Abstract
Background
Early detection and treatment of asymptomatic men with advanced and high-risk prostate cancer (PCa) may improve survival rates.
Objective
To determine outcomes for men diagnosed with advanced PCa following prostate-specific antigen (PSA) testing who were excluded from the ProtecT randomised trial.
Design, setting, and participants
Mortality was compared for 492 men followed up for a median of 7.4 yr to a contemporaneous cohort of men from the UK Anglia Cancer Network (ACN) and with a matched subset from the ACN.
Outcome measurements and statistical analysis
PCa-specific and all-cause mortality were compared using Kaplan-Meier analysis and Cox's proportional hazards regression.
Results and limitations
Of the 492 men excluded from the ProtecT cohort, 37 (8%) had metastases (N1, M0 = 5, M1 = 32) and 305 had locally advanced disease (62%). The median PSA was 17 μg/l. Treatments included radical prostatectomy (RP; n = 54; 11%), radiotherapy (RT; n = 245; 50%), androgen deprivation therapy (ADT; n = 122; 25%), other treatments (n = 11; 2%), and unknown (n = 60; 12%). There were 49 PCa-specific deaths (10%), of whom 14 men had received radical treatment (5%); and 129 all-cause deaths (26%). In matched ProtecT and ACN cohorts, 37 (9%) and 64 (16%), respectively, died of PCa, while 89 (22%) and 103 (26%) died of all causes. ProtecT men had a 45% lower risk of death from PCa compared to matched cases (hazard ratio 0.55, 95% confidence interval 0.38–0.83; p = 0.0037), but mortality was similar in those treated radically. The nonrandomised design is a limitation.
Conclusions
Men with PSA-detected advanced PCa excluded from ProtecT and treated radically had low rates of PCa death at 7.4-yr follow-up. Among men who underwent nonradical treatment, the ProtecT group had a lower rate of PCa death. Early detection through PSA testing, leadtime bias, and group heterogeneity are possible factors in this finding.
Patient summary
Prostate cancer that has spread outside the prostate gland without causing symptoms can be detected via prostate-specific antigen testing and treated, leading to low rates of death from this disease.
Keywords: Prostate cancer, Prostate-specific antigen screening, Survival
Take Home Message
Early detection and treatment of asymptomatic men with advanced and high-risk prostate cancer lead to good survival rates and are indicative of improved survival compared to men presenting clinically.
1. Introduction
Population-based prostate specific antigen (PSA) screening remains controversial [1]. Although screening in the European Randomised Study of Screening for Prostate Cancer (ERSPC) detected high numbers of prostate cancers (PCas) and lower mortality from that disease, the majority of cancers were indolent, leading to overdetection and overtreatment [2], [3]. The Prostate, Lung and Ovarian cancer screening study (PCLO) reported no survival benefit after 11.5 yr of follow-up, but there was widespread contamination in the control arm with previous PSA testing (up to 90%) [2], [3].
There is uncertainty regarding the effectiveness of treatments for PSA-detected clinically localised PCa. The Prostate Cancer Intervention Versus Observation Trial (PIVOT) reported no survival benefit after 12 yr of follow-up among men with mainly low-risk disease treated with surgery or observation, although there was high all-cause mortality in both arms, suggesting that men with major comorbidities were included [4]. No randomised trials have compared different radical treatments for men with advanced [5], [6] or high-risk disease, and retrospective studies have reported conflicting results [7], [8], [9], [10]. There is uncertainty regarding outcomes among men with higher-risk PCa detected via PSA screening, although a subgroup analysis of PIVOT suggested benefit in favour of radical treatment for intermediate- or high-risk disease [4].
Details of the ProtecT trial are reported elsewhere [11], [12], [13], [14], [15]. Men with metastatic or locally advanced disease (cT3–4) and/or PSA ≥20 μg/l were excluded from ProtecT, along with men considered by local urologists to be unsuitable for the trial because of their clinical features. These men excluded from the ProtecT randomised trial but diagnosed contemporaneously provide a unique opportunity to assess the outcomes of advanced and high-risk disease at diagnosis in a population with very low rates of opportunistic PSA screening (8–13%) [12], [16].
Here we present survival data for these men in comparison to data for a contemporaneous cohort from the UK Anglia Cancer Network (ACN), which has generally low rates of PSA testing, and with a matched ACN cohort with similar disease features.
2. Patients and methods
2.1. Case population
The ProtecT trial compares active monitoring, conformal external-beam radical radiotherapy (RT) with or without androgen deprivation therapy (ADT) and radical prostatectomy (RP) treatments for PSA-detected clinically localised PCa [12]. Between 2001 and 2009 there were 82 429 asymptomatic men aged 50 and 69 yr who underwent PSA testing, and those with PSA ≥3 μg/l proceeded to biopsy. Participants with initial PSA ≥20 μg/l or found to have locally advanced (T3–4) PCa or distant disease (N1 or M1) were ineligible and referred for standard care. The majority had locally advanced PCa; a small proportion (5%) were classed as at high risk of having non–organ-confined disease and were felt to be unsuitable for randomisation. In total, 513 men (PSA ≥20 μg/l, or locally advanced cT3–4 PCa, or Gleason ≥8, or N1/M1 disease) were excluded from ProtecT (Table 1). These men form the ProtecT advanced cases cohort reported here.
Table 1.
Demographic and clinicopathologic data for the study cohort
| Variable | Unmatched |
Matched |
||||
|---|---|---|---|---|---|---|
| ProtecT | ACN | p value a | ProtecT | ACN | p value a | |
| Patients (n) | 492 | 3978 | 401 | 401 | ||
| Year of diagnosis, n (%) | <0.0001 | 1 | ||||
| 1999–2003 | 178 (36) | 1109 (28) | 151 (38) | 151 (38) | ||
| 2004–2006 | 191 (39) | 1117 (28) | 157 (39) | 157 (39) | ||
| 2007–2010 | 123 (25) | 1752 (44) | 93 (23) | 93 (23) | ||
| Age band, n (%) | <0.0002 | 0.86 | ||||
| 50–59 yr | 102 (21) | 567 (14) | 83 (21) | 81 (20) | ||
| 60–72 yr | 390 (79) | 3411 (86) | 318 (79) | 320 (80) | ||
| Serum PSA, n (%) | <0.0001 | 0.48 | ||||
| <10 ng/ml | 160 (33) | 728 (18) | 149 (37) | 144 (36) | ||
| 10–20 ng/ml | 116 (24) | 752 (19) | 112 (28) | 117 (29) | ||
| 20–50 ng/ml | 141 (28) | 1086 (27) | 90 (22) | 75 (19) | ||
| 50–100 ng/ml | 49 (10) | 462 (12) | 24 (6) | 30 (7) | ||
| >100 ng/ml | 26 (5) | 769 (19) | 26 (6) | 35 (9) | ||
| Unknown | 0(0) | 181 (5) | 0 (0) | 0 (0) | ||
| Mean PSA, ng/ml (median) | 32.6 (16.7) | 201.1 (26.5) | 31.7 (14) | 217.2 (13) | ||
| Gleason score, n (%) | <0.0001 | 1 | ||||
| <7 | 112 (23) | 473 (12) | 93 (23) | 92 (23) | ||
| 7 | 259 (53) | 1300 (33) | 222 (55) | 223 (55) | ||
| >7 | 115 (23) | 1654 (42) | 86 (21)) | 86 (21) | ||
| Unknown | 6 (10 | 551 (14) | 0 (0) | 0 (0) | ||
| Mean Gleason score (median) | 7.1 (7) | 7.6 (7) | 7.1 (7) | 7.0 (7) | ||
| Clinical stage, n (%) | <0.0001 | 0.18 | ||||
| T1 | 17 (4) | 989 (25) | 16 (4) | 29 (7) | ||
| T2 | 42 (8) | 750 (19) | 42 (10) | 29 (7) | ||
| T3 | 305 (62) | 1063 (27) | 301 (75) | 298 (74) | ||
| T4 | 5 (10 | 44 (1) | 4 (1) | 5 (1) | ||
| M1 or N1 | 37 (8) | 1132 (28) | 37 (9) | 40 (10) | ||
| T stage unknown | 86 (18) | 0 (0) | 1 (1) | 0 (0) | ||
| Follow-up (yr) | ||||||
| Mean | 7.5 | 5.5 | 7.7 | 7.5 | ||
| Median | 7.4 | 5 | 7.6 | 7.6 | ||
| Interquartile range | 5.5–9.7 | 3.1–7.8 | 5.5–9.8 | 5.1–9.8 | ||
ACN = Anglia Cancer Network; PSA = prostate-specific antigen.
p value for χ2 test for heterogeneity between unmatched and matched ProtecT advanced cases and ACN controls.
Information on treatment and survival was obtained during annual ProtecT follow-up and checked using the English National Cancer Online Registration Environment database in the Eastern Office of the National Cancer Registration Service (NCRS-E) [15]. Cause of death was determined by review of certification by two independent clinicians blinded to study group and treatment.
2.2. Comparison population
Comparison patients (controls) were identified by the NCRS-E by interrogation of the Anglia Cancer Network (ACN) [10] for a contemporary cohort of men with comparable age and year of diagnosis and similarly advanced and high-risk disease features: PSA ≥20 ng/ml, locally advanced disease (cT3–4), Gleason score ≥8, or N1/M1 disease. The ACN cases were judged to be a suitable comparative cohort because of low rates of PSA testing (10–13%) in the ACN population [12], [17] (Supplementary material).
The ProtecT trial was approved by the East Midlands Ethics Committee (Derby, UK; record number 01/4/025).
2.3. Statistical analysis
We used the χ2 test for heterogeneity to assess baseline differences between cases and controls. The primary analysis compared risk of death from PCa and all causes between ProtecT cases and ACN controls with clinically detected PCa. Cases and controls were matched 1:1 according to age, year of diagnosis, PSA, Gleason score, and clinical stage. Survival estimates were carried out using Kaplan-Meier methods, with group differences (unmatched and matched) expressed as the hazard ratio (HR) with 95% confidence interval (CI) and compared using the log-rank test. Cox proportional hazards regression models (univariable and multivariable) were also fitted to estimate survival for the unmatched ProtecT cases and ACN controls adjusted for the above variables, with results expressed as HR with 95% CI. A sensitivity Cox regression survival analysis was performed for a subset of the unmatched groups separated for N0M0 and N1 or M1 disease, and was also fitted for the matched groups with further adjustment for treatment allocation. Fisher's exact test and a two-sample z-test of proportions were used to assess differences between treatments received in the matched groups. A secondary analysis assessed biochemical-free and castrate-resistant–free survival within treatment groups. Data for patients who died from PCa or other causes were censored at date of death. All tests were two-sided, with statistical significance set at p < 0.05. All analyses were performed using IBM SPSS for Windows, version 22.0, GraphPad Prism, version 6, and STATA version 14.
3. Results
3.1. ProtecT case and ACN control characteristics
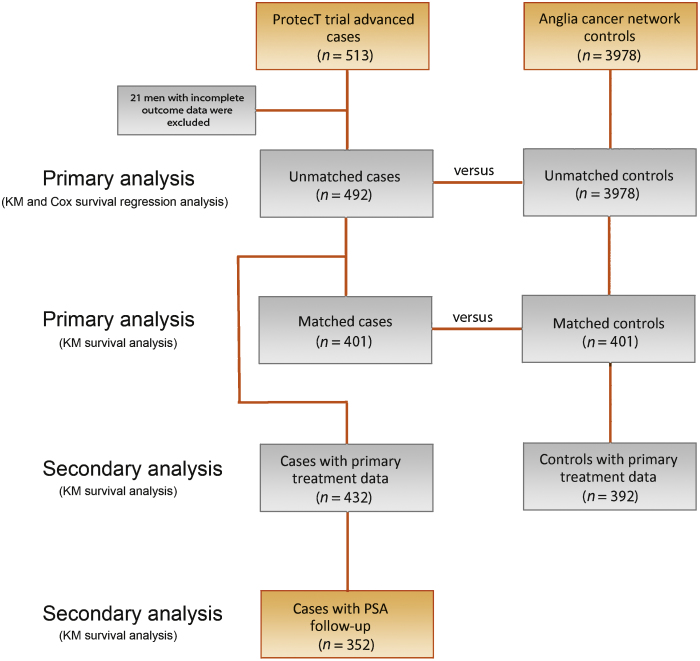
The flow of the patients through the study is summarised in Figure 1. There were 513 ProtecT advanced cases, of whom 21 were excluded because of incomplete data at presentation. For the remaining 492 cases, the mean age was 64 yr (interquartile range [IQR] 61–68); median PSA was 17 ng/ml (mean 33, IQR 8–32 ng/ml); 43% had PSA ≥20 ng/ml; 62% had clinical stage ≥T3; 23% had a Gleason score ≥8; and 8% had N1 or M1 disease. Median follow-up was 7.4 yr (IQR 5.5–9.7; Table 1). For analysis of biochemical recurrence, data on primary treatment were available for 432 out of 492 ProtecT cases (88%), and data on PSA follow-up and on neoadjuvant, adjuvant, or salvage therapies for 352 out of 492 cases (72%).
Fig. 1.
Diagram of patient flow through study. KM = Kaplan-Meier; PSA = prostate-specific antigen.
We identified 3978 ACN controls aged 50–72 yr with clinically detected PCa. Median follow-up was 5 yr (IQR 3.1–7.8). There were differences in baseline characteristics: ACN controls were older, had higher PSA levels, higher Gleason scores, and higher PCa stages (all p < 0.0002). Accordingly, we matched ProtecT cases (n = 401) to ACN controls (n = 401) across these variables (Table 1). The median follow-up for the matched cohorts was 7.6 yr (IQR 5.1–9.8). There were complete data on primary treatment for 352 of 401 (88%) matched ProtecT cases and 391 of 401 (98%) ACN controls (Table 2).
Table 2.
Primary treatments and death rates among matched ProtecT cases and Anglia Cancer Network (ACN) controls
| Treatment | Matched ProtecT cases |
Matched ACN controls |
||||
|---|---|---|---|---|---|---|
| N | Deaths, n (%) |
n | Deaths, n (%) |
|||
| PCS | AC | PCS | AC | |||
| RP | 47 | 1 (4) | 2 (4) | 150 | 5 (3) | 12 (8) |
| RT + ADT a | 200 | 11 (6) | 31 (16) | 127 | 6 (5) | 20 (16) |
| Nonradical b | 105 | 19 (18) | 37 (35) | 114 | 51 (45) | 68 (60) |
| Unknown | 49 | 6 (12) | 19 (38) | 10 | 1 (10) | 3 (3) |
| Total | 401 | 37 (9) | 89 (22) | 401 | 63 (16) | 103 (26) |
RP = radical prostatectomy; ADT = androgen deprivation therapy; PCS = prostate cancer–specific; AC = all causes.
Adjuvant ADT was given in combination with radical radiotherapy in 93% of ProtecT cases and 88% of ACN controls.
Nonradical treatment includes primary ADT, palliative chemotherapy, palliative radiotherapy, and monitoring.
3.2. Survival analysis
3.2.1. ProtecT advanced cases
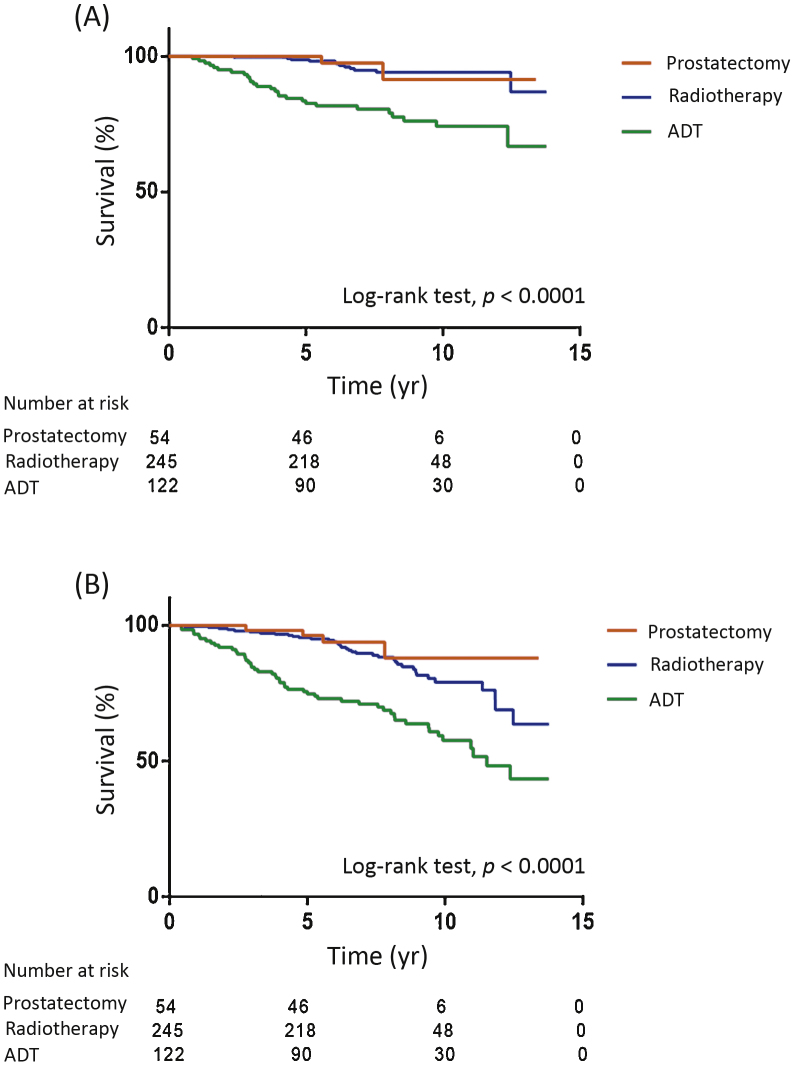
Of the 492 ProtecT men, 54 (11%) had radical prostatectomy (RP); 245 (50%) had RT, of whom 93% had neoadjuvant and adjuvant ADT; 122 (25%) had ADT alone; five (1%) had primary chemotherapy; six (1%) had other treatment (high-intensity focused ultrasound or monitoring); and for 60 (12%) the treatment was unknown. We were unable to demonstrate a difference in PCa-specific (HR 0.95, 95% CI 0.22–4.12; p = 0.94) or all-cause mortality (HR 0.69, 95% CI 0.29–1.67; p = 0.41) between the RP and RT groups (Fig. 2A,2B). Men who received RP were younger (p < 0.01) and had lower PSA (p < 0.0001) compared to the RT group, but no significant difference was observed in Gleason score (p = 0.84) or stage (p = 0.19; Supplementary Table 1).
Fig. 2.
(A) Prostate cancer–specific survival and (B) overall survival according to primary treatment groups among ProtecT cases. Death from prostate cancer occurred in two (4%) of the RP and 12 (5%) of the RT group (HR 0.95, CI 95% 0.22–4.12; p = 0.94). Death from all causes occurred in four (7%) of the RP and 37 (15%) of the RT group (HR 0.69, 95% CI 0.29–1.67; p = 0.41). A significantly greater proportion of the ADT group died from prostate cancer (n = 27, 22%) and all causes (n = 49, 40%) compared to men treated with radical therapy (p < 0.0001). RP = radical prostatectomy; RT = radical radiotherapy; ADT = androgen deprivation therapy; HR = hazard ratio; CI = confidence interval.
All-cause mortality was 7% (4/54) among men who underwent RP (2 died of PCa; 4%) and 15% (37/245) among those who received RT (12 died of PCa; 5%). All-cause mortality was higher among men who underwent nonradical treatment (51/133; 38%) and men whose treatments were unknown (25/60; 42%; all p < 0.0001; Fig. 2A,2B). Men treated using ADT were older (p = 0.01) and had higher PSA (p < 0.0001), Gleason score (p = 0.05), and stage (p < 0.0001) compared to men who received radical treatment (Supplementary Table 1).
3.2.2. Comparison with ACN controls: Kaplan-Meier survival analysis
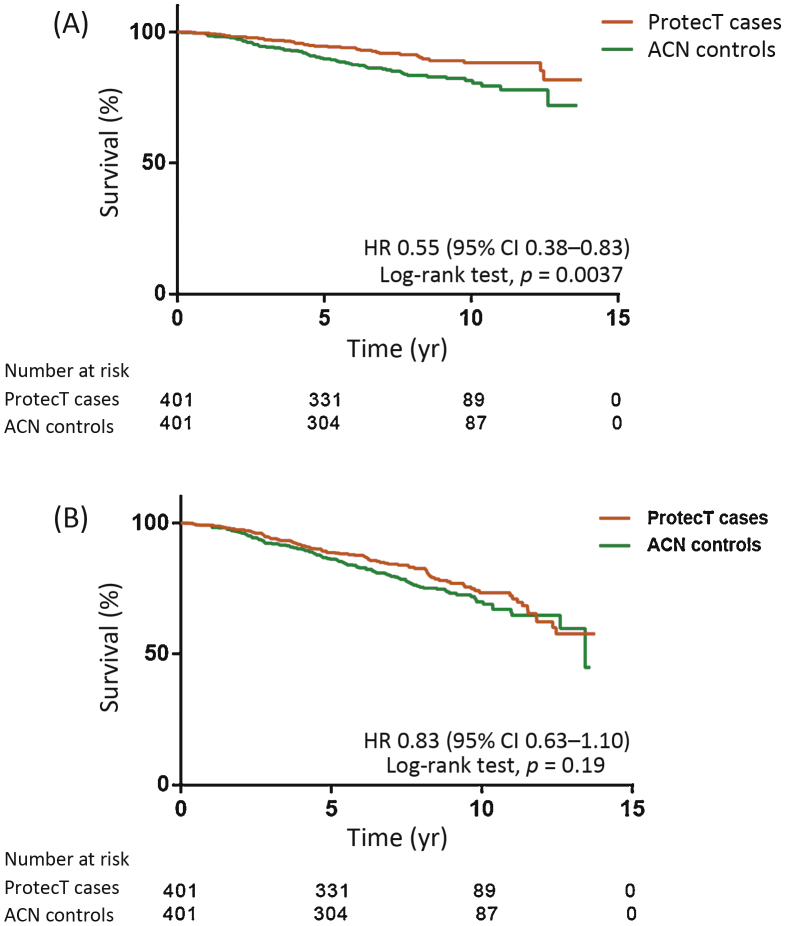
We found lower risks of death from PCa (HR 0.29, 95% CI 0.38–0.53; p < 0.0001) and from all causes (HR 0.45, 95% CI 0.48–0.63; p < 0.0001) among ProtecT cases compared to unmatched ACN controls (Supplementary Fig. 1A,1B). After matching (Table 1) we observed a 45% lower rate of death from PCa (HR 0.55, 95% CI 0.38–0.83; p = 0.0037), but were unable to demonstrate a difference in all-cause deaths (HR 0.83; 95% CI 0.63–1.1; p = 0.19) between matched ProtecT cases and ACN controls at 7.6 yr (Fig. 3A,3B).
Fig. 3.
Kaplan-Meier plots of (A) prostate cancer–specific survival and (B) overall survival among matched ProtecT cases and Anglia Cancer Network (ACN) controls. By the end of the study, 37 matched cases (9%) and 64 controls (16%) died from prostate cancer. Death from all causes occurred in 89 cases (22%) and 103 controls (26%). HR = hazard ratio; CI = confidence interval.
There was a similar proportion of men who received radical and nonradical treatments in the matched groups (p = 0.87), but more men in the ProtecT group received RT compared to the matched ACN controls (p < 0.0001; Table 2).
Among the ProtecT matched cases, 247 men received radical treatment (RP n = 47; RT n = 200) of whom 12 died from PCa (RP n = 1 [4%]; RT n = 11 [6%]) and 33 died of all causes [RP n = 2 [4%]; RT n = 31 [16%]).
Among the ACN matched controls, 277 men received radical treatment (RP n = 150; RT n = 127) of whom 11 died of PCa (RP n = 5 [3%]; RT n = 6 [5%]) and 32 died of all causes (RP n = 12 [8%]; RT n = 20 [16%]).
Among the matched men who received nonradical treatment, a significantly greater proportion died in the ACN control group (n = 114; 51 PCa deaths and 68 all-cause deaths) than in the ProtecT group (n = 105; 19 PCa deaths and 37 all-cause deaths; p < 0.0002; Table 2).
3.2.3. Comparison with ACN controls: Cox proportional hazards survival analysis
Multivariable analysis for the unmatched groups revealed that ProtecT cases (n = 404) had a 53% lower risk of death from PCa (HR 0.47, 95% CI 0.34–0.66; p < 0.0001) and a 30% lower risk of death from all causes (HR 0.70, 95% CI 0.56–0.88; p < 0.0001) compared to unmatched ACN controls (n = 3335; Table 3). Higher PSA, higher Gleason score, and higher stage all indicated a greater risk of death, whereas later years of diagnosis lowered the risk. There was also a higher risk of death from all causes in the oldest age group (67–72 yr). A subset analysis for men with N0, M0 disease did not demonstrate a difference in the risk of death from PCa (HR 0.69, 95% CI 0.45–1.06; p = 0.09) or all causes (HR 0.94, 95% CI 0.73–1.22; p = 0.65) between the unmatched groups. However, men with N1 or M1 disease had a much lower risk of death from PCa (HR 0.33, 95% CI 0.18–0.59; p < 0.0001) and all causes (HR 0.38; 0.22 - 0.63; P < 0.0001) in the ProtecT group than in the ACN group (Supplementary Table 2).
Table 3.
Cox proportional hazards survival analysis
| Variable | Reference | Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| category | Prostate cancer–specific survival |
Overall survival |
Prostate cancer–specific survival |
Overall survival |
|||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | ||
| ProtecT cases | ACN controls | 0.29 (0.21–0.38) | <0.0001 | 0.45 (0.38–0.55) | <0.0001 | 0.47 (0.34–0.66) | <0.0001 | 0.70 (0.56–0.88) | <0.002 |
| Year of diagnosis | 1999–2009 | ||||||||
| 2004–2006 | 0.63 (0.55–0.72) | <0.0001 | 0.71 (0.63–0.79) | <0.0001 | 0.55 (0.46–0.65) | <0.0001 | 0.69 (0.60–0.79) | <0.0001 | |
| 2007–2010 | 0.48 (0.41–0.58) | <0.0001 | 0.55 (0.48–0.64) | <0.0001 | 0.48 (0.38–0.60) | <0.0001 | 0.59 (0.50–0.71) | <0.0001 | |
| Age band | 50–59 yr | ||||||||
| 60–66 yr | 0.90 (0.75–1.07) | 0.24 | 1.09 (0.93–1.28) | 0.29 | 0.89 (0.71–1.10) | 0.28 | 1.06 (0.88–1.29) | 0.52 | |
| 67–72 yr | 1.12 (0.94–1.33) | 0.20 | 1.56 (1.34–1.81) | <0.0001 | 0.97 (0.79–1.19) | 0.75 | 1.43 (1.19–1.71) | <0.0001 | |
| PSA | 0–10 ng/ml | ||||||||
| 10–20 ng/ml | 1.23 (0.93–1.62) | 0.14 | 1.37 (1.12–1.67) | 0.002 | 1.05 (0.78–1.40) | 0.76 | 1.22 (0.99–1.50) | 0.065 | |
| 20–50 ng/ml | 1.58 (1.23–2.01) | <0.0001 | 1.57 (1.31–1.89) | <0.0001 | 1.48 (1.14–1.93) | <0.003 | 1.52 (1.25–1.85) | <0.0001 | |
| 50–100 ng/ml | 2.57 (1.97–3.36) | <0.0001 | 2.20 (1.80–2.71) | <0.0001 | 1.80 (1.35–2.43) | <0.0001 | 1.66 (1.32–2.08) | <0.0001 | |
| >100 ng/ml | 8.38 (6.70–10.5) | <0.0001 | 5.87 (4.93–6.98) | <0.0001 | 2.65 (2.02–3.45) | <0.0001 | 2.43 (1.96–2.98) | <0.0001 | |
| Combined Gleason | <7 | ||||||||
| 7 | 1.39 (1.05–1.84) | 0.02 | 1.04 (0.86–1.26) | 0.68 | 1.66 (1.22–2.26) | <0.001 | 1.18 (0.96–1.44) | 0.12 | |
| >7 | 4.13 (3.18–5.36) | <0.0001 | 2.47 (2.08–2.93) | <0.0001 | 4.01 (3.0–5.37) | <0.0001 | 2.45 (2.01–2.96) | <0.0001 | |
| Clinical stage | T1/T2 | ||||||||
| T3 | 0.71 (0.57–0.88) | <0.002 | 0.70 (0.60–0.81) | <0.0001 | 1.21 (0.96–1.53) | 0.11 | 1.08 (0.92–1.29) | 0.32 | |
| T4 | 2.10 (1.18–3.76) | 0.01 | 1.51 (0.95–2.43) | 0.08 | 2.74 (1.52–4.92) | <0.001 | 1.96 (1.22–3.15) | <0.005 | |
| M1 or N1 | 8.22 (7.08–9.55) | <0.0001 | 4.88 (4.36–5.47) | <0.0001 | 5.79 (4.82–6.95) | <0.0001 | 3.53 (3.07–4.10) | <0.0001 | |
HR = hazard ratio; CI = confidence interval; ACN = Anglia Cancer Network; PSA = prostate-specific antigen.
Multivariable analysis was performed for the matched groups after further adjusting for treatment received. We did not find evidence of a difference in the risk of death from PCa among men who received radical treatment (HR 1.91, 95% CI 0.73–5.02; p = 0.19). Men treated with RT had a higher risk of death from all causes compared to the RP group (HR 2.02, 95% CI 1.08–3.77, p = 0.03). There was a much higher risk of death from PCa (HR 6.70, 95% CI 2.64–16.9; p < 0.0001) and all causes (HR 4.55, 95% CI 2.42–8.52; p < 0.0001) among men who received nonradical treatment compared to those who underwent radical treatment (Supplementary Table 3).
3.2.4. Kaplan-Meier analysis of biochemical recurrence by primary treatment group in the ProtecT group
PSA follow-up was available for ProtecT cases and is reported in more detail in the Supplementary material. At a median of 7.4 yr, PCa-specific survival was 96% in the RP group and 96% in the RT group. There were no predictors of biochemical failure, PCa-specific mortality, or overall mortality among men treated with RP or RT on univariable or multivariable analysis, except for high Gleason score, which increased the risk of death from all causes in the RT group (HR 6.48, 95% CI 1.48–28.4; p = 0.01; Supplementary Table 4 and Supplementary Fig. 2).
4. Discussion
This study reports on asymptomatic men who were excluded from ProtecT because of advanced and high-risk PCa; their outcomes form an important component of the overall context of the ProtecT study and its generalisability with respect to treatment of PSA-detected PCa. In men who were excluded from ProtecT, but were treated radically, we found low rates of all-cause and PCa specific deaths (14% and 5%), with no differences between surgery and radiotherapy at a median of 7.4 years. Most deaths occurred among men receiving nonradical treatments, probably because they had more advanced disease and/or were not fit for radical treatment, although very unfit men were screened out from ProtecT by the general practitioner.
With respect to the main clinical outcome paper from ProtecT, all-cause mortality (∼10% at a median of 10 yr) [13] was lower than that noted here in the RT group (15%). This suggests that ProtecT men with advanced PCa treated by RT in the present study were less fit than those in the randomised group. Moreover, the group who received nonradical treatment and those whose treatment was unknown had significantly greater all-cause mortality (38% and 42%, respectively) compared with those undergoing radical treatment. The PCa-specific mortality among the ProtecT group receiving radical treatment (5%) was greater than that found in the randomised group (∼1%), but nevertheless indicates very good cancer survival.
The reduction in risk of death from PCa among advanced ProtecT cases (45%) compared to ACN controls persisted after careful case-control matching to attempt to compensate for leadtime bias and differences in baseline characteristics. However, other biases cannot be ruled out, including the greater number of men undergoing surgery in the ACN group than in the ProtecT group when comparing those who received radical treatment, and the fact that the ACN group were generally less fit. However, there were no differences in PCa-specific or all-cause mortality between the matched cases and controls when comparing those who received radical treatment. The higher death rates observed among ACN controls occurred mainly in men who received nonradical treatments, suggesting early detection may improve the life expectancy of this subgroup, although other explanations such as group heterogeneity, leadtime bias, and selection bias cannot be ruled out.
Cox regression results for survival analysis (53% lower risk of death from PCa and a 30% reduction in all-cause mortality in the ProtecT group) can probably be explained in part by leadtime bias in the ProtecT cohort [18], [19].
We found no difference in PCa-specific or overall survival between the RP and RT ProtecT groups. Only a small proportion of men who received radical treatment (RP 4%, RT 5%) died from PCa, which adds to increasing evidence that radical treatment of locally advanced or high-risk disease delivers good oncologic outcomes[8], [9]. The all-cause and PCa survival outcomes for the ProtecT group are better than in most studies on men clinically presenting with advanced disease [20], supporting the hypothesis that early detection of advanced and high-risk PCa may be of benefit. The wider context of the impact of PSA testing on community-based men will be presented in the findings of the CAP (Cluster randomised trial of prostate cancer) trial in 2017 [21].
The quality of data for the ACN group is likely to be good [15], [21]. We minimised misattribution of death by using two independent clinicians blinded to the study group and treatment, and by checking with the ProtecT recruitment centre of origin. Matching reduced the number of men for the matched analysis (n = 401) and there may be additional biases that our matching process was unable to take into account. Multidisciplinary teams reviewed the histopathology for ACN controls, whereas ProtecT cases were reviewed by the expert ProtecT histopathology group [22]. For surgically treated ACN cases, however, histology was reviewed centrally. Potential differences in grade and stage allocation may have had some impact on apparent survival benefits among the ProtecT cases. There was no information available on the comorbidity burden for the ACN controls, and therefore we were unable to match the two groups according to these factors. ProtecT participants were 98% Caucasian and patients with a prior history of cancer were excluded, which may have influenced overall survival. The natural history of PCa can be long and further follow-up is required, but such leadtime factors are likely to be of lesser magnitude among men with advanced disease [23], [24], [25].
5. Conclusions
PSA testing identifies asymptomatic men with advanced and high-risk PCa whose early treatment leads to good survival rates. We observed improved survival in the ProtecT men who received nonradical treatment compared to men presenting clinically without PSA testing, although leadtime and selection bias are difficult to exclude. It will be important to assess longer-term survival and add patient-reported outcomes among these men to assess the balance between treatment impact and survival.
Author contributions: Alastair D. Lamb had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Neal, Hamdy, Donovan, Lane.
Acquisition of data: Xiong, Johnston, Shaw, Lamb, Neal, Hamdy, Donovan, Davis.
Analysis and interpretation of data: Johnston, Parashar, Shaw, Lamb, Xiong.
Drafting of the manuscript: Johnston, Lamb, Neal.
Critical revision of the manuscript for important intellectual content: Johnston, Lamb, Neal, Hamdy, Donovan.
Statistical analysis: Johnston, Parashar.
Obtaining funding: Neal, Hamdy, Donovan.
Administrative, technical, or material support: None.
Supervision: Neal, Hamdy, Donovan.
Other (review of manuscript): All authors.
Financial disclosures: Alastair D. Lamb certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: The ProtecT trial is funded by the UK National Institute for Health Research (NIHR) Health Technology Assessment Programme (projects 96/20/06, 96/20/99) with the University of Oxford as sponsor (www.nets.nihr.ac.uk/projects/hta/962099). The sponsor played a role in the design and conduct of the study. Jenny L. Donovan is supported by the NIHR Collaboration for Leadership in Applied Health Research and Care West, hosted by University Hospitals Bristol NHS Foundation Trust. Freddie C. Hamdy is supported by the Oxford NIHR Biomedical Research Centre Surgical Innovation and Evaluation Theme, and the Cancer Research UK Oxford Centre.
Acknowledgments: The authors wish to acknowledge the tremendous contribution of all the ProtecT study participants, researchers, data monitoring committee (Chairs: Professors Adrian Grant and Ian Roberts; Prof. Deborah Ashby, Dr. Richard Cowan, Prof. Peter Fayers, Prof. Killian Mellon, Prof. James N’Dow, Mr. Tim O’Brien, Dr. Michael Sokal), and trial steering committee (Chair: Prof. Michael Baum; Prof. Anthony Zietman, Prof. David Dearnaley, Dr. Jan Adolfsson, Prof. Peter Albertsen, Prof. Fritz Schröder, Prof. Tracy Roberts).
Department of Health disclaimer: The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Department of Health.
Associate Editor: Stephen Boorjian
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eururo.2016.09.040.
Contributor Information
Thomas J. Johnston, Email: thomasjohnston1@nhs.net.
David E. Neal, Email: den22@cam.ac.uk.
Appendix A. Supplementary data
References
- 1.Schröder F.H., Hugosson J., Roobol M.J. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–2035. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andriole G.L., Crawford E.D., Grubb R.L., 3rd Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoag J.E., Mittal S., Hu J.C. Reevaluating PSA testing rates in the PLCO trial. N Engl J Med. 2016;374:1795–1796. doi: 10.1056/NEJMc1515131. [DOI] [PubMed] [Google Scholar]
- 4.Wilt T.J., Brawer M.K., Jones K.M. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widmark A., Klepp O., Solberg A. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373:301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 6.Warde P., Mason M., Ding K. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378:2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdollah F., Sun M., Thuret R. A competing-risks analysis of survival after alternative treatment modalities for prostate cancer patients: 1988-2006. Eur Urol. 2011;59:88–95. doi: 10.1016/j.eururo.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Boorjian S.A., Karnes R.J., Viterbo R. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011;117:2883–2891. doi: 10.1002/cncr.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sooriakumaran P., Nyberg T., Akre O. Comparative effectiveness of radical prostatectomy and radiotherapy in prostate cancer: observational study of mortality outcomes. Br Med J. 2014;348:1502–1515. doi: 10.1136/bmj.g1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg D.C., Lophatananon A., Wright K.A., Muir K.R., Gnanapragasam V.J. Trends and outcome from radical therapy for primary non-metastatic prostate cancer in a UK population. PLoS One. 2015;10:e0119494. doi: 10.1371/journal.pone.0119494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donovan J., Hamdy F., Neal D., Peters T., Oliver S. Prostate Testing for Cancer and Treatment (ProtecT) feasibility study. Health Technol Assess. 2003;7:42. doi: 10.3310/hta7140. [DOI] [PubMed] [Google Scholar]
- 12.Lane J.A., Donovan J.L., Davis M. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol. 2014;15:1109–1118. doi: 10.1016/S1470-2045(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 13.Hamdy FC Donovan JL, Athene JL, et al. Prostate cancer mortality and outcomes at 10 year follow-up in the ProtecT trial. N Engl J Med. http://dx.doi.org/10.1056/NEJMoa1606220
- 14.Donovan JL, Hamdy FC, Lane AJ, et al. Patient reported outcomes over six years in the ProtecT prostate cancer trial. N Engl J Med. http://dx.doi.org/10.1056/NEJMoa1696221
- 15.Greenberg D.C., Wright K.A., Lophathanon A., Muir K.R., Gnanapragasam V.J. Changing presentation of prostate cancer in a UK population — 10 year trends in prostate cancer risk profiles in the East of England. Br J Cancer. 2013;109:2115–2120. doi: 10.1038/bjc.2013.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melia J., Moss S., Johns L. Rates of prostate-specific antigen testing in general practice in England and Wales in asymptomatic and symptomatic patients: a cross-sectional study. BJU Int. 2004;94:51–56. doi: 10.1111/j.1464-4096.2004.04832.x. [DOI] [PubMed] [Google Scholar]
- 17.Williams N., Hughes L.J., Turner E.L. Prostate-specific antigen testing rates remain low in UK general practice: a cross-sectional study in six English cities. BJU Int. 2011;108:1402–1408. doi: 10.1111/j.1464-410X.2011.10163.x. [DOI] [PubMed] [Google Scholar]
- 18.Moore A.L., Dimitropoulou P., Lane A. Population-based prostate-specific antigen testing in the UK leads to a stage migration of prostate cancer. BJU Int. 2009;104:1592–1598. doi: 10.1111/j.1464-410X.2009.08652.x. [DOI] [PubMed] [Google Scholar]
- 19.Collin S.M., Martin R.M., Metcalfe C. Prostate-cancer mortality in the USA and UK in 1975 to 2004: an ecological study. Lancet Oncol. 2008;9:445–452. doi: 10.1016/S1470-2045(08)70104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason M.D., Parulekar W.R., Sydes M.R. Final report of the intergroup randomized study of combined androgen-deprivation therapy plus radiotherapy versus androgen-deprivation therapy alone in locally advanced prostate cancer. J Clin Oncol. 2015;33:2143–2150. doi: 10.1200/JCO.2014.57.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UK Department of Health. Delivering the cancer reform strategy. Report by the Controller and Auditor General. London, UK: National Audit Office; 2010. www.nao.org.uk/wp-content/uploads/2010/11/1011568.pdf.
- 22.Oxley J., Simpkin A., Goepel J. Gleason drift in the NIHR ProtecT study. Histopathology. 2015;66:438–446. doi: 10.1111/his.12549. [DOI] [PubMed] [Google Scholar]
- 23.Albertsen P.C., Hanley J.A., Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 24.Bill-Axelson A., Holmberg L., Ruutu M. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 25.Bill-Axelson A., Holmberg L., Filen F. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100:1144–1154. doi: 10.1093/jnci/djn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.