Abstract
It is known that, after a prolonged period of visual deprivation, the adult visual cortex can be recruited for nonvisual processing, reflecting cross-modal plasticity. Here, we investigated whether cross-modal plasticity can occur at short timescales in the typical adult brain by comparing the interaction between vision and touch during binocular rivalry before and after a brief period of monocular deprivation, which strongly alters ocular balance favoring the deprived eye. While viewing dichoptically two gratings of orthogonal orientation, participants were asked to actively explore a haptic grating congruent in orientation to one of the two rivalrous stimuli. We repeated this procedure before and after 150 min of monocular deprivation. We first confirmed that haptic stimulation interacted with vision during rivalry promoting dominance of the congruent visuo-haptic stimulus and that monocular deprivation increased the deprived eye and decreased the nondeprived eye dominance. Interestingly, after deprivation, we found that the effect of touch did not change for the nondeprived eye, whereas it disappeared for the deprived eye, which was potentiated after deprivation. The absence of visuo-haptic interaction for the deprived eye lasted for over 1 hr and was not attributable to a masking induced by the stronger response of the deprived eye as confirmed by a control experiment. Taken together, our results demonstrate that the adult human visual cortex retains a high degree of cross-modal plasticity, which can occur even at very short timescales.
Introduction
Neuroplasticity, the intrinsic capability of the nervous system to change and adapt as a function of physiologic changes, sensory experiences, and environmental pressures (Pascual-Leone, Amedi, Fregni, & Merabet, 2005), is maximal early in life, during the so-called critical period (Berardi, Pizzorusso, & Maffei, 2000; Hubel & Wiesel, 1970). During the critical period, neuroplasticity is so high that, in case of sensory loss, neural circuits can be dramatically reorganized (Merabet & Pascual-Leone, 2010; Pascual-Leone et al., 2005). For example, the primary visual cortex of early blind participants is recruited for tactile (Amedi, Raz, Azulay, Malach, & Zohary, 2010; Sathian, 2005; Hamilton & Pascual-Leone, 1998; Sadato et al., 1996) and auditory (Collignon et al., 2011; Gougoux, Zatorre, Lassonde, Voss, & Lepore, 2005) sensory processing, a phenomenon usually referred as cross-modal plasticity. Although neuroplasticity in higher-level association brain regions, like those important for learning and memory, remains high in adults (Fuchs & Flugge, 2014), it is very limited in low-level sensory cortices in adult brains (Berardi et al., 2000). However, some crossmodal plasticity is retained. For example, the primary visual cortex can be transiently recruited for tactile processing in normally sighted individuals after prolonged (5 days) blindfolding (Merabet et al., 2008; Kauffman, Theoret, & Pascual-Leone, 2002). This suggests that visual deprivation unmasks somatosensory signals in V1 rather than being a rewiring through the formation of new pathways (for this debate, see Qin & Yu, 2013; Striem-Amit, Cohen, Dehaene, & Amedi, 2012). In line with this hypothesis, primary visual cortical activity (BOLD) in the absence of visual stimulation has been reported during different haptic exploration tasks (Snow, Strother, & Humphreys, 2014; Merabet et al., 2007; Saito, Okada, Honda, Yonekura, & Sadato, 2006). Consistent with the suggestion of the unmasking hypothesis, a recent study by Convento, Vallar, Galantini, and Bolognini (2013) has shown increased visual cortical excitability (measured as decreased TMS phosphene thresholds) during tactile and auditory stimulation (Convento et al., 2013).
One efficient way of studying early cross-modal interactions is using ambiguous visual stimuli and, in particular, binocular rivalry, a specific form of perceptual bistability that takes place when two incompatible images are separately delivered to each eye (Alais & Blake, 2005; Blake & Logothetis, 2002; Levelt, 1965). During binocular rivalry, instead of merging the two monocular images, the observer perceives a periodic alternation of the two visual stimuli that compete against each other to gain access to the observer’s visual awareness. Whereas one image dominates observer’s perception, the other, albeit displayed on the retina, is suppressed from visual awareness. Binocular rivalry suppression occurs early in the visual system: Neural activity related to the suppressed visual stimulus is not detectable outside V1 or V2 (for a review, see Sterzer, Stein, Ludwig, Rothkirch, & Hesselmann, 2014). The existence of cross-modal connections to early visual areas is also supported by the findings that crossmodal signals can interact with the suppressed visual stimulus (for a review, see Deroy et al., 2016) during binocular rivalry or with the suppressed information during continuous flash suppression (Tsuchiya & Koch, 2005): Auditory (Lunghi, Morrone, & Alais, 2014; Alsius & Munhall, 2013; Conrad et al., 2013; Conrad, Bartels, Kleiner, & Noppeney, 2010), tactile (Lunghi & Alais, 2013, 2015; Lunghi & Morrone, 2013; Lunghi, Binda, & Morrone, 2010), combined auditory–tactile (Lunghi et al., 2014), olfactory (Zhou, Jiang, He, & Chen, 2010), proprioceptive (Salomon, Lim, Herbelin, Hesselmann, & Blanke, 2013), and vestibular (Salomon, Kaliuzhna, Herbelin, & Blanke, 2015) signals have been shown to interact with the suppressed visual signal during binocular rivalry, reinforcing the animal data showing direct cross-modal input in V1 (Clavagnier, Falchier, & Kennedy, 2004; Rockland & Ojima, 2003; Falchier, Clavagnier, Barone, & Kennedy, 2002). In particular, Lunghi et al. (2010) showed that actively exploring a haptic grating congruent in orientation with one of two rivalrous visual gratings could disambiguate visual perception during binocular rivalry, both prolonging dominance and shortening suppression of the visual grating congruent in orientation with the haptic one. To observe the effect, the haptic grating needed to be matched in spatial frequency (Lunghi et al., 2010), orientation (Lunghi & Alais, 2013), and spatial position (Lunghi & Morrone, 2013) to the visual stimulus. Taken together, these results support the idea that somatosensory signals can indeed reach early visual cortices, probably V1, given the high orientation and spatial frequency selectivity of the interaction that matches closely the selectivity of the primary visual cortex neurons. Binocular rivalry is a robust technique also to measure small plastic changes in ocular balance: 150 min of monocular deprivation considerably alter the dynamics of binocular rivalry, counterintuitively boosting the deprived eye signal (Lunghi, Burr, & Morrone, 2011). After deprivation, the deprived eye strongly dominates visual perception: The mean phase duration of the deprived eye becomes twice as long as that of the non-deprived eye, reflecting homeostatic plasticity. This effect decays over time, lasting up to 3 hr (Lunghi, Burr, & Morrone, 2013; Lunghi et al., 2011), and suggests that the adult visual cortex retains a high degree of experience-dependent plasticity (Lunghi, Berchicci, Morrone, & Di Russo, 2015; Lunghi et al., 2011, 2013).
Here, we investigated whether cross-modal plasticity can occur also at short timescales in the adult visual cortex of normally sighted humans. We combined the visuohaptic cross-modal paradigm described in Lunghi et al. (2010) with a short period of monocular deprivation. The data show that monocular deprivation interferes with the haptic modulation of binocular rivalry, but only for the deprived eye.
Methods
Participants
Eight participants (five women; mean age = 28.4 years, SD = 2.9 years), including two authors of this study, participated in the main experiment. Five of these participants also participated in the control experiment. All of the participants had normal or corrected-to-normal vision, no strong eye preference (measured as eye predominance in binocular rivalry), and normal stereo acuity (Frisby Stereotest; Sasieni, 1978). Except for the two authors, all participants were naive to the purposes of the experiment.
Ethic Statement
The experimental protocol was approved by the Tuscany Regional Ethics Committee of the Azienda Ospedaliero-Universitaria Meyer and was performed in accordance with the Declaration of Helsinki. All of the participants gave written informed consent.
Apparatus and Stimuli
The experiment took place in a quiet room in total darkness to remove any spatial frame of reference. The visual stimuli, developed in MATLAB (Version 7.11.0; The MathWorks, Inc., Natick, MA) using Psychtoolbox-3 (Kleiner et al., 2007; Brainard, 1997; Pelli, 1997), were two superimposed oblique orthogonal red and blue gratings (orientation = ±45°, size = 3°, SF = 2 c/deg, 50% of the maximum contrast) surrounded by a white smoothed circle, presented on a black uniform background (luminance = 0.17 cd/m2) in central vision. The peak luminance of the red grating was matched with the peak luminance of the blue one (1.7 cd/m2). Dichoptic stimulation was achieved by having participants wear red and blue anaglyph goggles.
The gratings contained a small (0.2°) white fixation dot in their center and were presented on a 24-in. LCD display (Acer LCD GD245HQ) positioned horizontally at 37 cm above a horizontal mirror (see Figure 1A). Observers viewed the stimuli reflected in the mirror at a distance of 35 cm from the observers’ eyes.
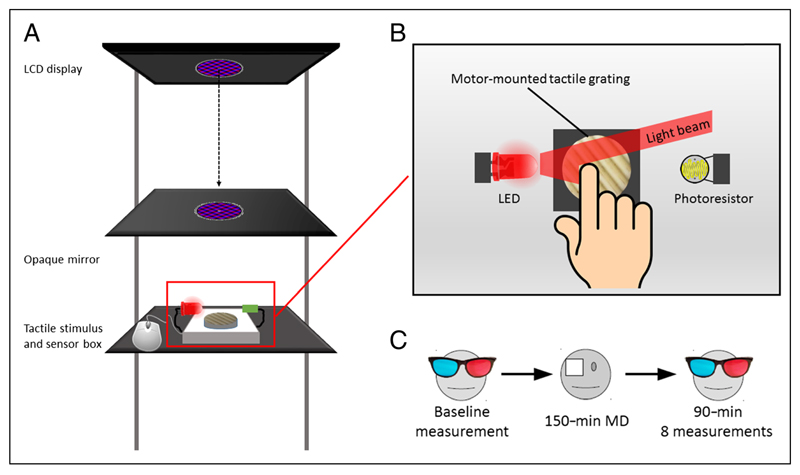
Figure 1.
Experimental setup and paradigm. (A) Diagram illustrating the experimental setup: The rivalrous gratings were presented on an LCD monitor and reflected by an opaque mirror onto the haptic stimulus (grooved grating) location. (B) The haptic grating orientation was varied at each touch period by a servomotor. The temporal dynamics of haptic exploration were finely tracked with an LED-photoresistor system. (C) Experimental paradigm: After baseline measurements, the observers wore a translucent eye patch on their dominant eye for 150 min. After the deprivation, 8 × 4 min measurements were collected at 0, 5, 10, 15, 30, 45, 60, and 90 min from the patch removal.
Placed 35 cm under the mirror, the haptic stimulus consisted of a 3-D-printed sinusoidal grating (diameter = 3 cm, 2 cycles/cm of spatial frequency). The grating was mounted on a servomotor, which could rotate it arbitrarily at any given orientation. The visual and haptic stimuli were spatially aligned so that observers experienced the illusion of a single visual and haptic object. The onset and offset of a touch period were measured monitoring an LED and a photoresistor positioned laterally to the 3-D grating (see Figure 1B). When the observer’s finger touched the grating patch, it occluded the LED light to the photoresistor, signaling the onset of touch. Vice versa, when the observer lifted the finger at the end of a touch period, the light from the LED activated the photoresistor again, signaling the offset of touch. The servomotor, LED, and photoresistor were controlled by an Arduino UNO R3 microcontroller (D’Ausilio, 2012) interfaced to MATLAB using the Arduino IO Package (www.mathworks.com/matlabcentral/fileexchange/32374-legacy-matlab-and-simulink-support-for-arduino).
Short-term monocular deprivation was achieved by having observers wear a translucent eye patch on their dominant eye, defined as the eye that perceptually dominated for most of the time during preliminary binocular rivalry measurements for each participant. The eye patch was made of translucent plastic material and blocked all patterned visual contrast without causing dark adaptation (attenuation = 15%; for details, see Lunghi et al., 2011).
Task and Procedure
Main Experiment
During each 240-sec experimental block, participants continuously reported which visual grating they were consciously perceiving: red, blue, or mixed. Observers reported the rivalrous perception by continuous alternate mouse button press with their nondominant hand. The association between grating color (red or blue) and orientation (+45° or −45° in relation to vertical orientation) was swapped after each trial to avoid adaptation and counterbalance for eye dominance. At randomized time intervals (time between two consecutive touch periods = 8.54 ± 0.49 sec), the fixation point shape changed from circular to squared, signaling to the observer the beginning of a touch period. Observers were instructed to actively explore the haptic grating by performing horizontal movements using their dominant hand’s index finger while reporting their visual perception by pressing the appropriate mouse button with the non-dominant hand. The duration of each touch period was randomized, lasting 2.63 ± 0.34 sec on average.
After at least four training experimental blocks, each observer performed 2 × 240 sec measurements before the beginning of deprivation, which served as baseline, and 8 × 240 sec measurements acquired at 0, 5, 10, 15, 30, 45, 60, and 90 min after the eye patch removal (see Figure 1C).
This procedure (2 × baseline measurements + 150-min monocular deprivation + 8 × after deprivation measurements) was repeated independently four times in different days for each participant.
Control Experiment
The visual stimuli contrast was varied to simulate the effect of monocular deprivation (simulated deprived eye contrast: mean = 0.77, SD = 0.1; nondeprived eye: mean = 0.22, SD = 0.1). For each participant, the appropriate contrasts were determined by increasing the contrast on the eye that was deprived in the previous session and decreasing the contrast of the nondeprived eye to achieve a difference in the perceptual dominance comparable with that induced by monocular deprivation. We collected 4 × 240 sec experimental blocks for each participant following the same experimental procedure of the main experiment.
Data Analyses
Before the deprivation, baseline measurements were acquired for each participant. Data from 240-sec blocks after deprivation were averaged in the intervals of 0–19, 30–49, and 60–94 min after eye patch removal.
We first computed for each touch and no-touch stimulation period the probability of maintaining the same visual percept for the whole period, switching perception once or switching more than once, conditioned to the type of visuo-haptic stimulation (congruent, incongruent orientation, no touch), separately for the two eyes (deprived and nondeprived). The touch periods that started during a period of mixed rivalry, which cannot be used to compute the visuo-haptic congruence given that both visual orientations were perceived simultaneously, were very rare (mixed rivalry: mean = 2.4%, SD = 1.3%).
We also computed the time course of the effect of haptic exploration on the dynamics of binocular rivalry by calculating the probability of seeing the visual stimulus congruent with the haptic one as a function of time elapsed from the onset of haptic stimulation. Each participant’s tracking of perceptual alternations for the 11 sec after the onset of each touch period was overlaid and averaged every 440 msec. Separate probability traces were obtained for the deprived and nondeprived eyes. To obtain a visual dominance index for each eye independently from the effect of touch, we averaged for each participant the last six bins (2.64 sec) of each probability trace. Calculating the index using the last 5 sec of the traces produced very similar results. Finally, we also calculated a touch effect index for each eye by computing the integral of the area of the probability trace from touch onset to the time in which the probability decayed to visual-only levels.
Statistics
To compare the effect of touch on binocular rivalry, we used paired-sample, two-tailed Student’s t tests (congruent, incongruent, and visual only). Each probability trace time bin was compared across eyes using paired-sample t tests. For the comparison between the areas of the probability curves during the haptic exploration (touch effect indexes) and the respective vision-only predominance indexes, we used bootstrap sign tests with 10,000 repetitions, and for the comparison between the touch effect indexes and the null touch effect, we used one-sample, two-tailed Student’s t tests.
Results
Main Experiment
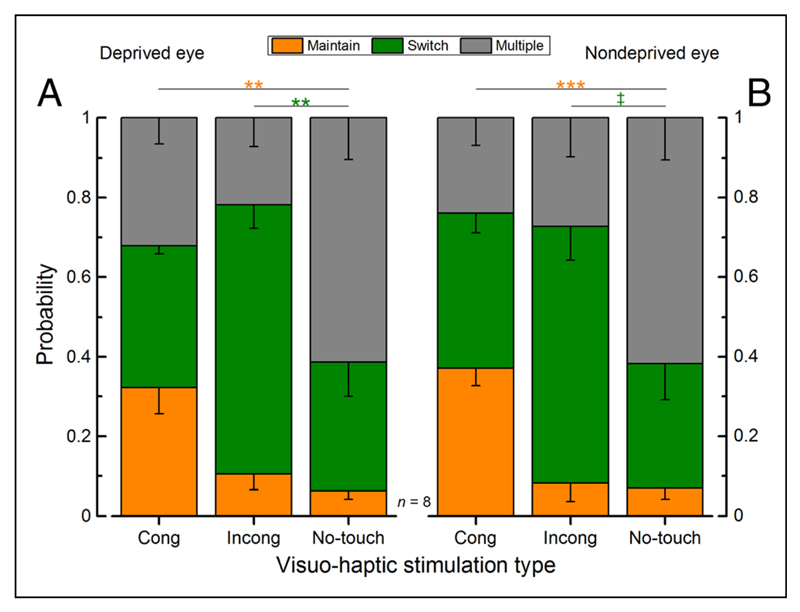
We measured the effect of haptic stimulation on the dynamics of binocular rivalry before and after 150 min of monocular deprivation (a schematic representation of the experimental setup and paradigm is shown in Figure 1). The probability of maintaining or switching perception during a touch period conditioned to the type of visuo-haptic stimulation (parallel, orthogonal, or visual only) was calculated separately for the two eyes. Consistent with Lunghi et al. (2010), before deprivation (Figure 2), the probability of maintaining was significantly higher for congruent visuo-haptic stimulation compared with vision-only stimulation (Figure 2A, deprived eye: paired-sample, two-tailed t test, t(7) = 4.91, Bonferroni–Holms corrected α = .0167, p = .0017; Figure 2B, nondeprived eye: paired-sample, two-tailed t test, Bonferroni–Holms corrected α = .0167, t(7) = 6.95, p = .0002). On the other hand, the probability of switching perception was significantly higher for incongruent visuo-haptic stimulation compared with vision-only stimulation for the deprived eye (Figure 2A, deprived eye: paired-sample, two-tailed t test, Bonferroni–Holms corrected α = .025, t(7) = 3.49, p = .01). For the nondeprived eye, the effect did not survive the correction for multiple comparisons (Figure 2B, nondeprived eye: paired-sample, two-tailed t test, t(7) = 3.01, Bonferroni–Holms corrected α = 0.0167, p = .02), although there was a clear trend. Both sets of data indicate that haptic stimulation interacted with binocular rivalry both by prolonging dominance and by shortening suppression of the congruent visual stimulus.
Figure 2.
Average probabilities across touch conditions, before monocular deprivation (baseline) separately for the two eyes. The average probabilities of maintaining the same visual percept (orange bar), switching visual perception once (green bar), or switching more than once during a touch period (gray bar), conditioned to the type of visuo-haptic stimulation (congruent, incongruent orientation, or no touch)—(A) deprived eye and (B) nondeprived eye—error bars represent 1 ± SEM. The probability of maintaining the same visual percept for the whole touch period was significantly higher for the congruent visuo-haptic stimulation for both eyes compared with the no-touch stimulation, whereas the probability of switching visual percept was significantly higher for the incongruent (orthogonal) visuo-haptic stimulation for both eyes compared with the no-touch stimulation (paired-sample, two-tailed t test: n = 8, *p ≤ .05, **p ≤ .01, ***p ≤ .001, ‡p ≤ .05; however, the test did not survive the correction for multiple comparisons).
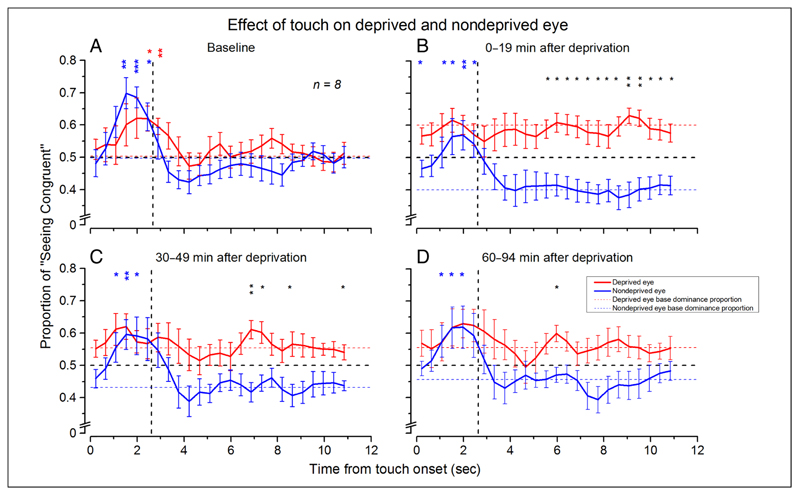
We then analyzed the dynamics of the visuo-haptic effect during binocular rivalry. Figure 3 (red symbols = deprived eye, blue symbols = nondeprived eye) shows the data compared with the average proportion of dominance of either the deprived (red dashed line) or nondeprived (blue dashed line) eye measured during the last 2.64 sec of visual-only stimulation. Before monocular deprivation (Figure 3A), haptic stimulation increased the probability to perceive the congruent visual grating for both the deprived and nondeprived eyes during the final phase of the touch period. In line with previous work (Lunghi et al., 2011, 2013), monocular deprivation increased the deprived eye dominance and decreased the nondeprived eye dominance. Eye dominance (mean ± SEM) before deprivation (Figure 3A) was 50 ± 3% for the deprived eye and 50 ± 2% for the nondeprived eye. During the first 19 min after eye patch removal (Figure 3B), the deprived eye dominance significantly increased compared with baseline measurements (+10%, paired-sample, two-tailed t test, Bonferroni–Holms corrected α = .0167, t(7) = 4.68, p = .002), whereas the nondeprived eye dominance significantly decreased (−10%, paired-sample, two-tailed t test, t(7) = −3.37, Bonferroni–Holms corrected α = .025, p = .012). The effect of deprivation was still significant 49 min after eye patch removal for the nondeprived eye (−7%, paired-sample, two-tailed t test, t(7) = −3.62, Bonferroni–Holms corrected α = .0167, p = .009), whereas it did not survive the correction for multiple comparisons for the deprived eye (+5%, paired-sample t test, t(7) = 2.52, Bonferroni–Holms corrected α = .025, p = .04). The effect was not significant for both eyes 94 min after deprivation, although a clear trend was present (+5% for the deprived eye, −4% for the nondeprived eye).
Figure 3.
Dynamics of the effect of touch on binocular rivalry. Proportion of reported dominance of the visual stimulus congruent in orientation with the haptic orientation plotted as a function of time from the touch onset for the deprived (red symbols) and nondeprived (blue symbols) eyes. These curves were obtained by overlaying and averaging each participant’s perceptual tracking for 11 sec after each touch onset. (A) Before deprivation. The probability of seeing the visual orientation congruent with the haptic orientation increases and slowly reverts to baseline levels for both eyes after touch offset. (B) At 0–14 min after the end of deprivation. (C) At 30–49 min after the end of deprivation. (D) At 60–94 min after the end of deprivation. After deprivation, the effect of touch is absent for the deprived eye. Horizontal red and blue lines are the average proportion of dominance during visual-only stimulation for the deprived and nondeprived eyes. Vertical dashed line shows the average of the touch interval. Bin width of 0.44 sec. Asterisks represent statistical significance: deprived eye versus baseline (red asterisks), nondeprived eye versus baseline (blue asterisks), and deprived eye versus nondeprived eye (black asterisks; *p ≤ .05, **p ≤ .01, ***p = .001). Error bars represent 1 ± SEM.
Together with the baseline effect, there was a differential effect of the touch for the deprived and nondeprived eyes. The probability of perceiving the congruent touch orientation with respect to the baseline significantly increased for the nondeprived eye as for the data before deprivation. However, we never observed any touch effect for the deprived eye, even after 94 min after the deprivation. At this time, the ocular balance is not statistically different from the one obtained before deprivation. Nevertheless, touch is not able to induce an increase in dominance of the congruent percept (Figure 3B–D).
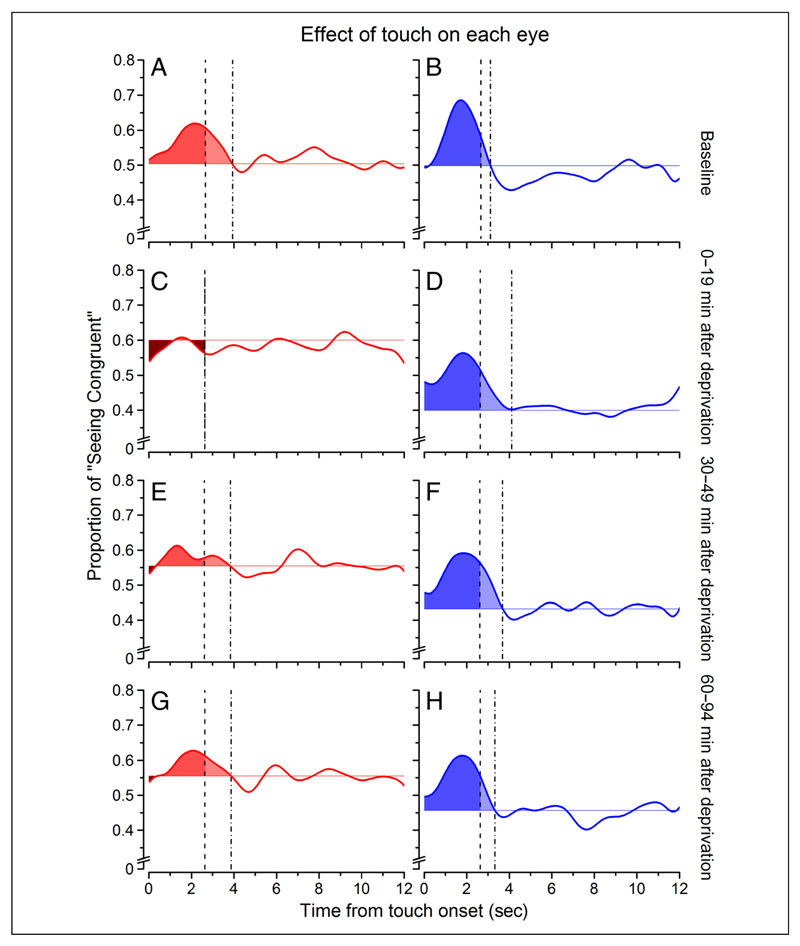
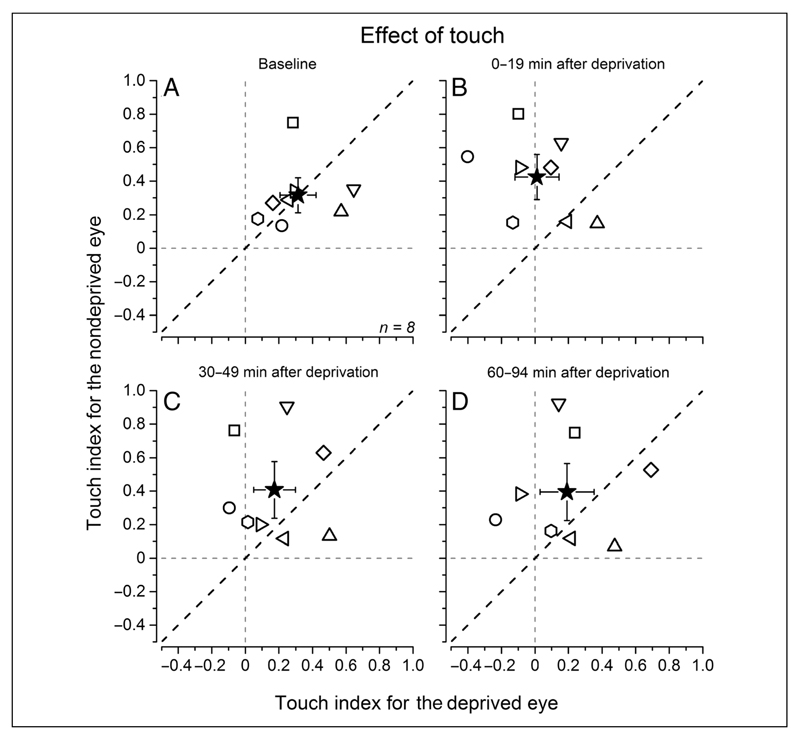
To quantify the effect of touch at different times before and after deprivation, we obtained an index computing the integral of the smoothed probability curves from touch onset to the decay time to baseline value (colored areas in Figure 4 showing, for visualization purposes only, the average probability traces after Gaussian temporal smoothing). Individual observers’ data are reported in Figure 5. Before deprivation (Figure 5A), the effect of touch was comparable for the deprived and nondeprived eyes (deprived eye touch effect index: 0.31 ± 0.11, nondeprived eye: 0.32 ± 0.1; two-tailed bootstrap sign test: n = 10000, α = .05, p = .98, ns). During the first 19 min after eye patch removal (Figure 5B), the effect of touch was significantly stronger for the nondeprived eye compared with the deprived eye (deprived eye: 0.01 ± 0.13, non-deprived eye: 0.42 ± 0.13; bootstrap sign test: n = 10000, α = .05, p = .001). A clear trend of a stronger effect of touch for the nondeprived eye was present up to 49 min after stimulus onset (Figure 5C; deprived eye: 0.17 ± 0.12, nondeprived eye: 0.41 ± 0.17; bootstrap sign test: n = 10000, α = .05, p = .064), decaying to predeprivation values 90 min after deprivation (Figure 5D; deprived eye: 0.19 ± 0.16, nondeprived eye: 0.39 ± 0.17; two-tailed bootstrap sign test: n = 10000, α = .05, p = .14, ns). The data for the deprived eye after deprivation are scattered around zero (Figure 5B–D), indicating no effect of touch (one-sample, two-tailed t test, H0: X = 0, all ps > .07), whereas for the nondeprived eye, the individual participants’ data were significantly different from zero (one-sample, two-tailed t test, H0: X = 0, all ps< .009).
Figure 4.
Average effect of touch. The average effect of touch on binocular rivalry is represented by the area subtended by the curve between touch onset and the time that the baseline values are reached. The left column (A, C, E, and G, in warm colors) refers to the deprived eye, whereas the right column (B, D, F, and H, in cold colors) refers to the nondeprived eye. The lighter colors represents the effect of touch extending after touch offset (dashed vertical line) until the curve decayed to baseline (dotted vertical line). The darker colors represent negative areas, that is, portions of the curve that lied below the vision-only base proportion. The probability curves are the same of those in Figure 3, after been smoothed with a Gaussian function with constant equal to 2.4 sec—this was done only for visualization purposes.
Figure 5.
Individual participants’ data. The touch index (computed for each participant as the area subtended by the probability curves as illustrated in Figure 4) on the deprived eye plotted for each participant against the touch index for the nondeprived eye measured before (A) and at different times after deprivation (B–D). The black star represents the average across all participants, and the error bars represent 1 ± SEM. After deprivation (B, C, and D), the magnitude of the visuo-haptic effect is higher for the nondeprived eye than the deprived eye (data points scattering above the equality dashed black line). The vertical and horizontal gray dashed lines represent the absence of visuo-haptic effect.
Deprivation Control Experiment
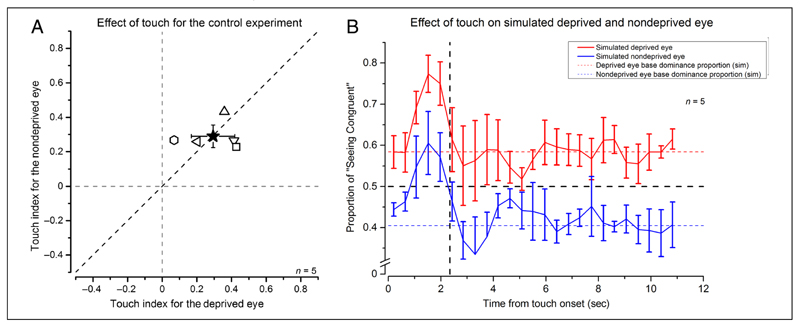
After deprivation, the baseline values of the two eyes are very different, with the deprived eye approaching 60% of predominance. This may generate a flooring effect that does not allow to measure reliably the influence of touch on the deprived eye. The stronger predominance of the deprived eye might also mask the presence of the haptic modulation. To investigate whether the absence of touch effect that we found for the deprived eye could be attributable to the different strength of the visual response, we performed a control experiment using stimuli of different contrast in the two eyes. The contrast difference was set to simulate the effect of deprivation in the unbalance of the phase duration (mean simulated deprived eye contrast: 0.78 ± 0.1, mean simulated nondeprived eye contrast: 0.22 ± 0.1). Figure 6A reports the individual participants’ data for the effect of touch on the simulated deprived (high-contrast stimulus) and nondeprived eye (low-contrast stimulus), whereas Figure 6B reports the probability traces for perceiving the touch congruent percept. Despite the factor of 3.67 difference in contrast and the factor of 1.38 difference in baseline eye dominance, the overall effect of touch is equal for the two eyes (simulated deprived eye = 0.29 ± 0.13, simulated nondeprived eye = 0.29 ± 0.07; two-tailed bootstrap sign test: n = 10000, α = .05, p = .99, ns). These indexes were also statistically different from zero (which indicates no effect of touch) for both eyes (one-sample, two-tailed t tests, simulated deprived eye: t(4) = 4.24, p = .013; simulated nondeprived eye: t(4) = 7.99, p = .0013). In addition, the temporal dynamics of the effect is very similar (Figure 6B) with both curves returning at baseline at the same time from touch onset.
Figure 6.
(A) Individual participants’ data for the control experiment. A is the same as Figure 5, but for the control experiment. B is the same as Figure 3, but for the control experiment. Error bars represent 1 ± SEM.
Discussion
We have demonstrated a new form of rapid cross-modal plasticity in adult normally sighted humans by showing that short-term monocular deprivation alters the interaction between visual and haptic signals during binocular rivalry. After deprivation, the effect of touch on the potentiated deprived eye disappears, whereas it remains unchanged for the nondeprived eye. This differential effect of touch for the two eyes lasts for over 1 hr after eye patch removal and is not reducible to a change in visual signal strength.
As reported by previous studies (Lunghi et al., 2011, 2013), short-term monocular deprivation counterintuitively boosts the deprived eye’s signal, prolonging dominance durations of the deprived eye, while shortening those of the nondeprived eye during binocular rivalry (Lunghi et al., 2011, 2013). This effect could be mediated by a homeostatic upregulation of gain control mechanisms in the attempt to compensate for the lack of visual input of the deprived eye. This is coherent with the observed increase in the apparent contrast for the images delivered to the deprived eye, which can appear as much as 36% higher compared with the nondeprived eye (Lunghi et al., 2011), pointing to fast, cortical gain adjustment mechanisms. Interestingly, the homeostatic plasticity induced by short-term monocular deprivation has been shown to arise in the primary visual cortex, as 150 min of monocular deprivation alter the earliest component of the visual evoked potential (Lunghi, Berchicci, et al., 2015) and the GABA resting level at the calcarine cortex (Lunghi, Emir, Morrone, & Bridge, 2015). This suggests that the differential visuo-haptic interactions that we found after deprivation arise early in the visual system. The suppression mechanisms acting during rivalry between two orthogonal orientations are thought to occur early in the visual processing hierarchy (reviewed in Sterzer et al., 2014). We found that the haptic signal is able to interact with the suppressed visual signal during binocular rivalry, again suggesting an early locus of signal interaction. The measured differences in the haptic effect between the deprived and nondeprived eyes cannot be explained as a result of a mere deprivation-induced change in low-level stimulus strength but are likely caused by a plastic change of the processing of the visual information induced by the deprivation. The control experiment showed that haptic stimulation affected equally low- and high-contrasted rivalrous stimuli, reinforcing the suggestion that the selective lack of cross-modal interactions observed after deprivation originates from a rapid plastic change of cross-modal processing, because changing the visual signal strength by varying the visual stimuli contrast did not alter the cross-modal interaction for both the deprived and nondeprived eyes.
Our results are consistent with a large literature of crossmodal plasticity: The ability of the nervous system to enhance the most appropriate sensory modalities/aspects given the past experiences is explained as an attempt to use the best information the system has reliable access to. For example, the occipital recruitment in blind people for tactile processing (Collignon et al., 2011; Amedi et al., 2010; Gougoux et al., 2005; Sathian, 2005; Hamilton & Pascual-Leone, 1998; Sadato et al., 1996) is explained as the request of the system to dedicate optimal processing to a modality that became critical for the exploration of the environment. Consistent with this hypothesis, many haptic performances in the blind people are better than those in sighted people (for a review, see Merabet & Pascual-Leone, 2010). Similar effects can be induced also in sighted people after training combined with prolonged blindfolding (Merabet et al., 2008; Kauffman et al., 2002). After 5 days of binocular deprivation, the primary visual cortex of normally sighted individuals is recruited for tactile processing (Merabet et al., 2008; Kauffman et al., 2002). These studies suggest that the occipital recruitment by other modalities can result from a rapid and adaptive rearrangement of the neural weights triggered by a strong enough perturbation of the sensory experience stream. They also point out that the haptic signals are probably already present in the occipital cortex, but their efficiency is low in normally sighted people. Long-term visual deprivation can reinforce these cross-modal signals in response to the decreased visual signal saliency. Here, we showed that cross-modal plasticity could occur also in the opposite direction and at short timescales: After short-term monocular deprivation, the deprived eye signal gain is homeostatically upregulated, and the haptic signal, which normally interacts with the visual one, is reduced. Whether this reduction is due to a change in the haptic signal strength or a change in the cross-modal combination mechanism is, at present, unknown. However, on the basis of the control experiment, we can dismiss the masking hypothesis, according to which the stronger deprived eye signal masks the haptic one without involving a change at the crossmodal level.
However, what are the possible mechanisms that induce this rapid rearrangement of cross-modal interactions? van Loon et al. (2013) recently showed that the dynamics of binocular rivalry are correlated to GABA concentrations in the primary visual cortex as measured by magnetic resonance spectroscopy, supporting the idea that bistable visual perception critically depends on excitatory/inhibitory balance in the visual cortex (van Loon et al., 2013; Alais, 2012; Tong, Meng, & Blake, 2006). By using the same technique, Lunghi et al. (2015) showed that a brief period of monocular deprivation induces a decrease of intracortical GABA concentration in the primary visual cortex and that the decrease strongly correlates with the perceptual ocular dominance changes caused by the monocular deprivation on binocular rivalry (Lunghi, Emir, et al., 2015). It is therefore possible that the short-term crossmodal plasticity that we observed here is also mediated by a transient decrease of intracortical inhibition induced by monocular deprivation. Studies on animal models also suggest that homeostatic plasticity is triggered by changes in the excitation/inhibition balance in the primary visual cortex (Maffei & Turrigiano, 2008), either mediated by a downregulation of GABAergic synapses (Maffei, Nelson, & Turrigiano, 2004) or an upregulation of excitatory neurons (Wang, Fontanini, & Maffei, 2012), and further animal and magnetic resonance spectroscopy work is needed to elucidate the exact molecular mechanisms.
In conclusion, the plastic and adaptive readjustment of the early visuo-haptic interactions demonstrated here strongly suggests that the adult nervous system retains a high degree of cross-modal plasticity that operates also at short timescales.
Acknowledgments
This research was funded by the European Research Council, under Grant Agreement No. 338866, ECSPLAIN. We thank David Charles Burr, Guido Marco Cicchini, Paola Binda, and Kyriaki Mikellidou for their helpful comments throughout the project.
Footnotes
Uncited References
Hubel & Wiesel, 1959
Maffei & Fiorentini, 1973
References
- Alais D. Binocular rivalry: Competition and inhibition in visual perception. Wiley Interdisciplinary Reviews: Cognitive Science. 2012;3:87–103. doi: 10.1002/wcs.151. [DOI] [PubMed] [Google Scholar]
- Alais D, Blake R. Binocular rivalry. 2005 [Google Scholar]
- Alsius A, Munhall KG. Detection of audiovisual speech correspondences without visual awareness. Psychological Science. 2013;24:423–431. doi: 10.1177/0956797612457378. [DOI] [PubMed] [Google Scholar]
- Amedi A, Raz N, Azulay H, Malach R, Zohary E. Cortical activity during tactile exploration of objects in blind and sighted humans. Restorative Neurology and Neuroscience. 2010;28:143–156. doi: 10.3233/RNN-2010-0503. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Current Opinion in Neurobiology. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Blake R, Logothetis NK. Visual competition. Nature Reviews Neuroscience. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Clavagnier S, Falchier A, Kennedy H. Long-distance feedback projections to area V1: Implications for multisensory integration, spatial awareness, and visual consciousness. Cognitive, Affective & Behavioral Neuroscience. 2004;4:117–126. doi: 10.3758/cabn.4.2.117. [DOI] [PubMed] [Google Scholar]
- Collignon O, Vandewalle G, Voss P, Albouy G, Charbonneau G, Lassonde M, et al. Functional specialization for auditory-spatial processing in the occipital cortex of congenitally blind humans. Proceedings of the National Academy of Sciences, USA. 2011;108:4435–4440. doi: 10.1073/pnas.1013928108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad V, Bartels A, Kleiner M, Noppeney U. Audiovisual interactions in binocular rivalry. Journal of Vision. 2010;10:27. doi: 10.1167/10.10.27. [DOI] [PubMed] [Google Scholar]
- Conrad V, Kleiner M, Bartels A, Hartcher O’Brien J, Bulthoff HH, Noppeney U. Naturalistic stimulus structure determines the integration of audiovisual looming signals in binocular rivalry. PLoS One. 2013;8:e70710. doi: 10.1371/journal.pone.0070710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convento S, Vallar G, Galantini C, Bolognini N. Neuromodulation of early multisensory interactions in the visual cortex. Journal of Cognitive Neuroscience. 2013;25:685–696. doi: 10.1162/jocn_a_00347. [DOI] [PubMed] [Google Scholar]
- D’Ausilio A. Arduino: A low-cost multipurpose lab equipment. Behavior Research Methods. 2012;44:305–313. doi: 10.3758/s13428-011-0163-z. [DOI] [PubMed] [Google Scholar]
- Deroy O, Faivre N, Lunghi C, Spence C, Aller M, Noppeney U. The complex interplay between multisensory integration and perceptual awareness. Multisensory Research. 2016 doi: 10.1163/22134808-00002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchier A, Clavagnier S, Barone P, Kennedy H. Anatomical evidence of multimodal integration in primate striate cortex. Journal of Neuroscience. 2002;22:5749–5759. doi: 10.1523/JNEUROSCI.22-13-05749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Flugge G. Adult neuroplasticity: More than 40 years of research. Neural Plasticity. 2014;2014:541870. doi: 10.1155/2014/541870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougoux F, Zatorre RJ, Lassonde M, Voss P, Lepore F. A functional neuroimaging study of sound localization: Visual cortex activity predicts performance in early-blind individuals. PLoS Biology. 2005;3:e27. doi: 10.1371/journal.pbio.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RH, Pascual-Leone A. Cortical plasticity associated with Braille learning. Trends in Cognitive Sciences. 1998;2:168–174. doi: 10.1016/s1364-6613(98)01172-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat’s striate cortex. Journal of Physiology. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. Journal of Physiology. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman T, Theoret H, Pascual-Leone A. Braille character discrimination in blindfolded human subjects. NeuroReport. 2002;13:571–574. doi: 10.1097/00001756-200204160-00007. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C. What’s new in Psychtoolbox-3. Perception. 2007;36:1. [Google Scholar]
- Levelt WJM. On binocular rivalry. Soesterberg, The Netherlands: IZF; 1965. [Google Scholar]
- Lunghi C, Alais D. Touch interacts with vision during binocular rivalry with a tight orientation tuning. PLoS One. 2013;8:e58754. doi: 10.1371/journal.pone.0058754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi C, Alais D. Congruent tactile stimulation reduces the strength of visual suppression during binocular rivalry. Scientific Reports. 2015;5:9413. doi: 10.1038/srep09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi C, Berchicci M, Morrone MC, Di Russo F. Short-term monocular deprivation alters early components of visual evoked potentials. Journal of Physiology. 2015;593:4361–4372. doi: 10.1113/JP270950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi C, Binda P, Morrone MC. Touch disambiguates rivalrous perception at early stages of visual analysis. Current Biology. 2010;20:R143–R144. doi: 10.1016/j.cub.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Lunghi C, Burr DC, Morrone C. Brief periods of monocular deprivation disrupt ocular balance in human adult visual cortex. Current Biology. 2011;21:R538–R539. doi: 10.1016/j.cub.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Lunghi C, Burr DC, Morrone MC. Long-term effects of monocular deprivation revealed with binocular rivalry gratings modulated in luminance and in color. Journal of Vision. 2013:13. doi: 10.1167/13.6.1. [DOI] [PubMed] [Google Scholar]
- Lunghi C, Emir UE, Morrone MC, Bridge H. Short-term monocular deprivation alters GABA in the adult human visual cortex. Current Biology. 2015;25:1496–1501. doi: 10.1016/j.cub.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi C, Morrone MC. Early interaction between vision and touch during binocular rivalry. Multisensory Research. 2013;26:291–306. doi: 10.1163/22134808-00002411. [DOI] [PubMed] [Google Scholar]
- Lunghi C, Morrone MC, Alais D. Auditory and tactile signals combine to influence vision during binocular rivalry. Journal of Neuroscience. 2014;34:784–792. doi: 10.1523/JNEUROSCI.2732-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nature Neuroscience. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. Journal of Neuroscience. 2008;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L, Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Research. 1973;13:1255–1267. doi: 10.1016/0042-6989(73)90201-0. [DOI] [PubMed] [Google Scholar]
- Merabet LB, Hamilton R, Schlaug G, Swisher JD, Kiriakopoulos ET, Pitskel NB, et al. Rapid and reversible recruitment of early visual cortex for touch. PLoS One. 2008;3:e3046. doi: 10.1371/journal.pone.0003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: The opportunity of change. Nature Reviews Neuroscience. 2010;11:44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Swisher JD, McMains SA, Halko MA, Amedi A, Pascual-Leone A, et al. Combined activation and deactivation of visual cortex during tactile sensory processing. Journal of Neurophysiology. 2007;97:1633–1641. doi: 10.1152/jn.00806.2006. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annual Review of Neuroscience. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Qin W, Yu C. Neural pathways conveying novisual information to the visual cortex. Neural Plasticity. 2013;2013:864920. doi: 10.1155/2013/864920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland KS, Ojima H. Multisensory convergence in calcarine visual areas in macaque monkey. International Journal of Psychophysiology. 2003;50:19–26. doi: 10.1016/s0167-8760(03)00121-1. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Ibañez V, Deiber MP, Dold G, et al. Activation of the primary visual cortex by Braille reading in blind subjects. 1996;380:526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Saito DN, Okada T, Honda M, Yonekura Y, Sadato N. Practice makes perfect: The neural substrates of tactile discrimination by Mah-Jong experts include the primary visual cortex. BMC Neuroscience. 2006;7:79. doi: 10.1186/1471-2202-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R, Kaliuzhna M, Herbelin B, Blanke O. Balancing awareness: Vestibular signals modulate visual consciousness in the absence of awareness. Consciousness and Cognition. 2015;36:289–297. doi: 10.1016/j.concog.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Salomon R, Lim M, Herbelin B, Hesselmann G, Blanke O. Posing for awareness: Proprioception modulates access to visual consciousness in a continuous flash suppression task. Journal of Vision. 2013;13:2. doi: 10.1167/13.7.2. [DOI] [PubMed] [Google Scholar]
- Sasieni L. The Frisby stereotest. Optician. 1978;176:7–10. [Google Scholar]
- Sathian K. Visual cortical activity during tactile perception in the sighted and the visually deprived. Developmental Psychobiology. 2005;46:279–286. doi: 10.1002/dev.20056. [DOI] [PubMed] [Google Scholar]
- Snow JC, Strother L, Humphreys GW. Haptic shape processing in visual cortex. Journal of Cognitive Neuroscience. 2014;26:1154–1167. doi: 10.1162/jocn_a_00548. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stein T, Ludwig K, Rothkirch M, Hesselmann G. Neural processing of visual information under interocular suppression: A critical review. Frontiers in Psychology. 2014;5:453. doi: 10.3389/fpsyg.2014.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striem-Amit E, Cohen L, Dehaene S, Amedi A. Reading with sounds: Sensory substitution selectively activates the visual word form area in the blind. Neuron. 2012;76:640–652. doi: 10.1016/j.neuron.2012.08.026. [DOI] [PubMed] [Google Scholar]
- Tong F, Meng M, Blake R. Neural bases of binocular rivalry. Trends in Cognitive Sciences. 2006;10:502–511. doi: 10.1016/j.tics.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Koch C. Continuous flash suppression reduces negative afterimages. Nature Neuroscience. 2005;8:1096–1101. doi: 10.1038/nn1500. [DOI] [PubMed] [Google Scholar]
- van Loon AM, Knapen T, Scholte HS, St John-Saaltink E, Donner TH, Lamme VAF. GABA shapes the dynamics of bistable perception. Current Biology. 2013;23:823–827. doi: 10.1016/j.cub.2013.03.067. [DOI] [PubMed] [Google Scholar]
- Wang L, Fontanini A, Maffei A. Experience-dependent switch in sign and mechanisms for plasticity in layer 4 of primary visual cortex. Journal of Neuroscience. 2012;32:10562–10573. doi: 10.1523/JNEUROSCI.0622-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Jiang Y, He S, Chen D. Olfaction modulates visual perception in binocular rivalry. Current Biology. 2010;20:1356–1358. doi: 10.1016/j.cub.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]