Summary
Background & aims
We implemented a prospective study among human immunodeficiency virus (HIV)-positive adults to examine the association between vitamin-D deficiency (VDD) and insufficiency (VDI) vs sufficiency (VDS) and CD4+T-cell improvement over 18 months of highly active antiretroviral therapy (HAART).
Methods
We used data from a randomized placebo-controlled micronutrient trial with 25-hydroxy vitamin-D (25(OH)D) measured at enrollment in 398 adults. CD4+T-cell count was measured repeatedly at months 0, 3, 6, 12 and 18. Linear mixed models quantified the vitamin-D-related differences in CD4+T-cell count and associated 99% confidence intervals at baseline and respective follow-up intervals.
Results
At baseline 23%, 60% and 17% of participants were VDS, VDI and VDD, respectively. Absolute CD4+T- cell counts recovered during follow-up were persistently lower for baseline VDD and VDI relative to VDS participants. The greatest deficit in absolute CD4+T-cells recovered occurred in VDD vs VDS participants with estimates ranging from a minimum deficit of 26 cells/μl (99% CI: −77, 26) to a maximum deficit of 65 cells/μl (99% CI: −125, −5.5) during follow-up. This VDD-associated lower absolute CD4+T-cell gain was strongest among patients 35 years old or younger and among participants with a baseline body mass index of less than 25 kg/m2.
Conclusions
VDD is associated with lower absolute CD4+T-cell count recovery in HIV-positive patients on HAART. Vitamin-D supplementation may improve CD4+T-cell recovery during HAART. However, future intervention studies are needed to definitively evaluate the effectiveness of this vitamin as an adjunct therapy during HAART.
Keywords: Vitamin-D, HIV, CD4+, T-cell count, Antiretroviral therapy, Immune recovery
1. Introduction
The prevalence of hypovitaminosis-D including vitamin-D deficiency (VDD) and insufficiency (VDI), varies from 35% to 75% in epidemiologic studies of human immunodeficiency virus (HIV)-infected African adults [1–5]. Vitamin-D status in HIV-positive adults maybe dysregulated by HIV-specific pathogenesis and by antiretroviral therapy with implications for the long-term health status of HIV-infected persons [6]. Epidemiologic studies suggest that certain highly active antiretroviral therapy (HAART) drugs (e.g., efavirenz, protease inhibitors and non-nucleoside reverse transcriptase inhibitors) may further lower vitamin-D levels independent of HIV-specific pathogenesis due to interactions in the cytochrome P450 pathway resulting in preferential hydroxylation of vitamin-D to biologically inactive forms [7,8]. In addition, vitamin-D modulates T-cells, cytokines and dendritic cells [9]. Thus, VDD in HIV-infected HAART-naive and HAART-experienced adults may adversely affect adaptive immune response. If confirmed, this deficiency may predict a range of adverse outcomes in HIV-infected patients on HAART and adds further urgency to the importance of understanding the role of this micronutrient and its deficiency in HIV-infected persons.
VDD has been associated with higher risk of wasting [6], increased risk of anemia, incidence of AIDS-defining conditions [10] and elevated risk of mortality [11] in HIV-infected adults. Among HIV-positive children from Cleveland, Ohio, higher levels of vitamin-D were associated with higher CD4+T-cell counts post-antiretroviral therapy (HAART) initiation [12]. Only one clinical trial of vitamin-D supplementation in HIV-infected persons has been implemented with immune restoration as the primary endpoint among 54 immune-competent HIV-infected children stable on HAART. The trial found no impact on CD4 cell count despite treatment-related improvements in vitamin-D levels [13].
A secondary analysis of HAART-naive patients from eight developing countries and the United States found elevated risk of HIV disease progression and mortality in relation to VDD/VDI post-HAART initiation in a random sub-sample of 250 patients originally recruited for an intervention study of once- vs twice-daily dosing of three HAART regimens [14]. More information is needed regarding the role of vitamin-D on HIV disease progression, particularly among HIV-infected adults from sub-Saharan Africa where micro-nutrient deficiencies are more common. To inform these gaps, we quantified the association between baseline vitamin-D status and absolute changes in CD4+T-cell counts from enrollment through 18 months in HIV-positive adults on HAART for less than 6 months at enrollment. Secondarily, we determined whether the type of HAART regimen, age at enrollment, nutritional status and multivitamin use modified any association between vitamin-D status and CD4+T-cell count recovery. We hypothesize that absolute CD4+T-cell gain will be higher among baseline VDS in comparison with VDD/VDI patients.
2. Methods
This is a longitudinal analysis of the effects of vitamin-D status-related differences on CD4+T-cell recovery among adult HIV-positive patients undergoing treatment at the Infectious Disease Institute (IDI) in Kampala, Uganda. The parent study was a randomized double-blind placebo-controlled trial of daily micro-nutrient supplementation including vitamins B-complex, C, and E vs placebo. Participants (N = 400) were HIV-positive men and non-pregnant women at least 18 years old starting HAART at enrollment or had been on HAART for no longer than 6 months. Each participant provided written informed consent, and resided within 20 km of IDI. Excluded from the trial at enrollment were pregnant women and the seriously ill (i.e. bed-ridden or in too critical a health condition to comprehend the study protocol), unable or unwilling to provide written informed consent and all HAART-ineligible (i.e. free of AIDS-defining conditions and had CD4 cell counts >350 cells/μl) HIV-positive adults. The parent trial was implemented between April 2010 and December 2012. Each patient was followed for 18 months, until loss to follow-up or death—whichever occurred first. Details of the design and methods used in the parent trial have been described elsewhere [15]. The present analysis includes 398 patients for whom baseline vitamin-D status was determined.
Ethical Approval for the trial was provided by the Scientific Review Committee of IDI; the Institutional Review Boards of Harvard School of Public Health and that of Makerere University School of Public Health. The sponsors of the parent study were not involved in the design, implementation and interpretation of results for this analysis.
2.1. Measurements & variable definitions
2.1.1. CD4+T-cell count
CD4+T-cell count was evaluated at months 0, 3, 6, 12 and 18 in all participants as the primary indicator of immune recovery using a FACS Calibur flow cytometer (Becton–Dickinson, San Jose, CA). For the purpose of this investigation, CD4+T-cell counts (in cells/μl) were evaluated as a linear response variable.
2.1.2. Vitamin-D status
Levels of 25-hydroxy vitamin-D (25(OH)D) were measured at baseline by high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) using an API-5000 (AB Sciex, Foster City, CA) at the Children's Hospital, Boston [18]. Serum samples were first extracted and ‘cleaned up’ using the Aria-TLX-2 (Thermo Fisher Scientific, Waltham, MA) after which 50 μl was mixed with acetonitrile containing internal standard of 25(OH)D3. Samples were then centrifuged and 50 μl of the supernatant was injected into the Aria-TLX-2, passed through a Cyclone-P column (Thermo Fisher Scientific), and then eluted through a Kinetex C column (Phenomenex, Torrance, CA). The eluate was injected into the API-5000 for atmospheric pressure chemical ionization and passed through the triple quadruple mass spectrometer for detection and quantified measurements. The assay is linear up to 100 ng/ml, and sensitive to 1 ng/ml. For analytic purposes, three vitamin-D thresholds were defined in light of prior studies [19,20] and a level of 25(OH)D was considered optimal for calcium homeostasis [21] as follows: VDD if 25(OH)D ≤ 20 ng/ml, VDI if 25(OH)D > 20 but < 32 ng/ml, and vitamin-D sufficient (VDS) if 25(OH)D ≥ 32 ng/ml with VDS as the reference group for all analyses.
2.1.3. Other covariates
Time was categorically defined based on timing of repeated CD4 assessment in months as: 0, 3, 6, 12 and 18. CD4+T-cell change was operationally defined as increases relative to month 0. Standardized questionnaires were used to collect socio-demographic, clinical and health data at enrollment and months 3, 6, 12 and 18.
2.2. Statistical analyses
We implemented multivariable random effects linear mixed regression models to quantify associations between vitamin-D status and changes in CD4+T-cell count over 18 months. Baseline CD4+T-cell was included in the outcome matrix; no assumption was made regarding the relationship between vitamin-D status and CD4+T-cell count at baseline given our observational study design [23]. An unstructured covariance matrix was assumed to account for non-independence of repeated CD4+T-cell counts within individuals. A random intercept was specified to allow within-subject differences in the trajectory of CD4+T-cell recovery for each participant. Empirical standard errors were used to derive robust estimates of effect and mitigate potential misspecification of the covariance matrix. We tested a global hypothesis of no vitamin-D-related difference in absolute CD4 over time at α = 0.05. In addition, we estimated VDD- and VDI-associated differences in absolute CD4 compared to VDS status at months 3, 6, 12 and 18 using a more conservative threshold of α = 0.01 to minimize the likelihood of obtaining spurious statistical significance in respective intervals by vitamin-D status.
To address confounders in estimating the relationship between baseline vitamin-D status and change in CD4 cell count, we adjusted for the following factors measured at baseline: baseline body mass index (BMI) in four categories (≤0.5,18.5 < BMI ≤ 25, 25 < BMI < 30, and BMI ≥ 30 kg/m2), anemia (defined as < 12 g/dl for women and <13 g/dl for men) [16], behavioral factors (smoking, drinking, self-reported multivitamin and bed-net use at enrollment), socio-demographic characteristics (age at enrollment, sex, income, marital status, and self-reported smoking and drinking), allocation to supplementation with vitamins B, C and E vs placebo in the parent study, and time. We examined heterogeneity in the association between vitamin-D status and change in CD4+T-cell count by the following baseline factors: micronutrient intervention status, sex, age, BMI, and baseline ART status using differences in likelihood ratio tests between nested models to evaluate heterogeneity. When the P-value associated with difference in likelihood ratio test between nested models was <0.05, we stratified by levels of the effect modifier. All analyses were implemented in Statistical Analyses Software (SAS) version 9.3.
3. Results
Our sample included 398 patients with baseline vitamin-D data and repeated CD4+T-cell assessments. Half of the participants were antiretroviral treatment naïve at enrollment. Of the 50% on HAART at enrollment, the median duration on ART was 0.2 (interquartile range: 0–1.9) months with most on nevirapine (62.8%) or efavirenz (35.7%) containing HAART regimen. The majority of enrollees were female (73%), had elementary or lower level of education (54%) and were VDI or VDD at enrollment (77%). Most participants were of normal weight and there was no difference by vitamin-D status with respect to CD4, BMI or hemoglobin at baseline. Self-reported multivitamin use was higher, whereas prevalent ART exposure was lower, in VDS patients at enrollment. Further details of the study population are provided in Table 1.
Table 1.
Socio-demographic description of adult AIDS patients starting antiretroviral therapy in Kampala, Uganda.
| Overall N = 398 (100%) | Vitamin-D sufficient 90 (22.67%) | Vitamin-D insufficient 241 (60.5%) | Vitamin-D deficient 67 (16.88%) | P-value | |
|---|---|---|---|---|---|
| % Female | 279 (69.3) | 62 (68.9) | 170 (70.5) | 44 (65.7) | 0.742 |
| Age (in years) | |||||
| <=25 | 57 (14.32) | 12 (13.33) | 32 (13.28) | 13 (19.40) | 0.636 |
| 26–35 | 144 (36.18) | 33 (36.67) | 90 (37.34) | 21 (31.34) | |
| 36–45 | 140 (35.18) | 36 (40.00) | 81 (33.61) | 23 (34.33) | |
| 46+ | 57 (14.32) | 9 (10.00) | 38 (15.77) | 10 (14.93) | |
| BMI (kg/m2) | |||||
| <18.5 | 22 (5.5) | 6 (6.7) | 13 (5.4) | 3 (4.48) | 0.821 |
| 18.5 < BMI <25 | 262 (65.8) | 58 (64.4) | 155 (64.3) | 49 (73.1) | |
| 25 ≤ BMI < 30 | 71 (17.8) | 16 (17.8) | 44 (18.3) | 11 (16.4) | |
| BMI ≥ 30 | 43 (10.8) | 10 (11.1) | 29 (12.0) | 4 (6.0) | |
| Educational status | |||||
| <Elementary | 163 (41.06) | 39 (43.33) | 99 (41.08) | 25 (37.88) | 0.920 |
| Elementary | 54 (13.60) | 9 (10.00) | 34 (14.11) | 11 (16.67) | |
| Some ordinary level | 79 (19.90) | 17 (18.89) | 48 (19.92) | 14 (21.21) | |
| Ordinary level & higher | 101 (25.44) | 25 (27.78) | 60 (24.90) | 16 (24.24) | |
| % Anemia | 154 (38.69) | 39 (43.33) | 87 (36.10) | 28 (41.79) | 0.412 |
| CD4 (cells/μl) | |||||
| Less than ≤ 50 | 64 (16.08) | 19 (21.11) | 38 (15.77) | 7 (10.45) | 0.171 |
| >50, <=100 | 82 (20.60) | 21 (23.33) | 40 (16.60) | 21 (31.34) | |
| >100, <=150 | 71 (17.84) | 13 (14.44) | 49 (20.33) | 9 (13.43) | |
| >150, <=200 | 82 (20.60) | 16 (17.78) | 53 (21.99) | 13 (19.40) | |
| >200 | 99 (24.87) | 21 (23.33) | 61 (25.31) | 17 (25.37) | |
| Baseline own income | |||||
| No | 104 (26.13) | 19 (21.11) | 62 (25.73) | 23 (34.33) | 0.171 |
| Yes | 294 (73.87) | 71 (78.89) | 179 (74.27) | 44 (65.67) | |
| Marital status | |||||
| Single/never married | 41 (10.30) | 8 (8.89) | 23 (9.54) | 10 (14.93) | 0.796 |
| Married monogamously | 33 (8.29) | 9 (10.00) | 18 (7.47) | 6 (8.96) | |
| Married polygamously | 32 (8.04) | 6 (6.67) | 20 (8.30) | 6 (8.96) | |
| Cohabitating | 102 (25.63) | 27 (30.00) | 63 (26.14) | 12 (17.91) | |
| Divorced/separated | 123 (30.90) | 25 (27.78) | 74 (30.71) | 24 (35.82) | |
| Widowed | 67 (16.83) | 15 (16.67) | 43 (17.84) | 9 (13.43) | |
| Baseline multivitamin use | 88 (22.11) | 29 (32.22) | 46 (19.09) | 13 (19.40) | 0.032 |
| Randomized to single recommended daily allowance of vitamins B,C and E | 198 (49.8) | 40 (44.4) | 119 (49.4) | 39 (58.2) | 0.229 |
| Currently on HAART | 199 (50.00) | 37 (41.11) | 120 (48.79) | 42 (62.69) | 0.028 |
| Ever smoked | 69 (17.34) | 20 (22.22) | 39 (16.18) | 10 (14.93) | 0.369 |
| Current drinker | 81 (20.35) | 25 (27.78) | 47 (19.50) | 9 (13.43) | 0.076 |
BMI, body mass index.
Patients were followed-up for a median of 18 months with no difference in follow-up duration by vitamin-D status. CD4+T-cell count rose steadily from a baseline average of 150.5 cells/μl (standard deviation (SD) = 99.9 cells/μl) to the 18th-month cohort-wide average of 296.8 cells/μl (SD = 158.4). However, absolute CD4+T-cell values were persistently lower for patients that were VDD/VDI relative to HIV+ patients that were VDS at study entry (P-value = 0.010, Fig. 1). Specifically, the overall absolute increase in CD4+T-cell count from enrollment to 18 months was 109.1 cells/μl (SD = 17.5), 143.8 cells/μl (SD = 15.5) and 178 cells/μl (SD = 20.7), respectively, for participants who were VDD, VDI and VDS at enrollment. The mean and standard errors of absolute CD4 T-cell count by vitamin-D status at baseline and each follow-up interval is presented in Table S1. The difference in CD4+T-cell recovery at each follow-up interval for patients with VDD/VDI compared to those who were VDS at enrollment is shown in Table 2. In general, the absolute CD4+T-cells recovered declined dose-dependently and significantly, in all follow-up periods as baseline vitamin-D levels worsened (vitamin-D*time, P-value < 0.001) (Table 2).
Fig. 1.
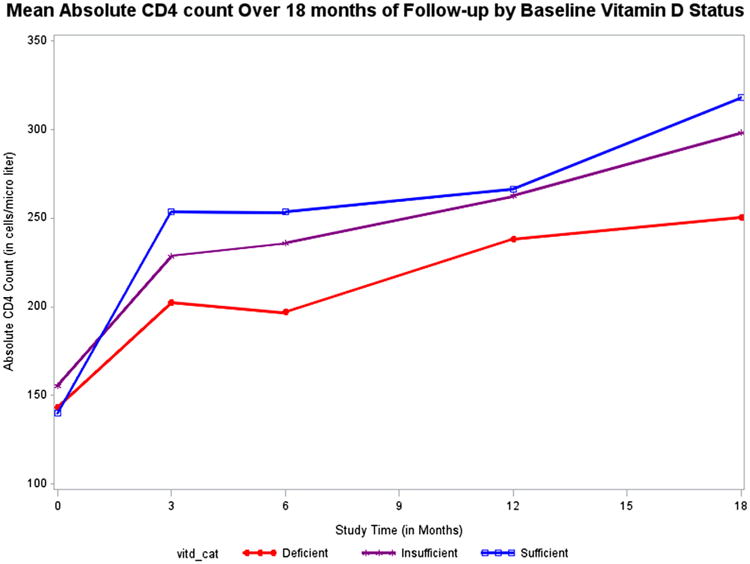
Average absolute CD4 cell counts at respective follow-up intervals by baseline vitamin-D status.
Table 2.
Differences in absolute CD4+T-cell count from enrollment to 18 months of follow-up in relation to baseline hypovitaminosis-D for adult human-immunodeficiency-virus-positive patients on highly active antiretroviral therapy.
| Vitamin-D-related contrast | Month 0 diff (99% CI) | Month 3 diff (99% CI) | Month 6 diff (99% CI) | Month 12 diff (99% CI) | Month 18 diff (99% CI) | P-valuea | |
|---|---|---|---|---|---|---|---|
| Crude modelb | Deficient vs sufficient | +3.3 (−35, 42) | −51.0 (−103, 1.2) | −55.6 (−100,-12) | −26.4 (−78.3, 25.5) | −66.6 (−128, −5.5) | <0.001 |
| Insufficient vs sufficient | +15.8 (−16, 21) | −24.8 (−73, 23) | −16.8 (−54.3, 20.8) | −2.4 (−43.8, 39.1) | -19.5 (-74.1, 35.2) | ||
| Sufficient (reference) | 140 (113, 167) | 254 (210, 297) | 253 (222, 286) | 266.3 (231, 301) | 318 (270, 365) | ||
| Multivariate modelc | Deficient vs sufficient | +3.7 (−36, 43) | −50.9 (−104, 2.5) | −55.2 (−99.3,-11) | −25.7 (−77, 26) | −65.2 (−125, −5.5) | <0.001 |
| Sufficient (reference) | +16.5 (−16, 49) | -23.6 (-64, 6.8) | −15.1 (−53,22.4) | −0.9 (−39.2, 23.0) | −17.7 (−71.1, 37) | ||
| Insufficient vs sufficient | 156 (117, 195) | 270.5 (218, 323) | 268.7 (226, 310) | 280.8 (237, 324) | 334.0 (281, 387) | ||
Analyses are implemented in SAS using linear mixed models SAS PROC MIXED to calculate differences and 95% confidence intervals (CI) for absolute CD4 cell counts at baseline and each follow-up period in relation to baseline vitamin-D-deficient or insufficient vs vitamin-D-sufficient patients. Average CD4 values and corresponding 99% confidence intervals are shown for the reference vitamin-D category. For hypovitaminosis D categories, mean difference in CD4 cell count and corresponding 99% CI relative to the vitamin-D-sufficient group is shown.
For vitamin-D status associated difference in trajectory of CD4+T-cell change during follow-up.
Crude Model adjusts for the following: Time + vitamin-D-category + vitamin-D-category *time; outcome = absolute CD4.
Multivariate model adjusted for the following covariates all measured at baseline: age, sex, ARV regimen, marital status, smoking history, alcohol use history, malaria diagnosis within 3 months of enrollment, daily bed-net use, baseline reported vitamin use, wealth index, educational status and perceived social standing.
The VDD/VDI-associated difference in absolute CD4+T-cells recovered varied significantly by age (age*vitamin-D*Time: P-value = 0.009) and BMI category (BMI*vitamin-D*time, P = 0.044) but not by randomization to multi-vitamins B,C and E vs placebo (supplementation*vitamin-D*time, P = 0.382; Table 3 and Figs. 2 and 3). The VDD/VDI-associated slower absolute CD4+T-cell recovery was similar for men and women (sex*vitamin-D*time, P = 0.522), persons with any vs no HAART experience at enrollment (ART status*vitamin-D*time, P = 0.949) and for persons that reported any vs no multivitamin use at enrollment (baseline multivitamin use*vitamin*time, P = 0.841; data not shown).
Table 3.
Baseline hypovitaminosis-D-related change in absolute CD4+T-cell recovery over 18 months among human-immunodeficiency-virus-infected Ugandan adults on highly active antiretroviral therapy within categories of age, micronutrient supplementation status and body mass index at enrollment.
| n/N | Month 0 | Month 3 | Month 6 | Month 12 | Month 18 | P-valuea | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||
| Difference (99% CI) | Difference (99% CI) | Difference (99% CI) | Difference (99% CI) | Difference (99% CI) | ||||
| Age categories Age*vitD*Time (P = 0.009) | Age ≤35 years | |||||||
| Vitamin-D deficient | 41/201 | +25.4 (−22.9, 73.7) | −58.3 (−134, 17.4) | −64.6 (− 127, −1.7) | −46.1 (−115, 23.3) | − 71.5 (−144, 1.74) | 0.002 | |
| Vitamin-D insufficient | 117/201 | +34.1 (−8.4, 76.6) | −33.8 (−106.6, 39) | −29.9 (−68.9, 12.3) | −3.3 (−62.3, 55.6) | − 18.7 (−84.9, 47.5) | ||
| Vitamin-D sufficient (ref.) | 43/201 | 125.9 (86.9, 164.9) | 260.4 (191.0, 329.8) | 259.2 (204.5, 313) | 266.8 (213, 320.9) | 310.9 (252.2, 369.5) | ||
| Age: ≥36 years | ||||||||
| Vitamin-D deficient | 26/197 | − 15.3 (−72, 42) | −42.0 (−98, 14.6) | −53.9 (− 106, −1.6) | −21.2 (−86, 43.1) | −74.1 (−157, 8.9) | 0.183 | |
| Vitamin-D insufficient | 124/197 | +0.9 (−41, 435) | − 14.3 (−63, 34) | +0.5 (−42, 43) | −0.6 (−47, 45) | −18.4 (−92, 51.6) | ||
| Vitamin-D sufficient (ref.) | 47/197 | 144.2 (104.4, 184) | 240 (197.7, 282.6) | 239 (203.1, 275.3) | 263 (225.1, 300.3 | 325 (261.9, 388.1) | ||
| Supplement Supplement* vitD*time (P = 0.382) | Placebo | |||||||
| Vitamin-D deficient | 28/199 | −24.8 (−80.4, 30.7) | − 79.6 (−144, −15.6) | −79.6 (− 145, −16) | −57.5 (−135, 20) | −104.8 (−180, −30) | 0.005 | |
| Vitamin-D insufficient | 122/199 | 7.3 (−37.9, 52.5) | −34.2 (−110, 42) | − 17.9 (−70, 34.2) | − 10.6 (−66.9, 45.8) | −34.2 (−103, 35) | ||
| Vitamin-D sufficient (ref.) | 50/199 | 144.5 (100.4, 189) | 261.1 (185.2, 337.1) | 260.4 (212, 308.8) | 273.8 (221, 326.6) | 325.0 (263.5, 386.6) | ||
| Vitamins B, C & E | ||||||||
| Vitamin-D deficient | 39/198 | +23.9 (−33, 80) | −28.7 (−89, 32) | −31.0 (−92, 30.4) | 3.9 (−64, 72) | −29.7 (−122, 63) | 0.065 | |
| Vitamin-D insufficient | 119/198 | +22.0 (−25, 69) | − 15.9 (−72, 40) | −6.2 (−58, 46) | 15.8 (−38, 70) | +6.2 (−577, 89) | ||
| Vitamin-D sufficient (ref.) | 40/198 | 149.0 (97.3, 200.7) | 256.0 (201.8, 314.1) | 249.5 (196, 302.9) | 260.3 (206.7, 314) | 315 (236.7, 394) | ||
| Body mass index (BMI) BMI cat*vitD*Time (P = 0.044) | BMI <25 kg/m2 | |||||||
| Vitamin-D deficient | 52/284 | 5.8 (39.6, 51.1) | 62.9 (124.1, 0.8) | 47.3 (95.8, 1.16) | 37.9 (91.9, 16.2) | 82.4 (150.0, 14.9) | 0.003 | |
| Vitamin-D insufficient | 168/284 | +7.8 (−30.8, 46.3) | −44.8 (−102.3, 13.4) | − 16.3 (−53.5, 20.9) | − 16.2 (−58.1, 25.7) | −36.3 (−95.6, 22.9) | ||
| Vitamin-D sufficient (ref.) | 64/284 | 136.7 (103, 171) | 266.4 (211, 321) | 242.7 (211, 275) | 264 (229, 299) | 323.8 (272, 376) | ||
| BMI ≥ 25 kg/m2 | ||||||||
| Vitamin-D deficient | 15/144 | +3.4 (65.9, 72.7) | −30.3 (−112.6, 52.0) | −31.4 (− 111.4, 48.5) | +53.3 (−39.7146.3) | +33.7 (−71.4, 138.8) | 0.05 | |
| Vitamin-D insufficient | 73/144 | +22.6 (−32.0, 77.1) | +3.9 (−59.4, 67.2) | +11.7 (−61.7, 85.1) | +65.6 (−5.4, 136.7) | +63.8 (−35.4, 162.9) | ||
| Vitamin-D sufficient (ref.) | 26/144 | 142 (93.5, 190.4) | 223 (178.5, 267.6) | 258 (197.2318.5) | 243 (186, 300) | 276.2 (200, 352) | ||
Analyses are implemented in SAS using linear mixed models SAS PROC MIXED to calculate differences and 95% confidence intervals (CI) for absolute CD4 cell counts at baseline and each follow-up period in relation to baseline vitamin-D deficient or insufficient vs vitamin-D sufficient patients by stratum of age, vitamin B, C, E or placebo. Results are from multivariable model adjusted for the following covariates all measured at baseline: age, sex, antiretroviral (ARV) regimen, marital status, smoking history, alcohol use history, malaria diagnosis within 3 months of enrollment, daily bed net use, baseline reported vitamin use, wealth index, educational status and perceived social standing.
For vitamin-D-status-associated difference in trajectory of CD4+T-cell change during follow-up; ref. = reference; n = number in vitamin-D category; N = total in stratum.
Fig. 2.
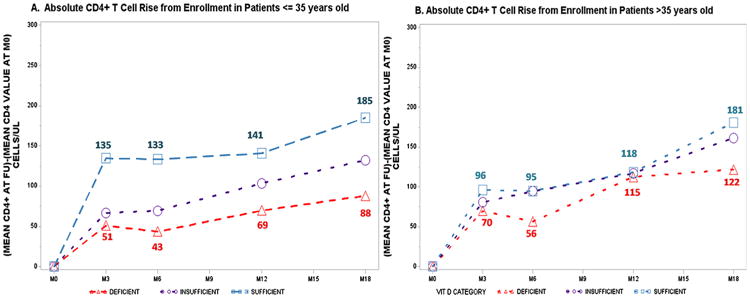
Age variation in absolute CD4+T-cell recovery post highly active antiretroviral therapy initiation.
Fig. 3.
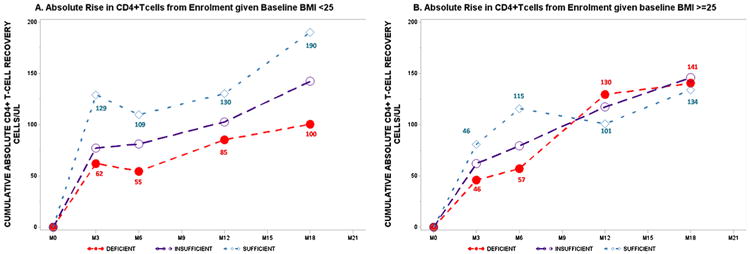
Body mass index variation in vitamin-D-related absolute rate of CD4+T-cell recovery post highly active antiretroviral therapy initiation.
In age-stratified analyses, the VDI/VDD-associated lower absolute CD4+T-cell recovery was strongest for participants 35 years old or younger among whom VDS patients had nearly 100 cells/ml CD4+T-cell advantage compared to VDD patients by month 18 (Fig. 2A). Among patients 36 years or older, the absolute number of CD4+T-cells recovered (Fig. 2B) did not differ by baseline vitamin-D status. However, the trend of lower CD4+T-cell recovery for VDD vs VDS patients was maintained and attained statistical significance in one of five follow-up intervals (Table 3, Fig. 2B).
Among vitamins B, C and E supplemented patients, those VDS vs VDD continued to have relative advantage with respect to increase in CD4+T-cells. However, the baseline VDD-associated absolute change in CD4+T-cell was muted and the overall vitamin-D-associated change in CD4 was statistically insignificant (vitamin-D*time, P-value = 0.065). Among patients randomized to placebo on the other hand, the impact of baseline VDD on change in CD4+T-cell count over 18 months was strong (vitamin-D*time, P-value = 0.005). The magnitude of CD4+T-cells recovered for VDS vs deficient patients in the placebo group is at least 2.5-times higher in comparison to the same contrast at comparable follow-up periods for patients randomized to micronutrient supplementation with vitamins B, C and E (Table 3).
The overall trend of VDD-related deficits in CD4+T-cell recovery is maintained among participants with BMI <25 kg/m2 at enrollment (vitamin-D*time, P-value = 0.003) with statistical significance attained in three of four follow-up intervals (Table 3). Among patients who were obese or overweight at enrollment, baseline vitamin-D-dependent change in CD4 cell count during follow-up was also evident (vitamin-D*time, P-value = 0.050). Within respective follow-up intervals, statistical significance was not attained for any contrast. Of note, the magnitude of CD4 recovery at months 3 and 6 were lower for patients who were VDD vs VDS at enrollment. Between months 12 and 18, however, average CD4 T-cell count was higher for VDD vs VDS; VDI participants on the other hand maintained higher mean CD4 cell counts in all intervals relative to VDS patients (Table 3). This trend of superior CD4+T-cell gain for baseline VDS compared to VDD patients was maintained among patients of baseline BMI < 25 kg/m2 with the absolute advantage in number of CD4+T-cells gained ranging from a minimum of 38 cells/μl at month 12 to a maximum of 82 cells/μl at month 18 (Fig. 3A). In patients overweight or obese at study entry, an early non-significant CD4+T-cell recovery advantage for VDS vs VDD enrollees was evident until month 6 with a reversal in absolute CD4 recovered evident by months 12 and 18 for overweight or obese VDS vs VDD patients (Fig. 3B).
4. Discussion
In this sample of HIV-positive adults, nearly eight out of 10 participants began HAART either VDD or VDI. The prevalence of hypovitaminosis-D observed in this study is comparable to the 72–75% previously reported prevalence in HIV-infected samples from South Africa and Malawi [1,2] but is considerably higher than the 37–57% prevalence previously reported for Uganda and other African settings [3–5]. In this study, the average CD4+T-cell values were comparable by vitamin-D status at enrollment. By Month 18, average CD4+T-cell counts doubled from a baseline average of 150 cells/μl to 300 cells/μl as patients either continued or were initiated on HAART from enrollment. In line with our study hypothesis, the absolute magnitude of CD4+T-cells recovered was persistently lower for baseline VDD and VDI in comparison with baseline VDS patients. This VDD-associated lower absolute CD4+T-cell recovery varied by age and nutritional status with the absolute difference in CD4+T-cell recovery most pronounced among patients 35 years or younger and those with BMI less than 25 kg/m2 at enrollment. Among patients overweight or obese at enrollment, baseline VDD status was variably associated with absolute CD4+T-cell gain during follow-up in comparison with HIV+ overweight or obese patients VDS at enrollment. Randomization to concurrent supplementation to vitamins B, C and E vs placebo did not independently predict CD4+T-cell recovery. However, the receipt of these micronutrients moderated, but did not eliminate, VDD-associated slower absolute CD4+T-cell recovery rate during follow-up.
Only a few epidemiologic studies (all observational with equivocal and inconsistent results), have evaluated associations between vitamin-D levels and CD4+T-cell count in HIV-infected adults [5,12,17–21]. Our finding of VDD-associated slower CD4+T-cell recovery is inconsistent with a previously reported lack of association between vitamin-D and CD4+T-cell recovery among HIV-infected African adults [5,17], and with a longitudinal study of an ethnically diverse sample of HIV-positive patients from The Netherlands [21]. Similarly, our study findings do not support the results from one longitudinal study [21] and two cross-sectional studies that found no association between VDD and CD4+T-cell levels in HIV-infected adults from Uganda [17] and the United States [19]. The exact reason for this divergence in results—particularly for the prospective studies including African HIV-positive results—is unclear. We speculate that sample variation by CD4 in baseline vitamin-D levels, HIV disease stage, local dietary patterns and ability to rigorously control for important confounders could partially contribute to these differences.
However, our finding of VDD-associated lower absolute CD4 recovery is similar to cross-sectional reports of VDD-associated severe immune deficiency among French HIV-infected adults [18] and directly corroborates findings from one longitudinal multinational [14] study and two prospective studies in HIV-infected adults from the United States [12,20]. In the multi-national study, VDD/VDI was associated with incidence of AIDS-defining events, virologic failure and death over 96 weeks of follow-up. They found associations-albeit not-statistically significant, between VDI/VDD and elevated risk of immunologic failure. Among 204 women in the Interagency HIV study, VDI was associated with 80% lower likelihood of high CD4+T-cell recovery after 2 years on ART [20] and higher baseline vitamin-D levels were associated with greater absolute increases in CD4+T-cell values during follow-up of 156 adults stable on HAART for at least 24 weeks at enrollment in another study [12]. Our larger sample size, comparable baseline CD4 values across vitamin-D categories, prospective follow-up design, the stability and dose-dependence of CD4 T-cell recovery according to the severity of hypovitaminosis-D, and the fact that our associations were robust to adjustment for multiple confounders are important strengths suggestive of VDD as a component cause of impaired CD4T + cell recovery in this sample. In spite of these strengths, we are unable to exclude the possibility of residual confounding by unmeasured factors given our observational design. Of note, vitamin-D status was assessed at baseline only and may evolve significantly once HIV-positive patients are started on HAART. The extra-skeletal impacts of vitamin-D including its role in maintaining immune system homeostasis through enhancement of innate immune responses, regulation of inflammatory cascade, direct impact on T-cell activation, and its capacity to modify the phenotype and function of antigen-presenting cells have been noted in the context of infectious and chronic morbidities [22]. In spite of the existing biologically plausible mechanisms described, whether vitamin-D deficiency further impinges on the general state of immune vulnerability in HIV, is predominantly a manifestation of HIV-related pathogenesis or both are important but yet unresolved questions. Therefore, specifically designed longitudinal and intervention studies in well-characterized populations with repeated vitamin-D assessment are needed to clarify the impact of vitamin-D status and its variation during HAART on CD4+T-cell recovery.
Our study provides new information regarding age and nutritional status as potential modifiers of any adverse VDD-related impact on immune recovery. The observations that VDD was associated with more dysregulation of absolute CD4+T-cell recovery in younger patients, and that the absolute amount and speed of improvement differed by pre-treatment nutritional status are similar to prior reports of age and BMI-related differences in immune recovery among HIV and tuberculosis co-infected Ugandan adults [23]. These age and nutritional status interactions in VDD-/VDI-associated lower CD4+T-cell recovery suggests that previously reported age-related immune recovery advantage in younger HIV-infected adults [24] maybe compromised by malnutrition [25,26]. The observation that the VDD-/VDI-associated slower rate of immune recovery was particularly strong in the absence of vitamins B, C and E supplementation suggests that the adverse impact of vitamin-D deficiency on absolute CD4 recovery could be partly moderated by repletion with these micronutrients as previously reported [27–30]. Overall, the presence of these age- and nutritional-status-related heterogeneities in VDD vs VDS association with immune recovery suggests that any future interventions to mitigate potentially adverse effects of hypovitaminosis-D could be enhanced by targeting participants who are 35 years old or younger and those who are normal or underweight at enrollment.
In summary, we report for the first time, a strong VDD-associated deficit in the absolute number of CD4+T-cells recovered over 18 months' follow-up in HIV-infected African adults on HAART. This relationship was strongest among participants 35 years old or younger and those who were underweight or normal weight at enrollment. Supplementation with vitamins B, C and E partially mitigated but did not eliminate these VDD-associated disadvantages in immune recovery. Supplementation with vitamin-D may improve immune recovery in HIV-infected adults on HAART. Multi-micronutrient cocktails including vitamins D, B, C and E may provide additive benefits. The safety and efficacy of such interventions should be evaluated in future randomized placebo controlled trials.
Supplementary Material
Acknowledgments
Disclaimer: We are grateful to the research participants who volunteered to take part in this trial. The authors also acknowledge the support provided by all the Infectious Diseases Institute staff who contributed in various ways to provide support to enable the smooth conduct of this trial. Research findings reported in this publication were supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD060333. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
Appendix A. Supplementary data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.clnu.2015.08.007.
References
- 1.Jarvis JN, Bicanic T, Loyse A, Meintjes G, Hogan L, Roberts CH, et al. Very low levels of 25-Hydroxyvitamin D are not associated with immunologic changes or clinical outcome in South African patients with HIV-associated Cryptococcal Meningitis. Clin Infect Dis. 2014 Aug 15;59(4):493–500. doi: 10.1093/cid/ciu349. http://dx.doi.org/10.1093/cid/ciu349. Epub 2014 May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banda R, Mhemedi B, Allain TJ. Prevalence of vitamin D deficiency in adult tuberculosis patients at a central hospital in Malawi. Int J Tuberc Lung Dis. 2011;15:408–10. [PubMed] [Google Scholar]
- 3.Conesa-Botella A, Goovaerts O, Massinga-Loembe M, Worodria W, Mazakpwe D, Luzinda K, et al. Low prevalence of vitamin D deficiency in Ugandan HIV-infected patients with and without tuberculosis. Int J Tuberc Lung Dis. 2012;16:1517–21. doi: 10.5588/ijtld.11.0146. [DOI] [PubMed] [Google Scholar]
- 4.Mastala Y, Nyangulu P, Banda RV, Mhemedi B, White SA, Allain TJ. Vitamin D deficiency in medical patients at a central hospital in Malawi: a comparison with TB patients from a previous study. PLoS One. 2013;8:e59017. doi: 10.1371/journal.pone.0059017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudfeld CR, Wang M, Aboud S, Giovannucci EL, Mugusi FM, Fawzi WW. Vitamin D and HIV progression among Tanzanian adults initiating antiretroviral therapy. PLoS One. 2012;7:e40036. doi: 10.1371/journal.pone.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev. 2006;64:226–33. doi: 10.1301/nr.2006.may.226-233. [DOI] [PubMed] [Google Scholar]
- 7.Lake JE, Adams JS. Vitamin D in HIV-infected patients. Curr HIV/AIDS Rep. 2011;8:133–41. doi: 10.1007/s11904-011-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havers FP, Detrick B, Cardoso SW, Berendes S, Lama JR, Sugandhavesa P, et al. Change in vitamin d levels occurs early after antiretroviral therapy initiation and depends on treatment regimen in resource-limited settings. PLoS One. 2014;9:e95164. doi: 10.1371/journal.pone.0095164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11:344–9. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 10.Viard JP, Souberbielle JC, Kirk O, Reekie J, Knysz B, Losso M, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25:1305–15. doi: 10.1097/QAD.0b013e328347f6f7. [DOI] [PubMed] [Google Scholar]
- 11.Mehta S, Mugusi FM, Spiegelman D, Villamor E, Finkelstein JL, Hertzmark E, et al. Vitamin D status and its association with morbidity including wasting and opportunistic illnesses in HIV-infected women in Tanzania. AIDS Patient Care STDS. 2011;25:579–85. doi: 10.1089/apc.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross AC, Judd S, Kumari M, Hileman C, Storer N, Labbato D, et al. Vitamin D is linked to carotid intima-media thickness and immune reconstitution in HIV-positive individuals. Antivir Ther. 2011;16:555–63. doi: 10.3851/IMP1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakalia S, Sochett EB, Stephens D, Assor E, Read SE, Bitnun A. Vitamin D supplementation and CD4 count in children infected with human immunodeficiency virus. J Pediatr. 2011;159:951–7. doi: 10.1016/j.jpeds.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Havers F, Smeaton L, Gupte N, Detrick B, Bollinger RC, Hakim J, et al. 25-Hydroxyvitamin D insufficiency and deficiency is associated with HIV disease progression and virological failure post-antiretroviral therapy initiation in diverse multinational settings. J Infect Dis. 2014;210:244–53. doi: 10.1093/infdis/jiu259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guwatudde D, Ezeamama AE, Bagenda D, Kyeyune R, Wabwire-Mangen F, Wamani H, et al. Multivitamin supplementation in HIV infected adults initiating antiretroviral therapy in Uganda: the protocol for a randomized double blinded placebo controlled efficacy trial. BMC Infect Dis. 2012;12:304. doi: 10.1186/1471-2334-12-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Department of Nutrition for Health and Development VaMNIS. Geneva: World Health Organization; 2011. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. [Google Scholar]
- 17.Nansera D, Graziano FM, Friedman DJ, Bobbs MK, Jones AN, Hansen KE. Vitamin D and calcium levels in Ugandan adults with human immunodeficiency virus and tuberculosis. Int J Tuberc Lung Dis. 2011;15:1522–7. i. doi: 10.5588/ijtld.10.0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legeai C, Vigouroux C, Souberbielle JC, Bouchaud O, Boufassa F, Bastard JP, et al. Associations between 25-hydroxyvitamin D and immunologic, metabolic, inflammatory markers in treatment-naive HIV-infected persons: the ANRS CO9 ≪COPANA≫ cohort study. PLoS One. 2013;8:e74868. doi: 10.1371/journal.pone.0074868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bearden A, Abad C, Gangnon R, Sosman JM, Binkley N, Safdar N. Cross-sectional study of vitamin D levels, immunologic and virologic outcomes in HIV-infected adults. J Clin Endocrinol Metab. 2013;98:1726–33. doi: 10.1210/jc.2012-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aziz M, Livak B, Burke-Miller J, French AL, Glesby MJ, Sharma A, et al. Vitamin D insufficiency may impair CD4 recovery among women's inter-agency HIV Study participants with advanced disease on HAART. AIDS. 2013;27:573–8. doi: 10.1097/QAD.0b013e32835b9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Den Bout-Van Den Beukel CJ, Fievez L, Michels M, Sweep FC, Hermus AR, Bosch ME, et al. Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: effects of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:1375–82. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- 22.Lang PO, Aspinall R. Can we translate vitamin D immunomodulating effect on innate and adaptive immunity to vaccine response? Nutrients. 2015;7:2044–60. doi: 10.3390/nu7032044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezeamama AE, Mupere E, Oloya J, Martinez L, Kakaire R, Yin X, et al. Age, sex, and nutritional status modify the CD4+ T-cell recovery rate in HIV-tuberculosis co-infected patients on combination antiretroviral therapy. Int J Infect Dis. 2015;35:73–9. doi: 10.1016/j.ijid.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gui J, Mustachio LM, Su DM, Craig RW. Thymus size and age-related thymic involution: early programming, sexual dimorphism, progenitors and stroma. Aging Dis. 2012;3:280–90. [PMC free article] [PubMed] [Google Scholar]
- 25.Marcos A, Nova E, Montero A. Changes in the immune system are conditioned by nutrition. Eur J Clin Nutr. 2003;57(Suppl. 1):S66–9. doi: 10.1038/sj.ejcn.1601819. [DOI] [PubMed] [Google Scholar]
- 26.Keith ME, Jeejeebhoy KN. Immunonutrition. Baillieres Clin Endocrinol Metab. 1997;11:709–38. doi: 10.1016/s0950-351x(97)80990-1. [DOI] [PubMed] [Google Scholar]
- 27.Papparella I, Ceolotto G, Berto L, Cavalli M, Bova S, Cargnelli G, et al. Vitamin C prevents zidovudine-induced NAD(P)H oxidase activation and hypertension in the rat. Cardiovasc Res. 2007;73:432–8. doi: 10.1016/j.cardiores.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Munteanu A, Ricciarelli R, Zingg JM. HIV protease inhibitors-induced atherosclerosis: prevention by alpha-tocopherol. IUBMB Life. 2004;56:629–31. doi: 10.1080/15216540400020387. [DOI] [PubMed] [Google Scholar]
- 29.Abrams B, Duncan D, Hertz-Picciotto I. A prospective study of dietary intake and acquired immune deficiency syndrome in HIV-seropositive homosexual men. J Acquir Immune Defic Syndr. 1993;6:949–58. [PubMed] [Google Scholar]
- 30.Tang AM, Graham NM, Kirby AJ, McCall LD, Willett WC, Saah AJ. Dietary micronutrient intake and risk of progression to acquired immunodeficiency syndrome (AIDS) in human immunodeficiency virus type 1 (HIV-1)-infected homosexual men. Am J Epidemiol. 1993;138:937–51. doi: 10.1093/oxfordjournals.aje.a116814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


