Abstract
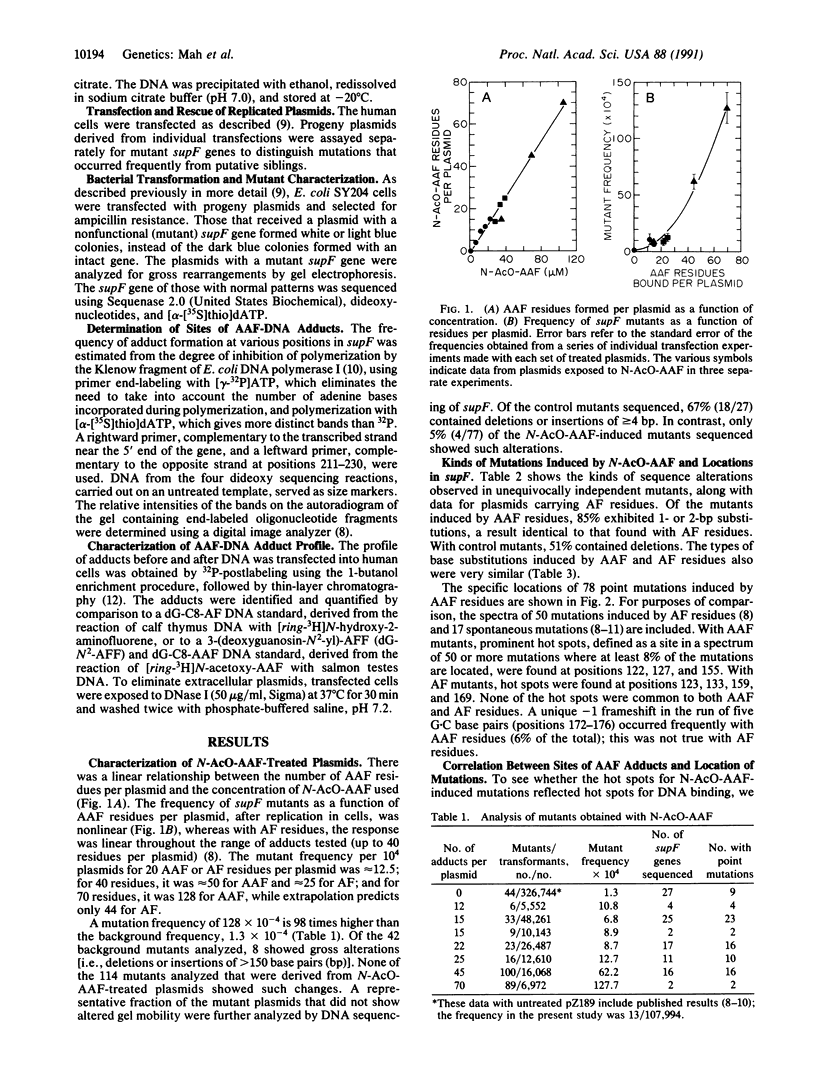
In rats fed the liver carcinogen 2-acetylaminofluorene (AAF), the two most abundant types of DNA adduct are N-(deoxyguanosin-8-yl)-2-acetylaminofluorene and its deacetylated derivative. When plasmids carrying AAF adducts replicate in bacteria, the predominant mutations are frameshifts, whereas with deacetylated (AF) adducts, they are mainly base substitutions, just as we found when plasmids carrying AF adducts replicated in human cells. We have investigated the frequency and spectrum of mutations induced when a shuttle vector carrying AAF adducts (85% bound to the C8 position of guanine, 15% to the N2 position) replicated in human cells. The frequency induced per initial AAF adduct was higher than with AF adducts, but the kinds of mutations were similar--i.e., 85% base substitutions, principally G.C----T.A transversions. There was good correlation between the "hot spots" for mutations and hot spots for AAF adduct formation, suggesting that mutational hot spots reflect preferential binding of the carcinogen to DNA. 32P-postlabeling analysis of the adducts before and after the DNA was transfected into the human cells showed that there was no deacetylation of AAF adducts and that 85% of both types of adducts were removed within 3.5 hr, most probably by excision repair.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bichara M., Fuchs R. P. DNA binding and mutation spectra of the carcinogen N-2-aminofluorene in Escherichia coli. A correlation between the conformation of the premutagenic lesion and the mutation specificity. J Mol Biol. 1985 Jun 5;183(3):341–351. doi: 10.1016/0022-2836(85)90005-1. [DOI] [PubMed] [Google Scholar]
- Boldt J., Mah M. C., Wang Y. C., Smith B. A., Beland F. A., Maher V. M., McCormick J. J. Kinds of mutations found when a shuttle vector containing adducts of 1,6-dinitropyrene replicates in human cells. Carcinogenesis. 1991 Jan;12(1):119–126. doi: 10.1093/carcin/12.1.119. [DOI] [PubMed] [Google Scholar]
- Brown T., Leonard G. A., Booth E. D., Chambers J. Crystal structure and stability of a DNA duplex containing A(anti).G(syn) base-pairs. J Mol Biol. 1989 May 20;207(2):455–457. doi: 10.1016/0022-2836(89)90268-4. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P. In vitro recognition of carcinogen-induced local denaturation sites native DNA by S1 endonuclease from Aspergillus oryzae. Nature. 1975 Sep 11;257(5522):151–152. doi: 10.1038/257151a0. [DOI] [PubMed] [Google Scholar]
- Grunberger D., Nelson J. H., Cantor C. R., Weinstein I. B. Coding and conformational properties of oligonucleotides modified with the carcinogen N-2-acetylaminofluorene. Proc Natl Acad Sci U S A. 1970 Jun;66(2):488–494. doi: 10.1073/pnas.66.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C. Enhanced sensitivity of 32P-postlabeling analysis of aromatic carcinogen:DNA adducts. Cancer Res. 1985 Nov;45(11 Pt 2):5656–5662. [PubMed] [Google Scholar]
- Koffel-Schwartz N., Verdier J. M., Bichara M., Freund A. M., Daune M. P., Fuchs R. P. Carcinogen-induced mutation spectrum in wild-type, uvrA and umuC strains of Escherichia coli. Strain specificity and mutation-prone sequences. J Mol Biol. 1984 Jul 25;177(1):33–51. doi: 10.1016/0022-2836(84)90056-1. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Mutational specificity of depurination. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1494–1498. doi: 10.1073/pnas.81.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah M. C., Maher V. M., Thomas H., Reid T. M., King C. M., McCormick J. J. Mutations induced by aminofluorene-DNA adducts during replication in human cells. Carcinogenesis. 1989 Dec;10(12):2321–2328. doi: 10.1093/carcin/10.12.2321. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Rabkin S. D., Osborn A. L., King C. M., Strauss B. S. Effect of acetylated and deacetylated 2-aminofluorene adducts on in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7166–7170. doi: 10.1073/pnas.79.23.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Moriya M., Takeshita M., Johnson F., Peden K., Will S., Grollman A. P. Targeted mutations induced by a single acetylaminofluorene DNA adduct in mammalian cells and bacteria. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1586–1589. doi: 10.1073/pnas.85.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm J., Turkington E., LaPointe D., Strauss B. Mutation induced in vitro on a C-8 guanine aminofluorene containing template by a modified T7 DNA polymerase. Biochemistry. 1989 Apr 4;28(7):2836–2843. doi: 10.1021/bi00433a014. [DOI] [PubMed] [Google Scholar]
- Santella R. M., Kriek E., Grunberger D. Circular dichroism and proton magnetic resonance studies of dApdG modified with 2-aminofluorene and 2-acetylaminofluorene. Carcinogenesis. 1980;1(11):897–902. doi: 10.1093/carcin/1.11.897. [DOI] [PubMed] [Google Scholar]
- Strauss B., Rabkin S., Sagher D., Moore P. The role of DNA polymerase in base substitution mutagenesis on non-instructional templates. Biochimie. 1982 Aug-Sep;64(8-9):829–838. doi: 10.1016/s0300-9084(82)80138-7. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Yang J. L., Maher V. M., McCormick J. J. Kinds and spectrum of mutations induced by 1-nitrosopyrene adducts during plasmid replication in human cells. Mol Cell Biol. 1988 Aug;8(8):3364–3372. doi: 10.1128/mcb.8.8.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. L., Maher V. M., McCormick J. J. Kinds of mutations formed when a shuttle vector containing adducts of (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9, 10-tetrahydrobenzo[a]pyrene replicates in human cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3787–3791. doi: 10.1073/pnas.84.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]