Summary
The endocannabinoids, 2-arachidonoylglycerol (2-AG) and arachidonylethanolamide (AEA) are endogenous ligands for the cannabinoid receptors (CB1 and CB2) and are implicated in a wide array of physiological processes. These neutral arachidonic acid (AA) derivatives have been identified as efficient substrates for the second isoform of the cyclooxygenase enzyme (COX-2). A diverse family of prostaglandin glycerol esters (PG-Gs) and prostaglandin ethanolamides (PG-EAs) is generated by the action of COX-2 (and downstream prostaglandin synthases) on 2-AG and AEA. As the biological importance of the endocannabinoid system becomes more apparent, there is a tremendous need for robust, sensitive and efficient analytical methodology for the endocannabinoids and their metabolites. In this chapter we describe methodology suitable for carrying out oxygenation of endocannabinoids by COX-2, and analysis of products of endocannabinoid oxygenation by COX-2 and of endocannabinoids themselves from in-vitro and cell assays.
Keywords: Cyclooxygenase-2, endocannabinoids, PG-Gs, PG-EAs, in-vitro assay, cell assay, LC-MS/MS
1. Introduction
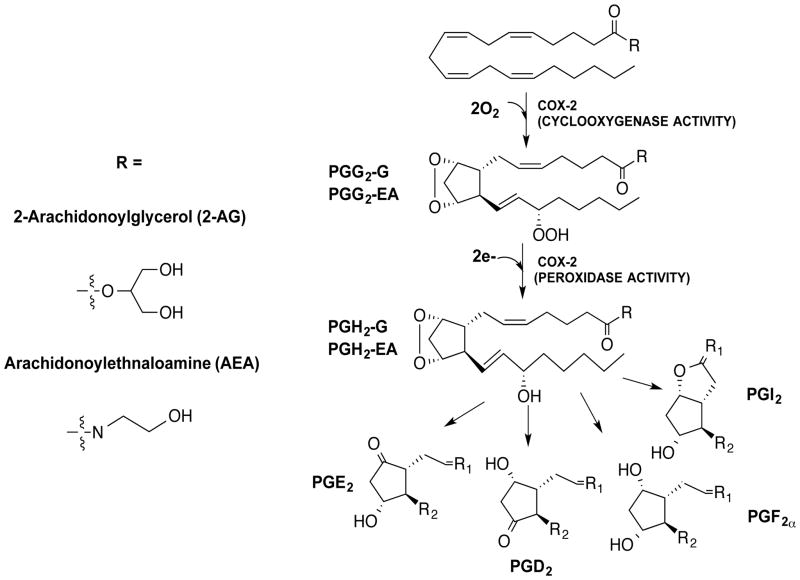
The endocannabinoids 2-archidonoylglycerol (2-AG) and arachidonylethanolamide (AEA) are neutral arachidonic acid (AA) derivatives that exert analgesic and anti-inflammatory effects via the activation of cannabinoid receptors, CB1 and CB2 (1,2). Much like arachidonic acid (AA), 2-AG and AEA are oxygenated by the second isoform of the cyclooxygenase enzyme, COX-2. The oxygenation of 2-AG and AEA by COX-2 produces prostaglandin H2-glycerol ester (PGH2-G) and prostaglandin H2-ethanolamide (PGH2-EA), respectively (3,4). Each PGH2 derivative undergoes further metabolism via prostaglandin synthases to a range of PG-glycerol esters (PG-Gs) and PG-ethanolamides (PG-EAs) that exhibit biological activities such as activation of calcium mobilization in tumor cells and macrophages, modulation of inhibitory synaptic transmission, induction of neurotoxicity by enhancement of excitatory glutamatergic synaptic transmission, and induction of hyperalgesia and anti-inflammatory responses (5–10) (Fig. 1). Additionally, when the macrophage cell line (RAW264.7) is treated with LPS and ionomycin, PG-Gs are produced which stimulate Ca2+ mobilization in the RAW264.7 cells (11,7), suggesting that PG-Gs may exert independent biological activities.
Fig. 1.
Structures of endocannabinoids, 2-arachidonoylglycerol (2-AG) and arachidonylethanolamide (AEA), and their conversion by COX-2 and various PG synthases to prostaglandin glyceryl esters (PG-Gs) and prostaglandin ethanolamides (PG-EAs), respectively.
Given the physiological importance of the endocannabinoids and the potential biological relevance of their COX-2-derived oxygenated products, dependable methods for investigating these interactions are indispensable. In this chapter, we provide step-wise instructions for (1) establishing reactions of 2-AG and/or AEA with COX-2 both in vitro and in the RAW264.7 macrophage cell line, and (2) LC-MS/MS methodology that enables quantitative analysis of the endocannabinoids and their oxygenated metabolites.
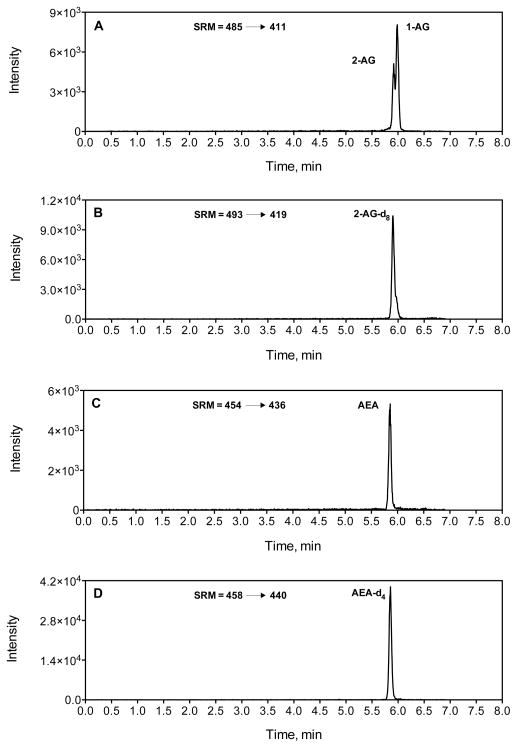
We have utilized two LC-MS/MS methods; one for the analysis of COX-2 substrates (2-AG and AEA) and a second for the analysis of COX-2 oxygenation products (PG-Gs and PG-EAs). A silver cation (Ag+) coordination, liquid-chromatography, electrospray-ionization, and tandem mass spectrometry (LC-ESI-MS-MS) method for analyzing 2-AG and AEA has been developed by our lab (12). In this method, the silver cation coordinates with the 4 double bonds of the arachidonate backbone of 2-AG and AEA, which is rich in π electrons. This coordination of silver to 2-AG and AEA forms an [M+Ag]+ complex that is amenable to electrospray ionization and tandem mass spectrometric techniques. Figure 2 shows the chromatograms of 2-AG, AEA and their respective internal standards coordinated with silver. In Figure 2A, two peaks are seen corresponding to 1-AG and 2-AG, which are two isomers of arachidonoylglycerol. While 2-AG is the biologically relevant isomer of arachidonoylglycerol, 2-AG readily undergoes acyl migration under biological settings to form 1-AG (13).
Fig. 2.
LC-MS/MS chromatograms of endocannabinoids and their deuterated internal standards, 2 and 1-AG (A), 2-AG-d8 (B), AEA (C) and AEA-d4 (D).
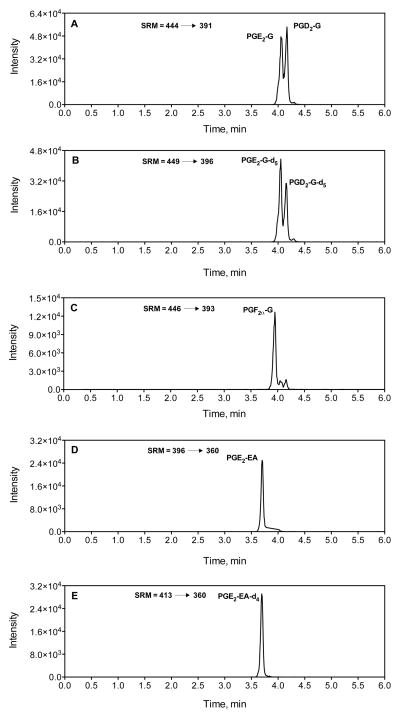
Our laboratory has also published a method for the simultaneous analysis of several PG-Gs and PG-EAs (14). A method describing the analysis of PGF2α-EA also exists in the literature (15). The methodology developed in our laboratory (14) involves complexing the neutral PG-Gs and PG-EAs with either ammonium (NH4+) or a proton (H+). The resultant [M+NH4]+ or [M+H]+ complexes yield multiple intense fragments upon collisionally-induced dissociation (CID), several of which may be employed in selected-reaction monitoring (SRM). Chromatograms of different species of PG-Gs and PG-EAs along with the respective internal standards are shown in Figure 3.
Fig. 3.
LC-MS/MS chromatograms of selected PG-Gs and PG-EAs; PGE2-G and PGD2-G (A), PGE2-G-d5 and PGD2-G-d5 (B), PGF2α-G (C), PGE2-EA (D) and PGE2-EA-d4 (E).
2. Materials
2.1 General
2.2 In vitro Assays
5 mM hematin stock solution in dimethyl sulfoxide (DMSO). Store at room temperature.
Reaction buffer: 100 mM Tris--HCL (pH 8.0) with 500 μM phenol. Store at room temperature.
Purified COX-2 enzyme (16).
Substrates or inhibitors of interest.
Quench solution: Internal standards dissolved in ethyl acetate with 0.5% acetic acid. Place on ice before use in assay. The concentration of the internal standards in the quench solution should be such that the amount of internal standard delivered to each sample upon quenching is within 10-fold of the amount of the analyte that the internal standard will be used to quantitate.
2.3 Cell Assay
RAW264.7 macrophages.
Dulbecco’s Modified Eagle Medium (DMEM) media supplemented with 10% fetal bovine serum.
100 ng/ml working solution of Kdo2-lipid A (KLA) in Ca2+/Mg2+ free sterile Dulbecco’s phosphate buffered saline (DPBS buffer). Make the working solution of KLA in serum-free medium.
2 mM ionomycin stock solution in DMSO. Our lab uses 2–5 μM of ionomycin as the final concentration.
Inhibitors of interest (see Note 2).
Extraction solution; similar to “Quench solution” in section 2.2.5.
2.4 LC-MS/MS
-
Silver complexation mobile phase components;
150 μM silver acetate in HPLC grade water plus 0.1% formic acid.
150 μM silver acetate in HPLC grade methanol plus 0.1 % formic acid.
-
Ammonium complexation mobile phase components;
5 mM ammonium acetate in HPLC grade water, pH adjusted to 3.2–3.4 with formic acid.
6% component A in HPLC grade acetonitrile with 0.1 % formic acid.
HPLC column: C18, 5 × 0.2 cm, either 3 or 5 um particle size.
LC-MS system: an HPLC system with a binary pump and autosampler in-line with a triple quadrupole mass spectrometer and appropriate data acquisition software (see Note 3).
Standard mixture of analytes in methanol at a concentration of 2 μM--store at −20°C.
Internal standard recovery solution (see Note 4).
3.0 Methods
3.1 In-vitro Assay
Prepare a 40X substrate stock solution whose concentration is 40 times the concentration of substrate in the reaction vessel. Prepare in DMSO and make enough to provide 5 μL for each 200 μL reaction.
Prepare a 40X inhibitor stock solution whose concentration is 40 times the concentration of inhibitor in the reaction vessel. Prepare in DMSO and make enough to provide 5 μL for each 200 μL reaction.
Prepare a COX-2 solution in 100 mM Tris--HCL with 500 μM phenol, pH.8.0 buffer (the usual concentration of COX-2 is 50–100 nM). Add 3 equivalents of hematin solution to this enzyme solution 10–15 mins prior to the experiment. Keep this enzyme solution on ice.
Prepare the quench solution and keep on ice.
For inhibition assays, aliquot 190 μL of the COX-2 solution into a 1.5 mL microfuge tube and incubate in heat block set at 37 °C for 3 mins. For assays with weak-reversible inhibitors, add 5 μL of 40X inhibitor stock solution to the enzyme solution and incubate at 37°C for an additional 3 mins. For assays with slow-tight binding inhibitors, add 5 μL of 40X inhibitor stock solution to 190 μL of the enzyme solution and incubate at 37°C for an additional 15 mins. After the incubation period, add 5 μL of stock substrate solution and wait 30 sec (see Note 5).
For assays with only COX-2 and substrate, aliquot 195 μL of the COX-2 solution into a 1.5 mL microfuge tube and incubate in a heat block set at 37 °C for 3 min. Then add 5 μL of 40X substrate stock solution and wait 30 sec (see Note 5).
After the 30 sec reaction period, quench the reaction by adding 200 μL of the quench solution. Vortex vigorously and keep on ice.
Collect the top layer from the quenched reaction and add it to a small glass tube. Dry the solution under a stream of N2.
Re-suspend the dried solution with 200 μL of 1:1 methanol:water (HPLC grade water) solution and vortex vigorously.
Aliquot ~200 μL of this solution to either auto-sampler vials or a 96-well plate.
Load the vials or plates into the autosampler of the LC-MS system; prepare a queue with an appropriate method and start the program as described in section 3.3.
3.2 Cells Assay
Plate 3 × 106 cells in 8mL DMEM (10% FBS).
After 24 h, co-treat with Kdo2-lipid A (KLA) and inhibitor solution for 6hrs (for COX-2 activation) (see Note 6).
Collect the media and transfer it to a Falcon tube (15 mL).
Extract PG-G or PG-EA species from the media by adding the extraction solution (2.3.6 -- using 2 times the media volume) to the Falcon tube containing the media. Vortex vigorously and keep on ice.
Transfer the top layer to a clean vessel and dry it down under N2
Re-suspend the dried solution with 200 μL of 1:1 methanol:water solution and vortex vigorously
Aliquot ~200 μL of this solution to either auto-sampler vials or a 96-well plate.
Load the vials or plates into the autosampler of the LC-MS system, prepare a queue with an appropriate method and start the program as described in section 3.3.
3.3 LC-MS analysis
This section will describe two LC-MS/MS method; one for the analysis of COX-2 substrates (2-AG and AEA) and a second for the analysis of COX-2 oxygenation products (PG-Gs and PG-EAs). Both methods employ reverse-phase chromatography and mass spectrometric detection where the mass spectrometer is equipped with an electrospray source, operated in positive ion mode and is configured for selected reaction monitoring (SRM). Obviously, not all analytes described here need be included in one’s assay--the choice of analytes depends on the experimental parameters and the interest of the investigator. These methods are based on methods described in the literature for endocannabinoids (12) and prostanoids (14).
Prime the LC system with appropriate mobile phase (see Section 2.4, 1 or 2) and establish mobile phase flow through the chosen column at the initial conditions.
Create (or modify an existing) instrument method containing the desired SRM transitions and chromatographic gradient profile, as specified in tables 1–4 (see Note 7)
Place samples in sample tray and create a run sequence. It is best to bracket all unknowns with standards and then randomize the order in which the unknowns are analyzed.
Inject the standard solution, verify that all analytes are observed
Inject the Internal Standard Recovery solution, verify that the retention times are very similar to Step 4, that no analyte peak is observed in the appropriate SRM transition, and that all internal standards are observed (see Note 4).
Inject a blank and ensure that no peaks appear in any transition.
Start the sequence.
After the samples have been successfully injected, prepare a processing method and process the resultant raw files.
Export data to Excel or other spreadsheet and calculate analyte amounts (see Note 8).
Table 1.
SRM transitions for endocannabinoids via silver complexation analysis
| Compound | M.W. | Q1 (m/z) | Q3 (m/z) | Collision Energy |
|---|---|---|---|---|
| 2-AG | 378.6 | 485 | 411 | 33 |
| 2-AG-d8 | 386.6 | 493 | 419 | 33 |
| AEA | 347.5 | 454 | 436 | 33 |
| AEA-d4 | 351.5 | 458 | 440 | 33 |
Table 4.
Gradient for oxygenated product LC-MS/MS.
| Time | %B |
|---|---|
| Initial | 30 |
| 0.5 | 30 |
| 2.0 | 80 |
| 3.70 | 80 |
| 3.74 | 30 |
| 3.75 | 30 |
The representative chromatograms of COX-2 substrates, 2-AG and AEA, and their oxygenated products are shown in figures 2 and 3. For SRM analysis of 2-AG and 2-AG-d8, the transitions of m/z 485 ➝ 411 and the m/z 493 ➝ 419 are used (Fig. 2A and 2B) (see note 12). For AEA, the m/z 454 ➝ 436 transition is employed for SRM analysis, while the m/z 462 ➝ 444 transition is used for detection of AEA-d8 (Fig. 2B and 2C). For PG-Gs and PG-G-d5 PGs m/z 444 ➝ 391 and m/z 449 ➝ 396 transitions are used for SRM analysis, respectively (Fig. 3A and 3B). A transition of m/z 446 ➝ 393 is used for PG-F2α-G (Fig. 3C). For SRM analysis of PG-EA and PG-EA-d4 m/z 396 ➝ 360 and m/z 400 ➝ 364 transitions are used (Fig. 3D and 3E).
Table 2.
SRM transitions for oxygenated products of COX-2
| Compound | M.W. | Q1 (m/z) | Q3 (m/z) | Collision Energy |
|---|---|---|---|---|
| PGE2-G & PGD2-G | 426.6 | 444 | 391 | 19 |
| PGF2α-G | 428.6 | 446 | 393 | 19 |
| PGE2-G -d5 | 431.6 | 449 | 396 | 19 |
| PGE2-EA | 395.6 | 396 | 360 | 13 |
| PGE2-EA-d4 | 399.6 | 400 | 364 | 13 |
Table 3.
Gradient for silver complexation LC-MS/MS.
| Time | %B |
|---|---|
| Initial | 70 |
| 0.5 | 70 |
| 4.5 | 100 |
| 5.5 | 100 |
| 6.0 | 70 |
| 7.5 | 70 |
Acknowledgments
We are grateful to Carol Rouzer for editorial assistance. This work was supported by a research grant from the national institute of health (GM 15431).
Footnotes
Many vendors sell deuterated internal standards listed in Section 2.1.3. It is in the researchers’ best interest to establish the isotopic purity of purchased internal standards before use with unknown samples. Our lab has found that some isotopically labeled compounds have a range of stable isotope incorporation and that some isotopically labeled compounds will give a signal in the SRM channel for the native compound of interest.
Stock solutions of inhibitors are made in DMSO.
Our lab has employed both a Thermo Quantum triple quadrupole (with Xcalibur software) and a SCIEX 3200 QTrap (with Analyst software) instruments. Any reasonably modern triple quadrupole or ion trap mass spectrometer should give reasonable results for the methods discussed here.
The recovery internal standard is a standard where the amount of internal standard in each sample is dissolved in the reconstitution volume used for each sample. This sample is important because it (a) establishes whether the instrument is working acceptably and (b) gives the experimenter the recovery level of his/her analytes, which is a useful parameter when assessing the experimental results.
It is important to limit the percent DMSO to ≤ 5 % to prevent protein precipitation.
Slow-tight binding inhibitors are added along with KLA for 6 hours. In contrast, weak-reversible inhibitors plus 5 μM of ionomycin are added after 6 hours of treatment with KLA. The addition of ionomycin releases 2-AG. Cells are incubated for an additional 45 mins before extracting PGs.
Flow rate of 0.3–0.4 mL/min is recommended for both the LC-MS-MS methods.
Stable isotope dilution is used for quantification of analytes in the assays described above. With stable isotope dilution, the amount of analyte in each sample = the response ratio (analyte peak area divided by the internal standard peak area) of the analyte multiplied by the amount of internal standard added to the sample.
References
- 1.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 2.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388(6644):773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 3.Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. Journal of Biological Chemistry. 2000;275(43):33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- 4.Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. Journal of Biological Chemistry. 1997;272(34):21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- 5.Sang N, Zhang J, Chen C. PGE2 glycerol ester, a COX-2 oxidative metabolite of 2-arachiclonoyl glycerol, modulates inhibitory synaptic transmission in mouse hippocampal neurons. Journal of Physiology-London. 2006;572(3):735–745. doi: 10.1113/jphysiol.2006.105569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sang N, Zhang J, Chen C. COX-2 oxidative metabolite of endocannabinoid 2-ag enhances excitatory glutamatergic synaptic transmission and induces neurotoxicity. Journal of Neurochemistry. 2007;102(6):1966–1977. doi: 10.1111/j.1471-4159.2007.04668.x. [DOI] [PubMed] [Google Scholar]
- 7.Nirodi CS, Crews BC, Kozak KR, Morrow JD, Marnett LJ. The glyceryl ester of prostaglandin E2 mobilizes calcium and activates signal transduction in RAW264.7 cells. P Natl Acad Sci USA. 2004;101(7):1840–1845. doi: 10.1073/pnas.0303950101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu SS-J, Bradshaw HB, Chen JSC, Tan B, Walker JM. Prostaglandin E-2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates nf kappa b activity. British Journal of Pharmacology. 2008;153(7):1538–1549. doi: 10.1038/bjp.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valdeolivas S, Pazos MR, Bisogno T, Piscitelli F, Iannotti FA, Allara M, Sagredo O, Di Marzo V, Fernandez-Ruiz J. The inhibition of 2-arachidonoyl-glycerol (2-AG) biosynthesis, rather than enhancing striatal damage, protects striatal neurons from malonate-induced death: A potential role of cyclooxygenase-2-dependent metabolism of 2-ag. Cell Death & Disease. 2013:4. doi: 10.1038/cddis.2013.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alhouayek M, Masquelier J, Cani PD, Lambert DM, Muccioli GG. Implication of the anti-inflammatory bioactive lipid prostaglandin d2-glycerol ester in the control of macrophage activation and inflammation by abhd6. P Natl Acad Sci USA. 2013;110(43):17558–17563. doi: 10.1073/pnas.1314017110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouzer CA, Marnett LJ. Glycerylprostaglandin synthesis by resident peritoneal macrophages in response to a zymosan stimulus. The Journal of biological chemistry. 2005;280(29):26690–26700. doi: 10.1074/jbc.M501021200. [DOI] [PubMed] [Google Scholar]
- 12.Kingsley PJ, Marnett LJ. Analysis of endocannabinoids by Ag+ coordination tandem mass spectrometry. Anal Biochem. 2003;314(1):8–15. doi: 10.1016/s0003-2697(02)00643-7. [DOI] [PubMed] [Google Scholar]
- 13.Rouzer CA, Ghebreselasie K, Marnett LJ. Chemical stability of 2-arachidonylglycerol under biological conditions. Chem Phys Lipids. 2002;119(1–2):69–82. doi: 10.1016/s0009-3084(02)00068-3. [DOI] [PubMed] [Google Scholar]
- 14.Kingsley PJ, Rouzer CA, Saleh S, Marnett LJ. Simultaneous analysis of prostaglandin glyceryl esters and prostaglandins by electrospray tandem mass spectrometry. Anal Biochem. 2005;343(2):203–211. doi: 10.1016/j.ab.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Weber A, Ni JS, Ling KHJ, Acheampong A, Tang-Liu DDS, Burk R, Cravatt BF, Woodward D. Formation of prostamides from anandamide in FAAH knockout mice analyzed by HPLC with tandem mass spectrometry. J Lipid Res. 2004;45(4):757–763. doi: 10.1194/jlr.M300475-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Marnett LJ, Rowlinson SW, Goodwin DC, Kalgutkar AS, Lanzo CA. Arachidonic acid oxygenation by COX-1 and COX-2 - mechanisms of catalysis and inhibition. Journal of Biological Chemistry. 1999;274(33):22903–22906. doi: 10.1074/jbc.274.33.22903. [DOI] [PubMed] [Google Scholar]