Abstract
Purpose
To evaluate the corneal epitheliotropic abilities of two commercialized human platelet lysates (HPLs) and to compare the results with other blood derivatives, including human peripheral serum (HPS) and bovine fetal serum (FBS).
Methods
In vitro, human corneal epithelial cells were incubated in various concentrations (0%, 3%, 5% and 10%) of blood derivatives. Two commercialized HPLs, including UltraGRO TM (Helios, Atlanta, GA) and PLTMax (Mill Creek, Rochester, MI), were tested and compared with HPS and FBS. Scratch-induced directional wounding assay was performed to evaluate cellular migration. MTS assay was used to evaluate cellular proliferation. Cellular differentiation was examined by scanning electron microscopy, inverted microscopy and transepithelial electrical resistance. Sprague-Dawley rats were used to evaluate the effects of the blood derivatives on corneal epithelial wound healing in vivo. Different blood derivatives were applied topically every 2 hours for 2 days after corneal epithelial debridement. The concentrations of epidermal growth factor (EGF), transforming growth factor -β1 (TGF-β1), fibronectin, platelet-derived growth factor-AB (PDGF-AB), PDGF-BB, and hyaluronic acid in different blood derivatives were evaluated by enzyme-linked immunosorbent assay (ELISA).
Results
In vitro experiments demonstrated statistically comparable epitheliotropic characteristics in cellular proliferation, migration, and differentiation for the two commercialized HPLs compared to FBS and HPS. Cells cultured without any serum were used as control group. The epitheliotropic capacities were statistically higher in the two commercialized HPLs compared to the control group (p<0.05). Among the different concentrations of blood derivatives, the preparations with 3% yielded better outcomes compared to 5% and 10%. In rats, HPLs also caused improved but not statistically significant wound healing compared to HPS. All the blood derivatives had better wound healing ratios than the control group (p<0.05). In the quantification of epitheliotropic factors, UltraGRO and PLTMax had significantly higher levels of EGF, TGF- β1, fibronectin than human peripheral serum (p<0.05).
Conclusions
Both commercialized HPLs showed comparable corneal epitheliotropic abilities and wound healing rates compared to HPS and FBS in the in vivo and in vitro studies. Our results suggest that HPLs may have the potential to replace HPS in the treatment of corneal epithelial problems.
Introduction
Human peripheral serum (HPS) has long been used as a topical treatment for ocular surface disorders such as recurrent corneal erosions, persistent epithelial defects, superior limbic keratoconjunctivitis, and dry eye syndrome. [1–13] However, HPS has several major disadvantages. The process of obtaining peripheral blood from the patients and processing this to serum eye drops may be inconvenient for clinical application. The quality of HPS may be inconsistent, especially in patients with poor health, and the epitheliotropic abilities may be unsatisfactory or unpredictable. No standardized dilution protocol has been reported. HPS contains proinflammatory agents such as matrix metalloproteinase (MMP) and acid hydrolase that are derived from leukocyte degranulation and may induce unwanted side effects. [14, 15] In addition, HPS can be inconvenient for the patient due to its need to be stored at -4°C and be used preferably within a week. [16]
Human platelet lysate (HPL) is known to contain a number of mitogenic growth factors, including platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), epidermal growth factor (EGF) and transforming growth factor (TGF) [17–19]. It is obtained from centrifugation and subsequent isolation of the platelet fraction from the platelet rich plasma (PRP). In order to induce platelet activation, the conversion of fibrinogen to fibrin clot, and growth factor release during the manufacturing process, platelet lysis is induced via either freeze-thaw cycles or the addition of CaCl2 [20] or thrombin[21–23]. Recently, it has been demonstrated that HPL can replace fetal bovine serum (FBS) and be used in mesenchymal stem cell (MSC) culture without adversely affecting the immunophenotype or MSC metabolism. [23–28] Since pharmaceutical grade HPLs are manufactured primarily from blood bank sources under strict Good Manufacturing Practices (GMP), the possibility of allogenic blood-derived infections is practically nonexistent. HPL can potentially replace HPS in treating ocular surface disorders, and be used as a substitute for FBS in MSC culture.
In this study, we hypothesized that HPL may provide comparable corneal epitheliotropic properties compared to HPS or FBS. We evaluated the rates of corneal epithelial proliferation, migration and differentiation in vitro with a human corneal epithelial cell line. The corneal epithelial wound healing abilities in an animal model were examined. We also compared the levels of several important corneal epitheliotropic factors in FBS, HPS, and HPL.
Materials and methods
Reagents and antibiotics
Dulbecco’s modified Eagle’s medium (DMEM), F12, Trypsin-EDTA, phosphate-buffered saline (PBS), FBS, and amphotericin B were purchased from Gibco (Rockville, MD). Dispase II was purchased from Roche Diagnostics Corporation (Indianapolis, IN). Enzyme-linked immunosorbent assay (ELISA) kit for TGF-β1 was purchased from RayBiotech, Inc. (Norcross, GA). ELISA kit for human EGF was obtained from eBioscience (San Diego, CA). Hyaluronic acid (HA) concentrations and PDGF-AB were measured using ELISA kits from R&D Systems (Minneapolis, MN). ELISA kit for fibronectin was purchased from Assaypro (Missouri, USA). ELISA kit for PDGF-BB was obtained from Peprotech (New Jersey, USA). All other reagents and chemicals were from Sigma-Aldrich (St. Louis, MO).
Preparations of blood derivatives
Preparation of FBS
FBS was purchased from Gibco (Rockville, MD) and stored in sterile tubes at -20°C before use. It was diluted to 3, 5, and 10% in DMEM for in vitro cell culture experiments, and 3, 5, and 10% with Refresh Tear (Allergan, Inc. Parsippany, NJ) for topical use in animal experiments.
Preparation of HPS
The procedure used to obtain HPS was approved by the Institutional Review Board for Human Studies at the National Taiwan University Hospital (201510123RINB). All participants provided their written informed consent to participate in this study and the consent procedure was approved by the ethics committee. All individuals were healthy volunteers who were not taking any medication. Samples of 20 ml whole blood were drawn from 10 healthy volunteers (mean age, 30.3 ± 10.2 years) by venipuncture, allowed to clot at room temperature (20–25°C) for 4 hours, and centrifuged at 3000g for 15 minutes. The serum was then heated for 30 minutes at 56°C to eliminate further complement activation, carefully filtered and aliquoted in a sterile manner. The serum was stored in sterile tubes at -20°C before use. HPS was diluted to 3%, 5%, and 10% using similar methods as the FBS.
Preparation of HPL
Two commercialized HPLs, including UltraGRO TM (Helios, Atlanta, GA) and PLTMax (Mill Creek, Rochester, MI), were stored in sterile tubes at -20°C before use. The methods of HPL dilution to 3%, 5%, and 10% were similar to those of FBS.
Culture of human corneal epithelial cell line (HCEC)
Human corneal epithelial cell line was purchased from ATCC (CRL-11515). The cells were centrifuged and then suspended in DMEM-F12 medium supplemented with antibiotic-antimycotic (100μg/ml penicillin/streptomycin and 1.25μg/ml amphotericin B) agents. Different percentages (3%, 5%, and 10%) of blood derivatives (HPS, HPLs, FBS) were added to the culture medium. The culture medium was replaced every 2 to 3 days. The cells were sub-cultured after reaching confluence. We used only cells from passages 2–3 of the initial culture. Cells cultured without any serum were used as control group.
Cell-migration: Scratch-induced directional wounding assay
HCECs were plated in 12-well tissue culture dishes at a concentration of 4 x 105 cells/ml and maintained in media with different concentrations (3%, 5%, and 10%) of different blood derivatives (HPLs, HPS, and FBS). After the cells reached confluence, a 200μl tip of the micropipette was used to wound the cells and create a linear scrape about 1 mm wide. Wound closure was recorded by photography at 0, 8, 12, and 16 hours after injury using an inverted microscope equipped with a digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI). The wound closure was quantified with an image processing and analysis software program (Image J 1.37v; Wayne Rasband at the Research Services Branch, National Institute of Mental Health, Bethesda, MD) by measuring the average residual gaps between migrating cells of opposing wound edges. Wound healing ratio was defined as the difference between the initial and current cell-free areas divided by the initial cell-free area. All experiments were repeated six times to ensure consistent results.
Cell proliferation: MTS assay
HCECs (5 x 103/well) were loaded in 96-well plates and maintained in different concentrations (3%, 5%, and 10%) of blood derivatives (HPLs, HPS, FBS) for 3 days. After incubating for 24, 48, and 72 hours, the numbers of viable cells were determined with MTS assay (Promega corp., Madison, WI). According to the manufacturer’s instructions, the assay system measures the reduction of a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS) into a soluble formazan product by the mitochondria of viable cells. Since the production of formazan is proportional to the number of living cells, the intensity of the produced color is a good indicator of the cellular proliferative ability. Absorbances at 490 nm (test wavelength) and 650nm (reference wavelength) were measured using an ELISA microplate reader (Model ELx 800; Bio-TEK instruments. Inc., Winooski, VT). Wells containing culture medium but no cells served as controls. All experiments were repeated six times to ensure consistent results. [29]
Cell differentiation-morphology: Inverted microscope and scanning electron microscopy (SEM)
4 x 105 cells were seeded on 24-well plastic cell culture dishes with cover glasses and incubated for 3 days after confluency in media with different concentrations (3%, 5%, and 10%) of blood derivatives (HPLs, HPS, and FBS). After washing with PBS, specimens were fixed in 2.5% glutaraldehyde solution in 0.1M cacodylate buffer (pH 7.4) for 120 minutes, then post-fixed in 1% osmium tetroxide for 60 minutes and progressively dehydrated through ascending alcohol concentrations, critical point dried, mounted, and sputter coated with gold before examining with SEM (JSM6510LV; JEOL, Ltd, Tokyo, Japan). The surface morphology of the cells was evaluated by 2 independent examiners. All experiments were repeated 6 times.
Cell differentiation-function: Measurement of transepithelial electrical resistance (TEER)
A total of 1 × 105 cells incubated in 3% of different blood derivatives (HPLs, HPS, and FBS) were seeded in the upper chamber of a Costar transwell (Corning Costar, Cambridge, MA) (1.12 cm2 diameter, 0.4μm pore size) and allowed to reach confluency. TEER was measured using a Millicell-ERS electrical resistance system (Millipore, Bedford, MA) after the cells reach 80% confluency (day 0) and 3 days later (day 3) when the cells were at full confluency. The TEER values were calculated as Ω cm2 by multiplying it with the surface area (1.12cm2) of the monolayer. The resistance of the supporting membrane in the transwell filter is substracted from all readings before calculations. All experiments were repeated 6 times to ensure consistent results [29].
Rat model of corneal epithelial wound healing
All animals in this study were handled according to the guidelines in the ARVO Statement for the Use of Animal in Ophthalmic and Vision Research. The protocol was approved by the Animal Care and Use Committee of National Taiwan University. All male Sprague-Dawley rats, 16–24 weeks, were purchased from Charles River Laboratories, Canada (n = 24). These animals were anesthetized with intraperitoneal injections of ketamine hydrochloride (2 mg/g body weight) and xylazine (0.4 mg/g body weight). The animals were monitored every 2 hour. After applying topical proparacaine (Alcaine; Alcon Laboratories, Inc., Fort Worth, TX) to each eye, the central cornea was marked by a trephine (4mm in diameter), and the epithelium was debrided by a corneal rust ring remover with a 0.5mm-burr (Algerbrush IITM; Alger Equipment Co., Inc., Lago Vista, TX) under the operating microscope (OPMI Pico I; Carl Zeiss Meditec, Jena, Germany). Blood derivatives (20% HPS, HPLs, FBS) were applied topically every 2 hours for 2 days. Rats treated with eye drops without blood derivatives were used as the control group. The area of corneal epithelial defect was checked at 0, 12, 24, and 48 hours with fluorescein staining and photographed under the operating microscope. Wound healing ratio was defined as the difference between the initial and current epithelial defect sizes divided by the initial epithelial defect area.
No animal died, appeared ill or suffered greatly prior to the experimental endpoints, although we had in place a protocol for early humane endpoints in cases where animals appeared irritable or in severe pain. Animals were maintained on a 12:12-hr light/dark cycle, and food and water were available ad libitum for the duration of experimentation.
Quantification of epitheliotrophic factors
Quantification of epitheliotrophic factors was modified from the previously published method and performed in 3 different human blood derivatives[29] (2 HPLs and HPS). EGF, TGF-β1, fibronectin, PDGF-AB, PDGF-BB, and HA were measured by ELISA according to the manufacturer’s instructions.
Chemical analysis
Contents of protein, glucose, chloride, sodium, potassium, calcium, phosphate, magnesium, iron, the total iron capacity, ferritin, vitamin B12, and folate in human peripheral serum and two commercialized human platelet lysates were determined using a Roche Module P800 Automatic Biochemical Analyzer (Roche Diagnstics, Mannheim, Germany).
Data evaluation and statistical methods
ANOVA, the Dunnett’s multiple comparison test and the Student’s t-test were performed for statistical analysis. P value < 0.05 was considered statistically significant.
Results
Cell migration: Scratch-induced directional wounding assay
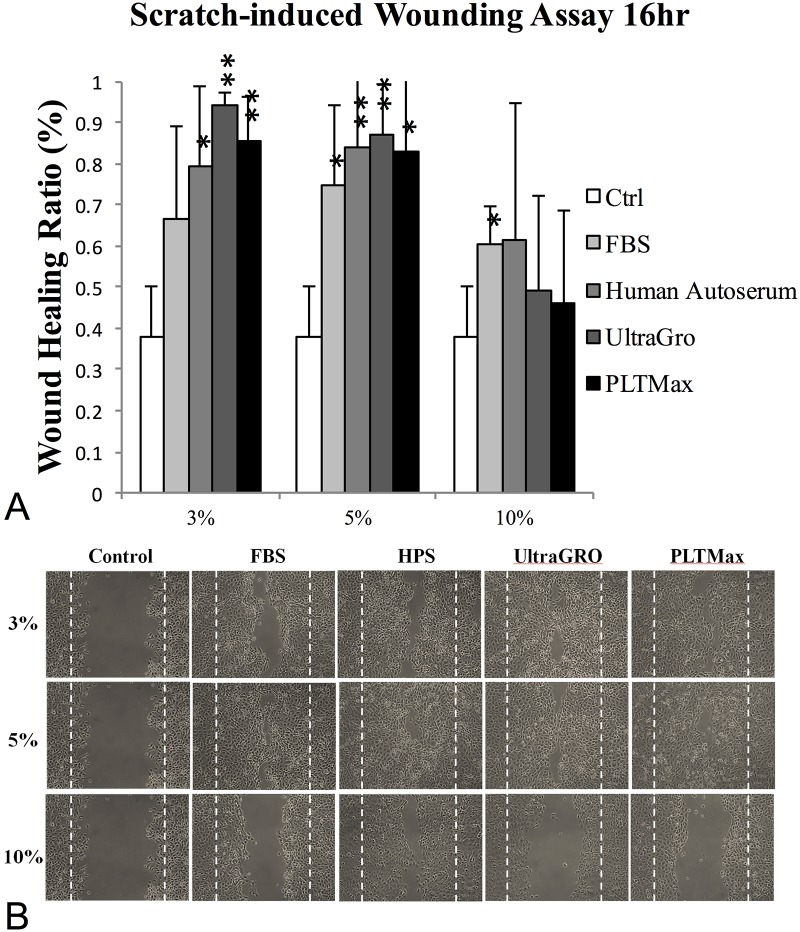
Fig 1 demonstrates the wound healing ratios at 16 hours. The healing ratios in 3% blood derivatives were 0.665±0.226, 0.791±0.195, 0.942±0.032, and 0.856±0.106 in FBS, HPS, UltraGRO, and PLTMax, respectively. The ratios at 16 hours in 5% blood derivatives were 0.745±0.197, 0.840±0.194, 0.868±0.122, and 0.827±0.203 in FBS, HPS, UltraGRO and PLTMax, respectively. In 3% and 5% blood derivative preparations, no significant differences were noted between HPS and the 2 commercialized HPLs. For the 10% blood derivatives, the wound healing ratios at 16 hours were 0.602±0.096, 0.612±0.336, 0.491±0.229, and 0.460±0.224 in FBS, HPS, UltraGRO, and PLTMax, respectively. A higher concentration (10%) appeared to retard wound healing for all 4 blood products. The inhibitory effect was more obvious in HPLs compared to that of HPS.
Fig 1. Cell migration: Scratch-induced directional wounding assay at 16 hours after wounding.
(A) 10% preparations of blood derivatives demonstrated poorer response compared to 3% and 5% preparations. There was no significant difference among the 4 blood derivatives in 3% and 5% preparations. * indicated p <0.05. ** indicated p <0.01 compared to the control group that was without blood derivatives in culture media. (B) Representative picture of the effects of different blood derivatives on epithelial scratch wound healing at 16 hours after wounding. FBS: fetal bovine serum, HPS: human peripheral serum. UltraGRO: Human platelet lysate from Helios pharmaceutical. PLTMax: Human platelet lysate from Mill Creek pharmaceutical.
Cell proliferation: MTS assay
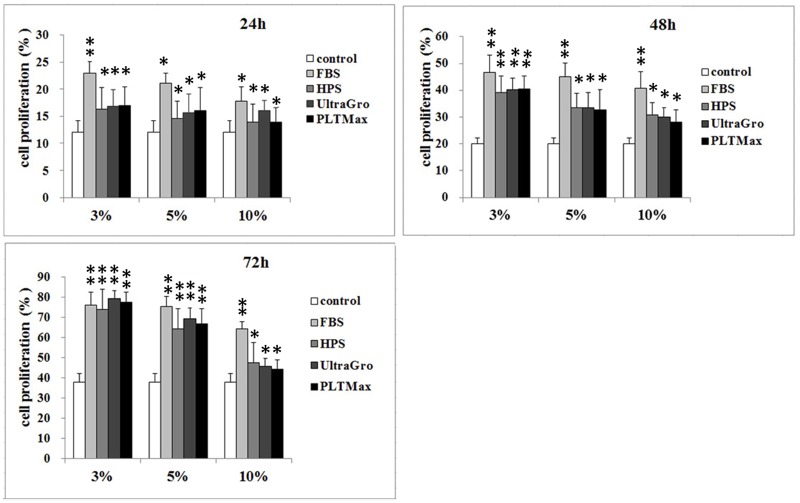
Fig 2. demonstrates the results of the MTS assay. Similar to the cellular migration assay, there were no significant differences between HPS and the 2 HPLs in 3%, 5% and 10% preparations at all 3 time points. However, FBS seemed to have more cell proliferative ability compared to HPS and the 2 HPLs. In addition, higher concentration (10%) of HPS and the 2 HPLs showed more inhibitory effects on cell proliferation compared to that of FBS.
Fig 2. Cell proliferation: The effects of different blood derivatives on cellular proliferation with MTS assay.
At 24 hours and 48 hours, corneal epithelial cells incubated with fetal bovine serum had significantly higher proliferative responses than those incubated in human peripheral serum, UltraGRO human platelet lysate (HPL), and PLTMax HPL. At 72 hours, there was no difference among these 4 products in 3% and 5% preparations. At 24, 48, and 72 hours, the proliferative responses showed no statistical difference between 3% and 5% blood derivative preparations. However, 10% preparation demonstrated poorer proliferative response compared to 3% and 5% preparations at all time points. FBS: fetal bovine serum, HPS: human peripheral serum. UltraGRO: Human platelet lysate from Helios pharmaceutical. PLTMax: Human platelet lysate from Mill Creek pharmaceutical. * indicated p <0.05. ** indicated p <0.01 compared to the control group that was without blood derivative in culture media.
Cell differentiation: Inverted microscopy, scanning electron microscopy (SEM) and transepithelial electric resistance (TEER)
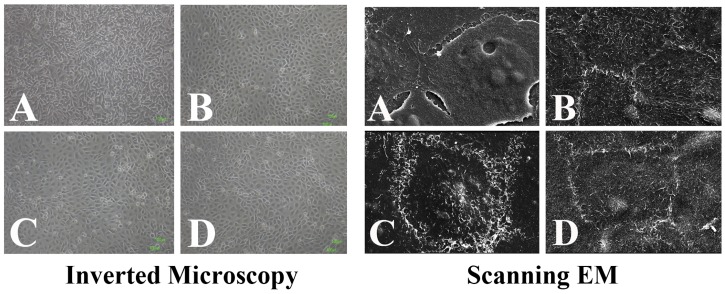
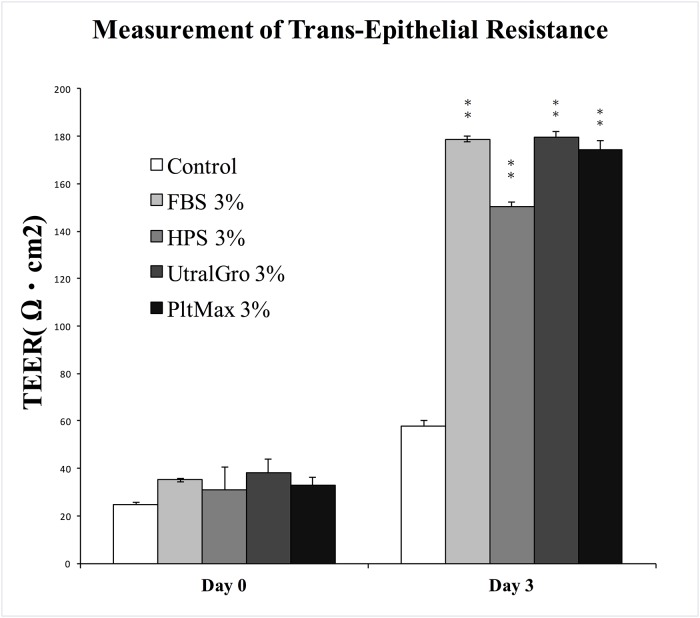
After incubating HCECs with 3% of various blood preparations for 3 days, cellular differentiation patterns were evaluated by inverted microscopy, SEM (Fig 3) and TEER (Fig 4). Under the inverted microscope, cells incubated without serum (Fig 3A) showed a coherent monolayer of irregularly shaped cells that were significantly different from normal cultivated epithelial cell morphology. In contrast, cells incubated with HPS and HPLs showed a coherent monolayer of cells with the regular polygonal morphology quite similar to that of normal cultivated epithelial cells (Fig 3B–3D). Under SEM, cells cultivated without serum showed increased cell-to-cell junctions without the formation of prominent microvilli on the cellular surface. Partial exfoliation of the cell borders from the culture dish was suspected (Fig 3A). Cells cultivated in HPS and the 2 HPLs demonstrated tight cell-to-cell junctions with prominent upright microvilli homogenously and densely distributed at the cellular surface (Fig 3B–3D). TEER is a functional differentiation assay which can reflect the epithelial tightness and functional integrity. In our study, cells cultivated in 3% of blood derivatives showed no significant difference in TEER values among HPS and the 2 HPLs on day 0 and day 3 of initial measurements. [30] (Fig 4)
Fig 3. Cell differentiation: Inverted microscopy and scanning electron microscopy.
Morphologies of human corneal epithelial cell (HCEC) lines cultivated for 48 hours with different blood derivatives, including (A) no serum, (B) human peripheral serum (HPS), (C) UltraGRO human platelet lysate (HPL), and (D) PLTMax HPL. Under inverted microscopy, cells incubated without serum formed a coherent monolayer of irregularly shaped cells (A) while cells cultivated in HPS, UltraGro, and PLTMax showed regular, polygonal flat cells (B-D). Under SEM, cells cultivated without serum showed increased cell-to-cell junctions without the formation of microvilli on the cell surface (A). The white arrow indicated the cell-to-cell junction and the white arrow heads represented the microvilli on the cell surface. Exfoliation of the cells from the culture dish was suspected in cells cultivated without blood derivatives. B-D) Cells cultivated in HPS, UltraGro, and PLTMax demonstrated tight cell-to-cell junction with upright microvilli homogenously and densely distributed at the cellular surface. Original magnifications of the inverted microscopy: 100X and SEM 1250X. UltraGRO: Human platelet lysate from Helios pharmaceutical. PLTMax: Human platelet lysate from Mill Creek pharmaecutical.
Fig 4. Cell differentiation: Measurement of trans-epithelial electrical resistance (TEER).
The effects of different blood derivatives on trans-epithelial electrical resistance. Cells incubated with fetal bovine serum (FBS), human peripheral serum (HPS), UltraGRO human platelet lysate (HPL), and PLTMax HPL demonstrated significantly higher TEER values compared to the control group. However, there was no significant differences among FBS, HPS, UltraGRO, and PLTMax on day 3 after the cells reached confluency. UltraGRO: Human platelet lysate from Helios pharmaceutical. PLTMax: Human platelet lysate from Mill Creek pharmaceutical. * indicated p <0.01. ** indicated p <0.01 compared to the control group that was without any blood derivative.
Rat model of corneal epithelial wound healing
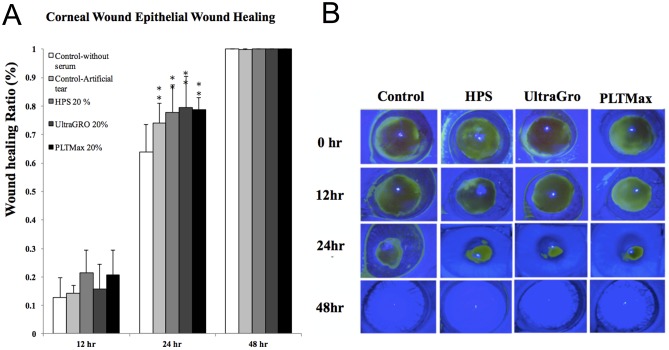
Fig 5 demonstrated the in vivo rat corneal epithelial wound healing after epithelial debridement and topical treatment with 20% of HPS and the 2 HPLs. The wound healing ratios at 24 hours were 0.639±0.095, 0.740±0.069, 0.778±0.096, 0.794±0.110, and 0.786±0.043 in control, FBS, HPS, UltraGRO, and PLTMax, respectively. There were significant increases in corneal epithelial wound healing in both the HPS and the 2 HPL groups compared to the control group 24 hours after injury (p<0.05).
Fig 5. Rat model of corneal epithelial wound healing.
(A) In vivo rat corneal epithelial wound healing after epithelial debridement and topical treatment with 20% of human peripheral serum (HPS) and the 2 different human platelet lysates (HPLs). There were significantly better wound healing abilities in HPS and the 2 HPL groups compared to the control group at 24 hours after wounding. (B) Representative pictures of corneal epithelial defects at 12, 24 and 48 hours after surgery in rat eyes that received epithelial debridement and different treatments. UltraGRO: Human platelet lysate from Helios pharmaceutical. PLTMax: Human platelet lysate from Mill Creek pharmaceutical. * indicated p<0.05 which was compared to the control group without any blood derivative.
Quantification of epitheliotropic factors and chemical analysis
Quantification of epitheliotropic factors was performed in HPS and the 2 HPLs. Table 1 illustrates the ELISA assay results of EGF, TGF-β1, PDGF-AB, PDGF-BB, HA, and fibronectin. The concentrations of EGF, TGF-β1, PDGF-AB, PDGF-BB, and HA were higher in the HPL groups compared to those in HPS, but fibronectin was significantly lower in the HPL groups. Chemical analysis (Table 2) revealed different concentrations of chemicals among the different groups, with significantly more glucose and significantly less ferritin in the PLTmax compared to HPS.(p<0.05)
Table 1. Concentrations of epitheliotropic factors in different human blood preparations (n = 3).
| Blood Preparations | HPS | UltraGRO | PLTMax |
|---|---|---|---|
| EGF (ng/ml) | 0.67±0.01*§ | 5.89±0.09*† | 7.53±0.42§† |
| TGF- β1 (ng/ml) | 57.79±0.85*§ | 98.35±0.26*† | 107.07±0.09§† |
| Fibronectin (μg/ml) | 566.54±63.31*§ | 76.60±20.03* | 147.06±3.71§ |
| PDGF-AB (ng/ml) | 0.80±0.05*§ | 1.47±0.01* | 1.72±0.01§ |
| PDGF-BB (ng/ml) | 0.87±0.38§ | 3.53±2.83 | 10.54±0.68§ |
| Hyaluronic Acid(ng/ml) | 32.23±0.22§ | 42.04±3.33† | 66.29±4.11§† |
*Significant difference between HPS and UltraGRO(p<0.05)
§ Significant difference between HPS and PLTMax (p<0.05)
†Significant difference between UltraGRO and PLTMax (p<0.05)
UltraGRO: Human platelet lysate from Helios pharmaceutical. PLTMax: Human platelet lysate from Mill Creek pharmaceutical.
Table 2. Comparative chemical analysis of fetal bovine serum (FBS), human peripheral serum (HPS), and 2 commercialized human platelet lysates (UltraGRO, PLTMax) (n = 3).
| FBS | HPS | UltraGRO | PLTMax | |
|---|---|---|---|---|
| Glucose(mg/dI) | 101.7±30.0 | 82.3±13.1§ | 129.0±33.1 | 200.0±3.6§ |
| Chloride(mEq/1) | 99.3±0.6 | 104.3±1.2*§ | 115.7±2.5*† | 83.3±0.6§† |
| Sodium(mEq/1) | 136.3±1.2 | 141.7±2.5*§ | 155.3±3.21* | 160.0±1.0§ |
| Potassium(mEq/l) | >10.0 | 4.3±0.2*§ | 5.0±0.1* | 4.8±0.1§ |
| Calcium(mg/dI) | 14.0±0.1 | 9.0±0.3* | 43.1±5.3*† | 7.8±0.3† |
| Phosphate(mg/dI) | 10.5±1.0 | 3.4±0.3 | 4.7±0.21 | 4.6±0.1 |
| Magnesium(mg/dI) | 3.4±0.1 | 1.9±0.3 | 2.2±0.1 | 2.0±0.0 |
| Iron(ug/dI) | 207.3±40.5 | 122.7±18.2 | 69.7±10.2 | 73.7±4.9 |
| Ferritin(ng/ml) | 0.8±0.2 | 70.2±24.4§ | 37.2±6.5 | 25.3±2.6§ |
| Vitamin B12(pg/ml) | 205.7±14.3 | 523.0±125.4 | 505.7±53.0 | 437.0±83.6 |
| Folate(ng/ml) | 8.5±6.5 | 5.8±1.6 | 3.9±2.3 | 7.5±1.9 |
*Significant difference between HPS and UltraGRO (p<0.05)
§ Significant difference between HPS and PLTMax (p<0.05)
†Significant difference between UltraGRO and PLTMax (p<0.05)
TIBC: total iron-binding capacity; UIBC: unsaturated iron-binding capacity
UltraGRO: human platelet lysate from Helios pharmaceutical. PLTMax: human platelet lysate from Mill Creek pharmaceutical
Discussion
In this study, we demonstrated two different commercialized HPLs with corneal epitheliotropic capacities not inferior to that of HPS both in vivo and in vitro. Commercialized HPLs had significantly higher concentrations of several important growth factors, such as EGF, TGF-β1, PDGF-AB and PDGF-BB, compared to HPS. As far as we know, this is the first study to evaluate commercialized HPLs and to compare their corneal epitheliotropic properties with those of other blood derivatives. We believed these two HPLs have the potential to be used as topical eye drops for facilitating corneal re-epithelialization and to replace blood derivatives like HPS.
Corneal wound healing is a complex process, which involves cellular migration, proliferation, differentiation, and deposition of extracellular substances.[29] In this study, we investigated the corneal epithelial migration, proliferation, and differentiation in 4 different blood-derived preparations, including two commercialized HPLs (UltraGRO and PLTMax), HPS, and FBS, in an in vitro cell culture model and in an in vivo rat corneal epithelial wound healing model. FBS, a well-known and widely used calf derived blood product, was used as control in our study. During the process of corneal epithelial wound healing, the initial migration of epithelial cells that cover the denuded area occurs before cellular proliferation and differentiation[31]. Scratch-induced directional wounding assay was designed to detect the effects of blood derivatives on cellular migration in vitro. HPS and the 2 HPLs had better capabilities to promote cellular migration than FBS (in 3% and 5% preparation), and the 2 HPLs were not inferior to HPS. Interestingly, a higher concentration (10%) produced significantly poorer cellular migration in all blood preparations compared to lower concentrations, especially in the 2 commercialized HPLs. These results seemed to imply that lower concentrations (3% and 5%) of HPLs promote better corneal epithelial cell migration, while higher concentrations may worsen cellular migration as shown in vitro. (Fig 1) This finding is of interest for clinical application since it may decrease the demands for a higher concentration of HPL supplies. We also performed the MTS assay to evaluate the cellular proliferating ability among different blood derivatives and found that the result was similar to that of the scratch-induced directional wound healing assay. There was no significant difference in cellular proliferating abilities between HPS and the 2 HPLs. Higher concentration (10%) once again resulted in poorer cellular proliferation. The reason why higher concentrations of HPS and HPLs cause poorer corneal epithelial cell migration and proliferation may be due to TGF-β, an anti-proliferative cytokine which can inhibit cell proliferation in a dose-dependent manner. [32] We found that HPLs contained higher concentrations of TGF-β than HPS, which may explain the higher inhibitory effect found in 10% HPLs than in 10% HPS. Some reports have shown satisfactory cornea epitheliotropic effects of 50% and 100% HPS in treating dry eye diseases. [33–35] Our study revealed that lower concentrations of HPS (3% and 5%) had better epitheliotropic abilities than a higher concentration (10%) in vitro. Further in vivo studies may be required to confirm this finding.
We also performed inverted microscopic imaging, SEM, and TEER to identify cellular differentiation in 3% HPS and HPLs. Under inverted microscopy and SEM, the cells cultured without any blood derivative did not differentiate well, while the cells cultured in the three human blood derivatives (HPS and 2 HPLs) had regular polygonal shapes, compact cell-to-cell junctions, and prominent upright microvilli homogenously and densely distributed at the cellular surface, showing signs of good differentiation. The TEER result of the cells cultivated with 3% blood derivatives also showed similar results as SEM. We demonstrated consistent and reliable results via cellular morphologies detectable by SEM, and functions of intercellular junctions as measured by TEER, reinforcing that 3% HPLs may be able to replace HPS.
In addition to the in vitro cell culture system, which investigated cellular migration, proliferation, and differentiation separately, we performed an in vivo corneal epithelial wound healing assay using Sprague-Dawley rats to reflect the physiological healing process. The in vivo results supported the in vitro results, showing that HPS and HPLs had comparable effects in promoting corneal epithelial wound healing.
The reason for using blood-derived products (e.g. HPS and HPL) as topical eye drops in corneal epithelial disorders is mainly due to the existence of abundant growth factors. [36–39]. Growth factors can be released by platelet activation and this can be reproduced in vitro to prepare growth factor-rich fluids like PRP. We compared several important epitheliotropic factors among HPS and the 2 commercialized HPLs. Our results showed that the 2 HPLs contained significantly higher concentrations of EGF, TGF-β1, PDGF-AB, PDGF-BB, and HA compared to HPS. EGF is normally secreted by lacrimal glands and corneal epithelial cells, and is well known to exert potent proliferative effects on the corneal epithelium [40, 41]. TGF-β1 can inhibit corneal epithelial cell proliferation but has been suggested to play an important role in cellular differentiation and migration [29, 42]. TGF-β1 also stimulates corneal epithelial cell migration via the activation of integrin-β1.[43, 44] Fibronectin is a glycoprotein that supports cell adhesion and is an important mediator for cellular migration. With the presence of fibronectin, PDGF isoforms can stimulate migration of rabbit corneal epithelial cells. [45] All the factors mentioned above are important for corneal wound healing. In this study, various concentrations of these epitheliotropic components were measured in the HPS and the 2 HPLs. We did not evaluate the concentrations in FBS due to the incomparability secondary to species differences. However, wound healing is a complex process and it can be difficult to tell which single epitheliotropic factor might predominantly control wound healing [29].
Topical application of HPS is commonly used in patients with poor epithelial healing. However, autologous HPS has laborious processing time and other drawbacks that limit its clinical use. [29]. Furthermore, the presence of autoantibodies in several ocular autoimmune diseases, such as systemic lupus erythematosus, Sjogren’s syndrome, and graft-versus-host disease, can decrease efficacy in treating ocular surface diseases. [46] In 2011, Shen EP et al. reported that human cord blood serum was superior to HPS in promoting corneal epithelial proliferation and differentiation. [29] However, the difficulty in obtaining human cord blood serum limits its applicability.
Platelets are specialized cells with biologically active substances such as various growth factors that are released from intracellular alpha granules when activated. They play an important role in the process of wound healing. [47, 48] The most well-known platelet-derived growth factors include PDGF, TGF-α, TGF-β, FGF, and vascular endothelial growth factors (VEGF). Although some of these growth factors are available in purified forms, wound healing is a complex process and cannot be mediated by a single agent. Growth factors obtained from platelets may play a role in regulating epithelial healing. [48, 49]. Recently, HPL has been proposed as an alternative for the treatment of various diseases and as a replacement for FBS during ex vivo stem cell expansion.[50] Human blood products are devoid of immunogenic risks due to their human origin. Secondly, the infrastructure for blood collection, as well as the quality and safety tests, are well-established in most developed countries. In countries like the United States, Germany, Switzerland, France, blood products are regulated as pharmaceutical/medicinal products and manufactured under the principle of GMP, which contributes to optimized product consistency, viral safety, and traceability. Since the World Health Organization (WHO) guidelines encourage the GMP implementation in blood establishments at a global level, this should increase the availability of qualified sources [50, 51].
Although autologous HPLs have been commonly reported in the treatment of corneal epithelial disorders, the use of commercialized HPLs can provide additional benefits. Firstly, a large amount of supply can be provided to many patients. Growth factors obtained from platelets may play a role in regulating epithelial healing. Secondly, conventional human peripheral serum needs to undergo 2 hours of precipitation for clotting, centrifugation for 15 minutes, and subsequent dilution of serum to 20 percent with BSS. The whole process may take hours. Commercialized HPL-derived eye drops can be prepared in advance and do not require blood draws or processing of the serum, thus shortening the patient waiting time. Finally, patients with Stevens-Johnson syndrome, bullous pemphigoid or severe graft-versus-host-disease often need long term use of human peripheral serum eye drops and can suffer from repeated blood draws for human peripheral serum. Commercialized HPLs will not require blood draws in these patients.
To our knowledge, this is the first study to systematically compare corneal epitheliotropic effects of commercialized HPLs with that of HPS. There are some limitations in our study. Firstly, the in vitro culture system itself is still a different environment compared to the in vivo system. The complex physiological and molecular interactions of the tear film and ocular surface in vivo cannot be completely replicated by cell culture models. Besides, the human corneal epithelial cell line used in this study may be tumorgenic and different from normal corneal epithelium since it was immortalized with SV-40 virus transformation. Further in vitro experiments using primary cultivated corneal epithelial cells or in vivo experiments are needed to support the current study. [52] Secondly, the concentrations and frequencies of autologous serum eye drops application may affect outcomes and these were different for in vitro models, which had continuous exposure to serum. Finally, we used denuded rat cornea as an in vivo model. However, the wound healing process of healthy rat corneas can be different from the diseased human corneas.
In conclusion, we found that commercialized HPLs can promote corneal epithelial wound healing in both in vivo and in vitro experimental systems. Commercialized HPLs may have the potential to replace HPS eye drops in the treatment of various ocular surface disorders.
Acknowledgments
The authors would like to thank the Integrated Core Facility for Functional Genomics of the National Core Facility Program for Biotechnology (NCFPB) for their technological support; and the technical supports on SEM and TEM by the Joint Center for Instruments and Researches, College of Bioresources and Agriculture at National Taiwan University.
The authors would also like to thank all colleagues and students who contributed to this study. We are grateful to our research assistants, who assisted with the ELISA and Rat eye model.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Noda-Tsuruya T, Asano-Kato N, Toda I, Tsubota K. Autologous serum eye drops for dry eye after LASIK. Journal of refractive surgery. 2006;22(1):61–6. [DOI] [PubMed] [Google Scholar]
- 2.Hartwig D, Herminghaus P, Wedel T, Liu L, Schlenke P, Dibbelt L, et al. Topical treatment of ocular surface defects: comparison of the epitheliotrophic capacity of fresh frozen plasma and serum on corneal epithelial cells in an in vitro cell culture model. Transfusion medicine. 2005;15(2):107–13. 10.1111/j.0958-7578.2005.00559.x [DOI] [PubMed] [Google Scholar]
- 3.Brown SM, Bradley JC. The effect of autologous serum eye drops in the treatment of severe dry eye disease: a prospective randomized case-control study. American journal of ophthalmology. 2005;140(3):565; author reply -6. 10.1016/j.ajo.2005.03.067 [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto Y, Dogru M, Goto E, Ohashi Y, Kojima T, Ishida R, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111(6):1115–20. 10.1016/j.ophtha.2003.10.019 [DOI] [PubMed] [Google Scholar]
- 5.Vajpayee RB, Mukerji N, Tandon R, Sharma N, Pandey RM, Biswas NR, et al. Evaluation of umbilical cord serum therapy for persistent corneal epithelial defects. The British journal of ophthalmology. 2003;87(11):1312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogawa Y, Okamoto S, Mori T, Yamada M, Mashima Y, Watanabe R, et al. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone marrow transplantation. 2003;31(7):579–83. 10.1038/sj.bmt.1703862 [DOI] [PubMed] [Google Scholar]
- 7.Takamura E, Shinozaki K, Hata H, Yukari J, Hori S. Efficacy of autologous serum treatment in patients with severe dry eye. Advances in experimental medicine and biology. 2002;506(Pt B):1247–50. [DOI] [PubMed] [Google Scholar]
- 8.Goto E, Shimmura S, Shimazaki J, Tsubota K. Treatment of superior limbic keratoconjunctivitis by application of autologous serum. Cornea. 2001;20(8):807–10. [DOI] [PubMed] [Google Scholar]
- 9.Rocha EM, Pelegrino FS, de Paiva CS, Vigorito AC, de Souza CA. GVHD dry eyes treated with autologous serum tears. Bone marrow transplantation. 2000;25(10):1101–3. 10.1038/sj.bmt.1702334 [DOI] [PubMed] [Google Scholar]
- 10.Tsubota K, Goto E, Shimmura S, Shimazaki J. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology. 1999;106(10):1984–9. 10.1016/S0161-6420(99)90412-8 [DOI] [PubMed] [Google Scholar]
- 11.Tsubota K, Goto E, Fujita H, Ono M, Inoue H, Saito I, et al. Treatment of dry eye by autologous serum application in Sjogren's syndrome. The British journal of ophthalmology. 1999;83(4):390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziakas NG, Boboridis KG, Terzidou C, Naoumidi TL, Mikropoulos D, Georgiadou EN, et al. Long-term follow up of autologous serum treatment for recurrent corneal erosions. Clinical & experimental ophthalmology. 2010;38(7):683–7. [DOI] [PubMed] [Google Scholar]
- 13.Shen EP, Hu FR, Lo SC, Chen YM, Sun YC, Lin CT, et al. Comparison of corneal epitheliotrophic capacity among different human blood-derived preparations. Cornea. 2011;30(2):208–14. 10.1097/ICO.0b013e3181eadb67 [DOI] [PubMed] [Google Scholar]
- 14.Kojima T, Higuchi A, Goto E, Matsumoto Y, Dogru M, Tsubota K. Autologous serum eye drops for the treatment of dry eye diseases. Cornea. 2008;27 Suppl 1:S25–30. [DOI] [PubMed] [Google Scholar]
- 15.Stenwall PA, Bergstrom M, Seiron P, Sellberg F, Olsson T, Knutson F, et al. Improving the anti-inflammatory effect of serum eye drops using allogeneic serum permissive for regulatory T cell induction. Acta ophthalmologica. 2015;93(7):654–7. 10.1111/aos.12801 [DOI] [PubMed] [Google Scholar]
- 16.Bradley JC, Simoni J, Bradley RH, McCartney DL, Brown SM. Time- and temperature-dependent stability of growth factor peptides in human autologous serum eye drops. Cornea. 2009;28(2):200–5. 10.1097/ICO.0b013e318186321e [DOI] [PubMed] [Google Scholar]
- 17.Golebiewska EM, Poole AW. Platelet secretion: From haemostasis to wound healing and beyond. Blood reviews. 2015;29(3):153–62. 10.1016/j.blre.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semple JW, Italiano JE Jr., Freedman J. Platelets and the immune continuum. Nature reviews Immunology. 2011;11(4):264–74. 10.1038/nri2956 [DOI] [PubMed] [Google Scholar]
- 19.Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Frontiers in bioscience: a journal and virtual library. 2008;13:3532–48. [DOI] [PubMed] [Google Scholar]
- 20.Van Pham P, Bui KH, Ngo DQ, Vu NB, Truong NH, Phan NL, et al. Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem cell research & therapy. 2013;4(4):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atashi F, Jaconi ME, Pittet-Cuenod B, Modarressi A. Autologous platelet-rich plasma: a biological supplement to enhance adipose-derived mesenchymal stem cell expansion. Tissue engineering Part C, Methods. 2015;21(3):253–62. 10.1089/ten.TEC.2014.0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Han Z, Liu D, Zhao P, Liang S, Xu K. Autologous platelet-rich plasma promotes neurogenic differentiation of human adipose-derived stem cells in vitro. The International journal of neuroscience. 2013;123(3):184–90. 10.3109/00207454.2012.742077 [DOI] [PubMed] [Google Scholar]
- 23.Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E, et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47(8):1436–46. 10.1111/j.1537-2995.2007.01220.x [DOI] [PubMed] [Google Scholar]
- 24.Perez-Ilzarbe M, Diez-Campelo M, Aranda P, Tabera S, Lopez T, del Canizo C, et al. Comparison of ex vivo expansion culture conditions of mesenchymal stem cells for human cell therapy. Transfusion. 2009;49(9):1901–10. Epub 2009/06/06. 10.1111/j.1537-2995.2009.02226.x [DOI] [PubMed] [Google Scholar]
- 25.Ben Azouna N, Jenhani F, Regaya Z, Berraeis L, Ben Othman T, Ducrocq E, et al. Phenotypical and functional characteristics of mesenchymal stem cells from bone marrow: comparison of culture using different media supplemented with human platelet lysate or fetal bovine serum. Stem cell research & therapy. 2012;3(1):6. Epub 2012/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia W, Li H, Wang Z, Xu R, Fu Y, Zhang X, et al. Human platelet lysate supports ex vivo expansion and enhances osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Cell Biol Int. 2011;35(6):639–43. Epub 2011/01/18. 10.1042/CBI20100361 [DOI] [PubMed] [Google Scholar]
- 27.Mojica-Henshaw MP, Jacobson P, Morris J, Kelley L, Pierce J, Boyer M, et al. Serum-converted platelet lysate can substitute for fetal bovine serum in human mesenchymal stromal cell cultures. Cytotherapy. 2013;15(12):1458–68. 10.1016/j.jcyt.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 28.Iudicone P, Fioravanti D, Bonanno G, Miceli M, Lavorino C, Totta P, et al. Pathogen-free, plasma-poor platelet lysate and expansion of human mesenchymal stem cells. Journal of translational medicine. 2014;12:28 10.1186/1479-5876-12-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen EP, Hu F-R, Lo S-C, Chen Y-M, Sun Y-C, Lin C-T, et al. Comparison of corneal epitheliotrophic capacity among different human blood—derived preparations. Cornea. 2011;30(2):208–14. 10.1097/ICO.0b013e3181eadb67 [DOI] [PubMed] [Google Scholar]
- 30.Reichl S. Cell culture models of the human cornea—a comparative evaluation of their usefulness to determine ocular drug absorption in-vitro. J Pharm Pharmacol. 2008;60(3):299–307. 10.1211/jpp.60.3.0004 [DOI] [PubMed] [Google Scholar]
- 31.Lu L, Reinach PS, Kao WW-Y. Corneal epithelial wound healing. Experimental Biology and Medicine. 2001;226(7):653–64. [DOI] [PubMed] [Google Scholar]
- 32.Honma Y, Nishida K, Sotozono C, Kinoshita S. Effect of transforming growth factor-beta1 and -beta2 on in vitro rabbit corneal epithelial cell proliferation promoted by epidermal growth factor, keratinocyte growth factor, or hepatocyte growth factor. Experimental eye research. 1997;65(3):391–6. 10.1006/exer.1997.0338 [DOI] [PubMed] [Google Scholar]
- 33.Semeraro F, Forbice E, Braga O, Bova A, Di Salvatore A, Azzolini C. Evaluation of the efficacy of 50% autologous serum eye drops in different ocular surface pathologies. BioMed research international. 2014;2014:826970 10.1155/2014/826970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussain M, Shtein RM, Sugar A, Soong HK, Woodward MA, DeLoss K, et al. Long-term use of autologous serum 50% eye drops for the treatment of dry eye disease. Cornea. 2014;33(12):1245–51. 10.1097/ICO.0000000000000271 [DOI] [PubMed] [Google Scholar]
- 35.Lekhanont K, Jongkhajornpong P, Choubtum L, Chuckpaiwong V. Topical 100% serum eye drops for treating corneal epithelial defect after ocular surgery. BioMed research international. 2013;2013:521315 10.1155/2013/521315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aggarwal S, Kheirkhah A, Cavalcanti BM, Cruzat A, Colon C, Brown E, et al. Autologous serum tears for treatment of photoallodynia in patients with corneal neuropathy: efficacy and evaluation with in vivo confocal microscopy. The ocular surface. 2015;13(3):250–62. 10.1016/j.jtos.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anitua E, Muruzabal F, Tayebba A, Riestra A, Perez VL, Merayo-Lloves J, et al. Autologous serum and plasma rich in growth factors in ophthalmology: preclinical and clinical studies. Acta ophthalmologica. 2015;93(8):e605–e14. 10.1111/aos.12710 [DOI] [PubMed] [Google Scholar]
- 38.Chen Y-M, Hu F-R, Huang J-Y, Shen EP, Tsai T-Y, Chen W-L. The effect of topical autologous serum on graft re-epithelialization after penetrating keratoplasty. American journal of ophthalmology. 2010;150(3):352–9. e2. 10.1016/j.ajo.2010.03.024 [DOI] [PubMed] [Google Scholar]
- 39.Soni NG, Jeng BH. Blood-derived topical therapy for ocular surface diseases. British Journal of Ophthalmology. 2015:bjophthalmol-2015-306842. [DOI] [PubMed] [Google Scholar]
- 40.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Progress in retinal and eye research. 2000;19(1):113–29. [DOI] [PubMed] [Google Scholar]
- 41.Klenkler B, Sheardown H, Jones L. Growth factors in the tear film: role in tissue maintenance, wound healing, and ocular pathology. The ocular surface. 2007;5(3):228–39. [DOI] [PubMed] [Google Scholar]
- 42.Haber M, Cao Z, Panjwani N, Bedenice D, Li WW, Provost PJ. Effects of growth factors (EGF, PDGF-BB and TGF-beta 1) on cultured equine epithelial cells and keratocytes: implications for wound healing. Vet Ophthalmol. 2003;6(3):211–7. [DOI] [PubMed] [Google Scholar]
- 43.Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J Biol Chem. 2001;276(50):46707–13. 10.1074/jbc.M106176200 [DOI] [PubMed] [Google Scholar]
- 44.Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, et al. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am J Pathol. 2004;164(2):651–63. 10.1016/S0002-9440(10)63153-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishida T. Translational research in corneal epithelial wound healing. Eye & contact lens. 2010;36(5):300–4. [DOI] [PubMed] [Google Scholar]
- 46.Hwang J, Chung SH, Jeon S, Kwok SK, Park SH, Kim MS. Comparison of clinical efficacies of autologous serum eye drops in patients with primary and secondary Sjogren syndrome. Cornea. 2014;33(7):663–7. 10.1097/ICO.0000000000000147 [DOI] [PubMed] [Google Scholar]
- 47.Geremicca W, Fonte C, Vecchio S. Blood components for topical use in tissue regeneration: evaluation of corneal lesions treated with platelet lysate and considerations on repair mechanisms. Blood Transfus. 2010;8(2):107–12. 10.2450/2009.0091-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandri G, Bonferoni MC, Rossi S, Ferrari F, Mori M, Del Fante C, et al. Thermosensitive eyedrops containing platelet lysate for the treatment of corneal ulcers. International journal of pharmaceutics. 2012;426(1):1–6. [DOI] [PubMed] [Google Scholar]
- 49.Anitua E, Sánchez M, Nurden AT, Nurden P, Orive G, Andía I. New insights into and novel applications for platelet-rich fibrin therapies. Trends in biotechnology. 2006;24(5):227–34. 10.1016/j.tibtech.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 50.Shih DT-B, Burnouf T. Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. New biotechnology. 2015;32(1):199–211. 10.1016/j.nbt.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guidelines on good manufacturing practices for blood establishments. Annex 4. WHO Tech Rep Series. 2011:148–214. [Google Scholar]
- 52.Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Investigative ophthalmology & visual science. 1995;36(3):614–21. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.