Abstract
Substance use disorders (SUD) are maladaptive patterns of substance use that are associated with psychiatric comorbidity, unhealthy lifestyle choices, and high rates of relapse. Exercise is associated with a wide range of acute and long-term benefits for both mental and physical health and is presently being investigated as a promising adjunctive treatment for SUD. Despite positive effects of regular physical activity on treatment outcomes and risk factors for relapse, low adherence and high attrition rates limit the benefits derived from exercise interventions. Lack of motivation is one of many perceived barriers to initiating exercise that contributes to poor adherence to interventions. In the present article, we describe the protocol for a novel, integrated exercise intervention that combines motivational interviewing (MI), a client-centered approach designed to enhance intrinsic motivation and resolve ambivalence towards change, and contingency management (CM), a behavioral treatment that provides monetary incentives for the completion of target behaviors. The protocol seeks to address the challenges surrounding initiation and maintenance of an exercise program at a level consistent with public health guidelines, particularly for sedentary patients. We conclude with considerations for the implementation of the intervention in SUD specific clinics.
Keywords: physical activity, incentives, addiction, sedentary
1.1 Introduction
Substance use disorders (SUD) are a pervasive set of conditions centered around continued substance use despite a host of negative consequences that affect nearly 20% of the U.S. population (Compton, Thomas, Stinson, & Grant, 2007; Grant et al., 2004) and negatively impact society in a variety of ways (Office of National Drug Control Policy, 2004; Rehm et al., 2009). Moreover, despite significant efforts, relapse after SUD treatment remains high with about 60% of individuals relapsing within one year (Agosti, Nunes, & O’Shea, 2012). In recent years, exercise has been suggested as an adjunctive intervention to SUD treatment due to its well-documented and varied physical and mental health benefits (Leasure, Neighbors, Henderson, & Young, 2015; Linke & Ussher, 2015; Read et al., 2001). This article provides a focal review surrounding the implementation of exercise as an adjunctive intervention for SUD, concerns surrounding adherence, and the development of an integrated intervention of motivational interviewing (MI) and contingency management (CM) that may be well-equipped to help overcome these limitations to exercise adherence. The article concludes with a description of the MI+CM exercise intervention we have developed, with recommendations and considerations for implementation in clinics serving individuals with SUD.
Exercise is physical activity that is done to improve or maintain physical fitness (American College of Sports Medicine [ACSM], 2013). Physical activity is an umbrella term that can refer to activity done at various intensities in relation to work and other day-to-day activities. Physical activity may be categorized as occupational, recreational, or transportation. Therefore, the same activity (e.g., biking) could be done for fitness or transportation purposes and could be done at a range of intensities. Throughout this manuscript we utilize the term exercise in reference to moderate intensity physical activity that is done for fitness. Moderate intensity exercise is defined as exercise that noticeably increases one’s heart rate and breathing; more objectively, it elicits a heart rate of 40–60% of heart rate reserve. Examples include brisk walking and bicycle riding slower than 10mph. A helpful guide for patients is that during moderate intensity exercise one should be able to talk, but not sing.
1.2 Rationale for Exercise
Exercise has been proposed as an adjunctive treatment for SUD due to its beneficial effects upon (1) comorbid psychiatric conditions, and (2) factors associated with SUD relapse. Psychiatric disorders and SUD commonly co-occur and frequently have a complex bidirectional relationship such that substance use can exacerbate symptoms related to psychiatric disorders while psychiatric disorders may conversely trigger substance use (Mueser, Drake, & Wallach, 1998). For example, symptoms of social anxiety may trigger drinking alcohol due to its ability to reduce uncomfortable feelings (Battista et al., 2015). Unfortunately, psychiatric comorbidity within SUD is frequently associated with greater impairment and poor treatment engagement (Greenfield et al., 1998; Weinstock, Alessi, & Petry, 2007), both of which can lead to poor treatment outcome and relapse to substance use. Conversely, exercise is an effective treatment for both major depression and certain anxiety disorders (e.g., Babyak et al., 2000; Broocks et al., 1998), which are disorders that frequently co-occur with SUD (Grant et al., 2004). Notably, exercise also does not come with side effects that are common to psychiatric medications. Thus, exercise positively impacts psychiatric symptoms that sustain substance use over time and represents a cost-efficient and non-pharmacological alternative.
Exercise also has a positive impact upon risk factors related to SUD relapse. Exercise reduces cravings for alcohol (Taylor, Oh, & Cullen, 2013; Ussher, Sampuran, Doshi, West, & Drummond, 2004), naturally induces positive mood both acutely (e.g., 2nd highest rated activity after intercourse out of 22 activities; Killingsworth & Gilbert, 2010) and over time by increasing feelings of vigor and reducing tension, fatigue, and confusion (Puetz, O’Connor, & Dishman, 2006). Moreover, exercise is positively associated with one’s ability to cope with daily stress without substance use (Medina et al., 2011). More specifically, exercise is a voluntary and predictable type of physiological stress, which in many cases improves the body’s biological regulation of psychological stress. One hypothesis regarding the stress-reducing effects of exercise suggests physical alterations brought about by exercise reinforce communication within the stress axes (e.g., Hypothalamus Pituitary Adrenal axis) such that the axes function more efficiently when activated by stress (Costa Rosa, 2004). In fact, exercise has been shown to decrease one’s reactivity to stress (Throne, Bartholomew, Craig, & Farrar, 2000) and can potentially replace substance use as a more appropriate and adaptive coping response to unpleasant or stressful situations (Read & Brown, 2003). Unfortunately, the experience of stress is a common and significant risk factor for SUD relapse (Sinha, 2008; Sinha, Garcia, Paliwal, Kreek, & Rounsaville, 2006). An important goal of SUD treatment is to find healthy ways to respond to and manage stress, and exercise appears to be such an approach. It addresses important facets that can lead to stable, long-term recovery from SUD.
1.3 Current Empirical Support of Exercise in SUD Treatment
Thus far, the studies examining the utility of exercise as an adjunctive SUD treatment are encouraging, although somewhat limited. Studies to date have used cross sectional designs (Read et al., 2001; Weinstock, Barry, & Petry, 2008), pre-post study designs (Palmer, Palmer, Michiels, & Thigpen, 1995; Sinyor, Brown, Rostant, & Seraganian, 1982; Walker et al., 2010), or small scale randomized clinical trials with follow-up periods ranging from no follow-up at all to 12 weeks (Brown et al., 2014; Brown et al., 2010; Cutter et al., 2014; Dolezal et al., 2013; Pérez-Moreno et al., 2007; Weinstock, Capizzi, Weber, Pescatello, & Petry, 2014). Exercise has been examined in a range of SUD diagnoses and treatment settings from prison methadone maintenance programs and residential methamphetamine treatment programs to outpatient alcohol use disorder treatment programs and non-treatment seeking hazardous drinking college students. Findings from these studies demonstrate improvements in physical health (e.g., physical fitness), mental health (e.g., depression), and overall quality of life. For example, Muller and Clausen (2015) reported on a 10-week adjunctive exercise program in a residential SUD treatment program. Patients who engaged in exercise reported significantly improved quality of life, an important predictor of sustained recovery (Laudet, Becker, & White, 2009). Pérez and colleagues (2007) also found quality of life improvements as a result of a four-month exercise program. Finally, Palmer and colleagues (1995) in an inpatient SUD sample found a four-week exercise training program led to reductions in depression symptomatology; while in a methadone maintenance sample Cutter et al. (2014) found significant reductions in stress for an intervention utilizing Wi-Fit. Thus, the many benefits of exercise can be experienced in individuals with SUD when exercise is incorporated as a component of treatment. Due to its flexibility, exercise can be implemented in a variety of SUD treatment settings.
Moving beyond the mental and physical health benefits of exercise, exercise interventions in SUD treatment populations have been found to reduce substance use. In three separate studies, Brown and colleagues (2009, 2010, 2014) incorporated exercise as an adjunctive treatment in an outpatient alcohol treatment program. In each study, over the course of 12 weeks, significant reductions in drinking outcomes occurred among those who engaged in regular exercise. These gains were not found in those who did not adhere to the exercise intervention (Brown et al., 2014; Brown et al., 2010). In a cross-sectional study, Weinstock and colleagues (2008) found that engagement in exercise activities during 12 weeks of intensive outpatient treatment predicted longer durations of abstinence. However, whether or not engagement in exercise or the other benefits gained according to the results of these studies (Brown et al., 2010; Brown et al., 2014; Weinstock et al., 2008; Cutter et al., 2014) endured over time remains an unanswered question due to the lack of follow-up with the study populations.
1.4 Exercise Guidelines and Prescription
Current public health guidelines call for 150 minutes per week of moderate intensity aerobic physical activity (American College of Sports Medicine, 2013; US Department of Health and Human Services, 2008). Within SUD treatment samples, at least 50% to 60% of patients do not meet these guidelines (Muller, Skurtveit, & Clausen, 2016; Read et al., 2001). While the benefits of exercise associated with these guidelines are well known, many people are sedentary or insufficiently physically active, and many of those who attempt to start exercising discontinue their participation prior to realizing its many potential benefits (Gordon-Larsen, Nelson, & Popkin, 2004; O’Brien et al., 2015). A commonly cited statistic is that approximately 50% dropout within six months of initiation or exercise insufficiently to garner its many benefits (Dishman, 1988). Within the few SUD treatment programs that incorporate exercise as an adjunctive treatment, most do not prescribe a sufficient dose for it to be therapeutically beneficial and/or suffer from poor adherence. For example, an outpatient methamphetamine treatment program offered group exercise classes twice per week that were poorly attended (i.e., poor adherence; Walker et al., 2010). The clinics introduced a CM intervention that reinforced attendance to the classes held each week. While CM intervention significantly improved attendance at the exercise classes, the frequency and intensity of the exercise classes offered were at an insufficient level to gain the many benefits of exercise. Other exercise intervention studies have utilized CM but adherence remains a problem in these studies, with dropout rates of 35% to 40% (Brown et al., 2014; Brown et al., 2010), albeit at rates less than if CM was not offered (e.g., 70%; Patel et al., 2016). Poor adherence and dropout is common even in residential SUD treatment settings where many of the barriers to exercise have been eliminated (e.g., time, access to equipment). Muller and Clausen (2015) found that about one-third of participants failed to engage in the exercise intervention. Those who did not adhere to the exercise protocol evidenced little to no gains in quality of life (e.g., decreased depression, reduced mental distress, improved physical condition) and reported greater substance use than those who did exercise regularly. Therefore, successful exercise interventions must address the critical issues of adherence and dropout.
Perceived barriers to exercise frequently contribute to poor adherence and attrition. Patients with a SUD frequently cite financial constraints that preclude purchasing exercise equipment or gym membership, low motivation to exercise, lack of transportation, lack of time, and limited social support as perceived barriers to exercise (Muller & Clausen, 2015; Read et al., 2001; Stoutenberg, Vidot, Jimenez, & Read, 2015). Conversely, several factors are associated with successful initiation and maintenance of an exercise program. These factors include social support, self-efficacy, motivation, having physical activity choices, goal setting and behavioral contracts, positive reinforcement, intervention duration, and feedback (Cress et al., 2005; Plotnikoff et al., 2015). More broadly, studies have found that while extrinsic motivation may be important for initiating exercise, intrinsic motivation is an important component for sustaining it (Buckworth, Lee, Regan, Schneider, & DiClemente, 2007). Interventions that are designed to enhance intrinsic and/or extrinsic motivation to start and maintain an exercise program demonstrate a range of positive effect sizes (Martins & McNeil, 2009; O’Halloran et al., 2014; Webb & Sheeran, 2006). With these factors in mind, we have developed an exercise intervention that combines MI with prize-based CM to help individuals with SUD start and maintain an exercise program. The MI component seeks to build intrinsic motivation that addresses ambivalence surrounding exercise initiation and factors associated with long-term adherence, while the CM component of the exercise intervention addresses difficulties associated with initiating an exercise program.
MI is defined as a patient-centered, directive method for enhancing intrinsic motivation to change by exploring and resolving ambivalence (Miller & Rollnick, 2013). A plethora of studies show the positive impact of MI on a myriad of outcomes related to SUD treatment at different points in treatment from initially seeking treatment to the maintenance of positive outcomes in outpatients who completed residential treatment programs (e.g., Brown & Miller, 1993; Connors, Walitzer, & Dermen, 2002; Stotts, Schmitz, Rhoades & Grabowski, 2001; Swanson, Pantalon & Cohen, 1999). Meta-analyses of MI find moderate effect sizes related to other areas of health such as diet and exercise, smoking cessation and medication adherence, suggesting that MI is a suitable intervention to use across a range of health conditions with no documented adverse effects, and is appropriate for a variety of practitioners in different healthcare settings (Burke, Arkowitz, & Menchola, 2003; Cushing, Jensen, Miller, & Leffingwell, 2014; VanBuskirk, & Wetherell, 2014). More specifically, it is efficacious as a stand-alone intervention or as a module of a larger intervention for exercise across a variety of populations, including healthy adults, with small to moderate effect sizes in comparison to no-treatment or placebo control groups (Lundahl et al., 2013; O’Halloran et al., 2014). Tailoring MI to include personalized feedback around self-reported exercise behaviors versus public health recommendations for exercise and consideration of the exercise’s benefits may help SUD patients resolve ambivalence about starting a regular exercise program, explore changes related to exercise over time, and renew commitment to regular exercise.
Prize-based CM is a behavioral treatment in which tangible reinforcement is provided to individuals when target behaviors are completed and objectively verified. A large body of literature supports the use of CM for treating SUD, as patients are typically reinforced with vouchers, exchangeable for retail goods and services, when objective evidence confirms abstinence from drugs (e.g., urine samples; Benishek et al., 2014; Prendergast, Podus, Finney, Greenwell, & Roll, 2006). Successful CM interventions are designed around three central tenets: (1) the environment is arranged such that target behaviors are frequently and easily monitored, (2) tangible reinforcers are provided whenever the target behavior is demonstrated, and (3) when the target behavior does not occur, rewards are systematically withheld (Stitzer & Petry, 2006). In fact, Lussier et al. (2006) found that immediacy and magnitude of rewards were significant moderators of CM interventions’ effect sizes with more immediate access to large reinforcement increasing the effect size.
Contingency management has also been adopted to reinforce other behaviors, including exercise. In a review of 11 studies offering financial incentives for exercise, 8 of the studies showed positive effects, while 3 were ineffective (Mitchell et al., 2013). This mix of findings, while overall is positive, highlights the importance of adherence to the principles of CM. Small magnitude and delayed provision of reinforcement (e.g., ≥ monthly basis) appears to limit the efficacy of CM exercise interventions (e.g., DeVahl, King, & Williamson, 2005; Jeffery, Wing, Thorson, & Burton, 1998; Patel et al., 2016). Conversely, studies showing more pronounced effects for incentives on exercise are those that adhere to the central tenets of CM (e.g., Brown et al., 2014; Irons, Pope, Pierce, Van Patten, & Jarvis, 2013; Patel et al., 2016). Thus, exercise behavior changes in response to CM interventions when it is monitored frequently, immediate and desirable rewards are offered, and reinforcement is withheld for non-adherence. Moreover, the generalizability of using CM to reinforce exercise and health behavior more broadly is already demonstrated in that positive reinforcement systems for health behavior change are already being widely implemented in the healthcare field. For example, Blue Shield of California and IBM provide monetary rewards for engaging in physical activity and completing health risk assessments.
Specifically as it relates to exercise, CM helps to instill extrinsic motivation in patients who often identify many barriers to beginning an exercise program. According to behavioral economics (Hursh, 1980), individuals are more likely to act in favor of immediate rewards and self-interest often to the detriment of long-term health. In terms of exercise, the perceived costs in time, energy and money are experienced immediately while the health benefits derived from continuous exercise are most often delayed. A CM intervention provides an immediate reward that may encourage adherence to exercise long enough for participants to experience delayed health benefits, particularly for individuals who are not intrinsically motivated to begin with. Interventions using CM have shown an increase in exercise adherence especially in the short term (Mitchell et al., 2013). Short-term adherence is important so that individuals experience the acute mental health benefits of exercise (e.g., positive mood induction, reductions in craving) that reduce the risk of SUD relapse.
Unfortunately, translation and dissemination of CM interventions to SUD clinics has been slow. While there has been some resistance in the SUD treatment field towards adopting CM, often the objections of professionals reflected a lack of exposure to CM training (Rash et al., 2012). Many professionals have cited perceived financial burden as an obstacle to adopting CM interventions; however, several studies have shown that even modest financial incentives improve treatment attendance (e.g., Weinstock et al., 2007), which in turn can increase overall reimbursement rates for SUD treatment services through revenue generated from consistent attendance over time (Lott & Jencius, 2009). The revenue increase may more than offset the cost of the intervention. Additional cost savings to the healthcare system more broadly may be achieved with an exercise intervention as it can negate the need for pharmacological treatment for co-occurring disorders and reduce the risk for a multitude of medical conditions.
2.1 Integrated Exercise Intervention
Below we provide a description of the integrated MI+CM intervention that we have developed for using exercise as an adjunctive treatment for SUD. Our description also includes discussion of potential issues that may arise around other factors that SUD treatment facilities may want to consider, such as safety and compliance, when developing an exercise intervention that prescribes moderate intensity exercise. This monitored exercise intervention has been used and refined in several completed and ongoing clinical trials in a variety of addiction populations and demonstrates adherence to exercise consistent with public health guidelines (Weinstock et al., 2014; Weinstock, Petry, Pescatello, & Henderson, in press). Our initial pilot study of the MI+CM intervention we found that the 8-week MI+CM intervention engendered a large effect size in terms of exercise frequency (d = 1.60) and moderate effect size in terms of estimated V02 maximum (d = 0.49) in sedentary hazardous drinking college students. Participants exercised about 2.5 times per week during the MI+CM intervention (Weinstock et al., 2014). No significant changes in drinking outcomes were detected, although small sample size (N=31) may have played a role. In Weinstock et al. (in press), by the end of an 8-week MI+CM intervention about 60% of sedentary hazardous drinking college students were exercising at or above the ACSM guidelines with an average of 3.1 bouts per week. We also found reductions in drinking; however, analyses suggest changes in drinking were unrelated to exercise engagement. Lastly, in our ongoing clinical trial (NCT01828307) that engages SUD patients in exercise as they are discharged into aftercare from a 14-day residential treatment program, preliminary data from 31 participants randomized to the MI+CM intervention found an average of 2.78 self-reported bouts of exercise per week with each bout lasting for approximately 38.5 minutes. Hence, across a range of studies the MI+CM intervention has shown significant promise in engaging individuals in exercise.
2.2 Pre-Participation Screening
Before implementing any exercise intervention patients must be screened to ensure that exercise is not contra-indicated. Screening is especially important in order to identify individuals who may be at risk for serious exercise-related cardiovascular problems. In 2015, the American College of Sports Medicine (ACSM) updated its pre-participation screening guidelines, which are used to determine if medical clearance by a physician or other qualified health care provider is needed before exercise begins (Riebe et al., 2015). The updated pre-participation health screening process focuses on (1) current physical activity levels, (2) presence of diagnosed cardiovascular, metabolic, or renal disease, or signs and/or symptoms of these diseases, and (3) the intensity of exercise that will be prescribed. For asymptomatic individuals who do not have a diagnosed disease, a low- to moderate-intensity exercise program can be initiated without additional screening/testing, even if they were previously inactive; if they were regularly performing vigorous physical activity, then vigorous exercise is also acceptable. See Riebe et al. (2015) for further information on pre-participation screening.
2.2 Exercise Supervision and Monitoring
In addition to pre-participation screening, another issue a SUD clinic must consider is whether exercise should be supervised by clinic personnel or unsupervised with an exercise monitoring plan. Both approaches have been used. Perri, and colleagues (1997) showed that in a sample of obese individuals, individuals assigned to unsupervised, monitored home-based exercises were more adherent than individuals assigned to supervised group exercises. Our studies have used unsupervised monitored exercise (Weinstock et al., 2014, in press), while Brown and colleagues (2009, 2010, 2014) used a combination of supervised exercise (once per week) and prescriptions for unsupervised moderate-intensity aerobic exercise on two to three additional occasions per week. Supervised exercise is exercise that takes place within the facility (e.g., exercise classes) and is supervised by an exercise specialist; it typically includes monitoring. Unsupervised, monitored exercise is exercise that takes place outside the SUD treatment facility (e.g., home, local gym) and is regularly monitored with exercise diaries or electronic devices (e.g., pedometers or heart rate monitors) by an exercise specialist via weekly check-in appointments. Each approach has pros and cons. Unsupervised, monitored exercise requires limited staff involvement, presents lower financial burden as it does not require the purchase of exercise equipment, has reduced liability if a person were to get hurt while exercising at the SUD treatment facility, and is potentially more generalizable as exercise gets integrated into a person’s daily routine outside the clinic. However, clinics have less control over exercise, must find ways to objectively verify that individuals complete the prescribed exercises, and issues of fabrication of verification can occur. With supervised exercise, clinics have more control over exercise activities, and it is easier to ensure exercise completion. However, this method requires fitness equipment be within the facility, and increased staffing and potential liability for the clinic.
2.3 General Overview of MI+CM for Exercise Intervention
Our current iteration of the MI+CM intervention occurs over six months, is individually delivered, and monitors exercise. We elected for a longer intervention period to ensure adequate time for sedentary individuals to develop a regular exercise routine and begin to experience the many benefits of exercise. Many long-term benefits of exercise take 12–16 weeks before they occur (American College of Sports Medicine, 2013). Shorter duration interventions can be adapted from this general protocol. The intervention consists of three MI sessions spaced every three months (beginning, middle, and end of the intervention) and weekly prized-based CM sessions that contract for specific exercise behaviors. The CM sessions could also be adapted for delivery in a group format. See Petry (2012) for details on group-based CM procedures.
While the MI sessions focus on promoting exercise, it is important to explicitly connect and discuss how exercise relates to a patient’s SUD recovery. This discussion can tie in some aspects about motivation to reduce substance use in the sense that substance use can adversely impact one’s motivation and ability to exercise. However, the focus of the intervention is to promote exercise. Broadly, the MI sessions have three components: (1) providing feedback about the individual’s lifestyle and normative information about exercise in adults using a personalized feedback report (available upon request); (2) exploring the individual’s current pattern of activity, highlighting both positive and negative consequences of this behavior, developing discrepancies or renewing commitment, discussing the possibility of change, and boosting self-efficacy; and finally, (3) discussing future goals and reasons for pursuing a regular exercise routine. A change plan worksheet may be utilized for the third component.
The MI sessions are approximately 50 minutes in length, although the initial MI session is longer as it incorporates an introduction to the CM component of the exercise intervention. The second MI session occurs halfway through the program and seeks to renew motivation and commitment, and the final MI session, at the conclusion of the program, explores progress made over the past six months and motivation to continue now that external reinforcers (i.e., CM intervention) are no longer available.
Introduction of the CM component at the first MI session consists of explaining weekly exercise contracts (see Figure 1 for an example contract), verification of exercise, the CM reinforcement schedule, the fish-bowl procedure, and possible reinforcers (e.g., prizes, gift certificates). At this time, the interventionist also provides the patient with a heart rate monitor and reviews how to use it. The interventionist explains that the heart monitor will serve as verification and verification is required to earn draws. Each exercise activity is defined in terms of duration and intensity required. If verification is provided for all three activities within one week, the patient will 3 draws plus bonus draws that start at 3 and escalate up to 10 over time with successful completion of all three exercise activities. Note the exercise contract shown in Figure 1 has spots for four activities because the fourth activity is used as a back-up in case one of the first three is not feasible (e.g., running outside when it snows or in excessive heat) or completed. In total, a patient can earn up to 279 draws from the prize bowl if the person completes 72 exercise activities across the 24 weeks of the intervention.
Figure 1.
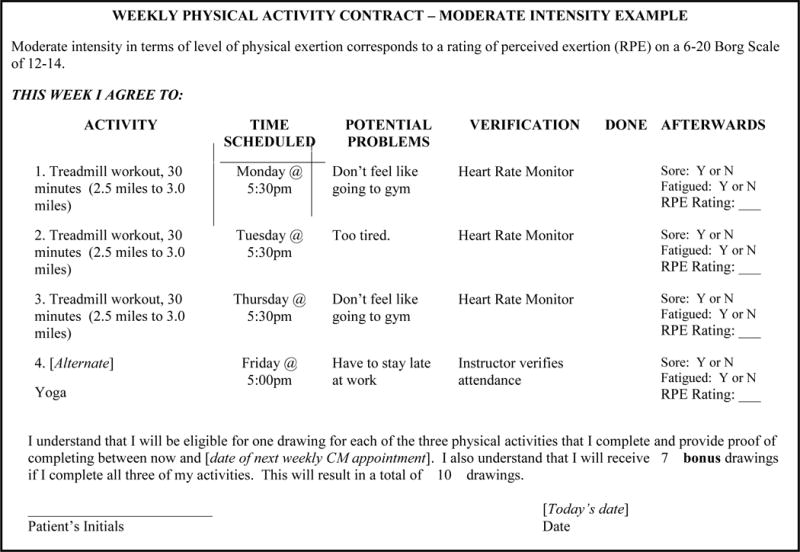
Example Exericse Contract
Our prize bowl for drawings contains 200 slips of paper. Half (100) state “Good job!” but are not associated with a prize. The other half are winning slips: 80 state “small prize”, 19 state “large prize”, and 1 states “jumbo prize”. When a patient draws a winning slip, the person chooses amongst the available gift certificates in that category: small, large, jumbo. The maximal costs of respective types of prizes are $1, $20, and $100 with average maximal reinforcement over the course of the intervention estimated at about $800 per patient (~$125/month). Efforts are made to have a large selection of desirable gift certificates available for each category and to have options consistent with a healthy lifestyle. At the end of the first session, patients are given three draws to introduce them to the concept of the CM draws, the possibility of winning prizes, and for constructing the first exercise contract. Exposure to the CM prize draws at the first session is important; some patients may never earn a draw otherwise which leads to not being exposed to the potential reinforcers (Petry et al., 2005). Prize bowl probabilities, magnitude of prizes and their associated costs can be varied according to budget. See Petry (2012) for guidance on how to construct CM reinforcement procedures and estimate budgets.
In the subsequent weeks, the interventionist meets briefly with the patient to review the prior week’s exercise contract, including verification of exercise and a discussion of whether or not a patient experienced excessive soreness or fatigue as a result of exercise, problem-solve any issues with exercising, provide any reinforcement earned, and collaborate on the creation of a new exercise contract for the upcoming week. Each session takes about 15–20 minutes, with later sessions being shorter due to the patient’s familiarity with the process and development of a consistent exercise routine. As many individuals are sedentary at the start of an exercise intervention, the goal of the exercise contracting is to build up gradually over time and maintain a level of exercise consistent with the current guidelines of 150–300 minutes of moderate intensity exercise (or as modified by a physician). For sedentary individuals, initial contracts should seek to maximize exercise frequency and gradually build up duration of those exercise bouts over time to meet public health guidelines. Overall, contracts are a useful tool to monitor exercise and adverse events (e.g., injury, excessive soreness) and ensure its prescription is appropriate to the individual.
Verification of exercise and its intensity is an important component of the CM intervention. Options for verifying exercise in monitored exercise protocols have increased dramatically due to advances in technology, and current options for verification include heart rate monitors, pedometers, before and after pictures/videos of exercise, fitness apps, activity trackers (e.g., Fitbit, Jawbone), and/or gym facility attendance sheets. Our current study, for example, uses a fitness watch that connects to a heart rate strap, and verification includes the patient providing the watch to the interventionist for confirmation that the contract specifics for the exercise bout were met (e.g., average heart rate between 120–150 bmp, 25 minute duration). Not only is verification in supervised exercise programs much easier with verification provided by the exercise specialists supervising the exercise sessions, but with monitored exercise, there is always a risk of faulty or fabricated data. Those activities that cannot be verified (e.g., sit-ups at home) can be encouraged for overall health, but not used for the purposes of the CM goals. When talking with patients, the interventionist should be aware of factors that facilitate initiation and maintenance of exercise (e.g., contracting, social support, feedback), and attempt to incorporate these factors into the physical activity goals (e.g., exercising with a friend).
2.4 Special Issues Related to Prescribing Exercise
2.4.1 Interventionist training
Throughout this manuscript we have used the word “interventionist” to describe the individual who works directly with patients to deliver the MI+CM intervention. Our studies have used a range of individuals from bachelor’s level ACSM certified exercise specialists to a licensed clinical psychologist. Depending upon an individual’s background, interventionists may need training in exercise prescription, MI and CM. Training for MI needs to follow current best practices of a training workshop, preferably led by a member of the Motivational Interviewing Network of Trainers, and ongoing supervision/coaching to sustain MI skills (Schwalbe, Oh, & Zweben, 2014). An additional day of training devoted to training in CM and exercise prescription led by an individual with expertise in these areas is helpful. We also recommend ongoing fidelity monitoring/supervision.
2.4.2 Physical activity in sedentary patients
Patients beginning this intervention may be sedentary, meaning they are not currently engaging in any regular physical activity. Research suggests that high intensity programs have greater non-adherence compared to low and moderate intensity programs (Dishman & Buckworth, 1996). Therefore, beginning with low intensity exercise activities and progressing to a moderate intensity program over time is recommended. The ACSM (2013) provides guidance on the rate of progression suggesting that frequency of exercise be established first then a gradual increase of 5 to 10 minutes be introduced every 1–2 weeks over the first 4 to 6 weeks. After the initial month exercise frequency, intensity, and time is to be gradually increased to public health guidelines. The recommendation also includes ongoing monitoring for adverse events associated with these increases in exercise volume (e.g., fatigue, soreness).
An additional consideration for sedentary individuals is the importance of self-efficacy (i.e., confidence in one’s ability to exercise), as it is one of the strongest predictors of adherence to a physical activity program (McAuley & Blissmer, 2000). Sedentary individuals often have low self-efficacy in regards to exercise due to a lack of knowledge and experience, lack of confidence, tenuous motivation, and unrealistic expectations. As an interventionist for a sedentary patient, it is important to foster and provide opportunities for the patient to develop self-efficacy. Conversely, some patients are extremely motivated and gung-ho about engaging in an exercise program and desire to immediately contract for exercise according to the public health guidelines. In this case, the interventionist is responsible for ensuring the patient is setting realistic goals that are not overwhelming or will lead acute injuries that can occur when one dramatically increases his or her level of physically activity in a short period time. Therefore, initially the idea with sedentary patients is to get them minimally physically active while enjoying themselves. The interventionist must also manage the patient’s expectations for physical activity and its subsequent consequences.
2.4.3 Preferences for exercise
Regarding the aim to get patients physically active, while enjoying themselves, the interventionist should, as much as possible, allow the patient to choose which exercises they would like to do. According to Self-Determination Theory (SDT), autonomy, including choice and opportunity for self-direction, is one of the three basic human needs that leads to intrinsic motivation (Deci & Ryan, 1985; Ryan & Deci, 2000). This theory applies to exercise (Barbeau, Sweet, & Fortier, 2009; Edmunds, Ntoumanis, & Duda, 2007; Silva et al., 2011; Wulf, Freitas, & Tandy, 2014). Intrinsic motivation has been shown to increase exercise participation and adherence (Barbeau et al., 2009; Edmunds et al., 2007; Silva et al., 2011; Teixeira, Carraça, Markland, Silva, & Ryan, 2012). Therefore, giving patients choice in their activity may help them to feel more in control and thus be more adherent. It is important, however, to ensure that the activities that patients pick are aerobic in nature and of an appropriate difficulty and intensity.
2.4.4 Non-adherence with physical activity contracts
When the patient does not complete contracted activities, draws for the non-completed activities are withheld, as are the bonus drawings. In addition, the bonus draws will be reset back to three on the next week’s contract (e.g., slight punisher). Two weeks after completed three activities per week, the bonus draws will be reinstated to the previous highest level. When resetting a patient, it is important to be firm, but sympathetic. The interventionist and patient should discuss reasons for non-adherence and problem solve around the issues. It may be helpful to reevaluate goals to ensure that they are challenging, yet attainable.
2.4.5 Illness and physical activity contracts
During the course of the intervention patients may become ill. For some illnesses or injuries, exercise is contraindicated (e.g., musculoskeletal injury unexamined by a physician, presence of a fever, or symptoms below the neck, such as a chest cold). In these cases, reinforcement is withheld for activities not completed, but the patient is not reset because the illness/injury is out of the patient’s control. If the illness/injury persists for longer than a week, the patient should be encouraged to seek medical attention. Furthermore, if illness/injury lasts more than a week, the patient should provide verification (e.g., doctor’s note). If a patient still wants to receive drawings, the interventionist can provide alternative activities (e.g., reading short articles about the benefits of exercise; icing an injury). We use readings from the New York Times fitness blog as they are written for a broad audience. After recovering, the patient may need to restart at a lower activity level than that which he/she had previously achieved, so the interventionist may need to help manage the patient’s expectations.
2.4.6 Fabrication and verification of physical activities
Unfortunately, sometimes patients try to provide false verification of exercise. For this reason supervised exercise may be a better alternative. It can be helpful at the outset to let the patient know that the interventionist is aware that cheating attempts may happen and is actively working to ensure that it does not occur. It can be helpful to provide all patients with pedometers or heart rate monitors to help ensure compliance with the intervention in a verifiable way. Additionally, interventionists should take time to ensure that patients know how to use the verification tools and provide them with information to take home regarding their use. If verification has obviously been fabricated, the interventionist should withhold drawings to prevent additional fabrication attempts in the future and discuss the issue with the patient. The interventionist should require objective verification. In other words, patients’ friend, family, etc. should not be allowed to verify activities. Another example of fabrication is having a friend wear the pedometer or fitness tracker and trying to use that data for verification. Overall, vigilance is required but unless a supervised exercise protocol is employed, fabrication can happen.
3.1 Conclusion
In conclusion, exercise appears to be a viable adjunctive treatment for SUD with many benefits for patients and clinics. Our MI+CM intervention addresses challenges associated with initiation and maintenance of exercise behaviors. Given the potential health and psychological benefits for individuals struggling with recovery from SUDs and the potential for increased clinic revenue, the integrated intervention presents a unique opportunity for improved treatment outcomes.
Highlights.
Exercise has great potential as an adjunctive treatment for substance use disorders
Exercise interventions suffer from attrition and poor adherence that limit benefits
An exercise intervention addressing intrinsic and extrinsic motivation is described
Acknowledgments
This research was supported in part by National Institutes of Health grants R21-AA-0177171, R03-AA-020194, and R01-DA-033411-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agosti V, Nunes EV, O’Shea D. Do manualized psychosocial interventions help reduce relapse among alcohol-dependent adults treated with naltrexone or placebo? A meta-analysis. American Journal on Addictions. 2012;21(6):501–507. doi: 10.1111/j.1521-0391.2012.00270.x. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Krishnan KR. Exercise treatment for major depression: Maintenance of therapeutic benefit at 10 months. Psychosomatic Medicine. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Barbeau A, Sweet SN, Fortier M. A Path-Analytic Model of Self-Determination Theory in a Physical Activity Context. Journal of Applied Biobehavioral Research. 2009;14(3):103–118. doi: 10.1111/j.1751-9861.2009.00043.x. [DOI] [Google Scholar]
- Battista SR, Mackinnon SP, Sherry SB, Barrett SP, MacNevin PD, Stewart SH. Does alcohol reduce social anxiety in daily life? A 22-day experience sampling study. Journal of Social and Clinical Psychology. 2015;34(6):508–528. doi: 10.1521/jscp.2015.34.6.508. [DOI] [Google Scholar]
- Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, Festinger DS. Prize-based contingency management for the treatment of substance abusers: A meta-analysis. Addiction. 2014;109(9):1426–1436. doi: 10.1111/add.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broocks A, Bandelow B, Pekrun G, George A, Meyer T, Bartmann U, Rüther E. Comparison of aerobic exercise, clomipramine, and placebo in the treatment of panic disorder. American Journal of Psychiatry. 1998;155(5):603–609. doi: 10.1176/ajp.155.5.603. [DOI] [PubMed] [Google Scholar]
- Brown JM, Miller WR. Impact of motivational interviewing on participation and outcome in residential alcoholism treatment. Psychology of Addictive Behaviors. 1993;7(4):211–218. [Google Scholar]
- Brown RA, Abrantes AM, Minami H, Read JP, Marcus BH, Jakicic JM, Stuart GL. A preliminary, randomized trial of aerobic exercise for alcohol dependence. Journal of Substance Abuse Treatment. 2014;47(1):1–9. doi: 10.1016/j.jsat.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, Stuart G. Aerobic exercise for alcohol recovery: Rationale, program description, and preliminary findings. Behavior Modification. 2009;33(2):220–249. doi: 10.1177/0145445508329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, Gordan AA. A pilot study of aerobic exercise as an adjunctive treatment for drug dependence. Mental Health and Physical Health. 2010;3:27–34. doi: 10.1016/j.mhpa.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckworth J, Lee RE, Regan G, Schneider LK, DiClemente LC. Decomposing intrinsic and extrinsic motivation for exercise: Application to stages of motivational readiness. Psychology of Sport and Exercise. 2007;8:399–410. [Google Scholar]
- Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: A meta-analysis of controlled clinical trials. Journal of Consulting and Clinical Psychology. 2003;71(5):843–861. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64(5):566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Connors GJ, Walitzer KS, Dermen KH. Preparing clients for alcoholism treatment: Effects on treatment participation and outcomes. Journal of Consulting and Clinical Psychology. 2002;70(5):1161–1169. doi: 10.1037/0022-006X.70.5.1161. [DOI] [PubMed] [Google Scholar]
- Costa Rosa LF. Exercise as a time-conditioning effector in chronic disease: A complementary treatment strategy. Evidenced-Based Complementary and Alternative Medicine. 2004;1:63–70. doi: 10.1093/ecam/neh018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress ME, Buchner DM, Prohaska T, Rimmer J, Brown M, Macera C, Chodzko-Zajko W. Best practices for physical activity programs and behavior counseling in older adult populations. Journal of Aging and Physical Activity. 2005;13:61–74. doi: 10.1123/japa.13.1.61. [DOI] [PubMed] [Google Scholar]
- Cushing CC, Jensen CD, Miller MB, Leffingwell TR. Meta-analysis of motivational interviewing for adolescent health behavior: Efficacy beyond substance use. Journal of Consulting and Clinical Psychology. 2014;82(6):1212–1218. doi: 10.1037/a0036912. [DOI] [PubMed] [Google Scholar]
- Cutter CJ, Schottenfeld RS, Moore BA, Ball SA, Beitel M, Savant JD, Barry DT. A pilot trial of a videogame-based exercise program for methadone maintained patients. Journal of Substance Abuse Treatment. 2014;47(4):299–305. doi: 10.1016/j.jsat.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deci EL, Ryan RM. Intrinsic motivation and self-determination in human behavior. New York: Plenum Press; 1985. [Google Scholar]
- DeVahl J, King R, Williamson JW. Academic incentives for students can increase participating in and effectiveness of a physical activity program. Journal of American College Health. 2005;53:295–298. doi: 10.3200/JACH.53.6.295-298. [DOI] [PubMed] [Google Scholar]
- Dishman RK. Exercise adherence: Its impact on public health. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- Dishman RK, Buckworth J. Increasing physical activity: A quantitative synthesis. Medicine and Science in Sports and Exercise. 1996;28(6):706–719. doi: 10.1097/00005768-199606000-00010. [DOI] [PubMed] [Google Scholar]
- Dolezal BA, Chudzynski J, Storer TW, Abrazado M, Penate J, Mooney L, Cooper CB. Eight weeks of exercise training improves fitness measures in methamphetamine-dependent individuals in residential treatment. Journal of Addiction Medicine. 2013;7(2):122. doi: 10.1097/ADM.0b013e318282475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds J, Ntoumanis N, Duda JL. Adherence and well-being in overweight and obese patients referred to an exercise on prescription scheme: A self-determination theory perspective. Psychology of Sport & Exercise. 2007;8:722–740. doi: 10.1016/j.psychsport.2006.07.006. [DOI] [Google Scholar]
- Gordon-Larsen P, Nelson MC, Popkin BM. Longitudinal Physical Activity and Sedentary Behavior Trends: Adolescence to Adulthood. American Journal of Preventive Medicine. 2004;27(4):277–283. doi: 10.1016/j.amepre.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Archives of General Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Weiss RD, Muenz LR, Vagge LM, Kelly JF, Bello LR, Michael J. The effect of depression on return to drinking: A prospective study. Archives of General Psychiatry. 1998;55(3):259–265. doi: 10.1001/archpsyc.55.3.259. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Economic concepts for the analysis of behavior. Journal of the Experimental Analysis of Behavior. 1980;34(2):219–238. doi: 10.1901/jeab.1980.34-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons JG, Pope DA, Pierce AE, Van Patten RA, Jarvis BP. Contingency management to induce exercise among college students. Behaviour Change. 2013;30(2):84–95. [Google Scholar]
- Jeffery RW, Wing RR, Thorson C, Burton LR. Use of personal trainers and financial incentives to increase exercise in a behavioral weight-loss program. Journal of Consulting and Clinical Psychology. 1998;66(5):777–783. doi: 10.1037//0022-006x.66.5.777. [DOI] [PubMed] [Google Scholar]
- Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330(6006):932–932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- Laudet AB, Becker JB, White WL. Don’t wanna go through that madness no more: Quality of life satisfaction as predictor of sustained remission from illicit drug misuse. Substance Use & Misuse. 2009;44(2):227–252. doi: 10.1080/10826080802714462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Neighbors C, Henderson CE, Young CM. Exercise and alcohol consumption: What we know, what we need to know, and why it is important. Frontiers in Psychiatry. 2015;6:156. doi: 10.3389/fpsyt.2015.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke SE, Ussher M. Exercise-based treatments for substance use disorders: Evidence, theory, and practicality. American Journal of Drug and Alcohol Abuse. 2015;41(1):7–15. doi: 10.3109/00952990.2014.976708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott DC, Jencius S. Effectiveness of very low-cost contingency management in a community adolescent treatment program. Drug and Alcohol Dependence. 2009;102:162–165. doi: 10.1016/j.drugalcdep.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Lundahl B, Moleni T, Burke BL, Butters R, Tollefson D, Butler C, Rollnick S. Motivational interviewing in medical care settings: A systematic review and meta-analysis of randomized controlled trials. Patient Education and Counseling. 2013;93(2):157–168. doi: 10.1016/j.pec.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Martins RK, McNeil DW. Review of motivational interviewing in promoting health behaviors. Clinical Psychology Review. 2009;29(4):283–293. doi: 10.1016/j.cpr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- McAuley E, Blissmer B. Self-efficacy determinants and consequences of physical activity. Exercise and Sport Sciences Reviews. 2000;28(2):85–88. [PubMed] [Google Scholar]
- Medina JL, Vujanovic AA, Smits JAJ, Irons JG, Zvolensky MJ, Bonn-Miller MO. Exercise and coping-oriented alcohol use among a trauma-exposed sample. Addictive Behaviors. 2011;36(3):274–277. doi: 10.1016/j.addbeh.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Helping people change. 3rd. New York, NY, US: Guilford Press; 2013. [Google Scholar]
- Mitchell MS, Goodman JM, Alter DA, John LK, Oh PI, Pakosh MT, Faulkner GE. Financial incentives for exercise adherence in adults: Systematic review and meta-analysis. American Journal of Preventive Medicine. 2013;45(5):658–667. doi: 10.1016/j.amepre.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Drake RE, Wallach MA. Dual diagnosis: A review of etiological theories. Addictive Behaviors. 1998;23(6):717–734. doi: 10.1016/S0306-4603(98)00073-2. [DOI] [PubMed] [Google Scholar]
- Muller AE, Clausen T. Group exercise to improve quality of life among substance use disorder patients. Scandinavian Journal of Public Health. 2015;43(2):146–152. doi: 10.1177/1403494814561819. [DOI] [PubMed] [Google Scholar]
- Muller AE, Skurtveit S, Clausen T. Many correlates of poor quality of life among substance users entering treatment are not addiction-specific. Healthand Quality of Life Outcomes. 2016;14(1):39. doi: 10.1186/s12955-016-0439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien N, McDonald S, Araujo-Soares V, Lara J, Errington L, Godfrey A, Sniehotta FF. The features of interventions associated with long-term effectiveness of physical activity interventions in adults aged 55 to 70 years: A systematic review and meta-analysis. Health Psychology Review. 2015:1–29. doi: 10.1080/17437199.2015.1012177. [DOI] [PubMed] [Google Scholar]
- O’Halloran PD, Blackstock F, Shields N, Holland A, Iles R, Kingsley M, Taylor NF. Motivational interviewing to increase physical activity in people with chronic health conditions: A systematic review and meta-analysis. Clinical Rehabilitation. 2014;28(12):1159–1171. doi: 10.1177/0269215514536210. [DOI] [PubMed] [Google Scholar]
- Office of National Drug Control Policy. The Economic Costs of Drug Abuse in the United States, 1992–2002. Washington, DC: Executive Office of the President; 2004. 207303. [Google Scholar]
- Palmer JA, Palmer LK, Michiels K, Thigpen B. Effects of type of exercise on depression in recovering substance abusers. Perceptual and Motor Skills. 1995;80:523–530. doi: 10.2466/pms.1995.80.2.523. [DOI] [PubMed] [Google Scholar]
- Patel MS, Asch DA, Rosin R, Small DS, Bellamy SL, Heuer J, Volpp KG. Framing financial incentives to increase physical activity among overweight and obese adults: A randomized, controlled trial. Annals of Internal Medicine. 2016;164:385–394. doi: 10.7326/m15-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Moreno F, Cámara-Sánchez M, Tremblay J, Riera-Rubio V, Gil-Paisan L, Lucia A. Benefits of exercise training in Spanish prison inmates. International journal of sports medicine. 2007;28(12):1046–1052. doi: 10.1055/s-2007-965129. [DOI] [PubMed] [Google Scholar]
- Perri MG, Martin AD, Leermakers EA, Sears SF, Notelovitz M. Effects of group- versus home-based exercise in the treatment of obesity. Journal of Consulting and Clinical Psychology. 1997;65(2):278–285. doi: 10.1037/0022-006X.65.2.278. [DOI] [PubMed] [Google Scholar]
- Petry NM. Contingency management for substance abuse treatment: A guide to implementing this evidence-based practice. New York, NY, US: Routledge/Taylor & Francis Group; 2012. [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, Li R. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: A National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2005;62:1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Plotnikoff RC, Costigan SA, Williams RL, Hutchesson MJ, Kennedy SG, Robards SL, Germov J. Effectiveness of interventions targeting physical activity, nutrition and healthy weight for university and college students: A systematic review and meta-analysis. International Journal of Behavioral Nutrition and Physical Activity. 2015;12(1):45. doi: 10.1186/s12966-015-0203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Puetz TW, O’Connor PJ, Dishman RK. Effects of chronic exercise on feelings of energy and fatigue: A quantitative synthesis. Psychological Bulletin. 2006;132:866–879. doi: 10.1037/0033-2909.132.6.866. [DOI] [PubMed] [Google Scholar]
- Rash CJ, Petry NM, Kirby KC, Martino S, Roll J, Stitzer ML. Identifying provider beliefs related to contingency management adoption using the contingency management beliefs questionnaire. Drug and Alcohol Dependence. 2012;121(3):205–212. doi: 10.1016/j.drugalcdep.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read JP, Brown RA, Marcus BH, Kahler CW, Ramsey SE, Dubreuil ME, Francione C. Exercise attitudes and behaviors among persons in treatment for alcohol use disorders. Journal of Substance Abuse Treatment. 2001;21:199–206. doi: 10.1016/s0740-5472(01)00203-3. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, T Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Riebe D, Franklin BA, Thompson PD, Garber CE, Whitfield GP, Magal M, Pescatello LS. Updating ACSM’s recommendations for exercise preparticipation health screening. Medicine & Science in Sports & Exercise. 2015;47(11):2473–2479. doi: 10.1249/MSS.0000000000000664. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American Psychologist. 2000;55(1):68–78. doi: 10.1037/0003-066X.55.1.68. [DOI] [PubMed] [Google Scholar]
- Schwalbe CS, Oh HY, Zweben A. Sustaining motivational interviewing: A meta-analysis of training studies. Addiction. 2014;109(8):1287–1294. doi: 10.1111/add.12558. [DOI] [PubMed] [Google Scholar]
- Silva MN, Markland D, Carraça EV, Vieira PN, Coutinho SR, Minderico CS, Teixeira PJ. Exercise autonomous motivation predicts 3-yr weight loss in women. Medicine & Science in Sports & Exercise. 2011;43(4):728–737. doi: 10.1249/MSS.0b013e31810818f. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic alcohol related neuroadaptations in stress responses: Effects on compulsive alcohol seeking and relapse outcomes. Alcoholism: Clinical and Experimental Research. 2008;32:341a. [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Archives of General Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinyor D, Brown T, Rostant L, Seraganian P. The role of a physical fitness program in the treatment of alcoholism. Journal of Studies on Alcohol. 1982;43:380–386. doi: 10.15288/jsa.1982.43.380. [DOI] [PubMed] [Google Scholar]
- Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annual Review of Clinical Psychology. 2006;2:411–434. doi: 10.1146/annurev.clinpsy.2.022305.095219. [DOI] [PubMed] [Google Scholar]
- Stotts AL, Schmitz JM, Rhoades HM, Grabowski J. Motivational interviewing with cocaine-dependent patients: A pilot study. Journal of Consulting and Clinical Psychology. 2001;69(5):858–862. doi: 10.1037//0022-006x.69.5.858. [DOI] [PubMed] [Google Scholar]
- Stoutenberg M, Warne J, Vidot D, Jimenez E, Read JP. Attitudes and preferences towards exercise training in individuals with alcohol use disorders in a residential treatment setting. Journal of Substance Abuse Treatment. 2015:4943–49. doi: 10.1016/j.jsat.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Swanson AJ, Pantalon MV, Cohen KR. Motivational interviewing and treatment adherence among psychiatric and dually diagnosed patients. Journal of Nervous And Mental Disease. 1999;187(10):630–635. doi: 10.1097/00005053-199910000-00007. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Oh H, Cullen S. Acute effect of exercise on alcohol urges and attentional bias towards alcohol related images in high alcohol consumers. Mental Health and Physical Activity. 2013;6(3):220–226. doi: 10.1016/j.mhpa.2013.09.004. [DOI] [Google Scholar]
- Teixeira PJ, Carraça EV, Markland D, Silva MN, Ryan RM. Exercise, physical activity, and self-determination theory: A systematic review. International Journal of Behavioral Nutrition & Physical Activity. 2012;9:78–107. doi: 10.1186/1479-5868-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throne LC, Bartholomew JB, Craig J, Farrar RP. Stress reactivity in fire fighters: An exercise intervention. International Journal of Stress Management. 2000;7:235–246. [Google Scholar]
- US Department of Health and Human Services [USDHHS] 2008 physical activity guidelines for American. Washington, DC: USDHHS; 2008. [Google Scholar]
- Ussher M, Sampuran AK, Doshi R, West R, Drummond DC. Acute effect of a brief bout of exercise on alcohol urges. Addiction. 2004;99:1542–1547. doi: 10.1111/j.1360-0443.2004.00919.x. [DOI] [PubMed] [Google Scholar]
- VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: A systematic review and meta-analysis. Journal of Behavioral Medicine. 2014;37(4):768–780. doi: 10.1007/s10865-013-9527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R, Rosvall T, Field CA, Allen S, McDonald D, Salim Z, Adinoff B. Disseminating contingency management to increase attendance in two community substance abuse treatment centers: Lessons learned. Journal of Substance Abuse Treatment. 2010;39:202–209. doi: 10.1016/j.jsat.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TL, Sheeran P. Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychological Bulletin. 2006;132:249–268. doi: 10.1037/0033-2909.132.2.249. [DOI] [PubMed] [Google Scholar]
- Weinstock J, Alessi SM, Petry NM. Regardless of psychiatric severity the addition of contingency management to standard treatment improves retention and drug use outcomes. Drug and Alcohol Dependence. 2007;87:288–296. doi: 10.1016/j.drugalcdep.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock J, Barry D, Petry NM. Exercise-related activities are associated with positive outcome in contingency management treatment for substance use disorders. Addictive Behaviors. 2008;33:1072–1075. doi: 10.1016/j.addbeh.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock J, Capizzi J, Weber SM, Pescatello LS, Petry NM. Exercise as an intervention for sedentary hazardous drinking college students: A pilot study. Mental Health and Physical Activity. 2014;7(1):55–62. doi: 10.1016/j.mhpa.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock J, Petry N, Pescatello L, Henderson C. Sedentary college student drinkers can start exercising and reduce drinking after intervention. Psychology of Addictive Behaviors. doi: 10.1037/adb0000207. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf G, Freitas HE, Tandy RD. Choosing to exercise more: Small choices increase exercise engagement. Psychology of Sport & Exercise. 2014;15:268–271. doi: 10.1016/j.psychsport.2014.01.007. [DOI] [Google Scholar]


