Abstract
Many viruses modulate calcium (Ca2+) signaling to create a cellular environment that is more permissive to viral replication, but for most viruses that regulate Ca2+ signaling, the mechanism underlying this regulation is not well understood. The hepatitis B virus (HBV) HBx protein modulates cytosolic Ca2+ levels to stimulate HBV replication in some liver cell lines. A chronic HBV infection is associated with life-threatening liver diseases, including hepatocellular carcinoma (HCC), and HBx modulation of cytosolic Ca2+ levels could have an important role in HBV pathogenesis. Whether HBx affects cytosolic Ca2+ in a normal hepatocyte, the natural site of an HBV infection, has not been addressed. Here, we report that HBx alters cytosolic Ca2+ signaling in cultured primary hepatocytes. We used single cell Ca2+ imaging of cultured primary rat hepatocytes to demonstrate that HBx elevates the cytosolic Ca2+ level in hepatocytes following an IP3-linked Ca2+ response; HBx effects were similar when expressed alone or in the context of replicating HBV. HBx elevation of the cytosolic Ca2+ level required extracellular Ca2+ influx and store-operated Ca2+ (SOC) entry and stimulated HBV replication in hepatocytes. We used both targeted RT-qPCR and transcriptome-wide RNAseq analyses to compare levels of SOC channel components and other Ca2+ signaling regulators in HBV-expressing and control hepatocytes and show that the transcript levels of these various proteins are not affected by HBV. We also show that HBx regulation of SOC-regulated Ca2+ accumulation is likely the consequence of HBV modulation of a SOC channel regulatory mechanism. In support of this, we link HBx enhancement of SOC-regulated Ca2+ accumulation to Ca2+ uptake by mitochondria and demonstrate that HBx stimulates mitochondrial Ca2+ uptake in primary hepatocytes. The results of our study may provide insights into viral mechanisms that affect Ca2+ signaling to regulate viral replication and virus-associated diseases.
Introduction
Viruses are obligate intracellular parasites, and many viruses have developed methods to subvert host cell signal transduction pathways and factors to support their own replication and survival [1]. Ca2+ is a universal and versatile intracellular second messenger, and Ca2+ signaling affects almost every cellular process, ranging from fertilization to cell proliferation and cell death [2–5]. The amplitude, frequency, and spatiotemporal patterning of an intracellular Ca2+ signal can elicit differential modulation of Ca2+-binding proteins and Ca2+-dependent effectors, thereby regulating numerous cellular responses and functions [5, 6]. Not surprisingly, many pathogens, including a large number of DNA and RNA viruses, encode proteins that alter normal cellular Ca2+ signaling; for viruses, altered Ca2+ signaling typically enhances viral replication [6, 7]. Due to the versatile nature of a Ca2+ signal, modulation of intracellular Ca2+ signaling is an ideal mechanism for viruses to create a cellular environment that is permissive to viral replication. Although Ca2+ signaling is a common target of many viruses, for most of these viruses, the mechanisms that underlie viral-mediated regulation of Ca2+ levels and signals remain undefined.
Intracellular Ca2+ signaling is extremely dynamic and tightly controlled. The cytosolic Ca2+ level ([Ca2+]c) is normally maintained at approximately 100 nM, and subtle changes in [Ca2+]c can have major effects within a cell [2, 3]. In non-excitable cells, such as hepatocytes, increases in [Ca2+]c mainly stem from two sources: Ca2+ release through IP3 receptors (IP3R) on the endoplasmic reticulum (ER), the major intracellular Ca2+ store, and Ca2+ influx from outside the cell. Excess Ca2+ is removed from the cytosol by ATPases such as plasma membrane (PM) Ca2+-ATPases (PMCA), which pumps Ca2+ out of the cell and into the extracellular matrix, and sarcoplasmic reticulum Ca2+-ATPases (SERCA), which pump Ca2+ from the cytosol into the ER [2, 3, 8]. Deregulation of [Ca2+]c and/or cytosolic Ca2+ signaling can affect development and progression of many diseases, including heart disease, schizophrenia, bipolar disorder, and Alzheimer’s disease. Moreover, disruption of Ca2+ signaling has been linked to cancer initiation, progression, metastasis, invasion, and tumor-associated angiogenesis [2, 5, 9]. Altered [Ca2+]c has also been implicated in the proliferation of human hepatoma cells [10, 11], and modified expression of Ca2+ signaling regulators has been associated with the development of hepatocellular carcinoma (HCC) [12]. Interestingly, altered Ca2+ signaling and elevated [Ca2+]c has been observed in cells with replicating hepatitis B virus (HBV) and hepatitis C virus (HCV); these viruses are currently the most common causes of HCC. These observations provide support for the notion that viral modulation of cellular Ca2+ signaling pathways could directly influence the development of viral-associated diseases.
Globally, approximately 350 million people are chronically infected with HBV. HBV infection is associated with life-threatening liver diseases, including cirrhosis and HCC [13–15]. HBV is a member of the Hepadnaviridae family, and its genome is a small, partially double-stranded DNA of 3.2 kb in length containing four overlapping open reading frames that encode just seven proteins [15]. The smallest HBV protein, HBx, is the main viral regulatory protein and stimulates viral replication both in vitro and in vivo [16–22]. HBx is localized mainly to the cytoplasm and nucleus of cells, with a small fraction present on the outer mitochondrial membrane (OMM), where HBx also interacts with the voltage-dependent anion channel (VDAC) [23–25]. HBx is thought to play a significant role in HBV pathogenesis, and many HBx activities, including its stimulation of viral replication and modulation of cell proliferation, apoptosis pathways, transcription, and other cell signaling pathways, are dependent on Ca2+ signaling [26].
The results of previously published studies have demonstrated that HBx expression causes an increase in the basal [Ca2+]c in some established cell lines [27, 28] and that cytosolic Ca2+ signaling is required for several steps in HBV replication in these cells, including capsid assembly, activation of the HBV polymerase, and replication of the HBV genome [29–32]. While some studies have identified activities of the HBx protein that are Ca2+-dependent in normal hepatocytes [30, 31, 33], the natural site of an HBV infection, whether HBx directly elevates [Ca2+]c in normal hepatocytes has not been assessed. We previously demonstrated that HBx enhances extracellular Ca2+ influx and mitochondrial Ca2+ uptake in human hepatoblastoma HepG2 cells [34]; however, HBx activities can have cell-specific consequences, necessitating a direct analysis of HBx effects on Ca2+ levels in normal hepatocytes [26, 35] For studies reported here, we have used cultured primary rat hepatocytes as a model system of normal hepatocytes; we previously showed that cultured primary rat hepatocytes can serve as a surrogate model for analyzing HBV effects in primary human hepatocytes [36, 37]. We now show that HBV, via HBx, increases the plateau [Ca2+]c in cultured primary rat hepatocytes following induction of an IP3-linked Ca2+ response and that HBx elevation of [Ca2+]c stimulates HBV replication in normal hepatocytes. We used both targeted and transcriptome-wide approaches to compare expression levels of store-operated Ca2+ (SOC) channel components and other Ca2+ signaling factors in HBV-expressing and control primary hepatocytes. HBV had no effect on the transcript level of any SOC channel component or Ca2+-signaling regulator that was detected in our analyses. Instead, we directly link HBx elevation of [Ca2+]c and HBx stimulation of HBV replication in hepatocytes to increased Ca2+ entry into hepatocytes through mitochondrial regulation of SOC entry (SOCE). Cumulatively, the results of our studies suggest that HBV (and HBx) does not affect the expression level or activity of SOC channel components but does increase, and likely prolong, Ca2+ entry through SOC channels to stimulate HBV replication in normal hepatocytes. The results from this study contribute to our current understanding of mechanisms that regulate HBV replication in normal hepatocytes and HBx effects that could influence the development of HBV-associated HCC. These studies may also provide insights into mechanisms that other viruses might employ to alter [Ca2+]c and Ca2+ signaling to enhance their own replication.
Materials and methods
Animal studies
Surgery and isolation of hepatocytes from rats, and this study, was approved by the Institutional Animal Care and Use Committee of Drexel University College of Medicine and complied with the Animal Welfare Act, the Policy on Humane Care and Use of Laboratory Animal, and the NIH Guide for the Care and Use of Laboratory Animals (2011).
Isolation and maintenance of cultured primary rat hepatocytes
Hepatocytes were isolated from male Sprague-Dawley rats by a two-step perfusion method, as previously described [100]. Cells were plated on collagen-coated tissue culture plates or slides (see below) and maintained in Williams E medium supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, 4 μg/ml insulin-transferrin-selenium, 5 μg/ml hydrocortisone, and 5 ng/ml epidermal growth factor at 37°C in 5% CO2. Hepatocytes were monitored for maintenance of hepatocyte morphology and expression of hepatocyte-specific mRNAs (see below) throughout the time course of our experiments.
Confirmation of differentiated hepatocytes
Levels of albumin, transferrin, and hepatocyte nuclear factor 4 alpha (HNF4α) mRNAs were monitored as markers of differentiated rat hepatocytes [38, 40]. RNA was isolated from rat hepatocytes immediately following isolation (0 hr) and following 48 hours of culture on collagen-coated glass coverslips (48 hr) using Trizol (Invitrogen), according to manufacturer’s protocols. RNA was treated with DNase and then converted to cDNA using M-MuLV reverse transcriptase (New England BioLabs, Inc). qPCR was performed with Power SYBR Green PCR master mix (Invitrogen), according to manufacturer’s instructions. Plasmids encoding gene portions for albumin, transferrin, and HNF4α (see below) were used to generate a standard curve for each target. These standard curves enabled us to convert Ct values to cDNA copy numbers, which we used in our comparisons. Levels of connexin 43, a marker present in other types of liver cells, including liver sinusoidal endothelial cells (LSECs), and in dedifferentiated hepatocytes, but not in differentiated hepatocytes [39], were also monitored; the absence of this marker in our hepatocytes confirmed both the purity of our preparation and the differentiated status of our cells. We also isolated RNA from primary rat LSECs and generated cDNA as described above. qPCR was performed on cDNA generated from LSECs and from hepatocytes at 0 hr and 48 hr for levels of connexin 43. A plasmid encoding a gene portion for connexin 43 (see below) was used to generate a standard curve, which enabled us to convert Ct values to cDNA copy numbers, which we used in our comparisons. Primers used for each target are listed in Table 1.
Table 1. Primer list.
| Primer | Sequence | ||
|---|---|---|---|
| Albumin-F | 5'- | AAA GCA CTG GTC GCA GCT GTC CG | -3' |
| Albumin-R | 5'- | TCG CTG GCT CAT ACG AGC TAC TGC C | -3' |
| HNF4a-F | 5'- | AGT GCT GCC TTG GAC CCA GCC T | -3' |
| HNF4a-R | 5'- | GGC ACA CAG GGC ACT GAC ACC C | -3' |
| Transferrin-F | 5'- | TTA CGG GTG CCC CCA AGG ATG GAC T | -3' |
| Transferrin-R | 5'- | ATT TCA CTG GCG CGC TGT CGA TGG | -3' |
| Connexin43 -F | 5'- | GAG GTG CCC AGA CAT GGG T | -3' |
| Connexin43-R | 5'- | AGC ACT GAC AGC CAC ACC T | -3' |
| Stim1-F | 5'- | GCC ACA GCA TGG CCT GGG | -3' |
| Stim1-R | 5'- | CCA GGA TTG TCT TCT TGG CC | -3' |
| Stim2-F | 5'- | AAT GCT GCT CTT CGG GCT GT | -3' |
| Stim2-R | 5'- | GCA TGG TGG ACT CAG AGA CAT | -3' |
| Orai1-F | 5'- | GCC TGG TCT TTA TCG TCT TTG CCG | -3' |
| Orai1-R | 5'- | CCT CTG TGG TCC ACG TGG TCC | -3' |
| Orai2-F | 5'- | GGA TTA CCG AGA CTG GGT CC | -3' |
| Orai2-R | 5'- | GGC TGA GGG TAC TGG TAC TTG | -3' |
| Orai3-F | 5'- | TAC CTC GAC CTT ATG GGG GC | -3' |
| Orai3-R | 5'- | TGC AGG CAC TAA ATG CCA CT | -3' |
| AldoB-F | 5'- | AGG TGC CCC GCT TGC AGG AAC | -3' |
| AldoB-R | 5'- | GCT GGC GTA GCG AGC CAG AGC | -3' |
| β-actin-F | 5'- | TGG CGT AGC GAG CCA GAG C | -3' |
| β-actin-R | 5'- | GGC CCA CGA TGG AGG GGA AG | -3' |
Plasmids and transfections
Plasmids encoding gene portions for albumin, transferrin, HNF4α, and connexin 43 were constructed as follows: a portion of each gene was PCR amplified and cloned into the pcDNA3.1- vector. Primary rat hepatocytes were transfected using Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions. Full-length HBx, containing N-terminally Flag-tagged HBx cloned into the pcDNA3.1(-) vector (pcDNAHBx), has been previously described [25]. pGEMHBV and pGEMHBV*7 have been previously described [41–43]. Briefly, pGEMHBV*7 is identical to pGEMHBV except that a point mutation in the codon for the seventh amino acid of HBx generates a stop codon, preventing HBx expression [42].
Reagents
Fura-4F/acetoxymethyl ester (Fura-4F/AM), pluronic acid, and ionomycin were purchased from Invitrogen; ATP was purchased from Amresco; 2-aminoethyldiphenyl borate (APB) was purchased from Cayman Chemical; vasopressin (Vp) was purchased from Calbiochem; lanthanide (La3+) and sulfobromophthalein (BSP) were purchased from Sigma; and thapsigargin (TG) was purchased from Acros Organics.
Antibodies
The anti-HBV core (HBcAg) antibody was purchased from Dako-Cytomation, the anti-ß actin antibody was purchased from Sigma, the anti-HBx antibody was purchased from ViroStat, Inc, and the antibodies for Stim1 and Stim2 were purchased from Cell Signaling.
Single cell [Ca2+]c measurements
Hepatocytes were plated on collagen-coated 15 mm glass coverslips immediately following isolation. 24 hours post plating, hepatocytes were transfected with 1 μg control vector (pGEM or pGEMHBV*7 or pcDNA3.1(-)) or 1 μg HBx expression vector (pGEMHBV or pcDNAHBx) and 0.2 μg dsRED. 24 hours post transfection, cells were washed twice with HEPES-buffered balanced salt solution (HBSS) (121 mM NaCl, 5 mM NaHCO3, 25 mM HEPES, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KPO4, 2 mM CaCl2, 10 mM glucose, 0.25% [w/v] bovine serum albumin [BSA], 200 μM BSP, pH 7.4). Hepatocytes were loaded with 5 μM Fura-4F-AM in HBSS supplemented with 0.02% pluronic acid for 40 min in 37°C shaker at 100 rpm, washed twice with HBSS, and then imaged using an Olympus 1X71 inverted microscope equipped with a 20X objective used for Fura imaging and a cooled charged-coupled-device camera. HBx-expressing and control cells were identified by dsRED expression and selected for [Ca2+]c measurements. To initiate Ca2+ release from the ER, 100 μM ATP or 100 nM Vp was added to the imaging buffer. Cytosolic Ca2+ signals were monitored by alternately exciting the dye at 340 nm and 380 nm and collecting emission at 510 nm with MetaFluor fluorescence ratio imaging software (Molecular Devices, Downingtown, PA). Fura-4F fluorescence images were recorded every 2 seconds, and the change in [Ca2+]c was calculated as the change in the Fura-4F 340/380 nm ratio. Following each experiment, auto-fluorescence was estimated by treating cells with 10 μM ionomycin and 20 mM MnCl2 in Ca2+-free ECM to quench the Fura signal. Any remaining signals at 340 or 380 nm were subtracted prior to calculating the 340/380 nm ratio.
SOCE measurements
To image SOCE, hepatocytes were plated, transfected, and loaded with Fura-4F, as described above. Following loading, hepatocytes were washed and imaged in Ca2+-free HBSS. Cells were treated with 2 μM TG for 10 min to drain the ER calcium store and activate SOCE. 1 mM CaCl2, 2 mM MnCl2, or 2 mM BaCl2 was then added to the buffer and cells were imaged an additional 10 min.
[Ca2+]m measurements
To measure [Ca2+]m, hepatocytes were plated, transfected, and loaded with Fura-4F, as described above. Following loading, hepatocytes were washed and imaged in HBSS. Cells were stimulated with 100 μM ATP to induce mitochondrial Ca2+ uptake. Following 3 min, cells were treated with 10 μM CCCP to release mitochondrial Ca2+ into the cytosol and imaged for an additional 5 min.
Statistical analysis
For all Ca2+ imaging studies, data are reported as population means ± standard error (SE). Statistical significance was determined using a two-tailed, nonparametric Student t test. A P value of ≤ 0.05 is considered statistically significant.
RT-qPCR of SOC channel and mitochondrial Ca2+ signaling components
Primary rat hepatocytes were transfected with the vector control (pGEM) or the pGEMHBV expression plasmid. Cells were collected 24 hours post transfection and total RNA was isolated using Trizol (Invitrogen), according to manufacturer’s instructions. RNA was treated with DNase and then converted to cDNA using M-MuLV reverse transcriptase (New England BioLabs, Inc). qPCR was performed with Power SYBR Green master mix (Invitrogen), according to manufacturer’s instructions. Fold differences in the expression of SOC channel and mitochondrial Ca2+ signaling component mRNAs were calculated by the delta-delta Ct method [101]. Primers used for each target are listed in Table 1.
Recombinant adenoviruses
Recombinant adenoviruses used in this study have been previously described [33, 37]. Briefly, recombinant adenoviruses that express GFP (AdGFP) or a greater-than unit length copy of the HBV genome (AdHBV) were used to infect cultured primary hepatocytes. All the recombinant adenoviruses express GFP, which was used to monitor infection efficiency and to ensure that 100% of hepatocytes were infected.
HBV replication assay
Primary rat hepatocytes were infected with AdHBV and treated with indicated SOCE inhibitors for 48 hours. HBV replication was analyzed by Southern blot analysis as previously described [29].
Northern blot analysis
Primary rat hepatocytes were infected with AdHBV and treated with indicated SOCE inhibitors for 48 hours. For control and APB-treated hepatocytes, total RNA was isolated using Trizol (Invitrogen) according to the manufacturer’s instructions. Poly(A) + RNA was isolated using oligo (dT)-cellulose columns (Molecular Research Center, Inc.) according to the manufacturer’s instructions, followed by northern blot analysis as previously described [86]. For control and La3+-treated hepatocytes, total RNA was isolated using the Ambion mirVana isolation kit (Life technologies) according to the manufacturers instructions. 7μg of total RNA was glyoxalated for 1 hour at 55°C, after which samples were separated for 1 hour on a 1% 0.01M NaPO4 agarose gel with vigorous buffer recirculation. The gel was transferred to a GENESCREEN membrane (Perkin Elmer) using 10X SSC overnight. The membrane was briefly rinsed in 2X SSC, then pre-hybridized according to the GENESCREEN membrane manufacturer’s instructions. An HBV- specific probe was generated using the Takara Random Primer DNA Labeling kit (Clonetech) and an HBV-genome length DNA fragment generated from digestion of the pGEM-HBV plasmid with the restriction enzyme AATII. Hybridization was carried out according to the GENESCREEN membrane manufacturer’s instructions. The membrane was washed 3 times in 2X SSPE/0.1% SDS, and then imaged using a Storm PhosphorImager.
Primary rat hepatocyte transcriptome analysis
Rat transcriptome experiments have been previously described [55], and associated data are available as a GEO SuperSeries using accession number GSE68113.
Results
HBV increases [Ca2+]c in cultured primary rat hepatocytes
We previously demonstrated that cultured primary rat hepatocytes, which are readily available, can serve as a surrogate model for studying HBV effects in primary human hepatocytes, which are not always available in large quantities. We have also reported that the HBV HBx protein has identical activities in cultured primary human and rat hepatocytes, providing further support for the use of primary rat hepatocytes in our studies [36, 37].
To validate the differentiated status of our cultured primary rat hepatocytes, we first confirmed that our cultured primary rat hepatocytes express markers of differentiation throughout the time course of our experiments. Reverse transcription-quantitative PCR (RT-qPCR) was performed to quantitate levels of the hepatocyte-specific markers albumin, transferrin, and hepatocyte nuclear factor 4 alpha (HNF4α) [38–40]. These markers were expressed at high levels in hepatocytes immediately following isolation and also in hepatocytes that were maintained in culture for 48 hours, the longest time frame for our experiments (Fig 1A). We also monitored levels of connexin 43, a protein that is expressed in liver sinusoid endothelial cells, Kupffer cells, stellate cells, and in dedifferentiated hepatocytes, but not in differentiated hepatocytes [39]. We were unable to detect connexin 43 in our hepatocytes (data not shown), confirming both the purity of the hepatocyte preparation and the differentiation status of our cells. While some level of dedifferentiation is both normal and expected, the results of our studies indicate that within the timeframe of our experiments, our cells retained a high level of differentiation.
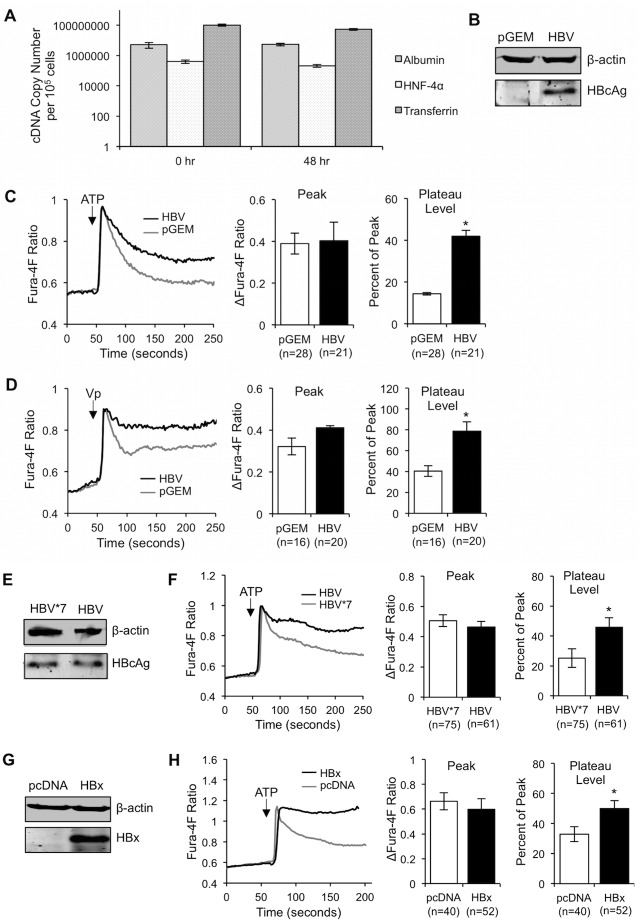
Fig 1. HBV/HBx increases [Ca2+]c in primary rat hepatocytes.
(A) RT-qPCR was performed on freshly isolated hepatocytes (0 hr) and on hepatocytes which had been plated for 48 hours (48 hr) for the hepatocyte-specific markers albumin, HNF4α, and transferrin. (B, C, and D) Primary rat hepatocytes were transfected with a control (pGEM) or HBV-expressing vector. 24 hrs post transfection, HBcAg expression was confirmed via western blot (B), and control and HBV-transfected cells were loaded with 5 μM Fura-4F and stimulated with 100 μM ATP (C) or 100 nM Vasopressin (Vp) (D). (E and F) Primary rat hepatocytes were transfected with an HBV-expressing or HBV(HBx-deficient) (HBV*7)-expressing vector. 24 hrs post transfection, equal levels of HBcAg expression were confirmed via western blot (E), and cells were loaded with 5 μM Fura-4F and stimulated with 100 μM ATP (F). (G and H) Primary rat hepatocytes were transfected with a control (pcDNA) or HBx-expressing vector. 24 hrs post transfection, HBx expression was confirmed via western blot (G), and cells were loaded with 5 μM Fura-4F and stimulated with 100 μM ATP (H). For all Ca2+-imaging experiments, the peak [Ca2+]c was calculated as the change in the peak Fura-4F ratio and the basal Fura-4F ratio (Rpeak−Rbasal). The plateau [Ca2+]c was calculated as a percentage of the peak [(Rplateau−Rbasal) / (Rpeak−Rbasal)]. The data represent the means ± SE and are taken from at least three experiments from different hepatocyte preparations. *P < 0.05
We first assessed whether HBV alters [Ca2+]c in cultured primary rat hepatocytes. Intracellular Ca2+-signaling studies are typically performed by the addition of a Ca2+-inducing agonist and subsequent measurement of the resultant intracellular Ca2+ response in cells that are loaded with a Ca2+ indicator; we utilized this technique to compare the intracellular Ca2+ responses of control and HBV-expressing hepatocytes. HBV has a very narrow host range and can only naturally infect primary human hepatocytes [15]; we therefore delivered a copy of the HBV genome into cultured primary rat hepatocytes by transfection of a plasmid encoding a greater-than full length HBV genome (pGEMHBV). Previous studies have demonstrated that HBV that is expressed from this plasmid recapitulates all aspects of the HBV life cycle, with the exception of viral cell-entry steps [41–43]. Cultured primary rat hepatocytes were transfected with pGEMHBV or the vector control (pGEM); we assessed expression of HBV core protein (HBcAg) as a marker of general HBV protein expression (Fig 1B). HBV-expressing and control hepatocytes were loaded with the ratiometric fluorescent cytosolic Ca2+ indicator Fura-4F. The excitation wavelength of Fura-4F shifts from 380 nm to 340 nm upon Ca2+ binding; by calculating the Fura-4F 340/380 ratio, changes in the [Ca2+]c in a single cell can be measured. We utilized ATP, a P2Y purinergic receptor agonist, to induce an IP3-linked cytosolic Ca2+ response in HBV-expressing and control hepatocytes (Fig 1C) [44–46]. ATP stimulation results in a biphasic Ca2+ response: the first phase, the release phase, reflects the initial release of Ca2+ from the ER and is represented by the initial peak in the Fura-4F ratio following ATP stimulation; the second phase, the recovery phase, occurs when the cell clears the excess Ca2+ from the cytosol at the same time that Ca2+ enters from outside the cell to refill the ER Ca2+ store, resulting in an elevated level of cytosolic Ca2+, referred to as the plateau [47]. When we compared this biphasic response between HBV-expressing and control hepatocytes, we observed no change in the initial Ca2+ peak following ATP stimulation. There was, however, a significant increase in the plateau Ca2+ level in HBV-expressing hepatocytes compared to control hepatocytes, indicating that HBV elevates [Ca2+]c following stimulation of Ca2+ release from the ER. To confirm that this result was not specific to activation of the P2Y receptor, we also exposed hepatocytes to vasopressin (Vp), which binds to the vasopressin receptor V1 and induces an IP3-linked cytosolic Ca2+ response [48]. When Fura-4F-loaded HBV-expressing and control hepatocytes were stimulated with Vp, there was no change in the initial Ca2+ peak, but there was a significant increase in the plateau Ca2+ level (Fig 1D), similar to results observed when we treated HBV-expressing hepatocytes with ATP.
HBV increases [Ca2+]c via HBx
Many viruses modulate Ca2+ signaling via a viral regulatory protein; we and others have linked HBV regulation of cytosolic Ca2+ in some established cell lines to expression of the HBx protein [27, 29, 34, 49]. To determine if HBx has a similar effect in normal hepatocytes, we compared the Ca2+ response in primary rat hepatocytes expressing either wildtype HBV or a mutant HBV that does not express HBx (HBV*7). Equal expression levels of HBcAg in cells expressing HBV and HBV*7 was confirmed via western blot analysis (Fig 1E). When we compared the Ca2+ response to ATP in HBV- and HBV*7-expressing cells, we observed no change in the peak Ca2+ level but did observe a significant increase in the plateau Ca2+ level in HBV-expressing cells as compared to HBV*7-expressing cells (Fig 1F), indicating that HBx does, indeed, mediate HBV modulation of [Ca2+]c. We confirmed this result using primary rat hepatocytes that were transfected with an HBx expression plasmid compared to hepatocytes expressing the vector control (pcDNA); similar HBx regulation of [Ca2+]c was observed when HBx was expressed alone or in the context of the full HBV genome (Fig 1H). HBx expression was confirmed by western blot analysis (Fig 1G).
The HBV/HBx-mediated increase in [Ca2+]c requires extracellular Ca2+ influx
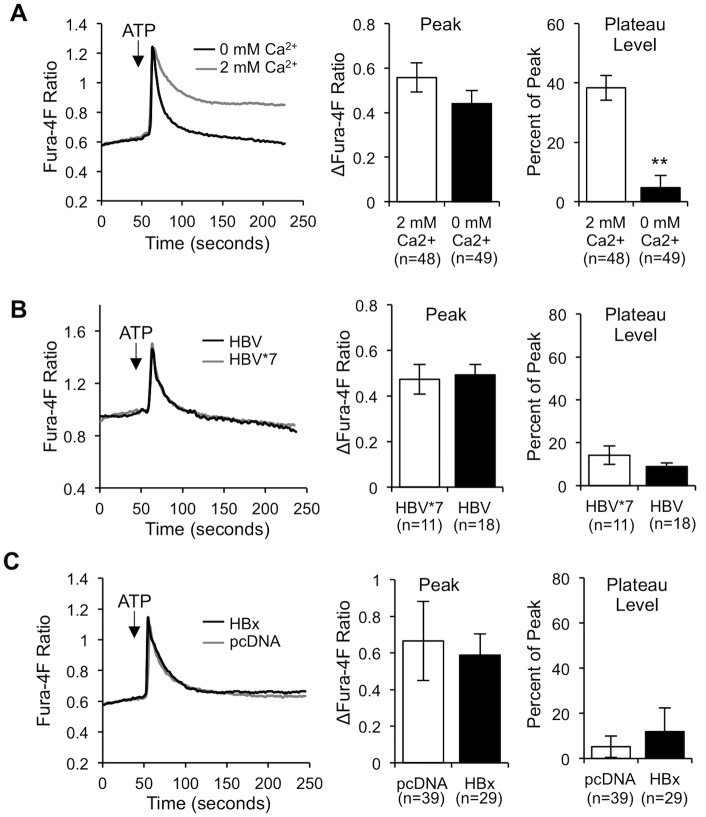
We next sought to define the mechanism underlying HBx elevation of the plateau Ca2+ level. It is well established that Ca2+ influx from outside the cell is responsible for the formation of the Ca2+ plateau following IP3-linked Ca2+ release from the ER [47, 50]. Indeed, when we stimulated control primary rat hepatocytes with ATP in Ca2+-free buffer, there was an extremely diminished Ca2+ plateau as compared to primary rat hepatocytes stimulated with ATP in Ca2+-containing buffer (Fig 2A). To determine whether Ca2+ influx mechanisms are involved in HBV elevation of [Ca2+]c, we treated Fura-4F-loaded HBx-expressing and control primary rat hepatocytes with ATP in Ca2+-free buffer, thus preventing any Ca2+ influx from outside the cell. Interestingly, the resulting plateau Ca2+ level in HBx-expressing primary rat hepatocytes was the same as in control primary rat hepatocytes (Fig 2B and 2C), indicating that HBV, via HBx, modulates Ca2+ influx mechanisms to elevate [Ca2+]c.
Fig 2. HBV/HBx requires extracellular Ca2+ influx to elevate [Ca2+]c.
(A) Primary rat hepatocytes were loaded with 5 μM Fura-4F and stimulated with 100 μM ATP in Ca2+-free buffer (0 mM Ca2+) or in Ca2+-containing buffer (2 mM Ca2+). (B and C) Control and HBx-expressing primary rat hepatocytes were loaded with 5 μM Fura-4F and stimulated with 100 μM ATP in Ca2+-free buffer (0 mM Ca2+). Calculations were performed as described for Fig 1. The data represent the means ± SE and are taken from at least three experiments from different hepatocyte preparations. **P < 0.01
HBV/HBx increases SOCE
The major mechanism of Ca2+ entry into hepatocytes is through SOC channels [51]. These channels consist of the ER transmembrane Stim proteins, Stim1 and 2, and the PM transmembrane Orai proteins, Orai1, 2, and 3. The Stim proteins contain an EF hand Ca2+-binding motif on the ER lumen side; upon depletion of the ER Ca2+ store, Ca2+ is no longer bound to this motif, resulting in aggregation of Stim near Orai, the pore-forming unit of the SOC channel. Stim binding to Orai causes the SOC channel to open, enabling Ca2+ to flow into the cell to refill the ER Ca2+ store [51, 52].
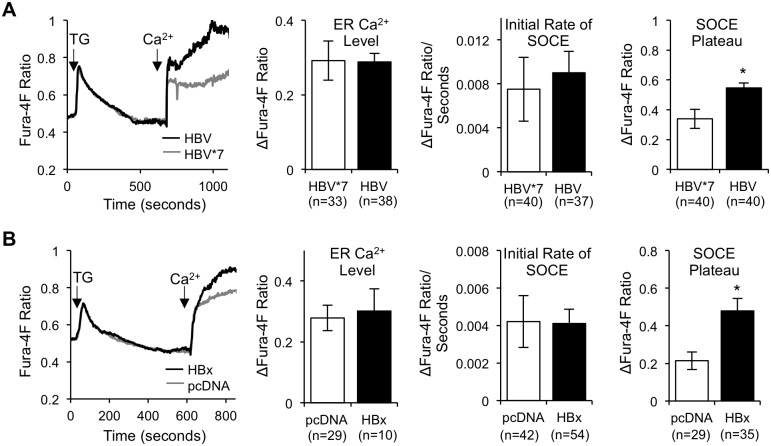
To determined if HBV altered Ca2+ entry through SOC channels to regulate [Ca2+]c, we utilized a commonly employed method for activating SOCE and subsequently measuring the flow of Ca2+ through the SOC channel. Fura-4F-loaded HBx-expressing and control primary rat hepatocytes were imaged in Ca2+-free buffer and treated with the SERCA inhibitor, thapsigargin (TG). The ER is naturally leaky, and the addition of TG causes a complete depletion of the ER Ca2+ store, thereby activating the SOC channel. Following ER store depletion, Ca2+ was added back to the imaging buffer; increased Fura-4F ratios reflect Ca2+ entering the cell through the open SOC channel [53]. When we compared the peak Ca2+ level following TG treatment, indicative of the ER Ca2+ level, and the initial rate of SOCE, we saw no change between HBx-expressing and control primary rat hepatocytes (Fig 3), suggesting that HBx does not affect the level of Ca2+ in the ER nor the rate of Ca2+ entering through the SOC channel. When we compared the SOCE plateau, however, there was a significant increase in the level of Ca2+ in HBx-expressing primary rat hepatocytes, as compared to control hepatocytes, indicating that HBx expression, both on its own and in the presence of other HBV proteins, does cause more SOCE-regulated Ca2+ accumulation in the cytosol of primary rat hepatocytes (Fig 3).
Fig 3. HBV/HBx stimulates SOCE.
(A and B) Control and HBx-expressing primary rat hepatocytes were loaded with 5 μM Fura-4F and treated with 2 μM TG in Ca2+-free buffer for 10 min. 1 mM CaCl2 (Ca2+) was then added back to the imaging buffer, and Fura-4F ratios were recorded during the following 10 min. The ER Ca2+ level was calculated as the difference in the peak Fura-4F ratio following TG treatment and the basal Fura-4F ratio (RTG peak−Rbasal). The initial rate of SOCE was calculated as the slope representing Ca2+ influx during the initial 15 seconds following Ca2+ addition. The SOCE plateau was calculated as the difference in the plateau Fura-4F ratio following Ca2+ addition and the baseline Fura-4F ratio prior to Ca2+ addition (Rplateau−Rbaseline). The data represent the means ± SE and are taken from at least three experiments from different hepatocyte preparations. *P < 0.05
SOCE is required for HBV replication
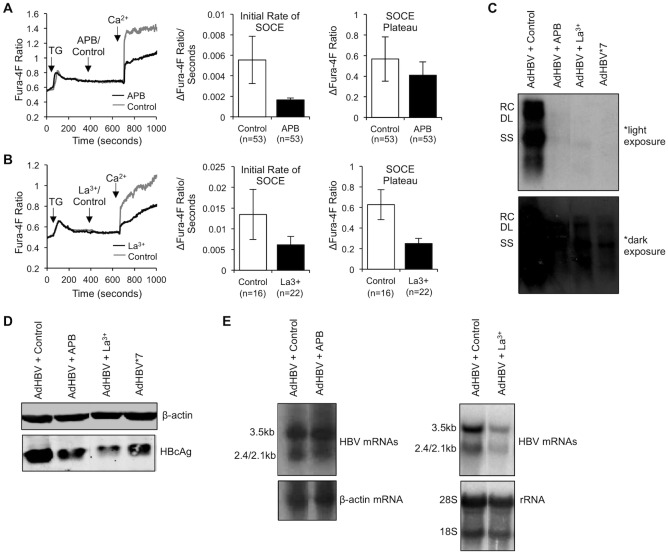
Previous studies have shown that Ca2+ signaling is required for HBV replication in various cell types [29, 30, 34]; therefore, we next tested whether SOCE was specifically required to stimulate HBV replication in normal hepatocytes. We infected primary rat hepatocytes with a recombinant adenovirus encoding HBV (AdHBV). We previously reported that to facilitate measurement of HBV replication in primary hepatocytes, a high percentage of the hepatocytes must express HBV [25]. Under optimal transfection conditions, we have observed that only 30–40% of hepatocytes are transfected; however, AdHBV infection is extremely efficient and can deliver the HBV genome to close to 100% of the cultured hepatocytes. We utilized two SOCE inhibitors, 2-aminoethoxydiphenyl (APB) and lanthanide (La3+), to assess the requirement for SOCE in HBV replication (Fig 4A and 4B) [47]. Upon AdHBV infection, primary rat hepatocytes were treated with either 50 μM APB or 1 μM La3+ for 48 hours, and HBV replication was then measured via Southern blot analysis as previously described [29]. Due to the partially double-stranded nature of the HBV genome, HBV replication appears as a smear with three distinct bands that represent three replicative intermediates: relaxed circular (RC), double-stranded linear (DL), and single-stranded linear (SS). Interestingly, treatment with both SOCE inhibitors significantly decreased levels of HBV replication to a similar level of replication that occurs with HBx-deficient HBV (HBV*7) (Fig 4C). We also assessed the effect of SOCE inhibition on expression levels of HBV core protein (Fig 4D) and HBV mRNAs (Fig 4E) in order to pinpoint the specific stage of HBV replication that SOCE influences. We found that while both APB and La3+ treatment resulted in a reduction in HBV core expression, detected via western blot analysis, only treatment with La3+ resulted in reduced levels of HBV mRNAs, detected via northern blot analysis. The significance of decreased HBV core expression and a reduction in HBV mRNA expression in the presence of SOCE inhibition is not entirely clear but suggests that HBx regulation of SOCE could be affecting expression of HBV mRNAs or proteins. Cumulatively, these results demonstrate that HBx affects HBV replication in primary rat hepatocytes by modulating Ca2+ influx through SOC channels to elevate [Ca2+]c.
Fig 4. SOCE is required for HBV replication.
(A and B) Primary rat hepatocytes were loaded with 5 μM Fura-4F and treated with 2 μM TG in Ca2+-free buffer, followed by treatment with DMSO or the SOCE inhibitor 50 μM APB (A) or 100 nM La3+ (B) before the addition of 1 mM CaCl2 (Ca2+). Calculations were performed as described for Fig 3. The data represent the means ± SE and are taken from at least three experiments from different hepatocyte preparations. (C-E) Primary rat hepatocytes were infected with a recombinant adenovirus expressing HBV (AdHBV) and treated with the SOCE inhibitors for 48 hrs or infected with a recombinant adenovirus expressing an HBx-deficient HBV (AdHBV*7). Levels of HBV replication (Southern blot) (C), HBV core protein expression (western blot) (D), and HBV mRNAs (northern blot) (E) were assessed.
HBV does not alter levels of SOC channel components
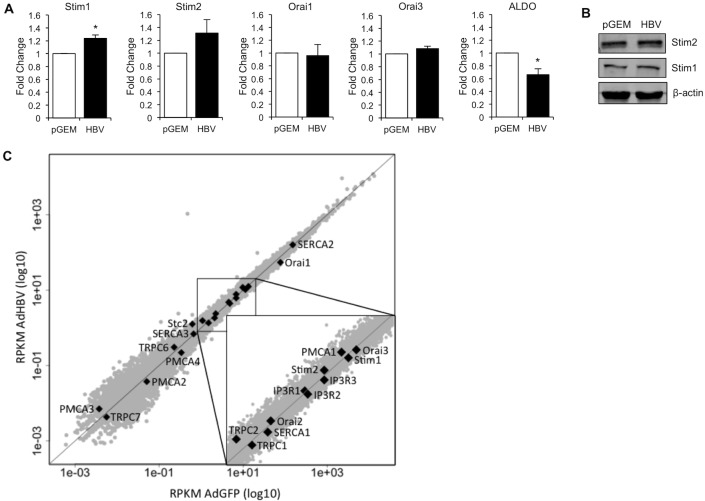
To further define the effect of HBV on SOCE, we monitored expression levels of SOC channel components in HBV-expressing and control primary rat hepatocytes. HBV regulates the expression of many proteins by activating transcription [22, 26], and studies have indicated that altered expression levels of Stim and Orai proteins can impact SOCE [51, 54]. We used RT-qPCR to assess the level of Stim1 and 2 and Orai1 and 3 mRNA transcripts in HBV-expressing and control primary rat hepatocytes (Fig 5A). Expression levels of Orai2 are extremely low in hepatocytes [54] and difficult to detect via RT-qPCR, and we therefore did not include Orai2 in this analysis. We previously showed that the levels of ALDO mRNA are decreased in the presence of HBV [36], and we included this target as a control for our assay. We observed equal Stim2, Orai1, and Orai3 mRNA expression levels in HBV-expressing and control cells and a slight increase in Stim1 mRNA (~1.2 fold). Although this slight increase in Stim1 mRNA was statistically significant, whether this increase is biologically relevant is unclear, as we did not detect an increase in Stim1 protein levels within the time frame of our studies (Fig 5B). It was not possible to measure protein expression for all SOC channel components because antibodies for detecting these components in rat cells are not available.
Fig 5. HBV does not alter levels of Ca2+ signaling-related genes.
(A) Primary rat hepatocytes were transfected with a control (pGEM) or HBV-expressing vector. 24 hrs post transfection, RNA was isolated and RT-qPCR was performed for expression of Stim1 and 2, Orai1 and 3, ALDO B, and Actin mRNAs. (B) Primary rat hepatocytes were transfected with a control (pGEM) or HBV-expressing vector. 24 hrs post transfection, hepatocytes were collected and western blot analysis was performed to analyze Stim1 and 2 and ß actin protein levels. (C) Primary rat hepatocytes were infected with AdGFP or AdHBV. 24 hrs post infection, total RNA was isolated and RNA-seq analysis was performed. Expressed genes were plotted on a log10 scale (grey). Ca2+ signaling-related genes were highlighted (black dots) to show that they do not deviate from the expected slope of 1 (black diagonal line), which would represent no difference in gene expression between AdGFP- and AdHBV-infected hepatocytes. Points along the middle of the plot were expanded (inset) for clearer representation.
We confirmed our RT-qPCR results and expanded our analysis to other factors that control Ca2+ signals in hepatocytes with data generated from an unbiased sequencing of the poly-A-selected RNA transcriptome (RNA-seq) of RNA isolated from primary rat hepatocytes that were infected with AdGFP or AdHBV (Fig 5C and Table 2). This analysis is described in detail in [55] and was conducted on two independent isolations of primary rat hepatocytes, referred to in Table 2 as Datasets I and II. Using this data, we confirmed our targeted RT-qPCR results by comparing levels of transcripts that code for the canonical SOC channel components and also investigated whether HBV alters levels of transcripts that code for other proteins that could influence SOCE and cytosolic Ca2+ signaling. We detected mRNA transcripts for Stim1 and 2 and Orai 1, 2, and 3 and, consistent with our qPCR results and with the findings of others, we observed equal mRNA expression of Stim1 and 2 in control hepatocytes, whereas Orai2 was expressed at much lower levels in control hepatocytes than Orai1 or 3 [54] (Table 2). Importantly, when we compared mRNA expression of these factors between control and HBV-expressing primary rat hepatocytes, we observed no change in expression, supporting our conclusion that HBV does not alter expression of SOC channel components to enhance SOCE (Fig 5C and Table 2). In addition to the canonical components of the SOC channel, members of the transient receptor potential cation (TRPC) channel family can bind to Stim or Orai proteins to facilitate Ca2+ influx into the cell [51, 56–59]. We detected TRPC1, 2, 6, and 7 mRNAs in control hepatocytes; our detection of expression and expression levels of specific members of the TRPC family in hepatocytes is consistent with previously reported observations [60, 61]. Importantly, when we compared expression levels of the detected members of the TRPC family in control and HBV-expressing primary rat hepatocytes, we detected no change (Fig 5C and Table 2), suggesting that HBV does not regulate mRNA transcript levels of canonical or noncanonical components of the SOC channel. We also detected PMCA-1, 2, 3, and 4 mRNAs and SERCA-1, 2, and 3 mRNAs. Interestingly, PMCA1 and SERCA2 mRNAs seem to have the highest expression levels in primary rat hepatocytes, as compared to PMCA-2, 3, and 4 or SERCA-1 and 3, respectively (Table 2) [62]; although it is important to note that mRNA levels might not directly correlate with protein levels [63]. HBV expression did not alter the levels of PMCA-1, 2, 3, or 4 mRNAs or SERCA-1, 2, or 3 mRNAs (Fig 5C and Table 2), suggesting that HBV does not regulate mechanisms that pump Ca2+ out of the cytoplasm, at least at the mRNA transcript level. Finally, we also detected mRNA transcripts that code for IP3R-1, 2, and 3 (Table 2). In Dataset 1, transcript levels for all three isoforms appear to be expressed at equivalent levels; however, in Dataset 2, transcript levels for IP3R-3 are expressed at much lower levels than IP3R-1 or IP3R-2, which is consistent with previous reports (Table 2) [64]. It is unclear why the expression levels of the IP3Rs are so different in the two datasets, as similar differences in expression levels between datasets were not observed for the other factors that were analyzed. Importantly, HBV expression did not alter levels of the mRNAs coding for any isoform of IP3R (Fig 5C and Table 2), supporting our finding that HBV does not affect the peak Ca2+ level released from the ER following an IP3-linked Ca2+ response (Fig 1C).
Table 2. Ca2+ signaling-related gene expression in control and HBV-expressing primary rat hepatocytes.
| Dataset 1 | Dataset 2 | ||||||
|---|---|---|---|---|---|---|---|
| Protein | Gene | Ensembl Gene ID | AdGFP RPKM | AdHBV RPKM | AdGFP RPKM | AdHBV RPKM | Avg Fold Change |
| Stim1 | Stim1 | ENSRNOG00000020425 | 11.279 | 10.334 | 9.152 | 9.579 | 0.981 |
| Stim2 | Stim2 | ENSRNOG00000002956 | 6.807 | 7.740 | 8.042 | 8.867 | 1.120 |
| Orai1 | Orai1 | ENSRNOG00000001336 | 77.046 | 54.370 | 36.921 | 25.958 | 0.704 |
| Orai2 | Orai2 | ENSRNOG00000001427 | 2.223 | 2.372 | 0.236 | 0.354 | 1.283 |
| Orai3 | Orai3 | ENSRNOG00000039730 | 13.320 | 12.443 | 12.672 | 12.583 | 0.964 |
| TRPC1 | Trpc1 | ENSRNOG00000009601 | 1.498 | 1.350 | 1.390 | 1.303 | 0.919 |
| TRPC2 | Trpc2 | ENSRNOG00000020188 | 1.078 | 1.549 | 1.661 | 2.857 | 1.579 |
| TRPC3 | Trpc3 | ENSRNOG00000016070 | - | - | - | - | N/A |
| TRPC4 | Trpc4 | ENSRNOG00000011133 | - | - | - | - | N/A |
| TRPC5 | Trpc5 | ENSRNOG00000027233 | - | - | - | - | N/A |
| TRPC6 | Trpc6 | ENSRNOG00000006324 | 0.228 | 0.306 | 0.136 | 0.171 | 1.296 |
| TRPC7 | Trpc7 | ENSRNOG00000012727 | 0.006 | 0.004 | 0.000 | 0.000 | 0.382 |
| PMCA1 | Atp2b1 | ENSRNOG00000004026 | 9.767 | 11.775 | 5.609 | 7.064 | 1.233 |
| PMCA2 | Atp2b2 | ENSRNOG00000030269 | 0.051 | 0.038 | 0.041 | 0.060 | 1.102 |
| PMCA3 | Atp2b3 | ENSRNOG00000017798 | 0.004 | 0.007 | 0.003 | 0.002 | 1.203 |
| PMCA4 | Atp2b4 | ENSRNOG00000003031 | 0.338 | 0.220 | 0.071 | 0.075 | 0.852 |
| SERCA1 | Atp2a1 | ENSRNOG00000047124 | 2.087 | 1.827 | 0.898 | 0.849 | 0.910 |
| SERCA2 | Atp2a2 | ENSRNOG00000001285 | 151.273 | 158.998 | 99.776 | 105.235 | 1.053 |
| SERCA3 | Atp2a3 | ENSRNOG00000017912 | 0.661 | 0.687 | 0.199 | 0.236 | 1.112 |
| IP3R-1 | Itpr1 | ENSRNOG00000007104 | 4.532 | 4.775 | 3.798 | 3.758 | 1.021 |
| IP3R-2 | Itpr2 | ENSRNOG00000001804 | 4.822 | 4.422 | 4.449 | 3.860 | 0.892 |
| IP3R-3 | Itpr3 | ENSRNOG00000026651 | 6.810 | 6.158 | 0.630 | 0.763 | 1.057 |
While no change in gene expression does not necessarily correlate to no change in the protein level, HBV regulates protein expression primarily at the level of transcription, and the results of our total transcriptome analyses and RT-qPCR studies suggest that HBV does not regulate [Ca2+]c and SOCE by altering expression levels of the Ca2+ regulators assessed here.
HBV/HBx alters a secondary regulatory mechanism to enhance SOCE
In addition to alterations in the expression level of SOC channel components, several mechanisms have been proposed to regulate SOCE. High [Ca2+]c in the vicinity of the SOC channel opening can initiate negative feedback signals that shut down SOCE; many SOCE regulatory mechanisms act by either augmenting or delaying negative feedback signals, resulting in dampened or enhanced SOCE, respectively [51, 65]. Thus far, we have shown that HBV expression enhances SOCE-regulated Ca2+ accumulation in the cytosol without altering the rate of SOCE (Fig 3). We have also shown that this enhanced Ca2+ accumulation is not due to altered levels of SOC channel components (Fig 5 and Table 2). These findings led us to hypothesize that HBV does not have a direct impact on the activity of the SOC channel but instead enables prolonged SOCE and enhanced cytosolic Ca2+ accumulation by affecting negative feedback mechanisms that would normally prevent further Ca2+ entry through the SOC channel.
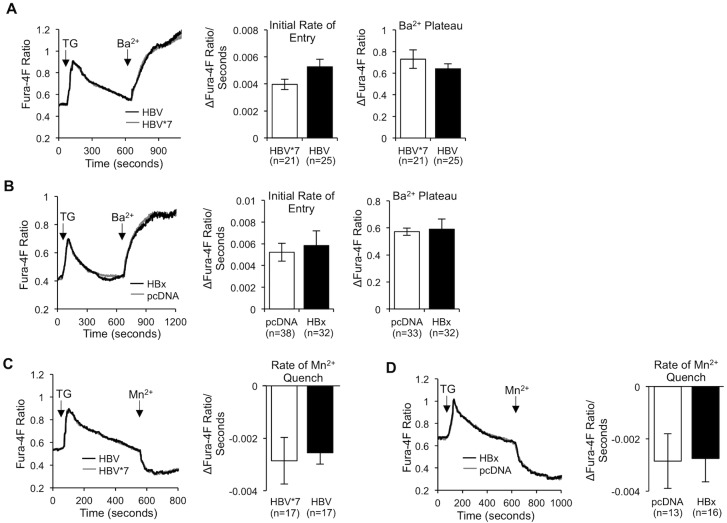
To begin to test this hypothesis, we first used two additional methods to measure changes in SOCE. In addition to Ca2+, both barium (Ba2+) and manganese (Mn2+) can selectively enter through the SOC channel and bind Fura-4F. Fura-4F binding to Ba2+ results in a similar Fura excitation wavelength shift as when Ca2+ binds; however, when Fura-4F binds Mn2+, this results in quenching of the Fura signal [53]. Importantly, neither Ba2+ nor Mn2+ are handled within the cell in the same way as Ca2+, and neither ion stimulates negative feedback mechanisms that turn off the influx signal. Consequently, the use of Ba2+ or Mn2+ allows us to directly measure the activity of the SOC channel without influence from SOC channel regulatory mechanisms [66]. When we treated primary rat hepatocytes with TG to activate the SOC channel and then added Ba2+ or Mn2+ to the medium, we observed no change in the rate of Ba2+ entry or in the rate of the Mn2+ quench between HBx-expressing and control primary rat hepatocytes (Fig 6), confirming that HBV, via HBx, does not affect the rate (and likely the activity) of the SOC channel. When we analyzed the Ba2+ plateau following addition of Ba2+ however, we observed that the increase that we had observed when Ca2+ was added back was now lost in HBx-expressing cells (Fig 6A and 6B). Because Ba2+ entry is not impacted by SOCE regulatory mechanisms, this result led us to conclude that a SOC channel regulatory mechanism is likely responsible for facilitating increased SOCE in HBx-expressing cells. It is not possible to calculate the plateau formed by Mn2+ addition because Mn2+ binding to Fura-4F quenches the Fura signal. In all, these results indicate that HBx, both when expressed on its own and in the context of the entire HBV genome, likely does not directly influence the activity of the SOC channel, but perhaps alters SOC channel regulatory mechanisms, such that negative feedback signals that would normally shut down SOCE are not activated, resulting in prolonged SOC channel activation and, subsequently, increased Ca2+ accumulation in the cytosol.
Fig 6. HBV/HBx does not directly affect SOC channel activity.
(A, B, C, and D) Control and HBx-expressing primary rat hepatocytes were loaded with 5 μM Fura-4F and treated with 2 μM TG in Ca2+-free buffer for 10 min. 2 mM BaCl2 (Ba2+) (A and B) or 2 mM MnCl2 (Mn2+) (C and D) was then added back to the imaging buffer and ratios were recorded during the following 10 min. (A and B) The initial rate of Ba2+ entry was calculated as the slope representing Ba2+ influx during the initial 15 seconds following Ba2+ addition. The Ba2+ plateau was calculated as the difference in the plateau Fura-4F ratio following Ba2+ addition and the baseline Fura-4F ratio prior to Ba2+ addition (Rplateau−Rbaseline). (C and D) The rate of Mn2+ quench was calculated as the slope representing Mn2+ influx during the initial 15 seconds following Mn2+ addition. (A, B, C, and D) The data represent the means ± SE and are taken from at least three experiments from different hepatocyte preparations.
HBV/HBx enhances [Ca2+]m to elevate SOCE
One SOCE regulatory mechanism that may be altered by HBV is mitochondrial Ca2+ uptake. Mitochondrial Ca2+ uptake is stimulated when there is a high local Ca2+ concentration in the vicinity of mitochondria, often the result of ER Ca2+ release or SOCE. By taking up this Ca2+, mitochondria can buffer negative feedback mechanisms and prolong a Ca2+ response [65, 67–71]. Altered or enhanced mitochondrial Ca2+ uptake during SOCE could result in increased Ca2+ accumulation in the cytosol. Interestingly, we previously reported that the mitochondrial membrane potential is increased in HBx-expressing primary rat hepatocytes [25], which is usually a precursor to increased mitochondrial Ca2+ uptake. Additionally, we also reported that mitochondrial Ca2+ levels ([Ca2+]m) were increased in HBx-expressing HepG2 cells [34]. These observations provide strong evidence that increased mitochondrial Ca2+ uptake likely occurs in HBV-expressing primary hepatocytes, which could potentially result in enhanced SOCE.
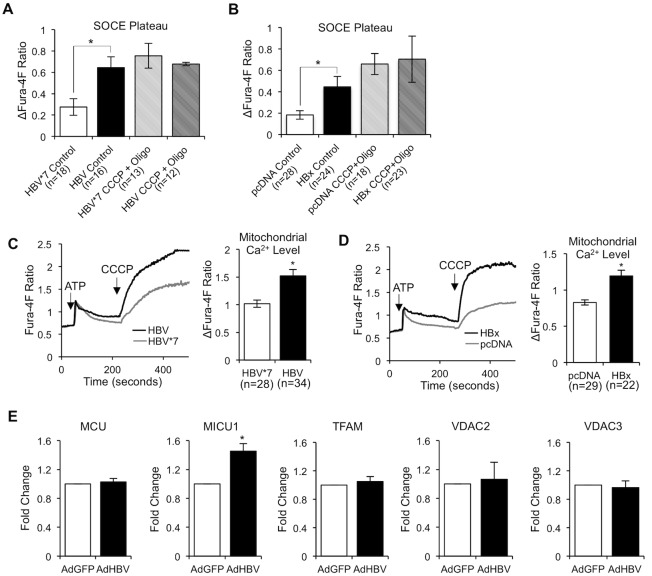
To first determine whether HBV does require mitochondrial Ca2+ uptake to enhance SOCE, we stimulated SOCE in HBx-expressing and control primary rat hepatocytes by exposing them to TG and simultaneously preventing mitochondrial Ca2+ uptake with the addition of the uncoupler carbonyl cyanide m-chlorophenyl hydrazine (CCCP), which disrupts the inner mitochondrial membrane potential, and oligomycin, an inhibitor of ATP synthase. Interestingly, when we prevented mitochondrial Ca2+ uptake, HBx-expressing cells no longer displayed enhanced SOCE (Fig 7A and 7B), indicating that mitochondria do play a critical role in mediating HBx regulation of SOCE. We next sought to determine whether HBx affects the [Ca2+]m in primary hepatocytes. We first stimulated mitochondrial Ca2+ uptake in HBx-expressing and control cells with ATP treatment and then released [Ca2+]m by the addition of CCCP. We observed an increase in [Ca2+]m in HBx-expressing cells compared to control cells (Fig 7C and 7D). These results suggest that HBx stimulation of SOCE likely requires mitochondrial Ca2+ uptake and that HBx increases [Ca2+]m following IP3R activation.
Fig 7. HBV/HBx enhances mitochondrial Ca2+ uptake to elevate SOCE.
(A and B) Control and HBx-expressing primary rat hepatocytes were loaded with 5 μM Fura-4F and treated with 2 μM TG in Ca2+-free buffer, followed by treatment with DMSO or 10 μM CCCP and 5 mg/ml Oligomycin (Oligo) before the addition of 1 mM CaCl2 (Ca2+). Calculations were performed as described for Fig 3 (C and D) Control and HBx-expressing primary rat hepatocytes were loaded with 5 μM Fura-4F and treated with 100 μM, followed by 10 μM CCCP. The mitochondrial Ca2+level was calculated as the difference in the plateau Fura-4F ratio following CCCP addition and the baseline Fura-4F ratio prior to CCCP addition (Rplateau−Rbaseline). (E) Primary rat hepatocytes were infected with a control (AdGFP) or HBV-expressing recombinant adenovirus (AdHBV). 24 hours post infection, RNA was isolated and RT-qPCR was performed for expression of MCU, MICU1, TFAM, VDAC2, and VDAC3 mRNAs. (A-E) The data represent the means ± SE and are taken from at least three experiments from different hepatocyte preparations. *P < 0.05
Taken together, these results support our hypothesis that HBx stimulates mitochondrial Ca2+ uptake during Ca2+ release from the ER and/or Ca2+ entry through the SOC channel. This mitochondrial uptake of Ca2+ dampens Ca2+-mediated inhibition of further Ca2+ release from the ER and/or Ca2+ entry through the SOC channel, thereby prolonging Ca2+ entry into the cytosol to elevate [Ca2+]c.
Lastly, in an effort to identify potential mechanisms underlying HBV modulation of [Ca2+]m, we performed an initial, targeted screen of mitochondrial Ca2+ regulators. Recent work has identified key regulators of mitochondrial Ca2+ signaling; alterations in the expression levels of these components has been shown to regulate mitochondrial Ca2+ uptake/efflux mechanisms, as well as [Ca2+]m [72–76]. In order to determine whether expression levels of some key mitochondrial Ca2+ regulators are altered in HBV-expressing primary rat hepatocytes, we monitored mRNA levels of the mitochondrial Ca2+ uniporter (MCU), mitochondrial Ca2+ uptake 1 (MICU1), VDAC2, VDAC3, and mitochondrial transcription factor A (TFAM) in AdHBV-infected primary rat hepatocytes. MCU is a highly selective Ca2+ uniporter that transports Ca2+ across the inner mitochondrial membrane (IMM); MCU activity is regulated by several proteins, including MICU1, a single-pass, transmembrane protein located within the IMM. MICU1 contains a pair of Ca2+-binding EF-hand domains and functions as a Ca2+-sensing regulatory subunit of MCU [75–77]. VDAC proteins are a component of the mitochondrial permeability transition pore (MPTP), which can regulate mitochondrial Ca2+ uptake and efflux [78, 79]. Interestingly, a fraction of HBx interacts with VDAC3 on the OMM [24]. TFAM is essential for mitochondrial DNA transcription and replication; while TFAM primarily regulates transcription of mitochondrial genes, recent evidence suggests that TFAM can also regulate expression of nuclear genes, including SERCA [80]. Altered expression levels of these various components could impact mitochondrial Ca2+ accumulation. Interestingly, we observed equal MCU, VDAC2, VDAC3, and TFAM mRNA expression levels in HBV-expressing and control hepatocytes and a 1.5-fold increase in MICU1 expression (Fig 7E). These results suggest that HBV likely elevates expression of MICU1; however, whether this increase directly relates to the HBx-mediated elevation of [Ca2+]m has yet to be determined. Additionally, these four factors are not the only regulators of mitochondrial Ca2+, and further studies will need to be performed to fully characterize HBx regulation of mitochondrial Ca2+.
Discussion
Ca2+ is a tightly controlled and very dynamic universal second messenger. Changes in [Ca2+]c can initiate signaling cascades that impact major cellular processes ranging from gene expression to cell growth and cell death [2–5]. Not surprisingly, it has been suggested that many human diseases may be caused by the remodeling or disruption of Ca2+ signaling pathways, resulting in inappropriate Ca2+ responses that are either too high or too low [12, 81]. Chronic infection with HBV is associated with various liver diseases and is the most common cause of HCC [13–15]. We have demonstrated that the HBV regulatory protein, HBx, alters normal cytosolic Ca2+ signaling in cultured, primary rat hepatocytes, and that HBx modulation of cytosolic Ca2+ is required for HBV replication.
HBx is the only regulatory protein encoded in the HBV genome; its expression stimulates HBV replication and is thought to influence the development of HBV-associated HCC [16–22, 26, 82–85]. We and others have shown that many HBx effects can be linked to HBx regulation of cytosolic Ca2+-dependent signal transduction pathways [28–31, 33, 49, 86–88]. Previous studies that have analyzed the direct effect of HBx on cytosolic Ca2+ levels were conducted in established cell lines, and most previous studies only assessed the effect of HBx that was expressed outside the context of the HBV genome [27, 28, 34, 87]. While these studies have provided valuable information regarding HBx effects in specific experimental contexts, it has become increasingly apparent that HBx effects can be context specific [26, 35]. In contrast, for the studies reported here, we used primary hepatocytes, the natural site of an HBV infection. We directly demonstrated that HBx elevates cytosolic Ca2+ levels in primary rat hepatocytes; this HBx effect was present when HBx was expressed alone or in the context of the HBV genome. Specifically, HBx altered a normal IP3-linked Ca2+ response, resulting in higher [Ca2+]c than in control hepatocytes (Fig 1B–1H). The HBx-induced elevation of cytosolic Ca2+ required influx of extracellular Ca2+ (Fig 2B and 2C) through SOC channels (Fig 3). Importantly, SOCE-dependent HBx elevation of Ca2+ was essential for HBV replication in normal hepatocytes (Fig 4C), thus highlighting the significance of our studies.
Modulation of Ca2+ signaling may also be a key event in the pathogenesis of many other viruses, including hepatitis C virus (HCV), human immunodeficiency virus (HIV), human T-lymphotropic virus-1 (HTLV-1), rotavirus, influenza A virus, enterovirus, and human herpesviruses (HHV) [6, 7]. Because changes in Ca2+ levels and Ca2+ signaling can stimulate a wide range of cellular effects, viral modulation of Ca2+ is an ideal mechanism to create a cellular environment that is permissive to viral replication. Many viruses increase [Ca2+]c, similar to what we report here for HBV. Virus-mediated elevation of [Ca2+]c is often linked to activities of a viral regulatory protein, although for many viruses, the mechanisms that underlie regulation of [Ca2+]c are not fully defined [6, 7]. The components and nature of the SOC channel have only recently been defined, and so only a handful of viruses have been shown to modify this process, including HBV, rotavirus, and enterovirus [34, 89, 90]. Interestingly, rotavirus has been shown to activate SOCE via increased permeability of the ER Ca2+ store and a resultant decrease in ER Ca2+ levels. This increased ER permeability was linked to the rotavirus regulatory protein NSP4, which contains a viroporin activity [90, 91]. Alternately, modulation of SOCE by enterovirus was linked to activities of the viral regulatory protein LMP-1, which increased expression of Orai1 [89]. Importantly, we did not see a change in ER Ca2+ levels and HBx does not exhibit viroporin characteristics [92]. We also did not observe altered expression levels of SOC channel components, indicating that the method of SOCE modulation by HBV is different from that of either rotavirus or enterovirus. The results of this study could provide insights into mechanisms underlying viral regulation of [Ca2+]c for other viruses that do not contain viroporins and that do not alter protein expression of SOC channel components.
We linked HBx elevation of cytosolic Ca2+ in normal hepatocytes to altered feedback mechanisms that negatively regulate SOCE, specifically mitochondrial regulation of SOC channels. By blocking mitochondrial uptake of Ca2+, we prevented HBx from elevating cytosolic Ca2+ levels that were associated with activation of SOCE (Fig 7A and 7B). HBx localizes to the OMM and interacts with the OMM protein VDAC [23–25]. Although not yet proven, the enhanced mitochondrial Ca2+ uptake seen in HBV- and HBx-expressing hepatocytes could be the result of HBx OMM localization and/or interaction with VDAC. Mitochondria normally take up cytosolic Ca2+ when the cytosolic Ca2+ level reaches 1 μM, which is 10 times the normal cytosolic concentration [76, 93, 94]. In HBV-infected hepatocytes, however, mitochondria may either have a lower threshold for taking up Ca2+, a larger capacity for Ca2+ storage, or are more sensitive or susceptible to taking up cytosolic Ca2+. When there is an IP3-linked Ca2+ response, mitochondria localized to the ER and/or SOC channels take up some of the released Ca2+, which dampens Ca2+-mediated Ca2+ inhibition of further Ca2+ release from the ER and/or Ca2+ entry through the SOC channel. This results in prolonged release of Ca2+ from the ER or prolonged entry of Ca2+ through SOC channels, causing a greater Ca2+ accumulation in the cytosol [65, 67–71]. Furthermore, we observed an HBV-mediated increase in MICU1 expression (Fig 7E) and elevated MICU1 could contribute to enhanced [Ca2+]m in HBV-expressing hepatocytes. Our observed link of HBx elevation of cytosolic Ca2+ to mitochondrial-dependent processes is contradictory to a previous study conducted in HeLa and HepG2 cells where HBx elevation of cytosolic Ca2+ was associated with altered PMCA activity [87]. PMCA pumps excess [Ca2+]c out of the cell; when PMCA activity is altered or impaired, [Ca2+]c could accumulate in the cytosol [95, 96]. The authors of this previous study proposed that HBx elevation of cytosolic Ca2+ was directly caused by stimulating the pro-apoptotic activation of caspase 3, which subsequently cleaved and inactivated PMCA, decreasing Ca2+ efflux from the cytosol to the extracellular environment, resulting in elevated [Ca2+]c [87]. It is important to note, however, that these studies were only conducted in a small number of HepG2 cells and HeLa cells with over-expressed HBx; the results were not confirmed in the context of the full HBV genome [87]. Moreover, cleavage of PMCA was only demonstrated in HeLa cells, which are derived from a cervical carcinoma. In contrast, we have demonstrated that HBx is normally anti-apoptotic in primary hepatocytes and does not activate caspase 3 [33, 36]. Overall, these contrasting results highlight the importance of directly assessing HBx activities in the context of the full HBV genome and in biologically relevant models systems such as cultured primary hepatocytes. Further defining the mechanism underlying HBV regulation mitochondrial Ca2+ uptake is the focus of our ongoing studies.
In addition to regulating HBV replication, HBx elevation of cytosolic Ca2+ may also be linked to the development, progression, and/or maintenance of HBV-associated diseases. Hepatocytes are constantly exposed to various insults, toxins, and growth hormones that could initiate an IP3-linked Ca2+ response [97–99]. In normal, non-HBV-infected hepatocytes, the resultant transient increase in [Ca2+]c is quickly corrected by shuttling this excess cytoplasmic Ca2+ out of the cell, through PMCA, or back into the ER, through SERCA. In HBV-infected hepatocytes, however, this regulation of Ca2+ levels and signals is altered, and HBV-infected hepatocytes are subjected to a more substantial and persistent increase in cytosolic Ca2+. Persistent elevation of cytosolic Ca2+ likely stimulates downstream Ca2+-dependent effector proteins and signaling pathways to create a cellular environment that is permissive to HBV replication, but consequently alters hepatocyte physiology, potentially influencing the development of HBV-associated diseases.
In summary, we have shown for the first time that HBV, via HBx, modulates SOCE to elevate [Ca2+]c in normal hepatocytes to stimulate HBV replication; we have linked this HBx effect to mitochondrial regulation of [Ca2+]c. HBx is the only HBV regulatory protein and is required for HBV replication in human hepatocytes [16–22]. HBx is also thought to play a major role in HBV pathogenesis [26]. HBV-related HCC develops after a decades-long HBV infection, and continuous deregulation of normal hepatocyte [Ca2+]c could sensitize cells to signals that promote liver disease and/or HCC development. Importantly, HBx modulation of cytosolic Ca2+ is required for many of its effects in cells, indicating that HBx modulation of cytosolic Ca2+ is likely an upstream, initiating event that stimulates numerous downstream cellular functions [22, 26, 29]. Our studies provide insight into mechanisms that underlie HBx activities and HBV replication; factors that regulate these HBx activities could be novel targets for inhibiting HBx activities, HBV replication, and HBV pathogenesis.
Acknowledgments
We thank members of the Bouchard lab, as well as Olimpia Meucci, Lawrence Gaspers, and Akhil Vaidya, for advice and many helpful discussions. We also thank Yi Guo for help in the construction of the plasmids used to confirm the differentiation status of the primary rat hepatocytes.
Data Availability
I have uploaded all the data for the figures into the website “Open Science Framework”. The address for the data is: https://osf.io/rtvhk/.
Funding Statement
This work was supported by the National Cancer Institute grant F31CA171850 to JCC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Condit R. Principles of Virology In: Knipe DaH, P., editor. Fields Virology. 1 6th ed: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P, et al. Calcium signalling—an overview. Semin Cell Dev Biol. 2001;12(1):3–10. Epub 2001/02/13. 10.1006/scdb.2000.0211 [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–29. Epub 2003/07/03. 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- 4.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. Epub 2001/06/20. 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- 5.Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–58. Epub 2007/12/18. 10.1016/j.cell.2007.11.028 [DOI] [PubMed] [Google Scholar]
- 6.Chami M, Oules B, Paterlini-Brechot P. Cytobiological consequences of calcium-signaling alterations induced by human viral proteins. Biochim Biophys Acta. 2006;1763(11):1344–62. 10.1016/j.bbamcr.2006.09.025 [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y, Frey TK, Yang JJ. Viral calciomics: interplays between Ca2+ and virus. Cell Calcium. 2009;46(1):1–17. Epub 2009/06/19. 10.1016/j.ceca.2009.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta 2009. p. 933–40. 10.1016/j.bbamcr.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 9.Parkash J, Asotra K. Calcium wave signaling in cancer cells. Life Sci. 2010;87(19–22):587–95. Epub 2010/09/30. 10.1016/j.lfs.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enfissi A, Prigent S, Colosetti P, Capiod T. The blocking of capacitative calcium entry by 2-aminoethyl diphenylborate (2-APB) and carboxyamidotriazole (CAI) inhibits proliferation in Hep G2 and Huh-7 human hepatoma cells. Cell Calcium. 2004;36(6):459–67. Epub 2004/10/19. 10.1016/j.ceca.2004.04.004 [DOI] [PubMed] [Google Scholar]
- 11.El Boustany C, Bidaux G, Enfissi A, Delcourt P, Prevarskaya N, Capiod T. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology. 2008;47(6):2068–77. Epub 2008/05/29. 10.1002/hep.22263 [DOI] [PubMed] [Google Scholar]
- 12.Yang N, Tang Y, Wang F, Zhang H, Xu D, Shen Y, et al. Blockade of store-operated Ca(2+) entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover. Cancer Lett. 2013;330(2):163–9. 10.1016/j.canlet.2012.11.040 [DOI] [PubMed] [Google Scholar]
- 13.Anzola M. Hepatocellular carcinoma: role of hepatitis B and hepatitis C viruses proteins in hepatocarcinogenesis. J Viral Hepat. 2004;11(5):383–93. 10.1111/j.1365-2893.2004.00521.x [DOI] [PubMed] [Google Scholar]
- 14.Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22(33):5093–107. Epub 2003/08/12. 10.1038/sj.onc.1206557 [DOI] [PubMed] [Google Scholar]
- 15.Seeger C, Zoulim F., and Mason W. Hepadnaviruses In: Knipe DaH, P., editor. Fields Virology. 2 6th ed Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 16.Chen H, Kaneko S, Girones R, Anderson R, Hornbuckle W, Tennant B, et al. The woodchuck helpatitis virus X gene is important for establishment of virus infection in woodchucks. J Virol. 1993;67:1218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoulim F, Saputelli J, Seeger C. Woodchuck hepatitis virus X protein is required for viral infection in vivo. J Virol. 1994;68:2026–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Z, Yen T, Wu L, Madden C, Tan W, Slagle B, et al. Enhancement of hepatitis B virus replication by its X protein in transgenic mice. J Virol. 2002;76:2579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keasler VV, Hodgson AJ, Madden CR, Slagle BL. Enhancement of hepatitis B virus replication by the regulatory X protein in vitro and in vivo. J Virol. 2007;81(6):2656–62. Epub 2006/12/22. 10.1128/JVI.02020-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuge M, Hiraga N, Akiyama R, Tanaka S, Matsushita M, Mitsui F, et al. HBx protein is indispensable for development of viraemia in human hepatocyte chimeric mice. J Gen Virol. 2010;91(Pt 7):1854–64. Epub 2010/03/12. 10.1099/vir.0.019224-0 [DOI] [PubMed] [Google Scholar]
- 21.Tsuge M, Hiraga N, Takaishi H, Noguchi C, Oga H, Imamura M, et al. Infection of human hepatocyte chimeric mouse with genetically engineered hepatitis B virus. Hepatology. 2005;42(5):1046–54. Epub 2005/10/27. 10.1002/hep.20892 [DOI] [PubMed] [Google Scholar]
- 22.Bouchard MJ, Schneider RJ. The enigmatic X gene of hepatitis B virus. J Virol. 2004;78(23):12725–34. Epub 2004/11/16. 10.1128/JVI.78.23.12725-12734.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huh KW, Siddiqui A. Characterization of the mitochondrial association of hepatitis B virus X protein, HBx. Mitochondrion. 2002;1(4):349–59. [DOI] [PubMed] [Google Scholar]
- 24.Rahmani Z, Huh K-W, Lasher R, Siddiqui A. Hepatitis B virus X protein colocalizes to mitochondria with human voltage -dependent anion channel, HVDAC3, and alters its transmembrane potential. J Virol. 2000;74:2840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clippinger AJ, Bouchard MJ. Hepatitis B virus HBx protein localizes to mitochondria in primary rat hepatocytes and modulates mitochondrial membrane potential. J Virol. 2008;82(14):6798–811. Epub 2008/05/02. 10.1128/JVI.00154-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouchard MJ, Navas-Martin S. Hepatitis B and C virus hepatocarcinogenesis: lessons learned and future challenges. Cancer Lett. 2011;305(2):123–43. Epub 2010/12/21. 10.1016/j.canlet.2010.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McClain SL, Clippinger AJ, Lizzano R, Bouchard MJ. Hepatitis B virus replication is associated with an HBx-dependent mitochondrion-regulated increase in cytosolic calcium levels. J Virol. 2007;81(21):12061–5. Epub 2007/08/19. 10.1128/JVI.00740-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh JC, Jeong DL, Kim IK, Oh SH. Activation of calcium signaling by hepatitis B virus-X protein in liver cells. Exp Mol Med. 2003;35(4):301–9. 10.1038/emm.2003.41 [DOI] [PubMed] [Google Scholar]
- 29.Bouchard MJ, Wang LH, Schneider RJ. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science. 2001;294(5550):2376–8. Epub 2001/12/18. 10.1126/science.294.5550.2376 [DOI] [PubMed] [Google Scholar]
- 30.Gearhart TL, Bouchard MJ. Replication of the hepatitis B virus requires a calcium-dependent HBx-induced G1 phase arrest of hepatocytes. Virology. 2010;407(1):14–25. Epub 2010/08/20. 10.1016/j.virol.2010.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gearhart TL, Bouchard MJ. The hepatitis B virus X protein modulates hepatocyte proliferation pathways to stimulate viral replication. J Virol. 2010;84(6):2675–86. Epub 2010/01/08. 10.1128/JVI.02196-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi Y, Gyoo Park S, Yoo JH, Jung G. Calcium ions affect the hepatitis B virus core assembly. Virology. 2005;332(1):454–63. 10.1016/j.virol.2004.11.019 [DOI] [PubMed] [Google Scholar]
- 33.Clippinger AJ, Gearhart TL, Bouchard MJ. Hepatitis B virus X protein modulates apoptosis in primary rat hepatocytes by regulating both NF-kappaB and the mitochondrial permeability transition pore. J Virol. 2009;83(10):4718–31. Epub 2009/03/13. 10.1128/JVI.02590-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang B, Bouchard MJ. The Hepatitis B Virus X Protein Elevates Cytosolic Calcium Signals by Modulating Mitochondrial Calcium Uptake. J Virol. 2011. Epub 2011/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casciano J, Bagga S, Yang B, Bouchard MJ. Modulation of Cell Proliferation Pathways by the Hepatitis B Virus X Protein: A Potential Contributor to the Development of Hepatocellular Carcinoma. Lau W-Y, editor. Croatia: InTech; 2012. [Google Scholar]
- 36.Rawat S, Bouchard MJ. The Hepatitis B Virus (HBV) HBx Protein Activates AKT To Simultaneously Regulate HBV Replication and Hepatocyte Survival. J Virol. 2015;89(2):999–1012. 10.1128/JVI.02440-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gearhart TL, Bouchard MJ. The hepatitis B virus HBx protein modulates cell cycle regulatory proteins in cultured primary human hepatocytes. Virus Res. 2011;155(1):363–7. Epub 2010/10/12. 10.1016/j.virusres.2010.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, et al. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132(6):1133–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piechocki MP, Burk RD, Ruch RJ. Regulation of connexin32 and connexin43 gene expression by DNA methylation in rat liver cells. Carcinogenesis. 1999;20(3):401–6. [DOI] [PubMed] [Google Scholar]
- 40.Runge D, Runge DM, Jager D, Lubecki KA, Beer Stolz D, Karathanasis S, et al. Serum-free, long-term cultures of human hepatocytes: maintenance of cell morphology, transcription factors, and liver-specific functions. Biochem Biophys Res Commun. 2000;269(1):46–53. 10.1006/bbrc.2000.2215 [DOI] [PubMed] [Google Scholar]
- 41.Scaglioni PP, Melegari M, Wands JR. Biologic properties of hepatitis B viral genomes with mutations in the precore promoter and precore open reading frame. Virology. 1997;233(2):374–81. 10.1006/viro.1997.8594 [DOI] [PubMed] [Google Scholar]
- 42.Melegari M, Scaglioni PP, Wands JR. Cloning and characterization of a novel hepatitis B virus x binding protein that inhibits viral replication. J Virol. 1998;72(3):1737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scaglioni PP, Melegari M, Wands JR. Posttranscriptional regulation of hepatitis B virus replication by the precore protein. J Virol. 1997;71(1):345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schofl C, Ponczek M, Mader T, Waring M, Benecke H, von zur Muhlen A, et al. Regulation of cytosolic free calcium concentration by extracellular nucleotides in human hepatocytes. Am J Physiol. 1999;276(1 Pt 1):G164–72. [DOI] [PubMed] [Google Scholar]
- 45.Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. Embo J. 1999;18(22):6349–61. 10.1093/emboj/18.22.6349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dixon CJ, White PJ, Hall JF, Kingston S, Boarder MR. Regulation of human hepatocytes by P2Y receptors: control of glycogen phosphorylase, Ca2+, and mitogen-activated protein kinases. J Pharmacol Exp Ther. 2005;313(3):1305–13. 10.1124/jpet.104.082743 [DOI] [PubMed] [Google Scholar]
- 47.Putney JW. Pharmacology of store-operated calcium channels. Mol Interv. 2010;10(4):209–18. Epub 2010/08/24. 10.1124/mi.10.4.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Birnbaumer M. Vasopressin receptors. Trends Endocrinol Metab. 2000;11(10):406–10. [DOI] [PubMed] [Google Scholar]
- 49.Geng X, Huang C, Qin Y, McCombs JE, Yuan Q, Harry BL, et al. Hepatitis B virus X protein targets Bcl-2 proteins to increase intracellular calcium, required for virus replication and cell death induction. Proc Natl Acad Sci U S A. 2012;109(45):18471–6. 10.1073/pnas.1204668109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7(1):1–12. [DOI] [PubMed] [Google Scholar]
- 51.Smyth JT, Hwang SY, Tomita T, DeHaven WI, Mercer JC, Putney JW. Activation and regulation of store-operated calcium entry. J Cell Mol Med. 2010;14(10):2337–49. Epub 2010/09/03. 10.1111/j.1582-4934.2010.01168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443(7108):230–3. Epub 2006/08/22. 10.1038/nature05122 [DOI] [PubMed] [Google Scholar]
- 53.Bird GS, DeHaven WI, Smyth JT, Putney JW Jr. Methods for studying store-operated calcium entry. Methods. 2008;46(3):204–12. Epub 2008/10/22. 10.1016/j.ymeth.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones BF, Boyles RR, Hwang SY, Bird GS, Putney JW. Calcium influx mechanisms underlying calcium oscillations in rat hepatocytes. Hepatology. 2008;48(4):1273–81. Epub 2008/09/20. 10.1002/hep.22461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lamontagne J, Mell JC, Bouchard MJ. Transcriptome-Wide Analysis of Hepatitis B Virus-Mediated Changes to Normal Hepatocyte Gene Expression. PLoS Pathog. 2016;12(2):e1005438 10.1371/journal.ppat.1005438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J, Barritt GJ. Evidence that TRPC1 (transient receptor potential canonical 1) forms a Ca(2+)-permeable channel linked to the regulation of cell volume in liver cells obtained using small interfering RNA targeted against TRPC1. Biochem J. 2003;373(Pt 2):327–36. Epub 2003/05/02. 10.1042/BJ20021904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aubart FC, Sassi Y, Coulombe A, Mougenot N, Vrignaud C, Leprince P, et al. RNA interference targeting STIM1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol Ther. 2009;17(3):455–62. Epub 2008/12/25. 10.1038/mt.2008.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol. 2009;11(6):669–77. Epub 2009/06/03. 10.1038/ncb0609-669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, et al. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42(2):205–11. Epub 2007/05/23. 10.1016/j.ceca.2007.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics. 2006;7:159 10.1186/1471-2164-7-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia RL, Schilling WP. Differential expression of mammalian TRP homologues across tissues and cell lines. Biochem Biophys Res Commun. 1997;239(1):279–83. 10.1006/bbrc.1997.7458 [DOI] [PubMed] [Google Scholar]
- 62.Delgado-Coello B, Bravo-Martinez J, Sosa-Garrocho M, Briones-Orta MA, Macias-Silva M, Mas-Oliva J. Plasma membrane calcium ATPase isoform 3 expression in single cells isolated from rat liver. Mol Cell Biochem. 2010;344(1–2):117–24. 10.1007/s11010-010-0535-1 [DOI] [PubMed] [Google Scholar]
- 63.Jovanovic M, Rooney MS, Mertins P, Przybylski D, Chevrier N, Satija R, et al. Immunogenetics. Dynamic profiling of the protein life cycle in response to pathogens. Science. 2015;347(6226):1259038 10.1126/science.1259038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dufour JF, Luthi M, Forestier M, Magnino F. Expression of inositol 1,4,5-trisphosphate receptor isoforms in rat cirrhosis. Hepatology. 1999;30(4):1018–26. 10.1002/hep.510300421 [DOI] [PubMed] [Google Scholar]
- 65.Parekh AB. Store-operated Ca2+ entry: dynamic interplay between endoplasmic reticulum, mitochondria and plasma membrane. J Physiol. 2003;547(Pt 2):333–48. 10.1113/jphysiol.2002.034140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prakriya M, Lewis RS. Potentiation and inhibition of Ca(2+) release-activated Ca(2+) channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. J Physiol. 2001;536(Pt 1):3–19. 10.1111/j.1469-7793.2001.t01-1-00003.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, et al. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40(5–6):553–60. 10.1016/j.ceca.2006.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hajnoczky G, Hager R, Thomas AP. Mitochondria suppress local feedback activation of inositol 1,4, 5-trisphosphate receptors by Ca2+. J Biol Chem. 1999;274(20):14157–62. [DOI] [PubMed] [Google Scholar]
- 69.Hajnocsky G, Robb-Gaspers L, Seitz M, Thomas A. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–24. [DOI] [PubMed] [Google Scholar]
- 70.Glitsch MD, Bakowski D, Parekh AB. Store-operated Ca2+ entry depends on mitochondrial Ca2+ uptake. Embo J. 2002;21(24):6744–54. 10.1093/emboj/cdf675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parekh AB. Store-operated channels: mechanisms and function. J Physiol. 2008;586(13):3033 10.1113/jphysiol.2008.156885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Logan CV, Szabadkai G, Sharpe JA, Parry DA, Torelli S, Childs AM, et al. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat Genet. 2014;46(2):188–93. 10.1038/ng.2851 [DOI] [PubMed] [Google Scholar]
- 73.Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, et al. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell. 2014;53(5):726–37. 10.1016/j.molcel.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca(2)(+) uniporter. Cell Metab. 2013;17(6):976–87. 10.1016/j.cmet.2013.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell. 2012;151(3):630–44. 10.1016/j.cell.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hajnoczky G, Csordas G. Calcium signalling: fishing out molecules of mitochondrial calcium transport. Curr Biol. 2010;20(20):R888–91. 10.1016/j.cub.2010.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467(7313):291–6. 10.1038/nature09358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochim Biophys Acta. 2007;1768(10):2510–5. Epub 2007/07/10. 10.1016/j.bbamem.2007.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gincel D, Zaid H, Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem J. 2001;358(Pt 1):147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Watanabe A, Arai M, Koitabashi N, Niwano K, Ohyama Y, Yamada Y, et al. Mitochondrial transcription factors TFAM and TFB2M regulate Serca2 gene transcription. Cardiovasc Res. 2011;90(1):57–67. 10.1093/cvr/cvq374 [DOI] [PubMed] [Google Scholar]
- 81.Berridge MJ. Calcium signalling remodelling and disease. Biochem Soc Trans. 2012;40(2):297–309. 10.1042/BST20110766 [DOI] [PubMed] [Google Scholar]
- 82.Zhang X, Zhang H, Ye L. Effects of hepatitis B virus X protein on the development of liver cancer. J Lab Clin Med. 2006;147(2):58–66. 10.1016/j.lab.2005.10.003 [DOI] [PubMed] [Google Scholar]
- 83.Kim C, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;353:317–20. [DOI] [PubMed] [Google Scholar]
- 84.Ng SA, Lee C. Hepatitis B virus X gene and hepatocarcinogenesis. J Gastroenterol. 2011;46(8):974–90. 10.1007/s00535-011-0415-9 [DOI] [PubMed] [Google Scholar]
- 85.Koike K. Hepatitis B virus X gene is implicated in liver carcinogenesis. Cancer Lett. 2009;286(1):60–8. Epub 2009/05/26. 10.1016/j.canlet.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 86.Bouchard MJ, Puro RJ, Wang L, Schneider RJ. Activation and inhibition of cellular calcium and tyrosine kinase signaling pathways identify targets of the HBx protein involved in hepatitis B virus replication. J Virol. 2003;77(14):7713–9. Epub 2003/06/28. 10.1128/JVI.77.14.7713-7719.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chami M, Ferrari D, Nicotera P, Paterlini-Brechot P, Rizzuto R. Caspase-dependent alterations of Ca2+ signaling in the induction of apoptosis by hepatitis B virus X protein. J Biol Chem. 2003;278(34):31745–55. 10.1074/jbc.M304202200 [DOI] [PubMed] [Google Scholar]
- 88.Tan C, Guo H, Zheng M, Chen Y, Huang W. Involvement of mitochondrial permeability transition in hepatitis B virus replication. Virus Res. 2009;145(2):307–11. Epub 2009/08/18. 10.1016/j.virusres.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 89.Dellis O, Arbabian A, Papp B, Rowe M, Joab I, Chomienne C. Epstein-Barr virus latent membrane protein 1 increases calcium influx through store-operated channels in B lymphoid cells. J Biol Chem. 2011;286(21):18583–92. 10.1074/jbc.M111.222257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hyser JM, Utama B, Crawford SE, Broughman JR, Estes MK. Activation of the endoplasmic reticulum calcium sensor STIM1 and store-operated calcium entry by rotavirus requires NSP4 viroporin activity. J Virol. 2013;87(24):13579–88. 10.1128/JVI.02629-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hyser JM, Collinson-Pautz MR, Utama B, Estes MK. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity. mBio. 2010;1(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nieva JL, Madan V, Carrasco L. Viroporins: structure and biological functions. Nat Rev Microbiol. 2012;10(8):563–74. 10.1038/nrmicro2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, et al. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell. 2010;38(2):280–90. 10.1016/j.molcel.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 94.Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell. 2010;39(1):121–32. 10.1016/j.molcel.2010.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paszty K, Caride AJ, Bajzer Z, Offord CP, Padanyi R, Hegedus L, et al. Plasma membrane Ca(2)(+)-ATPases can shape the pattern of Ca(2)(+) transients induced by store-operated Ca(2)(+) entry. Sci Signal. 2015;8(364):ra19 10.1126/scisignal.2005672 [DOI] [PubMed] [Google Scholar]
- 96.Philippe R, Antigny F, Buscaglia P, Norez C, Becq F, Frieden M, et al. SERCA and PMCA pumps contribute to the deregulation of Ca(2+) homeostasis in human CF epithelial cells. Biochim Biophys Acta. 2015;1853(5):892–903. 10.1016/j.bbamcr.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 97.Griffin DS, Segall HJ. Role of cellular calcium homeostasis in toxic liver injury induced by the pyrrolizidine alkaloid senecionine and the alkenal trans-4-OH-2-hexenal. Journal of biochemical toxicology. 1987;2:155–67. [DOI] [PubMed] [Google Scholar]
- 98.Mine T, Kojima I, Ogata E. Evidence of cyclic AMP-independent action of glucagon on calcium mobilization in rat hepatocytes. Biochim Biophys Acta. 1988;970(2):166–71. [DOI] [PubMed] [Google Scholar]
- 99.Rodrigues MA, Gomes DA, Andrade VA, Leite MF, Nathanson MH. Insulin induces calcium signals in the nucleus of rat hepatocytes. Hepatology. 2008;48(5):1621–31. 10.1002/hep.22424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.S P. Isolation of hepatocytes by collagenase perfusion, vol. 1 Academic Press, New York: 1993. [Google Scholar]
- 101.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
I have uploaded all the data for the figures into the website “Open Science Framework”. The address for the data is: https://osf.io/rtvhk/.