Abstract
We have found a significant upregulation of L-type voltage-operated Ca++ channels (VOCCs) in reactive astrocytes. To test if VOCCs are centrally involved in triggering astrocyte reactivity, we used in vitro models of astrocyte activation in combination with pharmacological inhibitors, siRNAs and the Cre/lox system to reduce the activity of L-type VOCCs in primary cortical astrocytes. The endotoxin lipopolysaccharide (LPS) as well as high extracellular K+, glutamate and ATP promote astrogliosis in vitro. L-type VOCC inhibitors drastically reduce the number of reactive cells, astrocyte hypertrophy and cell proliferation after these treatments. Astrocytes transfected with siRNAs for the Cav1.2 subunit that conducts L-type Ca++ currents as well as Cav1.2 knockout astrocytes showed reduce Ca++ influx by ~80% after plasma membrane depolarization. Importantly, Cav1.2 knock-down/out prevents astrocyte activation and proliferation induced by LPS. Similar results were found using the scratch wound assay. After injuring the astrocyte monolayer, cells extend processes toward the cell-free scratch region and subsequently migrate and populate the scratch. We found a significant increase in the activity of L-type VOCCs in reactive astrocytes located in the growing line in comparison to quiescent astrocytes situated away from the scratch. Moreover, the migration of astrocytes from the scratching line as well as the number of proliferating astrocytes was reduced in Cav1.2 knock-down/out cultures. In summary, our results suggest that Cav1.2 L-type VOCCs play a fundamental role in the induction and/or proliferation of reactive astrocytes, and indicate that the inhibition of these Ca++ channels may be an effective way to prevent astrocyte activation.
Keywords: astrocyte, calcium influx, voltage-operated Ca++ channels, astrogliosis
INTRODUCTION
Astrocytes exhibit excitability by way of ionic fluxes, and particularly in the form of intracellular Ca++ oscillations. Levels of intracellular Ca++ are critical for numerous homeostatic cellular functions in astrocytes, including migration and proliferation (Stanimirovic et al., 1995; Wang et al., 2010; Parnis et al., 2013). Cytoplasmic Ca++ levels in astrocytes are derived from multiple compartments, including endoplasmic reticulum (Kastritsis et al., 1992), mitochondrial sodium-calcium exchange (Parnis et al., 2013), and the extracellular space (Gao et al., 2013). One important mechanism by which intracellular Ca++ can be regulated in astrocytes is by voltage-operated Ca++ channels (VOCCs) present in the plasma membrane. VOCCs are an important route for Ca++ delivery into the cytoplasm of most excitable cells and additionally into many non-excitable cells such as astrocytes.
Several types of VOCCs have been characterized electrophysiologically and pharmacologically. The types fall into two groups: the high-voltage-operated Ca++ channels L-, P/Q-, N- and R-types, and the low-voltage-activated T-type (Akopian et al., 1996; MacVicar, 1984; Oh, 1997; Puro et al., 1996). Cultured astrocytes from different brain regions express a variety of ion channels, including both the L- and P/Q-type Ca++ channels. The first evidence for Ca++ channel activity in cultured astrocytes was the discovery of Ca++-dependent action potential firing on intracellular current injections (MacVicar, 1984). The presence of VOCCs was then demonstrated by recording voltage-operated Ca++ currents in cultured astrocytes (MacVicar and Tse, 1988; D'Ascenzo et al., 2004; Latour et al., 2003). Moreover, Ca++ dynamics mediated by VOCCs were detected in astrocytes from the subventricular zone (Young et al., 2010), the ventrobasal thalamus (Parri and Crunelli, 2003; Parri et al., 2001) and the hippocampus (Komuro and Rakic, 1992, 1998).
A number of different areas of research have discovered the importance of voltage-gated Ca++ signaling in glial development. For instance, we have found that L-type VOCCs regulate process extension and migration in oligodendrocyte progenitor cells (Paez et al., 2007; Paez et al., 2009a). Additionally, we have provided direct evidence that L-type VOCC activation increased oligodendrocyte progenitor cell morphological differentiation as well as the expression of mature oligodendrocyte markers (Cheli et al., 2015). On the contrary, inhibition of L-type Ca++ channels prevents oligodendrocyte development and myelination in vitro (Cheli et al., 2015). Since glial cells share a common origin as well as many features, it is tempting to speculate that VOCCs may play similar roles in astrocytes as in oligodendrocytes regarding regulation of morphological changes and migration.
Astrocytes respond to many types of pathological conditions and injuries to the central nervous system (CNS) by becoming reactive, a process called astrogliosis or astrocytosis (Sofroniew and Vinters, 2010). This process is characterized by astrocyte swelling, enlargement and characteristic morphological changes. It is well known that Ca++ influx and upregulation of different Ca++ channels play a key role in astrocytes signaling pathways following a damage to the CNS (Gurkoff et al., 2013). For example, the expression of L-type Ca++ channels was found to be augmented in reactive astrocytes in several models of brain injury, including mechanical and thermal lesions in the forebrain, hypomyelination in white matter, and ischemia (Westenbroek et al., 1998). However, little has been done to uncover the role of Ca++ inducing reactive astrogliosis. This work focused on the mechanisms of Ca++ signaling in astrocytes mediated by voltage-operated Ca++ channels, with a particular emphasis on the relevance of these Ca++ currents to astroglial activation. We present here evidence supporting a contribution of L-type Ca++ channels to astrogliosis in an in vitro model of inflammation and glial mechanical injury. We specifically tested the hypothesis that voltage-gated Ca++ entry promotes astrocyte reactivity and proliferation under pathological conditions and we have provided clues into molecular events that might be manipulated to encourage repair following different brain insults.
MATERIALS AND METHODS
Animals
All animals used in the present study were housed in the UB Division of Laboratory Animal Medicine vivarium, and procedures were approved by UB’s Animal Care and Use Committee, and conducted in accordance with the guidelines in “Guide for the Care and Use of Laboratory Animals” from the National Institutes of Health.
Primary culture of cortical astrocytes
Primary cultures of mouse cortical astrocytes were prepared as described by Amur-Umarjee et al. (1993). Brains from newborn (P1–P2) C57BL/6 mouse were dissected under sterile conditions. The cortex was isolated and tissue samples were mechanically dissociated and were plated on poly-D-lysine-coated flasks in Dulbecco′s modified Eagle′s medium and Ham′s F12 (1:1 v/v) (Life Technologies), containing 100μg/ml gentamycin and supplemented with 4mg/ml dextrose anhydrous, 3.75mg/ml HEPES buffer, 2.4mg/ml sodium bicarbonate and 10% fetal bovine serum (FBS) (Life Technologies). After 24h the medium was changed and the cells were grown in DMEM/F12 supplemented with insulin (5μg/ml), human transferrin (50μg/ml), sodium selenite (30nM), d-Biotin (10mM), 0.1% BSA (Sigma), 1% FBS (Omega Scientific) and 1% horse serum (Omega Scientific). After 14 days, oligodendrocytes and microglia were removed from the mixed glial culture by the differential shaking and adhesion procedure of Suzumura et al., (1984) and the astrocytes were collected from the flasks by trypsinization. The cells were plated on either 24-well glass bottom plates (100x103 cells/well) or 35mm dishes (500x103 cells/dish) coated with poly-D-lysine (Millipore). Cells were grown for different periods of time or until confluent in defined culture media G5: DMEM/F12 supplemented with hydrocortisone (10nM), sodium selenite (30nM), insulin (5μg/ml), human transferrin (50μg/ml), d-biotin (10ng/ml), bFGF (5ng/ml) and EGF (10ng/ml). To reduce microglial growth, the culture medium was changed every second day. The cultures routinely contained ≥98% astrocytes, as assessed by expression of the astrocyte marker GLT1, ≤0.5% microglia and ≤0.1% O4 or O1-positive oligodendrocytes and NeuN or βIII-tubulin-positive neurons (data not shown).
siRNA knock-down of Cav1.2 and Cav1.3
Three days after plating, astrocytes were transiently transfected with a combination of three different Stealth RNAiTM siRNA duplexes (Life Technologies) specific for Cav1.2 and Cav1.3 (see Cheli et al., 2015 for siRNA duplex sequences). Briefly, 6pmol of each siRNA duplex were mixed with LipofectamineTM RNAiMAX (Life Technologies) and the mixture was added to 24-well dishes containing ~70% confluent astrocytes. After 24h the medium was changed and the cells were further grown for 3 days in defined culture media (G5). Next, the cells were treated with LPS or used for the scratch wound assay. At the same time, total RNA and proteins were collected for RT-PCR and western blot experiments. Control cells were transfected with siRNA designed to minimize sequence homology to any known vertebrate transcript and with a similar GC content as our siRNAs. Additionally, following the transfection protocol described above, astrocytes were treated with fluorescein-labeled dsRNA oligomers (BLOCK-iT™ Fluorescent Oligos, Life Technologies) to determine siRNA transfection efficiency.
Adenovirus-mediated Cre deletion of floxed Cav1.2
Primary cultures of cortical astrocytes were prepared from homozygous floxed Cav1.2 mice as described above. In the floxed mutant Cav1.2 line (White et al., 2008), exon 2 of the wild-type Cav1.2 gene (cacna1c) was flanked with loxP sites and thus exon 2 is eliminated when Cre-recombinase is present. Cav1.2 floxed astrocytes were infected with type 5 adenoviruses expressing both Cre recombinase and GFP and control adenoviruses expressing the RFP (Vector Biolabs). Adenovirus infections were performed 3 days after plating, 50 MOI were added to 24-well dishes containing ~70% confluent astrocytes. Astrocytes were infected overnight for 12h with a reduced volume of culture medium containing the virus at the appropriate concentration. After infection, astrocytes were further cultured for 3 days in defined culture media (G5) and then used for LPS treatment or for the scratch wound assay. At the same time, total RNA and proteins were collected for RT-PCR and western blot experiments.
Scratch wound assay
Astrocytes were plated on 24-well glass bottom plates. When confluent, the cells were mechanically scratched with a pipette tip (20μl) similar to previous descriptions (MacFarlane and Sontheimer, 1997; Yu et al., 1993), yielding a linear cell-free area of approximately 300–400μm. Cells were washed twice with sterile PBS, and medium was replaced. After 24 and 48h of incubation, during which time the medium was not exchanged, cells were fixed with 4% paraformaldehyde prior to immunocytochemistry. For measurements of the area covered by cells expressing GFAP, vimentin and nestin, stained scratch wounds were analyzed using MetaMorph software (Molecular Devices). A threshold above background was set for each individual image and the pixel area above threshold was measured. For time lapse experiments, cultures were incubated in a stage top chamber with 5% CO2 at 37°C (Live-Cell Control Unit), which was placed on the stage of a spinning disc confocal microscope (Olympus, IX83-DSU) equipped with a motorized z-stage. A 10X objective was used for acquiring images. Bright-field and fluorescent images were taken every 6min over a period of 48h using a CCD camera (Hamamatsu ORCA-ER). For all the scratch wound experiments, data represent pooled results from at least 6 independent cultures.
Immunocytochemistry
Cells were stained with antibodies against several astrocyte markers and examined by confocal microscopy. Briefly, the cells were rinsed in PBS and fixed in 4% buffered paraformaldehyde for 20min at room temperature. After rinsing in PBS, the cells were permeabilized with 0.1% Triton X-100 in PBS for 2min at room temperature and then processed for immunocytochemistry following the protocol as outlined by Reyes and Campagnoni (2002). Essentially, fixed cells were incubated in a blocking solution (5% goat serum in PBS) followed by an overnight incubation at 4°C with the primary antibody. Cells were then incubated with the appropriate secondary antibodies (1:400; Jackson), nuclei were stained with the fluorescent dye DAPI (Life Technologies), mounted onto slides with Aquamount (Thermo Scientific), and fluorescent images were obtained using a spinning disc confocal microscope (Olympus, IX83-DSU). Quantitative analysis of the results was done counting the antigen-positive and DAPI-positive cells (total number of cells) in 20 randomly selected fields, which resulted in counts of >2000 cells for each experimental condition. For some antigens the average fluorescent intensity in 20 randomly selected fields was assessed by MetaMorph software (Molecular Devices). For all experiments involving quantification of positive cells and fluorescent intensity, data represent pooled results from at least 5 independent cultures. Quantification was performed blind to the experimental condition. The primary antibodies used for immunocytochemistry were against: caspase-3 (1:200; Cell Signaling), Cav1.2 (1:200; Alomone), Cav1.3 (1:200; Alomone), GFAP (1:2000; Millipore), Ki67 (1:500; Abcam), nestin (1:2000; Millipore), s100β (1:600; Thermo Scientific) and vimentin (1:200; BD Biosciences).
Incorporation of bromo-deoxyuridine
Bromo-deoxyuridine (BrdU) (10 μM) (BD Pharmingen) was applied at different time points after plating. After treatment, the cells were fixed in 4% paraformaldehyde in PBS and immunostained in order to determine the number of positive cells. To denature nuclear DNA, cell were treated with 6N HCl and 1% Triton X-100 and then with 0.1M sodium borate (in PBS and 1% Triton X-100) for 10min. Immunocytochemistry was done using anti-BrdU antibody (1:1000; BD Pharmingen) with the corresponding fluorescent secondary antibody. The percentage of BrdU positive cells was estimated on the basis of the total number of cells. Data represent pooled results from at least 5 independent cultures. Quantification was performed blind to the experimental condition.
MTT assay
The MTT survival assay was performed as described by Mosmann (1983). The sterile solution of MTT (Molecular Probes) was added to all wells and the micro plate was incubated at 37°C for 45min. The reaction was stopped by addition of SDS and the product was quantified by spectrophotometry at 570nm.
Western blot
Total protein was collected from primary culture of cortical astrocytes. The final protein pellet was homogenized in lysis buffer containing 50mM Tris-HCl, 0.25% (w/v) sodium deoxycholate, 150mM NaCl, 1mM EDTA, 1% (w/v) Triton X-100, 0.1% (w/v) SDS, 1mM sodium vanadate, 1mM AEBSF, 10μg/ml aprotinin, 10μg/ml leupeptin, 10μg/ml pepstatin and 4μM sodium fluoride. Western blots were performed as previously described (Ghiani et al., 2010). Twenty-five micrograms of total protein was loaded onto a 4–20% Tris-glycine gel (Life Technologies). Protein bands were detected by chemiluminescence using the Amersham ECL kit (GE Healthcare) with horseradish peroxidase-conjugated secondary antibodies (GE Healthcare). Relative intensities of the protein bands were quantified by scanning densitometry using the NIH Image Software Image J. Equal protein loading was verified by Ponceau S solution (Sigma) reversible staining of the blots and each extract was also analyzed for relative protein levels of β-actin and P84. Data represent pooled results from at least 4 independent cultures per experimental group. The primary antibodies used for Western blots were against: Cav1.2 (1:1000; Alomone), Cav1.3 (1:1000; Alomone), GAPDH (1:10000; Genetec), p84 (1:10000; Genetec) and β-actin (1:1000; Sigma).
RT-PCR
Total RNA was isolated from primary culture of cortical astrocytes using Trizol reagent (Life Technologies) according to the instructions of the manufacturer. RNA content was estimated by measuring the absorbance at 260nm and the purity was assessed by measuring the ratio of absorbance: 260/280nm. PCR primers for VOCC α subunits were designed based on published sequences by Badou et al., (2006), Chen et al., (2011), Omilusik et al., (2011), Rosati et al., (2011), White et al., (2008) and Xu et al., (2007), (see Table I for primer sequences). First-strand cDNA was prepared from 1μg of total RNA using SuperScriptTM III RNase H-reverse transcriptase (Life Technologies) and 1μg of oligo(dT). The mRNA samples were denaturated at 65°C for 5min. Reverse transcription was performed at 50°C for 55min and was stopped by heating the samples at 85°C for 5min. The cDNA was amplified by PCR using the Cav isoform-specific primers and PCR Platinum Supermix reagent (Life Technologies). PCR conditions were as follows: 94°C for 2min, 40 cycles of 94°C for 30s, 58°C for 30s followed by 68°C for 2min. After completion of the 40 cycles, samples were incubated at 72°C for 10min. A β-actin positive control was run alongside the experimental samples, as well as a negative control with no reverse transcriptase. The PCR products were visualized on a SYBR Safe stained agarose gel and the bands digitized using a Gel Doc™ EZ System (BioRad).
Table I.
Sequences of primers used for RT-PCR.
| Gene | α Subunit | Forward | Reverse | PCR fragment (bp) |
|---|---|---|---|---|
| cacna1s | CaV1.1 | 5’ - TGTGGTATGTCGTCACTTCCTCC | 5’ - CGTCAATGATGCTGCCGATG | 258 |
| cacna1c | CaV1.2 | 5’ - CAGCTCATGCCAACATGAAT | 5’ - TGCTTCTTGGGTTTCCCATA | 202 |
| cacna1d | CaV1.3 | 5’ - AATGGCACGGAATGTAGGAG | 5’ - GACGAAAAATGAGCCAAGGA | 207 |
| cacna1f | CaV1.4 | 5’ - GACGAATGCACAAGACATGC | 5’ - CAAGCACAAGGTTGAGGACA | 325 |
| cacna1a | CaV2.1 | 5’ - CATCATCATCGGCTCCTTTT | 5’ - GAAAAGCTCTCCGGTTCTCC | 100 |
| cacna1b | CaV2.2 | 5’ - GCATTTGCGTTCTCAGGATC | 5’ - CTTAGGCAGCCGCTTGATG | 104 |
| cacna1e | CaV2.3 | 5’ - AACCCACTTCAACACCCACG | 5’ - GCACGATCTGCAGGCTAGGT | 100 |
| cacna1g | CaV3.1 | 5’ - CGCTGACCATGAAATGCCT | 5’ - CAGAACTAGGCTCTGCATC | 134 |
| cacna1h | CaV3.2 | 5’ - TGGTTCGAGCACATTAGCATG | 5’ - CTGCAACGTTCTGAACGGC | 100 |
| cacna1i | CaV3.3 | 5’ - GGGCATCAGTGGCTGTAGTT | 5’ - GTGCACCCTGAATTGCTTCT | 100 |
| cacna1c | CaV1.2 floxed | 5’ - CCAACCATTGCGGAGGTAAGC | 5’ - CGGTGCTAAATTCTTGGAAGGG | WT ~800 KO ~350 |
Calcium imaging
Methods were similar to those described previously (Paez et al., 2007). Briefly, primary culture of cortical astrocytes were washed in serum and phenol red-free DMEM containing a final concentration of 4μM fura-2 (AM) (Life Technologies) plus 0.08% Pluronic F127 (Life Technologies) to load dye into the cells, incubated for 25min at 37°C, 5% CO2, then washed four times in DMEM and stored in DMEM for 10min before being imaged. Calcium influx and resting Ca++ levels were measured in serum and phenol red-free HBSS containing 1.3mM Ca++ and 1mM Mg++. The fluorescence of fura-2 was excited alternatively at wavelengths of 340 and 380nm every 2s by means of a high-speed wavelength-switching device (Lambda DG4; Sutter Instruments). A spinning disc confocal microscope (Olympus, IX83-DSU) equipped with a CCD camera (Hamamatsu ORCA-R2) measured the fluorescence. Calcium influx and resting Ca++ levels were measured on individual astrocytes using the image analysis software MetaFluor (Molecular Devices). More than 600 cells for each experimental condition were analyzed and the results from 5 separate experiments were pooled. To minimize bleaching, the intensity of excitation light and sampling frequency was kept as low as possible.
Enzyme-linked immunosorbent assays (ELISA)
Cell culture supernatants were collected after 3 days of LPS (1μg/ml) treatment. The release of chemokines IL1β, IL4, IL6, IL10, IL12, IL17A, IFNγ, TNFα, TGFβ1, MCP-1, MIP-1a and MIP-1b into the supernatant was measured using the Mouse Autoimmune Response Multi-Analyte ELISArray™ Kits (Qiagen). All ELISA procedures were performed according to the manufacturer's instructions and absorbance was measured at 450nm with subtraction of absorbance at 570nm using a microplate reader (BioRad).
Statistical Analysis
Normal distributions were tested in each data set using Kolmogorov-Smirnov tests. For data with normal distributions, single between-group comparisons were made by the Student paired t-test, and multiple comparisons were investigated by one-way ANOVA followed by Bonferroni’s multiple comparison tests to detect pair-wise between-group differences. All statistical tests were performed in Graphpad Prism (Graphpad Software). A fixed value of p<0.05 for one tailed tests was the criterion for reliable differences between groups. Data are presented as mean ± SEM unless otherwise noted.
RESULTS
Cortical astrocytes display voltage-operated Ca++ influx in vitro
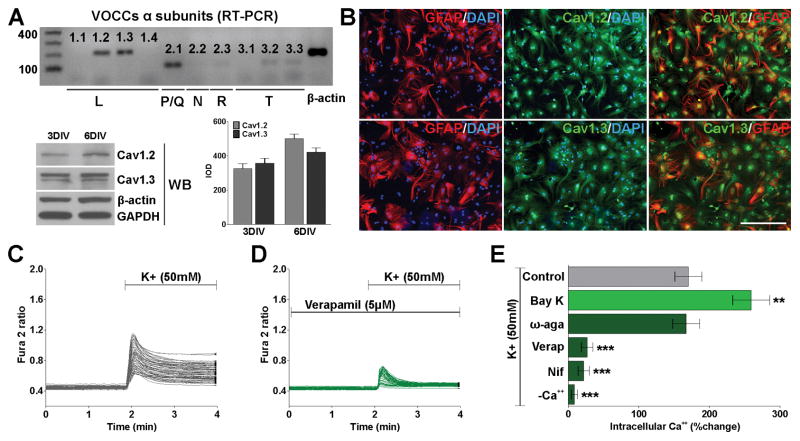
We have found by RT-PCR that both L- and P/Q-type voltage-operated Ca++ channels (VOCCs) are expressed in cultured cortical astrocytes (Figure 1A). The α1 subunit of the L-type Ca++ channels is encoded by Cav1-genes consisting of four different subtypes. Under normal physiological conditions the expression of Cav1.1 and Cav1.4 in astrocytes is very low to absent (Hell et al., 1993) (Figure 1A). In contrast, RT-PCR, western blot and immunocytochemistry experiments show that Cav1.2 and Cav1.3 are highly expressed in cortical astrocytes (Figure 1A and B). Figure 1B shows representative images of labeled cultured astrocytes at 6 days in vitro (DIV); while GFAP abundantly labeled cell processes and cell bodies, labeling with Cav1.2 and Cav1.3 antibodies displayed a punctate staining on the cells surface (Figure 1B). The presence of functional VOCCs in cortical astrocytes was then evaluated by ratiometric Ca++ measurements using fura-2 as intracellular Ca++ indicator. Calcium influx mediated by VOCCs was induced by depolarization via elevated external potassium (K+). Increasing external K+ to 50mM has been proven to be an effective way to fully activate VOCCs by plasma membrane depolarization (Paez et al., 2007; 2009a; 2010). High K+ induced a biphasic increase in astrocyte intracellular Ca++. The first phase consisted of a sharp peak characteristic of a transient, large increase in intracellular Ca++, which was followed by a second phase of slowly declining internal Ca++ concentrations (Figure 1C). Importantly, increases in fura-2 signal in these cells were abolished in the presence of zero Ca++, and were blocked by the specific L-type channels inhibitors, verapamil and nifedipine (Figure 1D and E). Additionally, the amplitude of Ca++ influx was enhanced by Bay K 8644, an L-type Ca++ channel agonist that prolongs single channel open time without affecting the close time (Figure 1E). Thus, the rise in intracellular Ca++ after high K+ stimulation in cortical astrocytes resulted from Ca++ influx via L-type VOCCs. Although the Cav2.1 α subunit was detected by RT-PCR (Figure 1A), Ca++ imaging experiments using an inhibitor for P/Q channels (ω-agatoxin IVA) demonstrate that these kind of VOCCs are not active in cortical astrocytes (Figure 1E).
FIGURE 1. L-type Ca++ channels are the predominant VOCCs in the plasma membrane of cortical astrocytes.
(A) Semi-quantitative RT-PCR and western blots analysis of VOCC α1 subunits expression in cortical astrocytes. The analysis was performed after 3 and 6 days in vitro (DIV) using β-actin and GAPDH as internal standards. Data from three independent western blots are summarized based on the relative spot intensities and plotted as percent of controls. (B) After 6DIV, astrocytes were stained with antibodies against GFAP, Cav1.2 and Cav1.3. Scale bar = 80μm. (C and D) VOCC activity was examined in cultured astrocytes at 6DIV using fura-2 as intracellular Ca++ indicator. Note that each trace corresponds to a single cell and the horizontal bars indicate the time of addition of external solution containing high K+ and verapamil. (E) The bar graph shows the average amplitude of the Ca++ response, calculated from the responding cells expressed as a percentage of change of the emission intensities. VOCC modulators were applied at the following final concentration: Bay K 8644 (5μM), ω-agatoxin IVA (1μM), verapamil (5μM) and nifedipine (5μM). Values are expressed as mean ± SEM of at least six independent experiments. **p<0.01, ***p<0.001 vs. control.
L-type Ca++ channels are involved in the effect of LPS on promoting astrogliosis in vitro
In order to explore the possible role of L-type VOCCs on astrocyte activation we mimicked a bacterial infection in vitro by treating primary cultures of cortical astrocytes with the endotoxin lipopolysaccharide (LPS) (Pistritto et al., 1999). Six days after plating, cortical astrocytes were treated with different concentration of LPS for 3 days. We found that LPS induced the expression of classical astrocyte reactivity markers, such as GFAP and s100β (Figure 2A and B). After 3 days of treatment, a significant increase in the expression of GFAP and s100β were observed at 0.75, 1 and 2μg/ml of LPS (Figure 2B), suggesting that LPS induced the activation of astrocytes in vitro in a concentration-dependent manner. Furthermore, individual GFAP-positive cells were scored according to their morphological complexity in two categories, based on the length and number of primary processes, the relative development of secondary and tertiary processes, and the overall size of the cell. At 1μg/ml of LPS, nearly all GFAP positive cells displayed morphological changes characteristic of reactive astrocytes (Figure 2A and C). For instance, LPS treated astrocytes showed an increase in the thickness of the primary processes and a significant reduction in the spatial coverage (Figure 2A and C). Then, we used specific antibodies and Ca++ imaging techniques to evaluate the expression and activity of L-type VOCCs in cortical astrocytes following administration of LPS. Immunocytochemical and western blot experiments showed a substantial upregulation of the L-type VOCC subunit Cav1.2 after 3 days of LPS treatment (Figure 2D and E). In contrast, LPS did not significantly modify the levels of Cav1.3 in cortical astrocytes (Figure 2D and E). Furthermore, we found a significant increase in the activity of L-type VOCCs in LPS treated astrocytes (Figure 2F). In control cells, bath application of a solution containing high K+ caused an average Ca++ increase of ~170% (Figure 2F and G), in LPS treated astrocytes, the Ca++ transient induced by high K+ was significantly higher (~270%) (Figure 2F and G). Importantly, the rise in intracellular Ca++ in these cells was blocked by the specific L-type VOCC inhibitors verapamil and nifedipine (Figure 2G).
FIGURE 2. LPS enhance the activity and the expression of Cav1.2 Ca++ channels in astrocytes.
(A) After 6DIV, astrocytes were treated with different concentrations of LPS for 3 consecutive days and were stained with antibodies against GFAP and s100β. Scale bar = 120μm. (B) The fluorescent intensity for each marker was measured by confocal microscopy and was plotted as integrated optical density (IOD). (C) Individual GFAP-positive cells were scored according to their morphological complexity in two categories. (D) After 3 days of LPS (1μg/ml) treatment, astrocytes were stained with antibodies against Cav1.2 and Cav1.3. The fluorescent intensity for each L-type Ca++ channel was measured by confocal microscopy and was plotted as IOD. Scale bar = 80μm. (E) Western blots analysis of Cav1.2/1.3 VOCC α1 subunits expression in cortical astrocytes. The analysis was performed after 3 days of LPS (1μg/ml) treatment using p84, β-actin and GAPDH as internal standards and data from three independent experiments are summarized based on the relative spot intensities. (F) After 3 days of LPS (1μg/ml) treatment, VOCC activity was examined in cultured astrocytes using fura-2 as intracellular Ca++ indicator. Note that each trace corresponds to a single cell and the horizontal bars indicate the time of addition of external solution containing high K+. (G) The bar graph shows the average amplitude of the Ca++ response, calculated from the responding cells expressed as a percentage of change of the emission intensities. Verapamil and nifedipine were applied at 5μM. Values are expressed as mean ± SEM of at least six independent experiments. *p<0.05, **p<0.01, ***p<0.001 vs. control; ###p<0.001 vs. LPS.
To determine if voltage-operated Ca++ entry was involved in LPS activation of astrocytes, cells were treated with LPS (1μg/ml) for 3 days in the presence of verapamil and nifedipine. As shown in Figure 3A-B, the presence in the culture medium of L-type VOCC inhibitors did not significantly modify the basal levels of GFAP and s100β positive cells but drastically reduces the number of these positive cells following LPS treatment. Furthermore, K+-induced Ca++ influx was significantly lower in astrocytes when verapamil or nifedipine were present in the culture medium during the complete LPS treatment (Figure 3C). Importantly, verapamil did not change Cav1.3 expression but was able to prevent Cav1.2 upregulation after LPS treatment (Figure 2D).
FIGURE 3. VOCC inhibitors block astrocyte activation in response to LPS.
After 6DIV, astrocytes were treated with LPS (1μg/ml) for 3 consecutive days in combination with verapamil (5μM) or nifedipine (5μM) and were stained with antibodies against GFAP and s100β (A and B), Ki67 (G and H) and caspase-3 (J). (C) VOCC activity was examined in cultured astrocytes after LPS treatment using fura-2 as intracellular Ca++ indicator. The bar graph shows the average amplitude of the Ca++ response, calculated from the responding cells expressed as a percentage of change of the emission intensities. (D, E and F) Astrocytes were exposed to BrdU during the last day (24h) (D and E), or during the complete LPS treatment (72h) (F). (I) Evaluation of astrocytes viability by the MTT assay 3 days after LPS treatment. Values are expressed as mean ± SEM of at least six independent experiments. **p<0.01, ***p<0.001 vs. control; #p<0.05, ##p<0.01, ###p<0.001 vs. LPS. Scale bar = 120μm (A), 100μm (D and H).
To determine the effect of LPS on astrocyte proliferation we labeled proliferating astrocytes with the thymidine analogue bromo-deoxyuridine (BrdU). A 24h pulse of 10μM BrdU was given after 2 days of LPS treatment. The average number of proliferating cells in LPS treated cultures (~26%) was significantly higher than that of control cells (~17%, P<0.01) (Figure 3D and E). Additionally, we exposed astrocytes to BrdU during the complete LPS treatment. Under this experimental condition we found that LPS increases the level of proliferation from ~24% to ~59%, suggesting that three days of LPS treatment promotes the proliferation of more than a half of the astrocyte population (Figure 3F). Importantly, no significant differences relative to controls were found when LPS was combined with verapamil or nifedipine (Figure 3D-F). Confirmation of the BrdU cell proliferation results was performed using Ki67 as markers of mitotic cells. There were more Ki67 positive astrocytes in LPS treated cultures than in controls (Figure 3G and H), but no significant differences vs. controls were found in the presence of VOCC specific inhibitors (Figure 3G and H). Cell survival was examined using the MTT quantitative colorimetric method for cell viability. The results revealed no statistically significant differences between experimental groups after 3 days of LPS treatment (Figure 3I). Furthermore, caspase-3 immunostaining showed no changes in the percentage of apoptotic cells relative to controls (Figure 3J).
We have also evaluated the release of several proinflammatory cytokines and chemokines by LPS treated astrocytes. The release of cytokines IL1β, IL4, IL6, IL10, IL12, IL17A, IFNγ, TNFα and TGFβ1, and chemokines MCP1, MIP-1α and MIP-1β into the supernatant was measured by ELISA after three days of LPS treatment (Table II). In agreement with previous publications (van Neerven et al., 2010; Tarassishin et al., 2014), we have found that astrocytes responded to LPS with a strong upregulation of proinflammatory cytokines such as IL1β, IL6, IL10 and TNFα (Table II). Importantly, treatment with the L-type VOCC blocker verapamil significantly reduce the release of these important cytokines (Table II). Similarly, the production of the chemokines MIP-1α and MIP-1β by LPS treated astrocytes was also decreased by verapamil (Table II).
Table II. Release of proinflammatory cytokines and chemokines by LPS treated astrocytes.
Cytokine and chemokine concentrations were measured by ELISA in astrocyte culture supernatants 3 days after LPS (1μg/ml) treatment. Verapamil was applied at 5μM. Values are expressed as mean ± SEM (pg/ml) of three independent experiments.
| Control | Verapamil | LPS | LPS + Verapamil | |
|---|---|---|---|---|
| Cytokines | ||||
| IL1β | 0.24 ± 0.03 | 0.23 ± 0.07 | 0.66 ± 0.02*** | 0.42 ± 0.07## |
| IL4 | n.d. | n.d. | n.d. | n.d. |
| IL6 | 0.60 ± 0.21 | 0.52 ± 0.44 | 256.50 ± 6.31*** | 134.21 ± 24.90### |
| IL10 | 0.37 ± 0.11 | 0.25 ± 0.03 | 39.81 ± 2.22*** | 26.68 ± 2.12### |
| IL12 | n.d. | n.d. | n.d. | n.d. |
| IL17A | n.d. | n.d. | n.d. | n.d. |
| IFNγ | n.d. | n.d. | n.d. | n.d. |
| TNFα | 0.02 ± 0.01 | 0.01 ± 0.01 | 32.67 ± 1.21*** | 23.48 ± 3.56# |
| TGFβ1 | n.d. | n.d. | n.d. | n.d. |
| Chemokines | ||||
| MCP1 | 20.15 ± 4.84 | 17.21 ± 9.03 | 613.42 ± 19.54*** | 593.07 ± 20.93 |
| MIP-1α | 0.09 ± 0.01 | 0.08 ± 0.04 | 180.94 ± 5.87*** | 133.05 ± 4.61### |
| MIP-1β | 0.65 ± 0.15 | 1.30 ± 0.26 | 180.06 ± 5.86*** | 150.13 ± 11.54# |
p<0.001 vs. control;
p<0.05,
p<0.01,
p<0.001 vs. LPS.
n.d.: non detected. Red denote significant increase relative to controls whereas blue represent significant decrease relative to LPS.
Together, these results indicate that LPS promote astrocyte activation and proliferation, i.e. an increase in the number of reactive astrocytes (GFAP and s100β), an increase in the number of proliferating cells (BrdU and Ki67) and a rise in the production of proinflammatory cytokines and chemokines. Moreover, LPS treatment upregulates the expression and the activity of L-type VOCCs containing the α1 subunit Cav1.2. These effects disappeared when L-type VOCC blockers were present in the culture media suggesting that L-type voltage-operated Ca++ influx is involved in the effect of LPS on promoting astrogliosis in vitro.
Cav1.2 knock-down/out inhibits astrocyte response to LPS
In this group of experiments, small interfering RNAs (siRNAs) for the Cav1.2 and Cav1.3 α1 subunits -the sole pore-forming subunits of the voltage-dependent L-type Ca++ channel found in astrocytes- were used to knock down the expression of L-type VOCCs in primary cultures of cortical astrocytes. Three days after plating, astrocytes were transiently transfected with three different siRNA duplexes specific for Cav1.2 and Cav1.3. siRNAs were selected to target three distinct Cav1.2/1.3 mRNA regions to enhance silencing (Cheli et al., 2015). siRNAs designed to minimize sequence homology to any known vertebrate transcript and with a similar GC content as our siRNA were used as negative controls for sequence independent effects in all the siRNA experiments performed in this work. To determine the percentage of siRNA-transfected cells, cortical astrocytes were treated with fluorescein-labeled dsRNA oligomers. We found that approximately all the cells displayed some degree of siRNA fluorescent signal after transfection (Figure 4A). The function of Cav1.2/1.3 siRNAs was then confirmed using semi quantitative RT-PCR and western blotting. Figure 4B shows representative RT-PCRs and western blots demonstrating that Cav1.2/1.3 siRNA decreased Cav1.2/1.3 mRNAs and proteins in cortical astrocytes. Importantly, siRNAs for Cav1.2 efficiently knock-down the expression of Cav1.2 but do not modify the levels of Cav1.3. On the contrary, siRNAs for Cav1.3 specifically decrease Cav1.3 expression without changing the levels of Cav1.2 (Figure 4B). Additionally, Figure 4C and D shows the effect of Cav1.2/1.3 siRNA on L-type Ca++ influx in astrocytes. Consistent with the RT-PCR and western blot data, Cav1.2 siRNA reduced L-type Ca++ influx by approximately 85% after plasma membrane depolarization, whereas Cav1.3 siRNA reduced Ca++ influx by ~15% (Figure 4C and D). These data suggest that Cav1.2 is the principal pore-forming subunit of the voltage-dependent L-type Ca++ channel in astrocytes.
FIGURE 4. Cav1.2 knock-down prevents astrocyte activation by LPS.
After 3DIV, astrocytes were transfected with siRNA duplexes specific for Cav1.2 and Cav1.3 (siCav1.2/1.3). (A) Cells were treated with fluorescein-labeled dsRNA oligomers to determine siRNA transfection efficiency. Scale bar = 60μm. (B) Three days after transfection, semi-quantitative RT-PCR and western blot analysis of Cav1.2 and Cav1.3 expression in astrocytes was performed using β-actin and GAPDH as internal standards. Data from three independent experiments are summarized based on the relative spot intensities and plotted as percent of controls. (C) At the same time, VOCC activity was examined in cultured astrocytes using fura-2 as intracellular Ca++ indicator. Note that each trace corresponds to a single cell and the horizontal bars indicate the time of addition of external solution containing high K+. (D) The bar graph shows the average amplitude of the Ca++ response, calculated from the responding cells expressed as a percentage of change of the emission intensities. (E–F) Three days after siRNA transfection, astrocytes were treated with LPS (1μg/ml) for 3 consecutive days and were stained with antibodies against GFAP, s100β and Ki67. The percentage of positive cells in each experimental condition was examined by confocal microscopy. Scale bar = 160μm. Values are expressed as mean ± SEM of at least six independent experiments. ***p<0.001 vs. corresponding controls.
Next, siRNAs transfected astrocytes were cultured in the presence of LPS for 3 days and immunostained for astrocyte reactivity markers. As shown in Figure 4E and F, LPS efficiently promotes the activation and proliferation of Cav1.3 transfected astrocytes but completely fails to trigger cell reactivity and proliferation in Cav1.2 deficient cultures. The percentage of GFAP, s100β and Ki67 positive cells in Cav1.3 deficient cultures was very similar to what we found in non-transfected cultures after 3 days of LPS treatment (compare Figure 3B and G with Figure 4F). On the contrary, astrocytes lacking Cav1.2 show low levels of reactivity and proliferation, close to non-treated cells, after LPS treatment (Figure 4F).
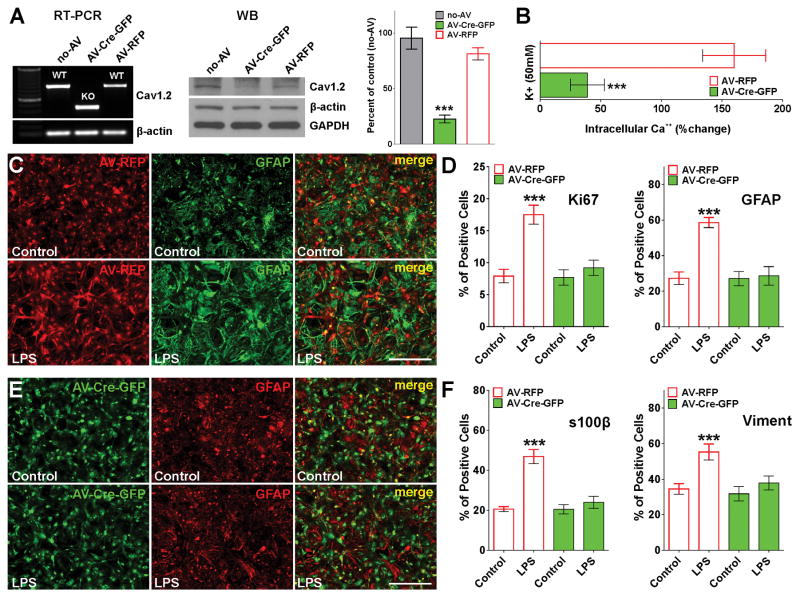
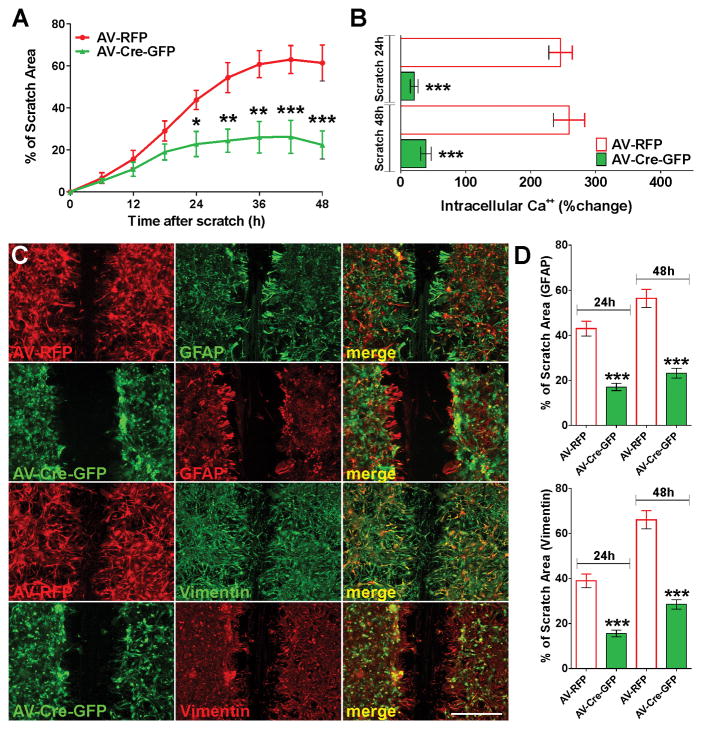
Similar results were found in primary cultures of Cav1.2 floxed astrocytes infected with adenovirus expressing the Cre-recombinase (Figure 5). In the floxed mutant Cav1.2 line (White et al., 2008), exon 2 of the wild-type Cav1.2 gene (cacna1c) was flanked with loxP sites and thus exon 2 is eliminated when Cre-recombinase is present. Removal of exon 2 leads to a truncated, non-functional protein and the absence of a specific L-type VOCC (White et al., 2008). Homozygous Cav1.2 floxed astrocytes were infected with adenoviruses expressing both Cre-recombinase and GFP (AV-Cre-GFP) as well as control adenoviruses expressing the RFP (AV-RFP). Three days after infection, more than 90% of the cells were positive for GFP or RFP (Figure 5C and E). RT-PCR and western blot experiments showed high recombination efficiency and decreased Cav1.2 protein levels in Cre expressing astrocytes (Figure 5A). Furthermore, Ca++ imaging experiments revealed a significant decrease in intracellular Ca++ concentrations after plasma membrane depolarization in Cre-GFP positive cells relative to astrocytes infected with the control virus (AV-RFP) (Figure 5B). In agreement with the siRNA knock-down experiments, Cav1.2 floxed astrocytes expressing the Cre enzyme (Cav1.2 KO cells) were not sensitive to LPS treatment (Figure 5C–F). Cav1.2 KO cells displayed control levels of reactivity and cell proliferation after LPS treatment (Figure 5D, E and F). In contrast, astrocytes infected with the control virus showed a substantial upregulation of GFAP, s100β and vimentin expression as well as high levels of proliferation and morphological changes characteristic of reactive astrocytes (Figure 5C, D and F). In summary, these data suggest that L-type VOCCs, particularly the channels containing the Cav1.2 α subunit, may play a role in the induction of reactive astrocytes initiated by LPS, and indicate that the inhibition of these Ca++ channels may be an effective way to prevent astrocyte activation.
FIGURE 5. Cav1.2 KO astrocytes are not sensitive to LPS.
At 3DIV, Cav1.2 floxed astrocytes were infected with type 5 adenoviruses expressing both Cre recombinase and GFP (AV-Cre-GFP), and control adenoviruses expressing the RFP (AV-RFP). (A) Three days after infection, RT-PCR and western blot analysis of Cav1.2 expression in astrocytes was performed using β-actin and GAPDH as internal standards. Data from three independent western blots are summarized based on the relative spot intensities and plotted as percent of controls. (B) At the same time, VOCC activity was examined in cultured astrocytes using fura-2 as intracellular Ca++ indicator. The bar graph shows the average amplitude of the Ca++ response, calculated from the responding cells expressed as a percentage of change of the emission intensities. (C–F) Three days after infection, astrocytes were treated with LPS (1μg/ml) for 3 consecutive days and were stained with antibodies against Ki67, GFAP, s100β and vimentin. (C and E) Examples of control (AV-RFP) and Cav1.2 KO (AV-Cre-GFP) cultures immunolabeled with GFAP. Scale bar = 120μm. (D and F) The percentage of positive cells in each experimental condition was examined by confocal microscopy. Values are expressed as mean ± SEM of at least six independent experiments. ***p<0.001 vs. corresponding controls.
L-type VOCCs activation stimulates astrocyte reactivity in vitro
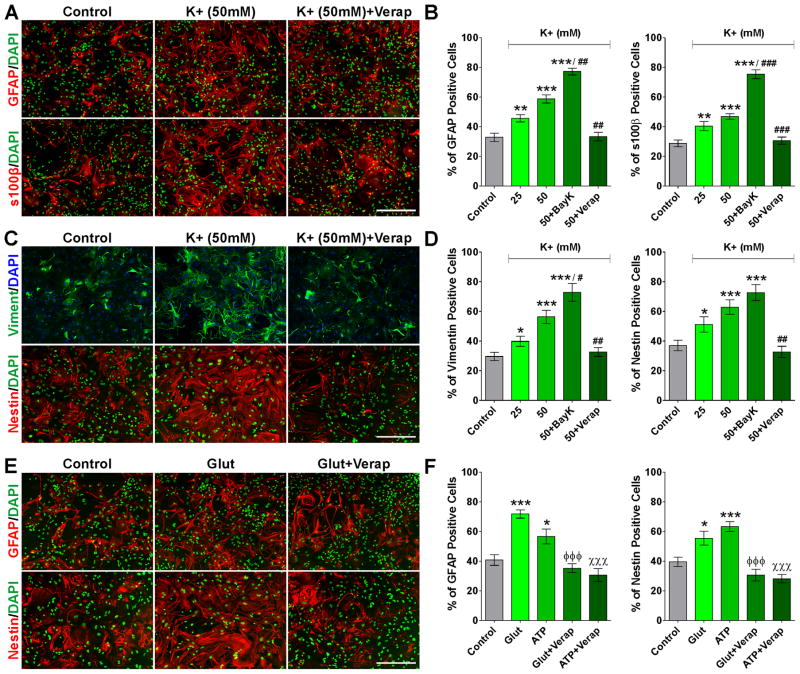
In order to test the ability of L-type VOCCs activation to induce astrocyte reactivity in vitro, we treated primary cultures of cortical astrocytes with high extracellular K+ (25 and 50mM). The proportion of GFAP, s100β, vimentin and nestin positive cells increases significantly after treating the cultures with 25 and 50mM of K+ (Figure 6), and positive cells for these reactive markers exhibit clear morphological changes consistent with astrocyte activation (Figure 6A and C). Importantly, all these changes were prevented by blocking L-type Ca++ influx with verapamil and were potentiated by the L-type agonist Bay K 8644 (Figure 6A–D). These data indicate that high K+ treatment is an effective approach to activate astrocytes in vitro and that Ca++ uptake across L-type VOCCs is essential for this effect.
FIGURE 6. High K+, glutamate and ATP induce astrocyte activation in vitro.
After 6DIV, astrocytes were treated with high extracellular K+ (25 and 50mM) for 3 consecutive days and were stained with antibodies against GFAP and s100β (A and B) and vimentin and nestin (C and D). Astrocytes were also treated with glutamate and ATP (10mM) for 3 consecutive days and were stained with antibodies against GFAP and nestin (E and F). Scale bar = 160μm. The percentage of positive cells in each experimental condition was examined by confocal microscopy. Verapamil and Bay K 8644 were applied at 5μM. Values are expressed as mean ± SEM of at least six independent experiments. *p<0.05, **p<0.01, ***p<0.001 vs. control; #p<0.05, ##p<0.01, ###p<0.001 vs. K+ (50mM); ϕϕϕp<0.001 vs. glutamate; χ χχp<0.001 vs. ATP.
Astrocytes express high levels of glutamate receptors such as the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors as well as ATP receptors like P2X purinoceptors (Lalo et al., 2006, 2008, 2011; Verkhratsky and Kirchhoff, 2007). Glutamate and ATP receptor-mediated Ca++ signaling in astrocytes is initiated by an increased influx of Na+ and Ca++ through the activated receptor. Subsequently Na+ influx can depolarize the astrocytic plasma membrane making ATP and glutamate an alternative way to stimulate L-type VOCCs. Since the extracellular concentration of glutamate and ATP in the brain increases drastically in several pathological conditions in which astrogliosis is a key feature, we treated primary cultures of cortical astrocytes with elevated concentrations of these molecules. Like high K+, glutamate and ATP notably increase the number of reactive astrocytes expressing GFAP and nestin (Figure 6E and F), and in agreement with our previous results, the L-type VOCC blocker verapamil significantly prevents astrocyte activation under these experimental conditions (Figure 6E and F).
Voltage-mediated Ca++ influx contributes to astrogliosis after mechanical trauma
To investigate the involvement of L-type VOCCs on astrocyte reactivity following mechanical trauma, we used the scratch wound assay (Etienne-Manneville and Hall, 2001). Mouse astrocytes were obtained from the postnatal cerebral cortex and grown to full confluence to allow for astrocyte maturation. Using this in vitro system of mechanical trauma in combination with time-lapse microscopy, we have evaluated for 48h the morphological changes that are characteristic for trauma-induced reactive astrogliosis, the expression of key reactive astrocyte markers and Ca++ influx mediated by VOCCs. In accordance with previous observations (Etienne-Manneville, 2006), injuring the monolayer results in astrocytes extending processes toward the cell-free scratch region and subsequently migrating and re-populating the scratch over a 48h period (Figure 7A). We first took a pharmacological approach and the effects of blocking VOCC dependent Ca++ influx were studied with the specific L-type channel inhibitors verapamil and nifedipine. Verapamil and nifedipine were added immediately after the scratch. Forty-eight hours following the mechanical injury, astrocytes extended and migrated into the injury area, resulting in 66% closure of the gap compared to the original wound size (Figure 7A and B). Upon addition of verapamil, the degree of closure was attenuated, resulting in significantly decreased closure of 30% of the initial injured area (Figure 7A and B). Similarly, addition of nifedipine significantly inhibited the closure of the wound area (38%) (Figure 7B). Calcium imaging experiments showed a significant increase in the activity of VOCCs in reactive astrocytes located in the growing line in comparison to quiescent astrocytes situated away from the scratch (Figure 7C, D and E). Moreover, astrocytes with scratch-oriented processes consistently displayed higher amplitude Ca++ signals than surrounding cells (Figure 7C, lower panel). In control experiments, fura-2 signals were blocked by nifedipine and verapamil, confirming that these changes in intracellular Ca++ result from Ca++ influx through L-type VOCCs (Figure 7E). We then examined the repair of the scratch by GFAP, nestin and vimentin positive astrocytes under the different experimental conditions. We expressed this as the area of the scratch covered by GFAP, nestin or vimentin at 24 and 48h using thresholding in image analysis to delineate the covered areas. At both time points, we found that control astrocytes expressing GFAP, nestin or vimentin covered a significantly larger area of the scratch than verapamil or nifedipine treated cells (Figure 7F and G). Additionally, suggesting a reduction in the number of proliferating astrocytes, the proportion of Ki67 positive cells within the scratch was below control levels in verapamil and nifedipine treated cultures (Figure 7F and G).
FIGURE 7. L-type Ca++ influx plays an important role in astrocyte activation after mechanical injury.
Immediately after the scratch, cortical astrocytes were incubated in a chamber with 5% CO2 at 37°C, which was placed on the stage of a spinning disc confocal microscope. (A) Time-lapse series of control and verapamil treated cultures. The original site of the scratch is marked by a yellow dotted line and time is denoted in hours in the upper right corner. Scale bar = 160μm. (B) The percentage of the initial scratched area covered by cells was measured in each experimental condition every 6h for a period of 48h. **p<0.01, ***p<0.001 vs. verapamil; ##p<0.01, ###p<0.001 vs. nifedipine. (C-E) VOCC activity was examined 24h after the scratch using fura-2 as intracellular Ca++ indicator. (C) Each frame represents a single section of a fura-2 time-lapse experiment. An increased fura-2 fluorescence ratio is indicated by warmer colors. Scale bar = 80μm. (D) Fura-2 imaging of Ca++ responses to 50mM K+ in control (central area) and scratched astrocytes (scratch). Note that each trace corresponds to a single cell and the horizontal bar indicates the time of high K+ addition. (E) The bar graph shows the average amplitude of the Ca++ response, calculated from the responding cells expressed as a percentage of change of the emission intensities. Verapamil and nifedipine were applied at 5μM. ***p<0.001 vs. control (central area); ###p<0.001 vs. scratch (24h). (F and G) Cortical astrocyte monolayers were scratched and immunostained for the astrocyte markers GFAP, nestin and vimentin or the proliferation marker Ki67 at 24 and 48h after the scratch. (F) Examples of control and verapamil treated cultures 48h after the scratch. Scale bar = 180μm. (G) The percentage of the initial scratched area covered by GFAP, nestin and vimentin positive cells and the percentage of Ki67-positive cells located in the scratch area were quantified in the different experimental conditions at 24 and 48h after the scratch. *p<0.05, **p<0.01, ***p<0.001 vs. respective controls. Values are expressed as mean ± SEM of at least six independent experiments.
Cav1.2 channels are necessary for astrocyte response to mechanical damage
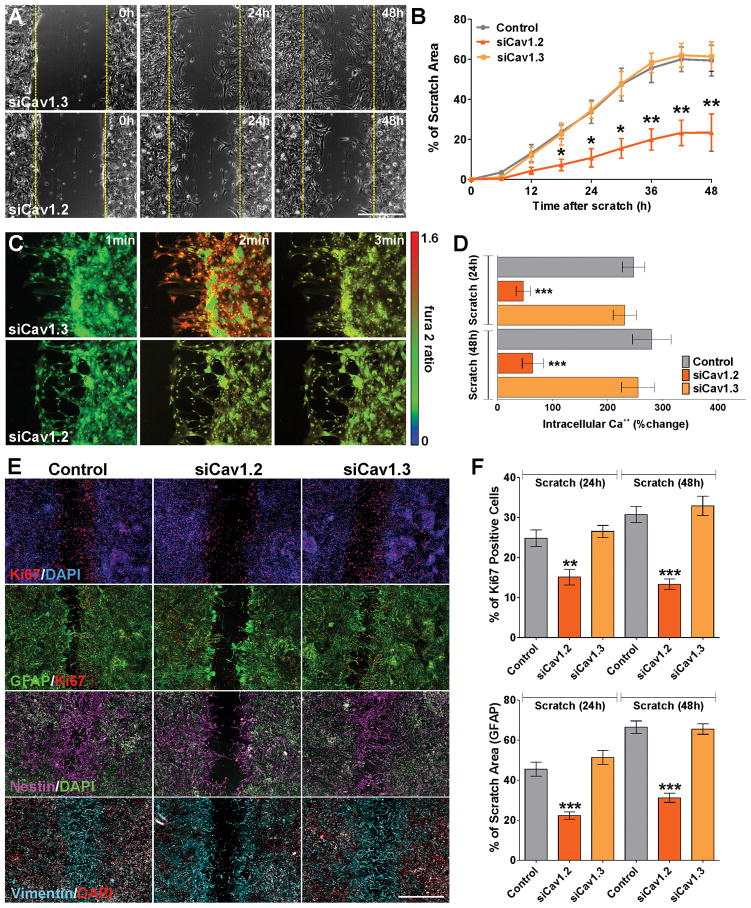
To study morphological changes and reactivity following mechanical trauma in cells lacking L-type VOCCs, scratch wound experiments were also performed using astrocytes transfected with siRNAs for the two α1 subunits of L-type VOCCs expressed in astrocytes. Similar to what we found in verapamil and nifedipine treated scratches, there was a significant delay in the closure of the wound area in cultures transfected with siRNAs for Cav1.2 (Figure 8A and B). Twenty-four and 48h after the scratch, we also found a decrease in voltage-gated Ca++ influx in Cav1.2 deficient cells relative to control or Cav1.3 transfected astrocytes located in the growing line (Figure 8C and D). Furthermore, the percentage of proliferating astrocytes as well as the area of the scratch covered by GFAP and vimentin positive cells was also reduced in Cav1.2 deficient cultures (Figure 8E and F). Importantly, in Cav1.3 knock-down cultures the level of cell proliferation as well as the speed of wound closure was similar to what we observed in control cells (Figure 8A, B, E and F).
FIGURE 8. Cav1.2 knock-down prevents astrocyte activation after scratch.
Immediately after the scratch, cortical astrocytes were incubated in a chamber with 5% CO2 at 37°C, which was placed on the stage of a spinning disc confocal microscope. (A) Time-lapse series of siCav1.2/1.3 transfected cultures. The original site of the scratch is marked by a yellow dotted line and time is denoted in hours in the upper right corner. Scale bar = 160μm. (B) The percentage of the initial scratched area covered by cells was measured in each experimental condition every 6h for a period of 48h. (C) VOCC activity was examined 24h after the scratch using fura-2 as intracellular Ca++ indicator. Each frame represents a single section of a fura-2 time-lapse experiment. An increased fura-2 fluorescence ratio is indicated by warmer colors. Scale bar = 100μm. (D) Fura-2 imaging of Ca++ responses to 50mM K+ in control and siCav1.2/1.3 transfected astrocytes at 24 and 48h after the scratch. (E and F) Control and siCav1.2/1.3 transfected astrocytes were scratched and immunostained for the astrocyte markers GFAP, nestin and vimentin or the proliferation marker Ki67 at 24 and 48h after the scratch. (E) Examples of control and siCav1.2/1.3 transfected cultures 48h after the scratch. Scale bar = 180μm. (F) The percentage of the initial scratched area covered by GFAP positive cells and the percentage of Ki67-positive cells located in the scratch area were quantified in each experimental conditions at 24 and 48h after the scratch. Values are expressed as mean ± SEM of at least six independent experiments. **p<0.01, ***p<0.001 vs. respective controls.
Parallel experiments were performed in primary cultures of Cav1.2 floxed astrocytes infected with adenovirus expressing the Cre-recombinase. In line with the previous results, Cav1.2 KO astrocytes (AV-Cre-GFP) repair the scratch much slower than control cells expressing the RFP (AV-RFP) (Figure 9A), and Cav1.2 KO cells located in the growing line display lower Ca++ influx than controls after plasma membrane depolarization (Figure 9B). Additionally, 48h after the scratch, most control astrocytes at the border of the scratch had developed long, unipolar cell protrusions pointing from the soma into the scratch (Figure 9C). On the other hand, the morphology of Cav1.2 KO astrocytes was more polygonal, with smaller protrusions than those of control cells, and the protrusions were less directed toward the scratch (Figure 9C). Moreover, the area of the scratch covered by GFAP and vimentin positive cells was reduced in Cav1.2 KO cultures (Figure 9C and D). In summary, these data suggest a key role for voltage-mediated Ca++ influx in astrocyte activation after mechanical trauma. Specifically, our results show that Ca++ influx triggered by Cav1.2 L-type Ca++ channels plays an important role in pathways regulating the response of astrocytes to injury.
FIGURE 9. Cav1.2 KO astrocytes show less reactivity following mechanical damage.
Immediately after the scratch, cortical astrocytes were incubated in a chamber with 5% CO2 at 37°C, which was placed on the stage of a spinning disc confocal microscope. (A) The percentage of the initial scratched area covered by cells was measured for control (AV-RFP) and Cav1.2 KO astrocytes (AV-Cre-GFP) every 6h for a period of 48h. (B) Fura-2 imaging of Ca++ responses to 50mM K+ in control and Cav1.2 KO astrocytes at 24 and 48h after the scratch. The bar graph shows the average amplitude of the Ca++ response, calculated from the responding cells expressed as a percentage of change of the emission intensities. (C and D) Control and Cav1.2 KO astrocytes were scratched and immunostained for the astrocyte markers GFAP and vimentin at 24 and 48h after the scratch. (C) Examples of control and Cav1.2 KO cultures immunolabeled with GFAP 48h after the scratch. Scale bar = 160μm. (D) The percentage of the initial scratched area covered by GFAP and vimentin positive cells was quantified in each experimental condition at 24 and 48h after the scratch. Values are expressed as mean ± SEM of at least six independent experiments. ***p<0.001 vs. respective controls (AV-RFP).
DISCUSSION
Voltage-operated Ca++ channels are upregulated in reactive astrocytes
The presence of VOCCs in astrocytes, although initially subjected to great controversy, is now widely accepted. The first evidence for VOCC activity in cultured astrocytes was the discovery of Ca++-dependent action potential firing on intracellular current injections (MacVicar, 1984). Then, the presence of VOCCs was demonstrated by recording voltage-operated Ca++ currents in cultured astrocytes (MacVicar and Tse, 1988; D'Ascenzo et al., 2004; Latour et al., 2003). Detailed investigation of membrane currents and Ca++ influx in rat hippocampus and visual cortex (Carmignoto et al., 1998) showed apparent lack of functional VOCCs, but different types of VOCCs were present in a population of immature mouse hippocampal astrocytes (Akopian et al., 1996). Similarly, Ca++ influx mediated by VOCCs was detected in astrocytes from the subventricular zone (Young et al., 2010) and the ventrobasal thalamus (Parri and Crunelli, 2003; Parri et al., 2001). In these brain regions, it has been demonstrated that astrocytes in acute slices prepared from young rats show voltage-operated Ca++ oscillations (Komuro and Rakic, 1993, 1998). Furthermore, a recently published RNA-sequencing transcriptome database of glial, neurons, and vascular cells of the cerebral cortex, reveal high expression of Cav1.2 and Cav1.3 L-type VOCC subunits in cortical astrocytes (Zhang et al., 2014).
We have found that L-type VOCCs are highly expressed by cortical astrocytes in vitro. Using ratiometric Ca++ imaging techniques, we have shown that depolarization induced a significant rise of intracellular Ca++ in the soma of cortical astrocytes and we have established that L-type Ca++ channels containing the Cav1.2 subunit are the most active VOCCs in the plasma membrane of these cells. Furthermore, we have demonstrated that Cav1.2 L-type Ca++ channels are upregulated following astrocyte activation by both LPS and mechanical trauma. In line with our findings, the expression of L-type Ca++ channels was increased in reactive astrocytes in several models of brain injury. For example, Westenbroek et al. (1998) found high levels of Cav1.2 Ca++ channels activity in reactive astrocytes in the CA1 section of the hippocampus, the striatum and the white matter after mechanical and thermal lesions in the forebrain, hypomyelination in white matter, and ischemia. Chung et al. (2001) detected increased expression of Cav1.2, but not Cav1.3, in reactive astrocytes located in the hippocampus and in the cerebral cortex following an ischemic injury in the rat brain. Also, reactive hippocampal astrocytes of mice submitted to pilocarpine-induced status epilepticus showed upregulation of expression of L- and P/Q-type channels (Xu et al., 2007). The fact that activated astrocytes from different CNS structures upregulate L-type VOCCs under different pathological conditions suggest that these Ca++ channels participate in critical cellular processes common to all reactive astrocytes.
Activation of L-type VOCCs promotes astrogliosis in vitro
During regular physiological activity in the CNS the extracellular K+ concentration rarely increases by more than 0.2 to 0.4mM. Nonetheless, under pathological conditions such as epileptic seizures, brain ischemia and trauma, K+ concentration can transiently peak at 50–60mM (Katayama et al., 1990, 1995; Nilsson et al., 1993). This transient increase in extracellular K+ might potentially depolarize the plasma membrane of quiescent astrocytes and generate Ca++ influx by VOCCs. We believe that this could be one of the first signals for astrocyte activation in the injured CNS. We have found a substantial astrogliosis after treating primary cultures of cortical astrocytes with high extracellular K+. Suggesting the participation of L-type Ca++ channels in this event, K+ stimulation of astrocyte activation was promoted by the L-type channel agonist Bay K and was blocked by VOCC antagonists. These data show that changes in intracellular Ca++ concentrations resulting from the modulation of voltage-gated Ca++ influx provide a powerful means by which astrogliosis may be induced in vitro as well as in vivo.
The scavenging activity of astrocytes is crucial in regulating excessive levels of glutamate, K+ and other ions. A close correlation between reactive gliosis and accumulation of excitotoxic levels of glutamate have been described (Rothstein et al., 2005). Excitotoxic levels of glutamate during neuroinflammation are also contributed by astrocytes through Ca++-dependent secretory pathways following intracellular Ca++ increase (Agulhon et al., 2012). Likewise, purinergic receptors are important modulators of reactive gliosis; ATP released from damaged cells can modulate several mechanisms of neuroinflammatory pathways, such as synthesis of cytokines, proliferation and glutamate release (Franke et al., 2012; Rodrigues et al., 2015). In this work, we have shown that astrocytes respond to high levels of glutamate and ATP by increasing the expression of reactive markers such as GFAP and nestin. Importantly, the presence of verapamil in the culture media decreased the percentage of reactive cells back to control levels. Thus, blocking L-type VOCCs in astrocytes attenuates astrogliosis induced by pathological concentrations of K+, glutamate and ATP, suggesting that these Ca++ channels participate in a molecular step common to all of these reactive stimulus.
Cav1.2 channels are essential for astrocyte activation in response to LPS
The feature of brain inflammation is the activation of glial cells (Ridet et al., 1997) and the secretion of cytokines. Astrocytes participate in the innate immune response of the brain and are capable of producing most inflammatory mediators (Eddleston and Mucke, 1993; Dong and Benveniste, 2001). LPS, a component of the cell wall of gram-negative bacteria (Pistritto et al. 1999), has been widely used experimentally to stimulate inflammatory responses in the CNS (Hauss-Wegrzyniak et al., 1998). Astrocytes have a toll-like receptor type 4 (TLR4), which belongs to TLR family receptors in the vertebrate immune system and specifically recognizes LPS (Carpentier et al., 2008). Recent studies have shown that astrocytes respond to LPS, decreasing expression of proteins such as gap junction proteins (Liao et al., 2010) and increasing expression of others such as GFAP, s100β, IL-1 and TNFα (Vergara et al., 2010; Guerra et al., 2011; van Neerven et al., 2010; Tarassishin et al., 2014). We have demonstrated that LPS treatment stimulates the expression and the activity of L-type VOCCs in primary cultures of cortical astrocytes. We have found that LPS increases the number of reactive astrocytes, the proportion of proliferating cells and promotes the production of proinflammatory cytokines and chemokines. Consistent with the hypothesis that functional L-type calcium channels are important for the activation of astrocytes in response to LPS, these effects disappeared when L-type VOCC blockers were present in the culture media. Moreover, these results were confirmed by knocking down/out the L-type VOCC subunit Cav1.2 in cortical astrocytes. Therefore, our data suggest that inhibition of L-type Ca++ channels can attenuate astrocyte activation started by LPS. Our results are in line with previous in vitro studies using human astrocytes (Hashioka et al., 2012), which showed that L-type Ca++ channel blockers conferred neuroprotection against astrocyte-mediated neurotoxicity by reducing IFNγ induced activation of STAT3 and the production of IFNγ-inducible T cell α chemo attractant (I-TAC), and with studies showing that L-type Ca++ channel inhibitors decrease neuronal damage through their anti-inflammatory activities on astrocytes in rat neuron-glia co-cultures (Liu et al., 2009; 2011).
The inflammatory response in the brain is primarily mediated by microglia (Farina et al., 2007), and like astrocytes, microglia cells respond to LPS by releasing TNFα and IL-1β (Hoogland et al., 2015). In this work, we used LPS to mimic a bacterial infection and to reproduce an inflammatory environment in vitro. Since our cultured astrocytes routinely contained a small proportion of microglia cells (≤0.5%), we can not exclude the possibility that some of the LPS effects on astrocytes were actually mediated by factors released by activated microglia. However, we believe that a small proportion of microglial cells essentially improved the response of astrocytes to LPS by creating an in vitro environment which is more similar to the in vivo situation.
L-type channels play an important role in astrogliosis induced by mechanical trauma
Our observations in cultured astrocytes establish a molecular mechanism involving L-type Ca++ channels that contributes to astroglial response to mechanical injury. We have found a significant increase in the activity of VOCCs in reactive astrocytes located in the growing line in comparison to quiescent astrocytes situated away from the scratch; and astrocytes with scratch-oriented processes consistently displayed higher amplitude Ca++ signals than surrounding cells. The number of proliferating astrocytes as well as the area of the scratch covered by reactive cells was reduced in Cav1.2 deficient cultures, and Cav1.2 KO astrocytes displayed shorter and less directed processes than control cells. Pappalardo et al., 2014 have recently showed that astrocytes display Ca++ influx after mechanical injury and that Na+/Ca++ exchanger blockers reduce this intracellular Ca++ response. Since the Na+/Ca++ exchanger is essential for plasma membrane depolarization in astrocytes (Kirischuk et al., 2012; Paluzzi et al., 2007), these data support our observations regarding a central role of VOCCs in astrocyte activation after mechanical trauma.
L-type Ca++ channel inhibition may reduce reactive astrocytosis directly, by attenuating biochemical changes in quiescent astroglia. For example, Ca++ entry through L-type VOCCs has been shown to increases GFAP expression, phosphorylation and assembly (Geisert, et al., 1990; Harrison and Mobley, 1992; Nakamura et al., 1992). The phosphorylation of GFAP is necessary for proper conformational changes and binding to the intermediate filaments, essential steps to induce morphological transformations in reactive cells (Steinert and Liem, 1990). On the other hand, inhibition of L-type Ca++ channels may decrease reactive astrogliosis indirectly by reducing migration and proliferation of reactive astrocytes after trauma. In this regard, the role of voltage-operated Ca++ influx in oligodendrocyte and neuronal migration and proliferation has been well documented (Paez et al., 2007; 2009a; 2009b; 2010; Cheli et al., 2015; Guo et al. 2010; Darcy and Isaacson, 2009). Moreover, we have recently reported that pharmacological inhibition of L-type channels as well as Cav1.2 knock-down negatively affects oligodendrocyte migration and proliferation (Paez et al., 2009a; 2009b; 2010; Cheli et al., 2015). In this work, we have established that pharmacological inhibition of VOCCs as well as Cav1.2 knockdown/out blocks astrocyte proliferation and migration after mechanical trauma and LPS treatment. Thus, it is possible that blocking L-type Ca++ channels disturbs astrogliosis by reducing migration and proliferation of reactive astrocytes after injury.
The significance of this work lies in our need to obtain a better understanding of the molecular and cellular mechanisms that control astrocyte activation in order to enhance repair and restore function after CNS damage. In summary, we have provided data suggesting that L-type VOCCs are key regulators of astrocyte reactivity in response to different insults. L-type Ca++ channels are upregulated following astrocyte activation by both LPS and mechanical trauma and blocking L-type VOCCs expression and activity prevent astrocyte reactivity. Therefore, modulation of astroglial VOCCs seems to have therapeutic potential for several CNS pathological conditions and injuries.
Main Points.
L-type Ca++ channels are important regulators of astrocyte activation.
Selective deletion of L-type Ca++ channels in astrocytes prevents astrogliosis in vitro.
Acknowledgments
NIH/NINDS grant 5R01NS078041-02 and National Multiple Sclerosis Society Grant RG4554-A-2.
References
- Agulhon C, Sun MY, Murphy T, Myers T, Lauderdale K, Fiacco TA. Calcium Signaling and Gliotransmission in Normal vs. Reactive Astrocytes. Front Pharmacol. 2012;3:139. doi: 10.3389/fphar.2012.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian G, Kressin K, Derouiche A, Steinhauser C. Identified glial cells in the early postnatal mouse hippocampus display different types of Ca++ currents. Glia. 1996;17:181–194. doi: 10.1002/(SICI)1098-1136(199607)17:3<181::AID-GLIA1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Amur-Umarjee S, Phan T, Campagnoni AT. Myelin basic protein mRNA translocation in oligodendrocytes is inhibited by astrocytes in vitro. J Neurosci Res. 1993;36:99–110. doi: 10.1002/jnr.490360111. [DOI] [PubMed] [Google Scholar]
- Badou A, Jha MK, Matza D, Mehal WZ, Freichel M, Flockerzi V, Flavell RA. Critical role for the beta regulatory subunits of Cav channels in T lymphocyte function. Proc Natl Acad Sci USA. 2006;103:15529–15534. doi: 10.1073/pnas.0607262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Pasti L, Pozzan T. On the role of voltage-dependent calcium channels in calcium signaling of astrocytes in situ. J Neurosci. 1998;18:4637–4645. doi: 10.1523/JNEUROSCI.18-12-04637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier PA, Duncan DS, Miller SD. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav Immun. 2008;22:140–147. doi: 10.1016/j.bbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheli VT, Santiago González DA, Spreuer V, Paez PM. Voltage-gated Ca++ entry promotes oligodendrocyte progenitor cells maturation and myelination in vitro. Experimental Neurology. 2015;265:69–83. doi: 10.1016/j.expneurol.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WC, Xue HZ, Hsu YL, Liu Q, Patel S, Davis RL. Complex distribution patterns of voltage-gated calcium channel α-subunits in the spiral ganglion. Hear Res. 2011;278:52–68. doi: 10.1016/j.heares.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YH, Shin CM, Kim MJ, Cha CI. Enhanced expression of L-type Ca2+ channels in reactive astrocytes after ischemic injury in rats. Neurosci Lett. 2001;302:93–96. doi: 10.1016/s0304-3940(01)01683-4. [DOI] [PubMed] [Google Scholar]
- D'Ascenzo M, Vairano M, Andreassi C, Navarra P, Azzena GB, Grassi C. Electrophysiological and molecular evidence of L-(Cav1), N- (Cav2.2), and R- (Cav2.3) type Ca++ channels in rat cortical astrocytes. Glia. 2004;45:354–363. doi: 10.1002/glia.10336. [DOI] [PubMed] [Google Scholar]
- Darcy DP, Isaacson JS. L-type calcium channels govern calcium signaling in migrating newborn neurons in the postnatal olfactory bulb. J Neurosci. 2009;29:2510–2518. doi: 10.1523/JNEUROSCI.5333-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Mucke L. Molecular profile of reactive astrocytes--implications for their role in neurologic disease. Neurosci. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S. In vitro assay of primary astrocyte migration as a tool to study Rho GTPase function in cell polarization. Methods Enzymol. 2006;406:565–578. doi: 10.1016/S0076-6879(06)06044-7. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Franke H, Verkhratsky A, Burnstock G, Illes P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012;8:629–657. doi: 10.1007/s11302-012-9300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K, Wang CR, Jiang F, Wong AY, Su N, Jiang JH, Chai RC, Vatcher G, Teng J, Chen J, Jiang YW, Yu AC. Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia. 2013;61:2063–2077. doi: 10.1002/glia.22577. [DOI] [PubMed] [Google Scholar]
- Geisert EE, Jr, Johnson HG, Binder LI. Expression of microtubule-associated protein 2 by reactive astrocytes. Proc Natl Acad Sci USA. 1990;87:3967–3971. doi: 10.1073/pnas.87.10.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN, Malvar JS, de Vellis J, Sabatti C, Dell'Angelica EC. The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry. 2010;15:204–215. doi: 10.1038/mp.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra MC, Tortorelli LS, Galland F, Da Ré C, Negri E, Engelke DS, Rodrigues L, Leite MC, Gonçalves CA. Lipopolysaccharide modulates astrocytic S100B secretion: a study in cerebrospinal fluid and astrocyte cultures from rats. J Neuroinflammation. 2011;8:128. doi: 10.1186/1742-2094-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Shi F, Zhang L, Zhang H, Yang J, Li B, Jia J, Wang X, Wang X. Critical role of L-type voltage-dependent Ca++ channels in neural progenitor cell proliferation induced by hypoxia. Neurosci Lett. 2010;478:156–160. doi: 10.1016/j.neulet.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Gurkoff G, Shahlaie K, Lyeth B, Berman R. Voltage-gated calcium channel antagonists and traumatic brain injury. Pharmaceuticals. 2013;26:788–812. doi: 10.3390/ph6070788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BC, Mobley PL. Phorbol myristate acetate and 8-bromo-cyclic AMP-induced phosphorylation of glial fibrillary acidic protein and vimentin in astrocytes: comparison of phosphorylation sites. J Neurochem. 1992;56:1723–1730. doi: 10.1111/j.1471-4159.1991.tb02073.x. [DOI] [PubMed] [Google Scholar]
- Hashioka S, Klegeris A, McGeer PL. Inhibition of human astrocyte and microglia neurotoxicity by calcium channel blockers. Neuropharmacology. 2012;63:685–6891. doi: 10.1016/j.neuropharm.2012.05.033. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Lukovic L, Bigaud M, Stoeckel ME. Brain inflammatory response induced by intracerebroventricular infusion of lipopolysaccharide: an immunohistochemical study. Brain Res. 1998;794:211–224. doi: 10.1016/s0006-8993(98)00227-3. [DOI] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastritsis CHC, Salm AK, McCarthy K. Stimulation of the P2Y purinergicreceptor on type 1 astroglia results in inositol phosphate formation and calciummobilization. J Neurochem. 1992;58:1277–1284. doi: 10.1111/j.1471-4159.1992.tb11339.x. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- Kawamata T, Katayama Y, Hovda DA, Yoshino A, Becker DP. Lactate accumulation following concussive brain injury: the role of ionic fluxes induced by excitatory amino acids. Brain Res. 1995;674:196–204. doi: 10.1016/0006-8993(94)01444-m. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: Another key to astroglial excitability? Trends Neurosci. 2012;35:407–506. doi: 10.1016/j.tins.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Selective role of N-type calcium channels in neuronal migration. Science. 1992;257:806–809. doi: 10.1126/science.1323145. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca++ fluctuations. J Neurobiol. 1998;37:110–130. [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci. 2006;26:2673–2683. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Wichert SP, Rossner MJ, North RA, Kirchhoff F, Verkhratsky A. P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J Neurosci. 2008;28:5473–5480. doi: 10.1523/JNEUROSCI.1149-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Parpura V, Verkhratsky A. Ionotropic receptors in neuronal-astroglial signalling: what is the role of ‘‘excitable’’ molecules in nonexcitable cells. Biochim Biophys Acta. 2011;1813:992–1002. doi: 10.1016/j.bbamcr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Latour I, Hamid J, Beedle AM, Zamponi GW, Macvicar BA. Expression of voltage-gated Ca++ channel subtypes in cultured astrocytes. Glia. 2003;41:347–353. doi: 10.1002/glia.10162. [DOI] [PubMed] [Google Scholar]
- Liao CK, Wang SM, Chen YL, Wang HS, Wu JC. Lipopolysaccharide-induced inhibition of connexin 43 gap junction communication in astrocytes is mediated by downregulation of caveolin-3. Int J Biochem Cell Biol. 2010;42:762–770. doi: 10.1016/j.biocel.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Liao CK, Wang SM, Chen YL, Wang HS, Wu JC. Lipopolysaccharide-induced inhibition of connexin 43 gap junction communication in astrocytes is mediated by downregulation of caveolin-3. Int J Biochem Cell Biol. 2010;42:762–770. doi: 10.1016/j.biocel.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu X, Liu Y, Bao Y, An L. Nimodipine protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. Neuropharmacology. 2009;56:580–589. doi: 10.1016/j.neuropharm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lo YC, Qian L, Crews FT, Wilson B, Chen HL, Wu HM, Chen SH, Wei K, Lu RB, Ali S, Hong JS. Verapamil protects dopaminergic neuron damage through a novel anti-inflammatory mechanism by inhibition of microglial activation. Neuropharmacology. 2011;60:373–380. doi: 10.1016/j.neuropharm.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane SN, Sontheimer H. Electrophysiological changes that accompany reactive gliosis in vitro. J Neurosci. 1997;17:7316–7329. doi: 10.1523/JNEUROSCI.17-19-07316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar BA. Voltage-dependent calcium channels in glial cells. Science. 1984;226:1345–1347. doi: 10.1126/science.6095454. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Tse FW. Norepinephrine and cyclic adenosine 3':5'-cyclic monophosphate enhance a nifedipine-sensitive calcium current in cultured rat astrocytes. Glia. 1988;1:359–365. doi: 10.1002/glia.440010602. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Takeda M, Aimoto S, Hojo H, Takao T, Shimonishi Y, Hariguchi S, Nishimura T. Assembly regulatory domain of glial fibrillary acidic protein. A single phosphorylation diminishes its assembly-accelerating property. J Biol Chem. 1992;267:23269–23274. [PubMed] [Google Scholar]
- Nilsson P, Hillered L, Olsson Y, Sheardown MJ, Hansen AJ. Regional changes in interstitial K+ and Ca++ levels following cortical compression contusion trauma in rats. J Cereb Blood Flow Metab. 1993;13:183–192. doi: 10.1038/jcbfm.1993.22. [DOI] [PubMed] [Google Scholar]
- Oh Y. Ion channels in neuroglial cells. Kaohsiung J Med Sci. 1997;13:1–9. [PubMed] [Google Scholar]
- Omilusik K, Priatel JJ, Chen X, Wang YT, Xu H, Choi KB, Gopaul R, McIntyre-Smith A, Teh HS, Tan R, Bech-Hansen NT, Waterfield D, Fedida D, Hunt SV, Jefferies WA. The Cav1.4 calcium channel is a critical regulator of T cell receptor signaling and naive T cell homeostasis. Immunity. 2011;35:349–360. doi: 10.1016/j.immuni.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Paez PM, Fulton DJ, Spreuer V, Handley V, Campagnoni CW, Macklin WB, Colwell C, Campagnoni AT. Golli myelin basic proteins regulate oligodendroglial progenitor cell migration through voltage-gated Ca++ influx. J Neurosci. 2009a;29:6663–6676. doi: 10.1523/JNEUROSCI.5806-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez PM, Fulton DJ, Spreuer V, Handley V, Campagnoni AT. Multiple kinase pathways regulate voltage-dependent Ca++ influx and migration in oligodendrocyte precursor cells. J Neurosci. 2010;30:6422–6433. doi: 10.1523/JNEUROSCI.5086-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez PM, Fulton DJ, Spreuer V, Handley V, Campagnoni CW, Campagnoni AT. Regulation of store-operated and voltage-operated Ca++ channels in the proliferation and death of oligodendrocyte precursor cells by Golli proteins. ASN-Neuro. 2009b;1:25–41. doi: 10.1042/AN20090003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez PM, Spreuer V, Handley V, Feng JM, Campagnoni C, Campagnoni AT. Increased expression of golli myelin basic proteins enhances calcium influx into oligodendroglial cells. J Neurosci. 2007;27:12690–12699. doi: 10.1523/JNEUROSCI.2381-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluzzi S, Alloisio S, Zappettini S, Milanese M, Raiteri L, Nobile M, Bonanno G. Adult astroglia is competent for Na+/Ca++ exchanger-operated exocytotic glutamate release triggered by mild depolarization. J Neurochem. 2007;103:1196–1207. doi: 10.1111/j.1471-4159.2007.04826.x. [DOI] [PubMed] [Google Scholar]
- Pappalardo LW, Samad OA, Black JA, Waxman SG. Voltage–gated sodium channel Nav 1.5 contributes to astrogliosis in an in vitro model of glial injury via reverse Na+/Ca++ exchange. Glia. 2004;62:1162–1175. doi: 10.1002/glia.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnis J, Montana V, Martinex-Delgado I, Matyash V, Parpura V, Kettenmann H, Sekler I, Nolte C. Mitochondrial exchanger NCLX plays a major role in the intracellular Ca++ signaling, gliotransmission, and proliferation of astrocytes. J Neurosci. 2013;33:7206–7219. doi: 10.1523/JNEUROSCI.5721-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri HR, Crunelli V. The role of Ca++ in the generation of spontaneous astrocytic Ca++ oscillations. Neuroscience. 2003;120:979–892. doi: 10.1016/s0306-4522(03)00379-8. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca++ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Pistritto G, Franzese O, Pozzoli G, Mancuso C, Tringali G, Preziosi P, Navarra P. Bacterial lipopolysaccharide increases prostaglandin production by rat astrocytes via inducible cyclo-oxygenase: evidence for the involvement of nuclear factor kappaB. Biochem Biophys Res Commun. 1999;263:570–574. doi: 10.1006/bbrc.1999.1413. [DOI] [PubMed] [Google Scholar]
- Puro DG, Hwang JJ, Kwon OJ, Chin H. Characterization of an L-type calcium channel expressed by human retinal Muller (glial) cells. Brain Res Mol Brain Res. 1996;37:41–48. doi: 10.1016/0169-328x(96)80478-5. [DOI] [PubMed] [Google Scholar]
- Reyes SD, Campagnoni AT. Two separate domains in the golli myelin basic proteins are responsible for nuclear targeting and process extension in transfected cells. J Neurosci Res. 2002;69:587–596. doi: 10.1002/jnr.10319. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Tomé AR, Cunha RA. ATP as a multi-target danger signal in the brain. Front Neurosci. 2015;9:148. doi: 10.3389/fnins.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati B, Yan Q, Lee MS, Liou SR, Ingalls B, Foell J, Kamp TJ, McKinnon D. Robust L-type calcium current expression following heterozygous knockout of the Cav1.2 gene in adult mouse heart. J Physiol. 2011;589:3275–3288. doi: 10.1113/jphysiol.2011.210237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanimirovic DB, Ball R, Mealing G, Morley P, Durkin JP. The role of intracellular calcium and protein kinase C in endothelin-stimulated proliferation of rat type I astrocytes. Glia. 1995;15:119–130. doi: 10.1002/glia.440150204. [DOI] [PubMed] [Google Scholar]
- Steinert PM, Liem RK. Intermediate filament dynamics. Cell. 1990;60:521–523. doi: 10.1016/0092-8674(90)90651-t. [DOI] [PubMed] [Google Scholar]
- Suzumura A, Bhat S, Eccleston PA, Lisak RP, Silberberg DH. The isolation and long-term culture of oligodendrocytes from newborn mouse brain. Brain Res. 1984;324:379–383. doi: 10.1016/0006-8993(84)90054-4. [DOI] [PubMed] [Google Scholar]
- Tarassishin L, Suh HS, Lee SC. LPS and IL-1 differentially activate mouse and human astrocytes: role of CD14. Glia. 2014;62:999–1013. doi: 10.1002/glia.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Neerven S, Nemes A, Imholz P, Regen T, Denecke B, Johann S, Beyer C, Hanisch UK, Mey J. Inflammatory cytokine release of astrocytes in vitro is reduced by all-trans retinoic acid. J Neuroimmunol. 2010;229:169–179. doi: 10.1016/j.jneuroim.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Vergara D, Martignago R, Bonsegna S, De Nuccio F, Santino A, Nicolardi G, Maffia M. IFN-beta reverses the lipopolysaccharide-induced proteome modifications in treated astrocytes. J Neuroimmunol. 2010;221:115–120. doi: 10.1016/j.jneuroim.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Kirchhoff F. NMDA receptors in glia. Neuroscientist. 2007;13:28–37. doi: 10.1177/1073858406294270. [DOI] [PubMed] [Google Scholar]
- Wang HH, Hsieh HL, Yang CM. Calmodulin kinase II-dependent transactivation of PDGF receptors mediates astrocytic MMP-9 expression and cell motility induced by lipoteichoic acid. J Neuroinflammation. 2010;7:84–100. doi: 10.1186/1742-2094-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek RE, Bausch SB, Lin RC, Franck JE, Noebels JL, Catterall WA. Upregulation of L-type Ca++ channels in reactive astrocytes after brain injury, hypomyelination, and ischemia. J Neurosci. 1998;18:2321–2334. doi: 10.1523/JNEUROSCI.18-07-02321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, McKinney BC, John MC, Powers PA, Kamp TJ, Murphy GG. Conditional forebrain deletion of the L-type calcium channel Cav1.2 disrupts remote spatial memories in mice. Learn Mem. 2008;15:1–5. doi: 10.1101/lm.773208. [DOI] [PubMed] [Google Scholar]
- Xu JH, Long L, Tang YC, Hu HT, Tang FR. Cav1.2, Cav1.3, and Cav2.1 in the mouse hippocampus during and after pilocarpine-induced status epilepticus. Hippocampus. 2007;17:235–251. doi: 10.1002/hipo.20263. [DOI] [PubMed] [Google Scholar]
- Young SZ, Platel JC, Nielsen JV, Jensen NA, Bordey A. GABA(A) Increases Calcium in Subventricular Zone Astrocyte-Like Cells Through L- and T-Type Voltage-Gated Calcium Channels. Front Cell Neurosci. 2010;4:8. doi: 10.3389/fncel.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AC, Lee YL, Eng LF. Astrogliosis in culture: I. The model and the effect of antisense oligonucleotides on glial fibrillary acidic protein synthesis. J Neurosci Res. 1993;34:295–303. doi: 10.1002/jnr.490340306. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]