Abstract
Global climate change not only leads to elevated seawater temperatures but also to episodic anomalously high or low temperatures lasting for several hours to days. Scleractinian corals are detrimentally affected by thermal fluctuations, which often lead to an uncoupling of their mutualism with Symbiodinium spp. (coral bleaching) and potentially coral death. Consequently, on many Caribbean reefs scleractinian coral cover has plummeted. Conversely, gorgonian corals persist, with their abundance even increasing. How gorgonians react to thermal anomalies has been investigated utilizing limited parameters of either the gorgonian, Symbiodinium or the combined symbiosis (holobiont). We employed a holistic approach to examine the effect of an experimental five-day elevated temperature episode on parameters of the host, symbiont, and the holobiont in Eunicea tourneforti, E. flexuosa and Pseudoplexaura porosa. These gorgonian corals reacted and coped with 32°C seawater temperatures. Neither Symbiodinium genotypes nor densities differed between the ambient 29.5°C and 32°C. Chlorophyll a and c2 per Symbiodinium cell, however, were lower at 32°C leading to a reduction in chlorophyll content in the branches and an associated reduction in estimated absorbance and increase in the chlorophyll a specific absorption coefficient. The adjustments in the photochemical parameters led to changes in photochemical efficiencies, although these too showed that the gorgonians were coping. For example, the maximum excitation pressure, Qm, was significantly lower at 32°C than at 29.5°C. In addition, although per dry weight the amount of protein and lipids were lower at 32°C, the overall energy content in the tissues did not differ between the temperatures. Antioxidant activity either remained the same or increased following exposure to 32°C further reiterating a response that dealt with the stressor. Taken together, the capability of Caribbean gorgonian corals to modify symbiont, host and consequently holobiont parameters may partially explain their persistence on reefs faced with climate change.
Introduction
Global climate change affects many ecosystems, including coral reefs [1]. One aspect of climate change is the rise of seawater temperatures that is anticipated to continue into the future [1, 2]. In addition, short-term fluctuations in prevailing temperatures over several hours or days are also projected to occur more frequently [3–5]. Exposure to seawater temperatures even 2°C above the mean summer maximum can adversely affect corals and their mutualistic endosymbiotic dinoflagellate algae, Symbiodinium spp. [3]. Numerous studies have investigated the predominantly detrimental effects of elevated seawater temperatures on scleractinian coral—Symbiodinium symbioses (reviewed in [6, 7, 8]), but such data on other abundant coral reef cnidarians, such as octocorals, lag behind.
In the Caribbean, for example, over the past few decades, scleractinian coral cover has dramatically declined [9, 10] concurrent with a rise in seawater temperatures by 0.2–0.4°C/decade between 1985 and 2006 [11]. On the other hand, the abundance of Caribbean octocorals, predominantly gorgonian corals, has remained the same or even increased [12–15]. In fact, gorgonian corals constitute the dominant benthic fauna on many Caribbean reefs [13, 14, 16, 17], where they provide food and shelter to a variety of invertebrates and fish [18–21]. Therefore, in order to understand the future of Caribbean reefs, it is imperative to determine the effects of potential stressors, such as elevated seawater temperatures, on gorgonian corals.
In corals, thermal stress often leads to a reduction in Symbiodinium numbers and/or the amount of chlorophyll within the remaining Symbiodinium, which is commonly referred to as coral bleaching [22]. The elevated temperatures can disrupt Symbiodinium photosynthesis by hindering the repair of damaged photosystems [23], increasing the production of reactive oxygen species (ROS) that impair the thylakoid membranes [24], and inhibiting enzymes responsible for carbon fixation [25]. In addition, the production of high levels of nitric oxide (NO) in thermally stressed Symbiodinium can result in apoptosis [26]. Sensitivity to thermal stress can vary between different Symbiodinium clades and sub-cladal types [27, 28].
Detrimental effects on the Symbiodinium may alter the nutrient exchange between the partners. Symbiodinium supply their host with carbohydrates, lipids, and essential and mycosporine-like amino acids [29–31], while the host provides Symbiodinium with carbon, nitrogen, nutrients, and an environment for photosynthesis [32–35]. Disruption of the symbiosis may alter nutrient exchange between the partners, the amount of energy required to maintain homeostasis, and drive the coral host and its symbionts to utilize their energy reserves [36]. For example, thermally stressed scleractinian corals and octocorals in the Indo-Pacific exhibit a drop in tissue reserves like lipids, proteins and carbohydrates [37–40]. In scleractinian corals, tolerance to, and the capacity to recover from, thermal stress is linked to the amounts of tissue reserves available [41, 42].
Faced with stressors, Symbiodinium and corals can utilize several mechanisms to mitigate dysfunction in their cells. By increasing the activities of antioxidant enzymes like superoxide dismutase (SOD) they can convert superoxide to H2O2, and then further break H2O2 down with peroxidase (POX) and catalase (CAT) to water and O2 [28, 43, 44]. Corals can also reduce damage to proteins by increasing the production of heat shock proteins (Hsp) [43–45]. As in Symbiodinium, the ability of corals to cope with thermal stress can vary between different host taxa [43, 46]. For example, Porites cylindrica, which possessed higher levels of SOD and Hsp than Stylophora pistillata, was better able to cope with, and recover from, thermal stress [43].
In contrast to the plethora of studies on the effects of elevated temperatures on scleractinian corals, only a handful of studies investigated the potential consequences of elevated seawater temperatures on Caribbean gorgonians. These studies focused only on a few parameters such as on the production of ROS, NO and Hsp90 [47, 48], the effects of pathogens on gorgonian corals at ambient and elevated temperatures [49–51] and the effects of ultraviolet radiation in conjunction with elevated temperatures [52]. We decided to employ a holistic approach to determine the effects of elevated temperature on multiple parameters of the gorgonian host, the Symbiodinium and the subsequent holobiont in representative species of these important Caribbean reef taxa.
Methods
Experimental setup
We assessed the effect of experimental short-term exposure to elevated temperature on the gorgonian species Eunicea tourneforti, E. flexuosa, and Pseudoplexaura porosa. From each species, 12 colonies located at a depth of 3–4m on a patch reef adjacent to the pier of the Instituto de Ciencas del Mar y Limnología (ICMyL), Universidad Nacional Autónoma de México (UNAM), at Puerto Morelos, México (20°52'5.23"N, 86°51'58.92"W) were sampled. Field permit was granted by Secretaria de Agricultura, Desarrollo Rural, Pesca y Alimentación (SAGARPA), permit No. GDOPA 08606.251011.3021. From each colony, two branches, 12–14cm long, were excised, attached vertically onto PVC stands, and separated into two outdoor flow-through aquaria. The temperature in both aquaria was controlled using an aquarium chiller (0.5hp Delta Star, Aqua Logic, USA) and two heaters (1000W and 1800W EasyPlug heater, Process Technologies, USA). To mimic light levels on the patch reef, garden shade cloth was placed over the tanks to reduce the incident irradiance by about 50%.
For a 14-day acclimation period, the temperature in both aquaria was held at 29.5°C. This temperature is similar to the ambient mean monthly summer (May-August) seawater temperature on local shallow reefs ranging from 29°C to 30°C [53, 54]. After the 14 acclimation days, the control aquarium was kept at the ambient 29.5°C, while the temperature in the treatment aquarium was raised, 1°C/day over three days, to 32°C. The 32°C treatment represented a 2–3°C increase above typical summer temperatures and the predicted average sea surface temperature by 2099 [55]. In addition, 32°C is 2°C greater than the bleaching threshold temperature of 30°C at this location (NOAA Coral Reef Watch Virtual Station Puerto Morelos, Mexico). Indeed, exposure to 31.5–32°C is stressful for scleractinian corals from this area and leads to coral bleaching [56–60]. When the treatment aquarium reached 32°C the experiment began. The gorgonian branches were kept in the ambient and elevated temperature treatments for five days following which the branches were processed.
Photochemical efficiency of photosystem II
Throughout the experiment, the maximum (at dusk, Fv/Fm) and effective (at local noon, ΔF/Fm`) photochemical yields were measured daily using a Diving PAM (Walz, Germany). Plastic tubing attached to the distal end of the probe ensured a fixed distance between it and the gorgonian tissue. For each branch, the photochemical yields were measured at three locations, in the upper one-third, middle, and lower one-third of the branch. The maximum excitation pressure over photosystem II (Qm) was determined by modifying the formula in Iglesias-Prieto et al. [61] to Qm = 1 –[(ΔF/Fm`at noon) / (Fv/Fm at dusk on the preceding day)].
Estimated absorbance
After the five experimental days, while each gorgonian branch was immersed in seawater maintained at its respective experimental temperature (29.5°C or 32°C), the reflectance spectrum of a region located 2-4cm from the branch tip was recorded using the protocol described in Ramsby et al. [62]. Reflectance was converted to estimated absorbance (De), and De at 675nm was used to calculate light absorbed by Chl a, and the Chl a specific absorption coefficient (a*Chl a) [63].
Sample processing
Following absorbance determination, a 2cm fragment, 2-4cm from the branch tip, was excised, and its length and diameter were recorded. Both this fragment and the remaining branch were flash-frozen in liquid nitrogen and stored at -80°C until further processing. To isolate Symbiodinium cells, the 2cm fragment was ground, and the cells were separated by filtration and a series of washes with 0.2μm-filtered seawater (FSW) [64]. The Symbiodinium cells were re-suspended in FSW and aliquots were taken for determination of density, chlorophyll content and genetic identification.
Symbiodinium cell density and chlorophyll content
From one Symbiodinium aliquot, in a minimum of three 100μl subsamples, Symbiodinium cells were counted using the FlowCAM Imaging Particle Analyzer (Fluid Imaging Technologies, Maine, USA), which pumps a liquid sample through a flow cell and captures images of the microscopic particles suspended in it [65]. Symbiodinium were distinguished from other particles (like cellular and sclerite debris) using a value filter based on their diameter, circle fit, and red:blue color ratio. Symbiodinium cell counts were standardized to surface area of the excised 2cm-long branch fragment. The Symbiodinium in a second aliquot were pelleted, the FSW removed, and a mixture of acetone and dimethyl sulfoxide 95:5 v/v [64] was added for 24h to extract Chlorophylls a (Chl a) and c2 (Chl c2), and their concentrations determined using the equations of Jeffrey and Humphrey [66]. The total amount of Chl a and c2 extracted was standardized to either the surface area of the excised 2cm-long fragment (Chl cm-2) or the number of Symbiodinium cells isolated from that branch fragment (Chl cell-1).
Genetic identification of Symbiodinium
The Symbiodinium in a third aliquot were also pelleted, their DNA extracted, and the internal transcribed spacer 2 region (ITS2) of the ribosomal DNA was PCR amplified using the protocol of LaJeunesse et al. [67], with an addition of a final extension step of 30min at 72°C to reduce the formation of heteroduplexes [68]. The amplified product was separated with Denaturing Gradient Gel Electrophoresis (DGGE) on a polyacrylamide gel containing a 45–80% gradient of urea [67], with modifications described in Shirur et al. [64]. Banding profiles were compared between samples, and prominent bands from unique profiles were excised, re-amplified, and sequenced [69]. Some DGGE profiles contained multiple prominent bands, and in E. flexuosa, with the exception of the lowest band, the others were heteroduplexes (Fig 1). These bands were re-amplified [67, 68], and their constituents were separated on a polyacrylamide gel with a 55–75% denaturant gradient. The resulting bands were then excised, re-amplified, and sequenced. In addition to the ITS2, the microsatellite locus Sym15 was amplified [70], and its flanker regions were used for Symbiodinium lineage identification [71–74]. Both the ITS2 and Sym15 loci were sequenced at the DNA Lab at Arizona State University. DNA sequences were compared to those in GenBank, and novel sequences deposited to GenBank.
Fig 1. DGGE gel of Symbiodinium types hosted by the Caribbean gorgonian corals Eunicea tourneforti, E. flexuosa and Pseudoplexaura porosa.
Bands characteristic of a particular Symbiodinium type are marked with white arrows. Black arrow heads denote heteroduplexes formed during the PCR process.
Biochemical composition
Using the protocols described in Shirur et al. [64], from ground lyophilized gorgonian branch pieces the amount of sclerites, protein, lipid, carbohydrate and refractory content per dry weight (%g DW) and the amount of protein, lipid, carbohydrate and refractory content per organic matter (%g OM) were determined. The protein, lipid and carbohydrate content within the organic matter and their specific enthalpies of combustion [75] were then used to calculate the total energy content of tissue reserves [76].
Enzyme activity
To quantify enzyme activity, proteins were extracted from 5cm-long frozen branch fragments using the protocol of Mydlarz and Harvell [77]. The activities of SOD, POX, and CAT in the final supernatant (crude protein extract) were measured using a Synergy HT microplate reader (Biotek, USA). To determine SOD activity, 5μl of the extract was diluted in 15ul of PBS (pH 7.8) and the activity was quantified at 37°C using the SOD assay kit (Sigma-Aldrich, USA). To measure POX activity, the protocol described in Shirur et al. [78] was modified, such that the reaction was initiated by adding 20mM H2O2 to E. tourneforti samples, and 25mM H2O2 to E. flexuosa samples. The optical density at 470nm was recorded every minute for 30min. To measure CAT activity, a 5μl aliquot of the crude extract was diluted in 150μl of 50mM H2O2 (in 50mM PBS, pH 7.0), and the breakdown of H2O2 was tracked at 240nm every 30s for 20min. Both POX and CAT assays were run at room temperature (25–27°C), and from the linear portion of the curves, their activities were calculated as the change in optical density per minute over an 8min and 10min interval, respectively. CAT activity was further converted to mM H2O2 scavenged during the assay from a standard curve generated using different concentrations of H2O2 [79–81]. Activities of all three enzymes were normalized to protein content of the aliquot used in each assay [77, 79, 82], and are reported as ΔAbs450nm mg protein-1 and ΔAbs470nm min-1 mg protein-1 for SOD and POX respectively, and mM H2O2 scavenged min-1 mg protein-1 for CAT [77, 79–85].
Protein content (mg/ml) of the extract was quantified using the RED660™ Protein Assay Kit (G-Biosciences). Enzyme activity in P. porosa branches could not be determined because the high mucus and lipid content interfered with the assays. Similarly, we could not obtain reliable values for CAT activity in E. tourneforti. Therefore, the activities of SOD and POX were determined for both Eunicea species, but CAT activity was only obtained for E. flexuosa.
Statistical analyses
We used linear mixed effects models fit by the restricted maximum likelihood method to analyze the data. For the majority of the data, the two fixed effects in the model were the gorgonian species (E. tourneforti, E. flexuosa and P. porosa) and temperature (ambient and elevated). Data on photochemistry was analyzed with a similar mixed effects model, with the time of sampling as the third fixed effect. Since multiple colonies were sampled per species, inter-colony variation was accounted for in the mixed model by nesting each colony within its parent species, and treating it as the random effect. For some parameters, samples from one of the two branches from the same colony were not measured (e.g. sample loss), therefore data from that particular colony were excluded from the statistical analysis of that parameter.
For all data, residuals were examined for normality and homogeneity of variances, and data were square, square root, log or reciprocal transformed when these assumptions were violated (S1 Table). When significant species by temperature interactions were detected, they were explored using six non-orthogonal planned contrasts. The first three contrasts tested the effect of elevated temperature on each species separately. The other three contrasts were pairwise comparisons testing whether the magnitude of the effect of temperature differed between the three gorgonian species. For the photochemical efficiency of photosystem II parameters, ΔF/Fm`and Qm had significant temperature by time interactions and they were explored with five orthogonal planned contrasts that tested the effect of elevated temperature on each day separately. For Fv/Fm, significant species by temperature by time interaction were explored with 27 non-orthogonal planned contrasts. The first 15 contrasts tested the effect of elevated temperature on each species on each experimental day. Then for each species, subsequent contrasts tested whether the magnitude of the effect of temperature differed between each consecutive day (day 1 versus 2, 2 versus 3, 3 versus 4, and 4 versus 5). P-values for all non-orthogonal planned contrasts were corrected using the method of Holm [86]. All analyses were performed using the packages “lme4” [87], “lmerTest” [88] and “multcomp” [89] with the R software version 3.0.2 [90].
Results
Symbiodinium parameters
Exposure to five days at the elevated temperature of 32°C did not lead to a significant change in Symbiodinium density (Fig 2A, Tables 1 and 2, S1 Table). On the other hand, Chl a and c2 contents per Symbiodinium cell were significantly lower after five days at elevated temperature than their levels in branches held at ambient temperature (Fig 2B, Table 1, S1 Table). This significant difference in Chl a per cell was primarily driven by P. porosa since the Chl a content per Symbiodinium cell in branches of the Eunicea species exposed to the elevated temperature did not significantly differ from levels in branches maintained at ambient temperature (Table 2). In conjunction with no significant change in Symbiodinium density but a decrease in chlorophyll per Symbiodinium cell, the amounts of Chl a and c2 per surface area of gorgonian branches were significantly lower after five days at elevated temperature compared to their content in branches held at ambient temperature (Fig 2C, Table 1, S1 Table). The effect of temperature on Chl a and c2 content was significantly less in the Eunicea species than in P. porosa (Table 2). Consequently, the Chl a:c2 ratio in the Eunicea species did not differ between ambient and elevated temperatures while it did in P. porosa (Tables 1 and 2, S1 Table).
Fig 2. Symbiodinium parameters in branches of the Caribbean gorgonian corals Eunicea tourneforti, E. flexuosa and Pseudoplexaura porosa exposed to ambient and elevated temperatures.
Symbiodinium (Sym) density (A), and chlorophyll a (Chl a) content per Symbiodinium cell (B), and per surface area (C) after five days at ambient, 29.5°C (gray) or elevated, 32°C (white) temperatures. Data are mean ± SE. Mixed model analyses that yielded significant species (species), temperature (temp) and/or interaction (int) effects are noted in the panels, (*) denotes comparisons in which the interaction term was significant and the planned contrast analyses detected significant temperature effects. Sample sizes in panels A, B, C were 11, 9, and 10 in E. tourneforti, 12, 11 and 11 in E. flexuosa, and 10 in A-C in P. porosa.
Table 1. Symbiodinium and holobiont parameters in branches of the Caribbean gorgonian corals Eunicea tourneforti, E. flexuosa and Pseudoplexaura porosa after exposure to ambient (29.5°C) or elevated (32°C) temperatures for five days.
| Parameter | E. tourneforti (10) | E. flexuosa (11) | P. porosa (10) | |||
|---|---|---|---|---|---|---|
| Ambient | Elevated | Ambient | Elevated | Ambient | Elevated | |
| Symbiodinium density (106 cells cm-2) | 1.73 ± 0.23 (%) | 1.70 ± 0.28 (%) | 1.35 ± 0.25 (^) | 1.34 ± 0.30 (^) | 4.22 ± 0.68 | 6.48 ± 1.01 |
| Chlorophyll a content (pg cell-1) | 2.88 ± 0.24 (#) | 3.31 ± 0.98 (#) | 4.85 ± 1.29 | 2.69 ± 0.59 | 4.77 ± 1.71 | 1.20 ± 0.11 |
| Chlorophyll c2 content (pg cell-1) | 0.78 ± 0.06 (#) | 0.82 ± 0.21 (#) | 1.55 ± 0.44 | 0.82 ± 0.17 | 1.25 ± 0.44 | 0.35 ± 0.03 |
| Chlorophyll a content (μg cm-2) | 4.77 ± 0.53 | 3.49 ± 0.37 | 3.84 ± 0.46 | 2.53 ± 0.37 | 12.92 ± 0.60 | 6.87 ± 0.47 |
| Chlorophyll c2 content (μg cm-2) | 1.30 ± 0.15 | 0.93 ± 0.11 | 1.15 ± 0.11 | 0.78 ± 0.11 | 3.43 ± 0.15 | 2.03 ± 0.14 |
| Chlorophyll a:c2 ratio | 3.69 ± 0.08 | 3.86 ± 0.13 | 3.29 ± 0.13 | 3.24 ± 0.06 | 3.78 ± 0.06 | 3.39 ± 0.09 |
| De, estimated absorbance of Chl a | 0.44 ± 0.04 (^) | 0.38 ± 0.04 (^) | 0.56 ± 0.03 (^) | 0.43 ± 0.04 (^) | 0.74 ± 0.04 | 0.56 ± 0.03 |
| a*Chl a (m2 mg-1 Chl a) | 0.02 ± 0.004 | 0.03 ± 0.002 | 0.04 ± 0.003 | 0.04 ± 0.003 | 0.01 ± 0.001 | 0.02 ± 0.001 |
| SOD activity | 782.91 ± 33.46 (%) | 787.84 ± 43.97 (%) | 485.19 ± 26.27 (^) | 423.57 ± 15.55 (^) | NA | NA |
| POX activity | 0.23 ± 0.03 (!) | 0.52 ± 0.10 (!) | 0.56 ± 0.15 ($) | 0.36 ± 0.06 ($) | NA | NA |
| CAT activity | NA | NA | 196.47 ± 33.86 (^) | 173.61 ± 19.77 (^) | NA | NA |
| Sclerite content (%g DW) | 84.12 ± 1.18 (^) | 85.62 ± 1.40 (^) | 80.88 ± 1.22 (^) | 81.71 ± 1.25 (^) | 50.97 ± 1.81 | 56.18 ± 2.31 |
| Refractory content (%g DW) | 12.13 ± 1.25 | 11.47 ± 1.57 | 13.10 ± 1.14 | 12.62 ± 1.38 | 21.09 ± 0.71 | 19.44 ± 1.24 |
| Protein content (%g DW) | 0.65 ± 0.06 (^) | 0.48 ± 0.04 (^) | 1.84 ± 0.16 (^) | 1.53 ± 0.14 (^) | 9.97 ± 0.56 | 8.37 ± 0.59 |
| Lipid content (%g DW) | 2.44 ± 0.21 | 2.15 ± 0.21 | 2.96 ± 0.25 | 2.71 ± 0.26 | 14.81 ± 0.98 | 12.89 ± 0.92 |
| Carbohydrate content (%g DW) | 0.95 ± 0.10 (^) | 0.93 ± 0.05 | 1.60 ± 0.12 (^) | 1.48 ± 0.07 | 3.16 ± 0.21 | 3.12 ± 0.31 |
| Refractory content (%g OM) | 73.94 ± 2.25 | 74.81 ± 2.32 | 66.33 ± 2.43 | 67.26 ± 3.10 | 43.27 ± 1.31 | 44.47 ± 1.59 |
| Protein content (%g OM) | 4.14 ± 0.26 (^) | 3.55 ± 0.33 (^) | 9.95 ± 0.89 (^) | 8.75 ± 0.89 (^) | 20.28 ± 0.78 | 19.15 ± 0.60 |
| Lipid content (%g OM) | 15.64 ± 1.44 | 15.33 ± 1.77 | 15.57 ± 1.23 | 15.53 ± 1.82 | 29.94 ± 1.07 | 29.30 ± 1.40 |
| Carbohydrate content (%g OM) | 6.15 ± 0.65 (^) | 7.04 ± 0.65 (^) | 8.65 ± 0.73 (^) | 8.38 ± 0.58 (^) | 6.51 ± 0.42 | 7.31 ± 0.78 |
| Energy content (kJ g-1 OM) | 8.26 ± 0.72 | 8.00 ± 0.80 | 9.94 ± 0.74 | 9.70 ± 0.99 | 17.81 ± 0.45 | 17.38 ± 0.58 |
Values are mean ± SE. (n) = number of different colonies with branches both at ambient and elevated temperatures and n = 8 (!), n = 9 (#), n = 10 ($), n = 11 (%), n = 12 (^) designating other sample sizes. a*Chl a = Chlorophyll (Chl) a specific absorption coefficient, SOD = Superoxide dismutase (ΔAbs450 mg protein-1), CAT = Catalase (mM H2O2 scavenged min-1 mg protein-1), POX = Peroxidase (ΔAbs470 mg protein-1), DW = Dry weight, OM = Weight of organic matter, NA = Not available and pertains to assays that could not be conducted.
Table 2. Summary of the results of a linear mixed effects model analyses testing the effect of elevated temperature on Symbiodinium parameters in the Caribbean gorgonian corals Eunicea tourneforti (ET), E. flexuosa (EF) and Pseudoplexaura porosa (PP).
| Parameter | Species (P<0.05) |
Temp (P<0.05) |
Species * Temp (P<0.05) | |||||
|---|---|---|---|---|---|---|---|---|
| ET | EF | PP | Magnitude of the effect | |||||
| A vs E | A vs E | A vs E | A vs E | ET vs EF | ET vs PP | EF vs PP | ||
| Symbiodinium density (106 cells cm-2) | (ET-EF)<PP | - | ||||||
| Chlorophyll a content (pg cell-1) | - | > | - | - | > | - | < | - |
| Chlorophyll c2 content (pg cell-1) | - | > | ||||||
| Chlorophyll a content (μg cm-2) | (ET-EF)<PP | > | > | > | > | - | < | < |
| Chlorophyll c2 content (μg cm-2) | (ET-EF)<PP | > | > | > | > | - | < | < |
| Chlorophyll a:c2 ratio | (ET-PP)>EF | - | - | - | > | - | < | - |
| De, estimated absorbance of Chl a | ET<EF<PP | > | ||||||
| a*Chl a (m2 mg-1 Chl a) | EF>ET>PP | < | - | - | < | - | - | < |
Comparisons where P < 0.05 are further delineated with ‘<‘ or ‘>‘ to denote the trend in the differences, and those where P > 0.05 are indicated with ‘-’. In case of a significant interaction, planned contrast analyses were run to test the effect of temperature (A = Ambient, 29.5°C, E = Elevated, 32°C) on each species, and the magnitude of the effect on each possible species pair combinations. P values were adjusted using the Holm’s method [86]. Chl = Chlorophyll, Temp = Temperature, a*Chl a = Chl a specific absorption coefficient.
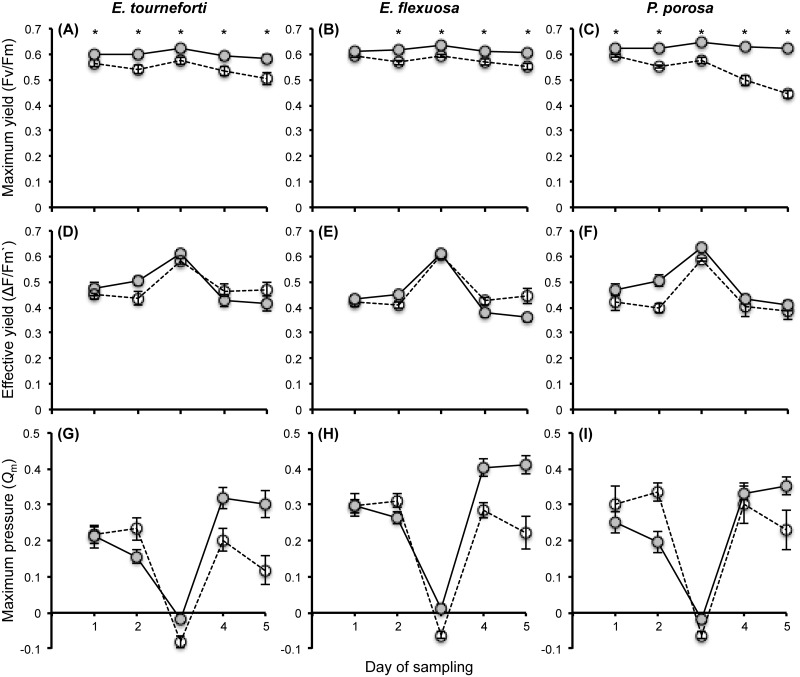
Fv/Fm, ΔF/Fm`and Qm did not significantly differ between the upper, middle and lower regions of each branch, and therefore the values from these regions were pooled. In the three gorgonian species, Fv/Fm in branches exposed to elevated temperature were significantly lower than in those maintained at ambient temperature (Fig 3A–3C, S1 Table). In P. porosa, Fv/Fm at elevated temperature declined over time, such that there was a larger reduction in Fv/Fm after four and five days than during the first three days of exposure to elevated temperature (Fig 3C). In contrast to Fv/Fm, ΔF/Fm`in the Eunicea species did not differ between the two temperatures, while in P. porosa a 10% reduction in ΔF/Fm`at the elevated temperature was significant compared to the control (Fig 3D–3F, S1 Table). This significant difference was probably driven by the first days at the elevated temperature. Examining ΔF/Fm`of the three gorgonian species throughout the experiment showed that on days 1, 3, and 4 there were no differences in ΔF/Fm`between branches at ambient and elevated temperatures, on day 2 there was a reduction in ΔF/Fm`at elevated temperature, but on day 5 ΔF/Fm`was actually significantly higher at the elevated compared to the ambient temperature (Fig 3D–3F, S1 Table). Mirroring the changes in photochemical efficiency, Qm after two and three days at elevated temperature was significantly higher than the Qm in branches held at ambient temperature (Fig 3G–3I, S1 Table). But after four and five days, Qm in branches exposed to the elevated temperature was actually significantly lower than Qm in branches maintained at ambient temperature (Fig 3G–3I).
Fig 3. Symbiodinium photochemical parameters in branches of the Caribbean gorgonian corals Eunicea tourneforti, E. flexuosa and Pseudoplexaura porosa exposed to ambient and elevated temperatures.
The maximum (A-C) and effective (D-F) photochemical yields, and the maximum pressure over photosystem II (G-I) of Symbiodinium in branches exposed to ambient, 29.5°C (solid line, gray circles) and elevated, 32°C (dashed line, open circles) temperatures for five days. Data are mean ± SE, with n = 8 for E. tourneforti and E. flexuosa and n = 6 for P. porosa. (*) indicate significant temperature effects detected in the planned contrast analyses of the three-way interaction for Fv/Fm.
Gorgonian branches exposed to elevated temperature exhibited significantly lower estimated absorbance, De, and greater a*Chl a compared to branches maintained at ambient temperature (Tables 1 and 2, S1 Table). The pattern in a*Chl a was driven by the significant differences in P. porosa a*Chl a since the a*Chl a in branches of the Eunicea species did not significantly differ between branches at ambient or elevated temperatures (Table 2).
Symbiodinium genotypes
Based on the ITS2 region of the ribosomal DNA, five different Symbiodinium types within clade B resided in the three gorgonian species (Fig 1). All 12 E. tourneforti colonies hosted a newly named Symbiodinium type B41 (Fig 1, Genbank accession no. KX344963). E. flexuosa also hosted new Symbiodinium types, with four colonies containing Symbiodinium type B41a (Fig 1, Genbank accession no. KX344964), and the remaining eight colonies associating with Symbiodinium type B41b (Fig 1, Genbank accession no. KX344965). Among P. porosa colonies, nine colonies harbored the previously characterized Symbiodinium type B1i (Fig 1, [72]: Genbank accession no. GU907636), one colony hosted the new Symbiodinium, type B42 (Fig 1, Genbank accession no. KX344981), while the remaining two colonies simultaneously associated with types B1i and B42 (Fig 1). All sequence files are available from the Genbank database (accession numbers KX344963, KX344964, KX344965, KX344981). Hosting different Symbiodinium types within a gorgonian species did not affect the parameters measured and therefore all branch pairs (ambient and elevated) were used in the analyses. Regardless of the Symbiodinium type hosted, in all three gorgonian species, the elevated temperature did not cause a change in Symbiodinium type.
In addition to the three gorgonian species hosting different Symbiodinium types, these Symbiodinium belonged to three different lineages. Symbiodinium type B41 in E. tourneforti belonged to one Symbiodinium lineage (Genbank accession no. KX344969). Although E. flexuosa hosted both Symbiodinium types B41a and B41b, the Sym15 flanker sequences, and hence the lineage, were identical for both types (Genbank accession no. KX344973). Similarly, even though P. porosa hosted Symbiodinium type B1i, B42, or a mixture of both, the Sym15 flanker sequences of both types were identical (Genbank accession no. KX344977). As with the ITS2 types, elevated temperature did not alter the microsatellite Sym15 flanker regions in the respective colonies and species.
Biochemical composition of tissues
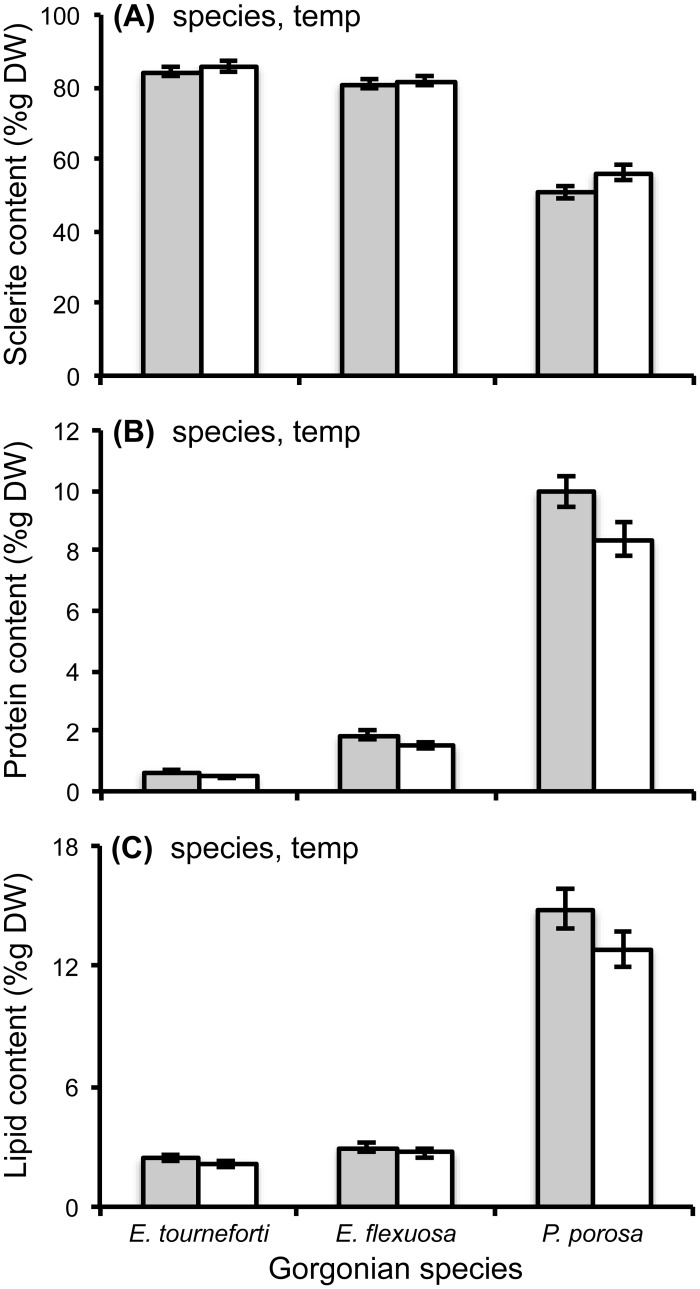
Per dry weight (%g DW), sclerite content was significantly higher, while protein and lipid contents were significantly lower, in gorgonian branches exposed to 32°C than in those maintained at ambient temperature (Fig 4A–4C, Table 1, S1 Table). Protein content per organic matter (%g OM) was also significantly lower in gorgonian branches exposed to elevated temperature than in those held at the ambient temperature (Table 1, S1 Table). On the other hand, the carbohydrate content per dry weight and per organic matter, the lipid content per organic matter, and the total energy content of the tissues did not significantly differ between branches held at ambient and elevated temperatures (Table 1, S1 Table).
Fig 4. Biochemical parameters in branches of the Caribbean gorgonian corals Eunicea tourneforti, E. flexuosa and Pseudoplexaura porosa exposed to ambient and elevated temperatures.
Sclerite (A), protein (B) and lipid (C) contents per dry weight (%g DW) after five days at ambient, 29.5°C (gray) and elevated, 32°C (white) temperatures. Data are mean ± SE. Mixed model analyses that yielded significant species (species) and/or temperature (temp) effects are noted in the panels. Sample sizes in panels A, B, C were 12, 12, and 10 in E. tourneforti, 12, 12 and 11 in E. flexuosa, and 10 in A-C in P. porosa.
Enzyme activity
Compared to ambient levels, exposure to 32°C for five days did not cause a significant change in the SOD activity in branches of both Eunicea species (Table 1, S1 Table), and CAT and POX activity in E. flexuosa branches (Table 1, S1 Table, CAT: Paired t test, t11 = 0.74, P = 0.475). In contrast, five days at 32°C significantly increased POX activity in branches of E. tourneforti compared to the branches held at the ambient temperature (Table 1, S1 Table).
Discussion
Thermal fluctuations that expose coral reefs to anomalously high or low seawater temperatures for several hours or days are projected to occur more frequently in the future [3–5]. In scleractinian corals subjected to experimental conditions simulating such events, a 50–80% reduction in Symbiodinium density often occurs [39, 43, 91–96]. Subsequently, scleractinian corals may recover from the bleaching event. Conversely, the loss of Symbiodinium, compounded with the other stress responses, may lead to the demise of the host [39, 43, 94–96]. While we mimicked a short term thermal event by exposing branches of three gorgonian species, Eunicea tourneforti, E. flexuosa, and Pseudoplexaura porosa, to an elevated 32°C seawater temperature, the Symbiodinium densities in these branches did not significantly differ from Symbiodinium densities in branches from the same colonies maintained at the ambient temperature of 29.5°C. Furthermore, in P. porosa, Symbiodinium densities at the elevated temperature were actually higher, not lower, compared to those at the ambient temperature, although not significantly so (Fig 2). The ability to continue hosting the same Symbiodinium density at elevated temperatures may be one reason why Caribbean gorgonians are maintaining or increasing their abundance on Caribbean coral reefs while scleractinian coral cover is declining [9, 10, 12–15].
Although the elevated temperature did not significantly alter the Symbiodinium density in the three gorgonian species, the Symbiodinium in branches of the gorgonian corals did react to the change in environmental conditions by modifying other Symbiodinium parameters. For example, at 32°C there was less Chl a and Chl c2 per Symbiodinium cell which, in turn, affected the amount of chlorophyll per surface area (Fig 2). Absorbance was less and, concomitantly, a* was more at the elevated temperature. Chlorophyll content can be altered over short timescales in response to changes in the environment (reviewed in [97]), and is a quicker response than Symbiodinium re-population, following symbiont loss, which can take from six weeks [58] to over two years [98].
The adjustments in pigments and subsequent light capture could in turn affect photochemical efficiency. In scleractinian corals, thermal stress can hamper symbiont photochemistry and photosynthesis [57, 91, 92], resulting in reductions in both Fv/Fm and ΔF/Fm`[43, 99, 100], leading to Qm values of 0.8 or above [99, 101, 102]. In the three gorgonian species, Fv/Fm at the elevated temperature was reduced, but Fv/Fm can also be lower due to activation of photoprotective processes [103]. In the Eunicea species, ΔF/Fm`did not mirror the reduction in Fv/Fm and was not affected by the elevated temperatures (Fig 3). In P. porosa a 10% significant reduction in ΔF/Fm`occurred, but this reduction was much smaller than the >50% reduction in ΔF/Fm`recorded in thermally stressed scleractinian corals [43, 99, 100]. Furthermore, looking at daily changes in ΔF/Fm`in all three gorgonian species demonstrated that by day 5, ΔF/Fm`was actually higher at the elevated than at the ambient temperature. Lastly, in all three gorgonian species, the Qm in branches exposed to 32°C was either similar to or lower than the Qm in branches held at the 29.5°C ambient temperature, with Qm values being lower than 0.5 (Fig 3). Given the numerous parameters related to photosynthesis, in addition to the maintenance of symbiont densities, the Symbiodinium appeared to not be photosynthetically compromised.
Concomitantly, the elevated temperature did not lead to a change in the Symbiodinium genotypes in any of the three gorgonian symbioses. Lack of symbiont turnover either over time [104, 105] or following environmental perturbation or disease in gorgonian corals ([49, 78], Ramsby et al. unpubl., McCauley et al. unpubl.), other octocorals [106] and in numerous studies on scleractinian coral species [107–111] and sea anemones [27] has been demonstrated repeatedly. Although the gorgonians did not change their symbiont complement, the three gorgonian species did host different Symbiodinium types (Fig 1), and analysis of the microsatellite Sym15 flanker region indicated that these Symbiodinium belonged to three distinct lineages of the “B1” radiation [72, 112]. Symbiodinium type B41 in E. tourneforti in our study (previously referred to as B1l in [64]) fell within the same Symbiodinium lineage as the Symbiodinium hosted by E. flexuosa at >20m depth [73] and Symbiodinium endomacracis that associate with the scleractinian coral Madracis sp. [74] in the Caribbean. Symbiodinium types B41a and B41b (previously referred to as B1b in [64]) which we found in E. flexuosa, belong to a separate lineage that includes the Symbiodinium inhabiting E. flexuosa found at <5m depth [73]. Symbiodinium types B1i and B42 found in P. porosa belong to a novel lineage. Given the lack of a change in Symbiodinium, any response and potential acclimation to the stressor was accomplished by modifying parameters within the existing host/symbiont genotypic combination. The gorgonian species hosting different Symbiodinium, with these symbionts exhibiting different physiologies [62], may have contributed to the differences between the response of the gorgonian species to the elevated temperature.
In addition to elevated temperature potentially affecting Symbiodinium, the entire symbiosis, including the host may be detrimentally affected [113]. Activation of cellular mechanisms to deal with stressors could increase the amount of energy required to maintain homeostasis [45, 114], and thereby alter metabolism, and consequently the biochemical composition of tissues. In our study, compared to branches maintained at the ambient temperature, gorgonian branches exposed to elevated temperature exhibited higher sclerite content driven by lower protein and lipid contents per dry weight (Fig 4), and also lower protein content when protein was assessed per organic matter. Thus, as seen in scleractinian corals [39, 115], exposure to elevated temperature led to a reduction in the amount of tissue biomass present within gorgonian branches. Compared to scleractinian corals, however, this reduction was relatively small. For example, in bleached scleractinian corals, total biomass, mean protein, lipid and carbohydrate contents can be 40–70% lower [98, 116], and mean energy content can be 22–37% lower [115] compared to tissues of unbleached corals. In our study, in the branches of the Eunicea species and P. porosa, protein per organic matter at the elevated temperature was only 6.5–14.3% lower compared to branches at ambient temperature. Furthermore, lipid, carbohydrate, and total energy content in tissues did not significantly differ between the gorgonian branches at ambient and elevated temperatures. Thus, despite some changes in biomass and protein content, exposure to elevated temperature did not affect the amount of energy available to these gorgonian species.
An integral part of maintaining the symbiosis under thermal stress involves managing the levels of ROS produced in the chloroplasts of Symbiodinium and the mitochondria of the host [26, 44, 117]. Oxidative outbursts after exposure to elevated temperature have been recorded in Caribbean gorgonian corals, and their magnitude can vary between species [47]. Both Symbiodinium and their host cnidarian possess antioxidant enzymes that neutralize ROS [26, 28, 43, 44]. In this study, SOD activity did not significantly vary between branches of the Eunicea species at ambient and elevated temperatures. Therefore, despite the nearly two-fold difference in SOD activity between the Eunicea species, basal levels of SOD in both species were sufficient to convert any excess O2- to H2O2 [44, 45]. H2O2 itself is damaging because it can readily diffuse across membranes from one partner to the other, affect distant cell organelles, and trigger apoptosis [26, 44]. The enzymes POX and CAT neutralize H2O2. POX activity in E. tourneforti was two times higher in branches exposed to elevated temperature than in those maintained at ambient temperature while in E. flexuosa branches, POX and CAT activity did not differ between the two temperatures. Therefore, the Eunicea species maintained or increased the activities of antioxidant enzymes when exposed to elevated temperature indicating that they managed oxidative stress.
Looking at Symbiodinium, holobiont and enzymatic parameters, the three gorgonian-Symbiodinium symbioses examined dealt with the potential stress of elevated temperature, although the way in which they did so differed. In P. porosa many Symbiodinium parameters were modified in response to the elevated temperature. Not only did the largest reduction in Fv/Fm occur in P. porosa but Fv/Fm also progressively declined with the duration of exposure to elevated temperature. A reduction in chlorophyll content of symbiont cells also only occurred in P. porosa. Among the three species P. porosa has the highest symbiont density and pigment content in tissues, and the lowest a*Chl a (this study, [62, 64]). These parameters along with attributes of the photosynthesis-irradiance curves, led Ramsby et al. [62] to hypothesize that the symbionts in P. porosa were comparatively less efficient at absorbing and utilizing light than those in E. tourneforti. Since high light levels can exacerbate thermal stress, the inefficient utilization of light may alleviate the negative effects of elevated temperature, and promote photoacclimation through adjusting photochemistry over losing symbiont cells from tissues. Furthermore, net photosynthesis in P. porosa is two to three times higher than in E. tourneforti [62], and P. porosa possess significantly greater amounts of tissue reserves than the Eunicea species (this study, [64]). Thus, the low efficiency of photosynthesis per symbiont cell coupled with high net photosynthesis and tissue resources may enable P. porosa to tolerate disruptions in symbiont photosynthesis that may occur when exposed to elevated temperature.
In the Eunicea species, the modifications that occurred in the symbioses were predominantly at the holobiont rather than the symbiont level. Exposure to elevated temperature led to greater reductions in mean protein content per organic matter in tissues of E. tourneforti (14.30%) and E. flexuosa (12.11%) than in those of P. porosa (6.51%) and to a doubling of POX activity in E. tourneforti. Even under ambient conditions, the Eunicea species had lower Symbiodinium density, pigment content, and energy reserves than P. porosa (this study, [64]). Due to the lower tissue resources at their disposal, the Eunicea species may attenuate changes in symbiont parameters by maintaining or increasing antioxidant activity to survive unfavorable conditions.
In the literature, bleaching of Caribbean gorgonian corals is seldom reported [118–121] and the three gorgonian species in our study did not exhibit a decline in Symbiodinium density although a reduction in the amount of chlorophyll per gorgonian surface area did occur, with P. porosa having a larger drop than within the Eunicea species. The varied responses of the gorgonian corals in our study match the inter-species differences in a visual assessment of bleaching on the reef [121]. In a 2005 bleaching event in Puerto Rico, 22% of Pseudoplexaura spp. colonies bleached [121]. In contrast, bleaching was observed in only a few E. flexuosa colonies and none of the other Eunicea species bleached [121]. Our study, however, suggests that even with a reduction in chlorophyll at 32°C, Symbiodinium photosynthesis in P. porosa was not compromised, and therefore the changes in pigment content were potentially part of an acclimatory response. This may explain why, with the exception of Muricea sp., the gorgonian species that were observed bleached in 2005 survived the thermal event [121]. Furthermore, in the 2005 bleaching event, bleaching in the Caribbean gorgonian species occurred much after most scleractinian corals, hydrocorals and zoanthids had bleached [121]. Taken together, the response of the gorgonian symbioses to elevated temperature in this study, and the few reports on bleaching in gorgonian corals [118–121], suggest that in the Caribbean, gorgonian corals may be comparatively more tolerant to thermal stress than many scleractinian coral species.
Supporting information
(DOCX)
Acknowledgments
We thank the staff and students at the Unidad Académica de Sistemas Arrecifales, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México and D. Goulet, H. Pearson and L. Camp for their assistance in the field. We thank M. Slattery, S. Ankisetty, C. Jackson at the University of Mississippi (UM) and L. Mydlarz and W. Mann at the University of Texas at Arlington for their help with the biochemical assays, T. LaJeunesse at the Pennsylvania State University for his help with naming Symbiodinium, and S. Brewer and J. Hoeksema at UM for their statistical guidance.
Data Availability
All sequence files are available from the GenBank database (accession numbers KX344963, KX344964, KX344965, KX344981, KX344969, KX344973, KX344977).
Funding Statement
Funding for this work was provided by the Graduate Student Council at the University of Mississippi (http://gsc.olemiss.edu/research-grants/), the Society for Integrative & Comparative Biology (Fellowship for Graduate Student Travel, http://www.sicb.org/index.php3), Sigma Xi (Grants-in-Aid of Research, G20101015155231, https://www.sigmaxi.org/programs/grants-in-aid), and Richard Gilder School at the American Museum of Natural History (Lerner Gray Fund for Marine Research, http://www.amnh.org/our-research/richard-gilder-graduate-school/academics-and-research/fellowship-and-grant-opportunities/research-grants-and-student-exchange-fellowships/#grants) to KPS, and the National Science Foundation under Grant No. IOS 0747205 and REU supplement to TLG. The FlowCAM particle analyzer was funded by the National Science Foundation under MRI Grant No. DBI 1126379. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation (https://www.nsf.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hoegh-Guldberg O. Coral reef ecosystems and anthropogenic climate change. Reg Environ Change. 2011;11(S1):215–27. [Google Scholar]
- 2.McWilliams J, Côté I, Gill J, Sutherland W, Watkinson A. Accelerating impacts of temperature-induced coral bleaching in the Caribbean. Ecology. 2005;86(8):2055–60. [Google Scholar]
- 3.Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Mar Freshwater Res. 1999;50(8):839–66. [Google Scholar]
- 4.Li A, Reidenbach MA. Forecasting decadal changes in sea surface temperatures and coral bleaching within a Caribbean coral reef. Coral Reefs. 2014;33(3):847–61. [Google Scholar]
- 5.Magris RA, Heron SF, Pressey RL. Conservation planning for coral reefs accounting for climate warming disturbances. PLoS ONE. 2015;10(11):e0140828 10.1371/journal.pone.0140828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas AE. Coral bleaching—How and why? Mar Poll Bull. 2003;46(4):385–92. [DOI] [PubMed] [Google Scholar]
- 7.Baker AC, Glynn PW, Riegl B. Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci. 2008;80(4):435–71. [Google Scholar]
- 8.Ban SS, Graham NAJ, Connolly SR. Evidence for multiple stressor interactions and effects on coral reefs. Glob Change Biol. 2014;20(3):681–97. [DOI] [PubMed] [Google Scholar]
- 9.Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301(5635):958–60. 10.1126/science.1086050 [DOI] [PubMed] [Google Scholar]
- 10.Alvarez-Filip L, Côté IM, Gill JA, Watkinson AR, Dulvy NK. Region-wide temporal and spatial variation in Caribbean reef architecture: Is coral cover the whole story? Glob Change Biol. 2011;17(7):2470–7. [Google Scholar]
- 11.Strong AE, Liu G, Eakin CM, Christensen TRL, Skirving WJ, Gledhill DK, et al. Implications for our coral reefs in a changing climate over the next few decades—Hints from the past 22 years. Proc 11th Int Coral Reef Symp; 7–11 July; Ft. Lauderdale, USA 2008. p. 1324–8.
- 12.Colvard NB, Edmunds PJ. Decadal-scale changes in abundance of non-scleractinian invertebrates on a Caribbean coral reef. J Exp Mar Biol Ecol. 2011;397(2):153–60. [Google Scholar]
- 13.Ruzicka R, Colella M, Porter J, Morrison J, Kidney J, Brinkhuis V, et al. Temporal changes in benthic assemblages on Florida Keys reefs 11 years after the 1997/1998 El Niño. Mar Ecol Prog Ser. 2013;489:125–41. [Google Scholar]
- 14.Villamizar E, Díaz MC, Rützler K, de Nóbrega R. Biodiversity, ecological structure, and change in the sponge community of different geomorphological zones of the barrier fore reef at Carrie Bow Cay, Belize. Mar Ecol. 2014;35(4):425–35. [Google Scholar]
- 15.Lenz EA, Bramanti L, Lasker HR, Edmunds PJ. Long-term variation of octocoral populations in St. John, US Virgin Islands. Coral Reefs. 2015;34(4):1099–109. [Google Scholar]
- 16.Cary LR. The Alcyonaria as a factor in reef limestone formation. Proc Natl Acad Sci USA. 1915;1(5):285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordán-Dahlgren E. Gorgonian community structure and reef zonation patterns on Yucatan coral reefs. Bull Mar Sci. 1989;45(3):678–96. [Google Scholar]
- 18.Voss GL. Protective coloration and habitat of the shrimp Tozeuma carolinensis Kingsley, (Caridea: Hippolytidae). Bull Mar Sci. 1956;6(4):359–63. [Google Scholar]
- 19.Birkeland C, Neudecker S. Foraging behavior of two Caribbean chaetodontids: Chaetodon capistratus and C. aculeatus. Copeia. 1981;1:169–78. [Google Scholar]
- 20.Lasker HR, Coffroth MA, Fitzgerald L. Foraging patterns of Cyphoma gibbosum on octocorals: The roles of host choice and feeding preference. Biol Bull. 1988;174(3):254–66. [Google Scholar]
- 21.Vreeland HV, Lasker HR. Selective feeding of the polychaete Hermodice carunculata Pallas on Caribbean gorgonians. J Exp Mar Biol Ecol. 1989;129(3):265–77. [Google Scholar]
- 22.Glynn P. Coral reef bleaching: Ecological perspectives. Coral Reefs. 1993;12(1):1–17. [Google Scholar]
- 23.Takahashi S, Whitney SM, Badger MR. Different thermal sensitivity of the repair of photodamaged photosynthetic machinery in cultured Symbiodinium species. Proc Natl Acad Sci USA. 2009;106(9):3237–42. 10.1073/pnas.0808363106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tchernov D, Gorbunov MY, Vargas Cd, Yadav SN, Milligan AJ, Häggblom M, et al. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci USA. 2004;101(37):13531–5. 10.1073/pnas.0402907101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones RJ, Hoegh-Guldberg O, Larkum AWD, Schreiber U. Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ. 1998;21(12):1219–30. [Google Scholar]
- 26.Weis VM. Cellular mechanisms of cnidarian bleaching: Stress causes the collapse of symbiosis. J Exp Biol. 2008;211(19):3059–66. [DOI] [PubMed] [Google Scholar]
- 27.Goulet TL, Cook CB, Goulet D. Effect of short-term exposure to elevated temperatures and light levels on photosynthesis of different host-symbiont combinations in the Aiptasia pallida / Symbiodinium symbiosis. Limnol Oceanogr. 2005;50(5):1490–8. [Google Scholar]
- 28.McGinty ES, Pieczonka J, Mydlarz LD. Variations in reactive oxygen release and antioxidant activity in multiple Symbiodinium types in response to elevated temperature. Microb Ecol. 2012;64(4):1000–7. 10.1007/s00248-012-0085-z [DOI] [PubMed] [Google Scholar]
- 29.Wang JT, Douglas AE. Essential amino acid synthesis and nitrogen recycling in an alga-invertebrate symbiosis. Mar Biol. 1999;135(2):219–22. [Google Scholar]
- 30.Shick JM, Dunlap WC. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu Rev Physiol. 2002;64:223–62. 10.1146/annurev.physiol.64.081501.155802 [DOI] [PubMed] [Google Scholar]
- 31.Kopp C, Domart-Coulon I, Escrig S, Humbel BM, Hignette M, Meibom A. Subcellular investigation of photosynthesis-driven carbon assimilation in the symbiotic reef coral Pocillopora damicornis. mBio. 2015;6(1):e02299–14. 10.1128/mBio.02299-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salih A, Larkum AWD, Cox G, Kühl M, Hoegh-Guldberg O. Fluorescent pigments in corals are photoprotective. Nature. 2000;408(6814):850–3. 10.1038/35048564 [DOI] [PubMed] [Google Scholar]
- 33.Hughes AD, Grottoli AG, Pease TK, Matsui Y. Acquisition and assimilation of carbon in non-bleached and bleached corals. Mar Ecol Prog Ser. 2010;420:91–101. [Google Scholar]
- 34.Imbs AB, Yakovleva IM, Dautova TN, Bui LH, Jones P. Diversity of fatty acid composition of symbiotic dinoflagellates in corals: Evidence for the transfer of host PUFAs to the symbionts. Phytochemistry. 2014;101:76–82. 10.1016/j.phytochem.2014.02.012 [DOI] [PubMed] [Google Scholar]
- 35.Tanaka Y, Grottoli AG, Matsui Y, Suzuki A, Sakai K. Partitioning of nitrogen sources to algal endosymbionts of corals with long-term 15N-labelling and a mixing model. Ecol Model. 2015;309–310:163–9. [Google Scholar]
- 36.Hoadley KD, Pettay DT, Grottoli AG, Cai W-J, Melman TF, Schoepf V, et al. Physiological response to elevated temperature and pCO2 varies across four Pacific coral species: Understanding the unique host+symbiont response. Sci Rep. 2015;5:18371 10.1038/srep18371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michalek-Wagner K, Willis BL. Impacts of bleaching on the soft coral Lobophytum compactum. II. Biochemical changes in adults and their eggs. Coral Reefs. 2001;19(3):240–6. [Google Scholar]
- 38.Slattery M, Paul VJ. Indirect effects of bleaching on predator deterrence in the tropical Pacific soft coral Sinularia maxima. Mar Ecol Prog Ser. 2008;354:169–79. [Google Scholar]
- 39.Rodrigues LJ, Grottoli AG. Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol Oceanogr. 2007;52(5):1874–82. [Google Scholar]
- 40.Imbs AB, Yakovleva IM. Dynamics of lipid and fatty acid composition of shallow-water corals under thermal stress: An experimental approach. Coral Reefs. 2012;31(1):41–53. [Google Scholar]
- 41.Anthony KRN, Hoogenboom MO, Maynard JA, Grottoli AG, Middlebrook R. Energetics approach to predicting mortality risk from environmental stress: A case study of coral bleaching. Funct Ecol. 2009;23(3):539–50. [Google Scholar]
- 42.Schoepf V, Grottoli AG, Levas SJ, Aschaffenburg MD, Baumann JH, Matsui Y, et al. Annual coral bleaching and the long-term recovery capacity of coral. Proc R Soc B. 2015;282(1819):20151887 10.1098/rspb.2015.1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fitt WK, Gates RD, Hoegh-Guldberg O, Bythell JC, Jatkar A, Grottoli AG, et al. Response of two species of Indo-Pacific corals, Porites cylindrica and Stylophora pistillata, to short-term thermal stress: The host does matter in determining the tolerance of corals to bleaching. J Exp Mar Biol Ecol. 2009;373(2):102–10. [Google Scholar]
- 44.Lesser MP. Coral bleaching: Causes and mechanisms In: Dubinsky Z, Stambler N, editors. Coral Reefs: An ecosystem in transition. Berlin, Heidelberg, New York: Springer Verlag; 2011. p. 405–19. [Google Scholar]
- 45.Downs CA, Fauth JE, Halas JC, Dustan P, Bemiss J, Woodley CM. Oxidative stress and seasonal coral bleaching. Free Radical Biol Med. 2002;33(4):533–43. [DOI] [PubMed] [Google Scholar]
- 46.Hawkins TD, Krueger T, Becker S, Fisher PL, Davy SK. Differential nitric oxide synthesis and host apoptotic events correlate with bleaching susceptibility in reef corals. Coral Reefs. 2014;33(1):141–53. [Google Scholar]
- 47.Mydlarz LD, Jacobs RS. An inducible release of reactive oxygen radicals in four species of gorgonian corals. Mar Freshwater Behav Physiol. 2006;39(2):143–52. [Google Scholar]
- 48.Ross C. Nitric oxide and heat shock protein 90 co-regulate temperature-induced bleaching in the soft coral Eunicea fusca. Coral Reefs. 2014;33(2):513–22. [Google Scholar]
- 49.Kirk NL, Ward JR, Coffroth MA. Stable Symbiodinium composition in the sea fan Gorgonia ventalina during temperature and disease stress. Biol Bull. 2005;209(3):227–34. 10.2307/3593112 [DOI] [PubMed] [Google Scholar]
- 50.Ward JR, Kim K, Harvell CD. Temperature affects coral disease resistance and pathogen growth. Mar Ecol Prog Ser. 2007;329:115–21. [Google Scholar]
- 51.Mann WT, Beach-Letendre J, Mydlarz LD. Interplay between proteases and protease inhibitors in the sea fan—Aspergillus pathosystem. Mar Biol. 2014;161(10):2213–20. [Google Scholar]
- 52.Drohan AF, Thoney DA, Baker AC. Synergistic effect of high temperature and ultraviolet-B radiation on the gorgonian Eunicea tourneforti (Octocorallia: Alcyonacea: Plexauridae). Bull Mar Sci. 2005;77(2):257. [Google Scholar]
- 53.Rodríguez-Martinez RE, Ruíz-Rentería F, van Tussenbroek B, Barba-Santos G, Escalante-Mancera E, Jordán-Garza G, et al. Environmental state and tendencies of the Puerto Morelos CARICOMP site, Mexico. Rev Biol Trop. 2010;58(3):23–43. [PubMed] [Google Scholar]
- 54.Kemp DW, Thornhill DJ, Rotjan RD, Iglesias-Prieto R, Fitt WK, Schmidt GW. Spatially distinct and regionally endemic Symbiodinium assemblages in the threatened Caribbean reef-building coral Orbicella faveolata. Coral Reefs. 2015;34(2):535–47. [Google Scholar]
- 55.Donner SD. Coping with commitment: Projected thermal stress on coral reefs under different future scenarios. PLoS ONE. 2009;4(6):e5712 10.1371/journal.pone.0005712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colombo-Pallotta MF, Rodríguez-Román A, Iglesias-Prieto R. Calcification in bleached and unbleached Montastraea faveolata: Evaluating the role of oxygen and glycerol. Coral Reefs. 2010;29(4):899–907. [Google Scholar]
- 57.DeSalvo MK, Sunagawa S, Fisher PL, Voolstra CR, Iglesias-Prieto R, Medina M. Coral host transcriptomic states are correlated with Symbiodinium genotypes. Mol Ecol. 2010;19(6):1174–86. 10.1111/j.1365-294X.2010.04534.x [DOI] [PubMed] [Google Scholar]
- 58.Grottoli AG, Warner ME, Levas SJ, Aschaffenburg MD, Schoepf V, McGinley M, et al. The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Glob Change Biol. 2014;20(12):3823–33. [DOI] [PubMed] [Google Scholar]
- 59.Levas S, Grottoli AG, Schoepf V, Aschaffenburg M, Baumann J, Bauer JE, et al. Can heterotrophic uptake of dissolved organic carbon and zooplankton mitigate carbon budget deficits in annually bleached corals? Coral Reefs. 2015;35(2):495–506. [Google Scholar]
- 60.Schoepf V, Levas SJ, Rodrigues LJ, McBride MO, Aschaffenburg MD, Matsui Y, et al. Kinetic and metabolic isotope effects in coral skeletal carbon isotopes: A re-evaluation using experimental coral bleaching as a case study. Geochim Cosmochim Acta. 2014;146:164–78. [Google Scholar]
- 61.Iglesias-Prieto R, Beltrán VH, LaJeunesse TC, Reyes-Bonilla H, Thomé PE. Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc R Soc B. 2004;271(1549):1757–63. 10.1098/rspb.2004.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramsby BD, Shirur KP, Iglesias-Prieto R, Goulet TL. Symbiodinium photosynthesis in Caribbean octocorals. PLoS ONE. 2014;9(9):e106419 10.1371/journal.pone.0106419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodríguez-Román A, Hernández-Pech X, Thomé PE, Enríquez S, Iglesias-Prieto R. Photosynthesis and light utilization in the Caribbean coral Montastraea faveolata recovering from a bleaching event. Limnol Oceanogr. 2006;51(6):2702–10. [Google Scholar]
- 64.Shirur KP, Ramsby BD, Iglesias-Prieto R, Goulet TL. Biochemical composition of Caribbean gorgonians: Implications for gorgonian—Symbiodinium symbiosis and ecology. J Exp Mar Biol Ecol. 2014;461(C):275–85. [Google Scholar]
- 65.Brown L. Characterizing biologics using dynamic imaging particle analysis. BioPharm Int. 2011;24(8):s1–s8. [Google Scholar]
- 66.Jeffrey SW, Humphrey GF. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanzen. 1975;167:191–4. [Google Scholar]
- 67.LaJeunesse TC, Loh WKW, van Woesik R, Hoegh-Guldberg O, Schmidt GW, Fitt WK. Low symbiont diversity in southern Great Barrier Reef corals relative to those of the Caribbean. Limnol Oceanogr. 2003;48(5):2046–54. [Google Scholar]
- 68.Janse I, Bok J, Zwart G. A simple remedy against artifactual double bands in denaturing gradient gel electrophoresis. J Microbiol Methods. 2004;57:279–81. 10.1016/j.mimet.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 69.LaJeunesse TC. Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol. 2002;141(2):387–400. [Google Scholar]
- 70.Pettay DT, LaJeunesse TC. Microsatellites from clade B Symbiodinium spp. specialized for Caribbean corals in the genus Madracis. Mol Ecol Notes. 2007;7(6):1271–4. [Google Scholar]
- 71.LaJeunesse TC, Parkinson JE, Reimer JD. A genetics-based description of Symbiodinium minutum sp. nov. and S. psygmophilum sp. nov. (Dinophyceae), two dinoflagellates symbiotic with cnidaria. J Phycol. 2012;48(6):1380–91. 10.1111/j.1529-8817.2012.01217.x [DOI] [PubMed] [Google Scholar]
- 72.Finney JC, Pettay DT, Sampayo EM, Warner ME, Oxenford HA, LaJeunesse TC. The relative significance of host–habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodinium. Microb Ecol. 2010;60(1):250–63. 10.1007/s00248-010-9681-y [DOI] [PubMed] [Google Scholar]
- 73.Prada C, McIlroy SE, Beltrán DM, Valint DJ, Ford SA, Hellberg ME, et al. Cryptic diversity hides host and habitat specialization in a gorgonian-algal symbiosis. Mol Ecol. 2014;23(13):3330–40. 10.1111/mec.12808 [DOI] [PubMed] [Google Scholar]
- 74.Parkinson JE, Coffroth MA, LaJeunesse TC. New species of Clade B Symbiodinium (Dinophyceae) from the greater Caribbean belong to different functional guilds: S. aenigmatum sp. nov., S. antillogorgium sp. nov., S. endomadracis sp. nov., and S. pseudominutum sp. nov. J Phycol. 2015;51(5):850–8. 10.1111/jpy.12340 [DOI] [PubMed] [Google Scholar]
- 75.Gnaiger E, Bitterlich G. Proximate biochemical composition and caloric content calculated from elemental CHN analysis: A stoichiometric concept. Oecologia. 1984;62(3):289–98. [DOI] [PubMed] [Google Scholar]
- 76.Lesser MP. Using energetic budgets to assess the effects of environmental stress on corals: Are we measuring the right things? Coral Reefs. 2013;32(1):25–33. [Google Scholar]
- 77.Mydlarz LD, Harvell CD. Peroxidase activity and inducibility in the sea fan coral exposed to a fungal pathogen. Comp Biochem Physiol A. 2007;146(1):54–62. [DOI] [PubMed] [Google Scholar]
- 78.Shirur KP, Jackson CR, Goulet TL. Lesion recovery and the bacterial microbiome in two Caribbean gorgonian corals. Mar Biol. 2016;163(12). [Google Scholar]
- 79.Palmer CV, McGinty ES, Cummings DJ, Smith SM, Bartels E, Mydlarz LD. Patterns of coral ecological immunology: Variation in the responses of Caribbean corals to elevated temperature and a pathogen elicitor. J Exp Biol. 2011;214(24):4240–9. [DOI] [PubMed] [Google Scholar]
- 80.Pinzón C JH, Beach-Letendre J, Weil E, Mydlarz LD. Relationship between phylogeny and immunity suggests older Caribbean coral lineages are more resistant to disease. PLoS ONE. 2014;9(8):e104787 10.1371/journal.pone.0104787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pinzón C JH, Dornberger L, Beach-Letendre J, Weil E, Mydlarz LD. The link between immunity and life history traits in scleractinian corals. PeerJ. 2014;2(4):e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Couch CS, Mydlarz LD, Harvell CD, Douglas NL. Variation in measures of immunocompetence of sea fan coral, Gorgonia ventalina, in the Florida Keys. Mar Biol. 2008;155(3):281–92. [Google Scholar]
- 83.Mydlarz LD, Couch CS, Weil E, Smith GW, Harvell CD. Immune defenses of healthy, bleached and diseased Montastraea faveolata during a natural bleaching event. Dis Aquat Org. 2009;87:67–78. 10.3354/dao02088 [DOI] [PubMed] [Google Scholar]
- 84.Mydlarz LD, Palmer CV. The presence of multiple phenoloxidases in Caribbean reef-building corals. Comp Biochem Physiol A. 2011;159(4):372–8. [DOI] [PubMed] [Google Scholar]
- 85.Palmer CV, Bythell JC, Willis BL. A comparative study of phenoloxidase activity in diseased and bleached colonies of the coral Acropora millepora. Dev Comp Immunol. 2011;35(10):1098–101. 10.1016/j.dci.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 86.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 87.Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1. 1.0–5. ed2013.
- 88.Kuznetsova A, Brockhoff PB, Bojesen Christensen RH. lmerTest: Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package). R package version 2.0. 2.0–11 ed2014.
- 89.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical J. 2008;50(3):346–63. [DOI] [PubMed] [Google Scholar]
- 90.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 91.Fitt WK, Warner ME. Bleaching patterns of four species of Caribbean reef corals. Biol Bull. 1995;189(3):298–307. [DOI] [PubMed] [Google Scholar]
- 92.Warner ME, Fitt WK, Schmidt GW. The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: A novel approach. Plant Cell Environ. 1996;19(3):291–9. [Google Scholar]
- 93.Kemp DW, Oakley CA, Thornhill DJ, Newcomb LA, Schmidt GW, Fitt WK. Catastrophic mortality on inshore coral reefs of the Florida Keys due to severe low-temperature stress. Glob Change Biol. 2011;17(11):3468–77. [Google Scholar]
- 94.Dove SG. Scleractinian corals with photoprotective host pigments are hypersensitive to thermal bleaching. Mar Ecol Prog Ser. 2004;272:99–116. [Google Scholar]
- 95.Downs CA, McDougall KE, Woodley CM, Fauth JE, Richmond RH, Kushmaro A, et al. Heat-stress and light-stress induce different cellular pathologies in the symbiotic dinoflagellate during coral bleaching. PLoS ONE. 2013;8(12):e77173 10.1371/journal.pone.0077173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lutz A, Raina J-B, Motti CA, Miller DJ, van Oppen MJH. Host coenzyme Q redox state Is an early biomarker of thermal stress in the coral Acropora millepora. PLoS ONE. 2015;10(10):e0139290 10.1371/journal.pone.0139290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roth MS. The engine of the reef: Photobiology of the coral—algal symbiosis. Front Microbiol. 2014;5(422):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fitt WK, Spero H, Halas J, White M, Porter JW. Recovery of the coral Montastrea annularis in the Florida Keys after the 1987 Caribbean “bleaching event”. Coral Reefs. 1993;12(2):57–64. [Google Scholar]
- 99.Roth MS, Goericke R, Deheyn DD. Cold induces acute stress but heat is ultimately more deleterious for the reef-building coral Acropora yongei. Sci Rep. 2012;2:240 10.1038/srep00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hawkins TD, Krueger T, Wilkinson SP, Fisher PL, Davy SK. Antioxidant responses to heat and light stress differ with habitat in a common reef coral. Coral Reefs. 2015;34(4):1229–41. [Google Scholar]
- 101.Fisher PL, Malme MK, Dove SG. The effect of temperature stress on coral–Symbiodinium associations containing distinct symbiont types. Coral Reefs. 2012;31(2):473–85. [Google Scholar]
- 102.Pontasch S, Hill R, Deschaseaux ESM, Fisher PL, Davy SK, Scott A. Photochemical efficiency and antioxidant capacity in relation to Symbiodinium genotype and host phenotype in a symbiotic cnidarian. Mar Ecol Prog Ser. 2014;516:195–208. [Google Scholar]
- 103.Warner ME, Lesser MP, Ralph PJ. Chlorophyll fluorescence in reef building corals In: Suggett DJ, Prášil O, Borowitzka MA, editors. Chlorophyll a fluorescence in aquatic sciences: Methods and applications. Developments in applied phycology. Dordrecht, New York: Springer; 2010. p. 209–22. [Google Scholar]
- 104.Goulet TL, Coffroth MA. Stability of an octocoral-algal symbiosis over time and space. Mar Ecol Prog Ser. 2003;250:117–24. [Google Scholar]
- 105.Baker DM, Weigt L, Fogel ML, Knowlton N, Goldstien S. Ancient DNA from coral-hosted Symbiodinium reveal a static mutualism over the last 172 Years. PLoS ONE. 2013;8(2):e55057 10.1371/journal.pone.0055057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goulet TL, LaJeunesse TC, Fabricius KE. Symbiont specificity and bleaching susceptibility among soft corals in the 1998 Great Barrier Reef mass coral bleaching event. Mar Biol. 2008;154(5):795–804. [Google Scholar]
- 107.Thornhill DJ, LaJeunesse TC, Kemp DW, Fitt WK, Schmidt GW. Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Mar Biol. 2005;148(4):711–22. [Google Scholar]
- 108.Thornhill DJ, Fitt WK, Schmidt GW. Highly stable symbioses among western Atlantic brooding corals. Coral Reefs. 2006;25(4):515–9. [Google Scholar]
- 109.Stat M, Loh WKW, LaJeunesse TC, Hoegh-Guldberg O, Carter DA. Stability of coral–endosymbiont associations during and after a thermal stress event in the southern Great Barrier Reef. Coral Reefs. 2009;28(3):709–13. [Google Scholar]
- 110.Thornhill DJ, Xiang Y, Fitt WK, Santos SR. Reef endemism, host specificity and temporal stability in populations of symbiotic dinoflagellates from two ecologically dominant Caribbean corals. PLoS ONE. 2009;4(7):e6262 10.1371/journal.pone.0006262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.LaJeunesse TC, Smith RT, Walther M, Pinzón JH, Pettay DT, McGinley M, et al. Host–symbiont recombination versus natural selection in the response of coral–dinoflagellate symbioses to environmental disturbance. Proc R Soc B. 2010;277(1696):2925–34. 10.1098/rspb.2010.0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.LaJeunesse TC. "Species” radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene-Pliocene transition. Mol Biol Evol. 2005;22(3):570–81. 10.1093/molbev/msi042 [DOI] [PubMed] [Google Scholar]
- 113.Baird AH, Bhagooli R, Ralph PJ, Takahashi S. Coral bleaching: The role of the host. Trends Ecol Evol. 2008;24(1):16–20. 10.1016/j.tree.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 114.Gates RD, Edmunds PJ. The physiological mechanisms of acclimatization in tropical reef corals. Amer Zool. 1999;39(1):30–43. [Google Scholar]
- 115.Grottoli AG, Rodrigues LJ, Palardy JE. Heterotrophic plasticity and resilience in bleached corals. Nature. 2006;440(7088):1186–9. 10.1038/nature04565 [DOI] [PubMed] [Google Scholar]
- 116.Porter JW, Fitt WK, Spero HJ, Rogers CS, White MW. Bleaching in reef corals: Physiological and stable isotopic responses. Proc Natl Acad Sci USA. 1989;86(23):9342–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dunn SR, Pernice M, Green K, Hoegh-Guldberg O, Dove SG. Thermal stress promotes host mitochondrial degradation in symbiotic cnidarians: Are the batteries of the reef going to run out? PLoS ONE. 2012;7(7):e39024 10.1371/journal.pone.0039024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lasker HR, Peters EC, Coffroth MA. Bleaching of reef coelenterates in the San Blas islands, Panama. Coral Reefs. 1984;3(4):183–90. [Google Scholar]
- 119.Lasker HR. Zooxanthella densities within a Caribbean octocoral during bleaching and non-bleaching years. Coral Reefs. 2003;22(1):23–6. [Google Scholar]
- 120.Hannes AR, Barbeitos MS, Coffroth MA. Stability of symbiotic dinoflagellate type in the octocoral Briareum asbestinum. Mar Ecol Prog Ser. 2009;391:65–72. [Google Scholar]
- 121.Prada C, Weil E, Yoshioka PM. Octocoral bleaching during unusual thermal stress. Coral Reefs. 2010;29(1):41–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All sequence files are available from the GenBank database (accession numbers KX344963, KX344964, KX344965, KX344981, KX344969, KX344973, KX344977).