scholar]
- 3.Curry WT, Jr, Butler WE, Barker FG., 2nd Rapidly rising incidence of cerebrospinal fluid shunting procedures for idiopathic intracranial hypertension in the United States, 1988–2002. Neurosurgery 2005;57:97–108;discussion 197–108 [DOI] [PubMed] [Google Scholar]
- 4.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology 2002;59:1492–1495 [DOI] [PubMed] [Google Scholar]
- 5.Liu GT, Volpe NJ, Galetta SL. Optic disc swelling: papilledema and other causes. Neuro-ophthalmology: diagnosis and management. Philadelphia: Saunders-Elsevier; 2010 [Google Scholar]
- 6.Keltner JL, Johnson CA, Cello KE, Edwards MA, Bandermann SE, Kass MA, Gordon MO. Classification of visual field abnormalities in the ocular hypertension treatment study. Arch Ophthalmol 2003;121:643–650 [DOI] [PubMed] [Google Scholar]
- 7.Frisen L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry 1982;45:13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spoor TC, Ramocki JM, Madion MP, Wilkinson MJ. Treatment of pseudotumor cerebri by primary and secondary optic nerve sheath decompression. Am J Ophthalmol 1991;112:177–185 [DOI] [PubMed] [Google Scholar]
- 9.Feldon SE. Visual outcomes comparing surgical techniques for management of severe idiopathic intracranial hypertension. Neurosurg Focus 2007;23:E6. [DOI] [PubMed] [Google Scholar]
- 10.Banta JT, Farris BK. Pseudotumor cerebri and optic nerve sheath decompression. Ophthalmology 2000;107:1907–1912 [DOI] [PubMed] [Google Scholar]
- 11.Alsuhaibani AH, Carter KD, Nerad JA, Lee AG. Effect of optic nerve sheath fenestration on papilledema of the operated and the contralateral nonoperated eyes in idiopathic intracranial hypertension. Ophthalmology 2011;118:412–414 [DOI] [PubMed] [Google Scholar]
- 12.Burgett RA, Purvin VA, Kawasaki A. Lumboperitoneal shunting for pseudotumor cerebri. Neurology 1997;49:734–739 [DOI] [PubMed] [Google Scholar]
- 13.Eggenberger ER, Miller NR, Vitale S. Lumboperitoneal shunt for the treatment of pseudotumor cerebri. Neurology 1996;46:1524–1530 [DOI] [PubMed] [Google Scholar]
- 14.Johnston I, Besser M, Morgan MK. Cerebrospinal fluid diversion in the treatment of benign intracranial hypertension. J Neurosurg 1988;69:195–202 [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg ML, Corbett JJ, Smith C, Goodwin J, Sergott R, Savino P, Schatz N. Cerebrospinal fluid diversion procedures in pseudotumor cerebri. Neurology 1993;43:1071–1072 [DOI] [PubMed] [Google Scholar]
- 16.Woodworth GF, McGirt MJ, Elfert P, Sciubba DM, Rigamonti D. Frameless stereotactic ventricular shunt placement for idiopathic intracranial hypertension. Stereotact Funct Neurosurg 2005;83:12–16 [DOI] [PubMed] [Google Scholar]
- 17.McGirt MJ, Woodworth G, Thomas G, Miller N, Williams M, Rigamonti D. Cerebrospinal fluid shunt placement for pseudotumor cerebri-associated intractable headache: predictors of treatment response and an analysis of long-term outcomes. J Neurosurg 2004;101:627–632 [DOI] [PubMed] [Google Scholar]
- 18.Bynke G, Zemack G, Bynke H, Romner B. Ventriculoperitoneal shunting for idiopathic intracranial hypertension. Neurology 2004;63:1314–1316 [DOI] [PubMed] [Google Scholar]
- 19.Uretsky S. Surgical interventions for idiopathic intracranial hypertension. Curr Opin Ophthalmol 2009;20:451–455 [DOI] [PubMed] [Google Scholar]
- 20.Killer HE, Jaggi GP, Flammer J, Miller NR, Huber AR, Mironov A. Cerebrospinal fluid dynamics between the intracranial and the subarachnoid space of the optic nerve. Is it always bidirectional? Brain 2007;130:514–520 [DOI] [PubMed] [Google Scholar]
- 21.Friedman DI, Jacobson DM. Idiopathic intracranial hypertension. J Neuroophthalmol 2004;24:138–145 [DOI] [PubMed] [Google Scholar]
- 22.Spoor TC, McHenry JG. Long-term effectiveness of optic nerve sheath decompression for pseudotumor cerebri. Arch Ophthalmol 1993;111:632–635 [DOI] [PubMed] [Google Scholar]