ABSTRACT
Plants respond to hypoxic stress through either acclimation to the stress or avoidance of it, as they do to most environmental stresses. The hypothesis that has general consensus among the community is that ethylene response factors (ERFs) are central elements that control both types of responses to hypoxia. Recent studies suggest that this may not be the case for all cells experiencing hypoxic stress. Mature maize root cells undergoing hypoxic stress were found to undergo acclimation and avoidance mechanisms involving ERFs, whereas meristematic root cells and cells still undergoing differentiation acclimated to the response without the involvement of ethylene synthesis or ERFs. Phytoglobins (PGBs) and NO were demonstrated to be components critical to the acclimation response. These findings are discussed relative to the possibility that PGBs may be acting as molecular switches controlling cellular stress responses and hormonal changes and responses in cells.
KEYWORDS: Ethylene response factors, hypoxia, MYC2, NO, PCD, phytoglobin, ROS
Introduction
Plants respond to hypoxic stress, as they do to with most environmental stresses, by altering their metabolism and growth to avoid the stress and/or acclimate to it.1,2 Avoidance mechanisms generally involve altering growth patterns or sacrificing cells and/or tissues that are not critical to the survival of the organism to gain access to non-stress environments, or to await a more hospitable environment. Acclimation comes into play to ensure that cells and/or organs survive the hostile environment.
These concepts are best exemplified during freezing stress, when plants enter a day length driven growth cessation and dormancy period to avoid the stress 3 and further acclimate to it through a low temperature-driven process that reduces the potential for ice crystal formation in vital cells and organs.4 While the 2 triggering processes, light and temperature have interacting effects, the processes that affect growth cessation and dormancy are distinct from those that influence survival to ice crystal formation.
One of the more studied hypoxia-avoidance mechanisms used by some plants is the formation of aerenchyma that permits oxygen movement within hypoxic roots.5 After prolonged exposure to low oxygen levels, cortical cells within the mature region of the root initiate program cell death (PCD), a process that leads to the formation of aerenchyma. The death program is precluded in the root apical meristem (RAM) harboring the “stem” cells. Retention of a functional (RAM) is an acclimation mechanism that allows hypoxic roots to grow and escape conditions of low oxygen levels, and to produce vigorous root systems upon the re-establishment of normoxic conditions. Decision on whether a cell dies, as is the case for aerenchyma-forming cortical cells, or survive, as is the case for meristematic cells, appears to be controlled by cell and tissue-specific mechanisms managing ethylene synthesis and response.6
Ethylene: A common denominator in avoidance or acclimation to hypoxic stress
There is strong evidence that processes associated with ethylene are critical in the plant's capability to tolerate hypoxic stress.1,2 Of the myriad of physiologic responses resulting from low oxygen stress, most have ethylene as a common denominator.2 Thus, ethylene has long been recognized as a factor during hypoxic avoidance strategies including adventitious rooting and aerenchyma formation in maize,7 hyponastic growth and petiole elongation in Rumex,8,9 and in stem elongation in deep-water rice.10
Ground-breaking studies have demonstrated the importance of the Sub1A and Sub1C alleles of the Sub1 locus in affecting submergence survival of lowland rice,11 while the same locus has been shown to be involved in internode elongation during submergence of deep-water rice.12 Ethylene response factors (ERFs) are the central elements within the Sub1 locus regulating these events, resulting in considerable attention to the role of these factors in the hypoxic response. The involvement of the N-end rule pathway in the turnover of ERFs,13,14 has resulted in proposals that N-cysteine oxidase, a main component of the N-end rule pathway, may act as an oxygen sensor 14 and/or a nitric oxide (NO) sensor 15 in regulating the hypoxic response. The N-end rule pathway is likely one of several mechanisms regulating the hypoxic response,16 but it only possesses the ability to control the ethylene response pathway by regulating the catabolism of ERFs, presumably determined by the availability of O2 and/or NO.
Besides participating in avoidance responses, ethylene is considered to be involved in acclimation strategies.1,17 In examining the response of hypoxic maize roots 6 the execution of the avoidance or acclimation pathway was dependent on how cells managed ethylene synthesis and response, and was associated with the developmental stage of the cells along the root profile. Meristematic or early differentiating cells in proximity of the root apical meristem suppressed pathways associated with ethylene synthesis and response that resulted in acclimation, i.e. improved cell survival and root growth during hypoxia. This was in contrast to fully differentiated cells in more mature sections of the root where hypoxia induced ethylene synthesis and response genes, accompanied by increased levels of NO and reactive oxygen species (ROS) with evidence of PCD, processes normally associated with the formation of aerenchyma.
Do phytoblobins act as a molecular switch for acclimation or avoidance responses?
Phytoglobins (Pgbs) are highly expressed in the root tip18,19 and are effective scavengers of NO,20 a signal molecule integrated in ethylene signaling. It has been established that NO modulates ethylene production in the hypersensitive response 21 and that Pgbs mediate that response.22 Many hypoxic responses are also regulated by Pgbs through NO;6,23,24,25 some of these responses are linked to ethylene. For example, suppressing either Pgb1 or Pgb2 expression in Arabidopsis resulted in increased production of both ethylene and NO during hypoxia 25 It was also noted that root flooding resulted in increased shoot Pgb1 expression that correlated with ethylene-induced hyponastic growth. The authors conclude that Pgbs may influence hyponasty through both ethylene-dependent and ethylene-independent pathways and hypothesize that this occurs through the Pgb-scavenging of NO. Suppression of Pgb in maize suspension cultures was found to increase ethylene levels in either normoxic or hypoxic conditions, while imposing hypoxic conditions actually reduced ethylene production compared with normoxia in the suspension cultures.24 Suppressing Pgb enhanced ACC oxidase enzyme activity as opposed to affecting genes associated with ethylene synthesis.
The link among Pgb, NO and ethylene holds true also for acclimation and avoidance responses of hypoxic roots. Aerenchyma formation, a hypoxia-avoidance strategy, occurs in fully differentiated cortical cells as a consequence of reactive oxygen species (ROS) and ethylene-induced PCD.1 In maize, ethylene-induced aerenchyma formation requires the up regulation of respiratory burst oxidase genes, producing ROS and culminating with PCD.26 Execution of the death program was precluded by the use of an NADPH oxidase inhibitor. Reducing Pgb expression during hypoxia resulted in elevated NO levels that induced the expression of respiratory burst oxidase homologs (RBOHs), ethylene-associated genes, and PCD.6 Phytoglobins, like other hemoglobins, are only known to sequester a few gaseous ligands, like oxygen and NO, and react in the oxygenated form in redox reactions, such as the conversion of NO to nitrate reviewed in.20 The most immediate explanation for the above observations would, therefore, be related to oxygen binding and/or reaction with NO, upstream of ethylene synthesis and response.
If conditions of low Pgb expression increase NO, ethylene synthesis and responses, and PCD in aerenchyma-forming cortical cells, elevated Pgb levels have opposite effects. This is the case of meristematic and differentiating cells of hypoxic RAMs where the high levels of Pgbs reduce NO, the expression of ethylene synthesis and responses, and ultimately protect cells from dying.6 Therefore, presence or absence of Pgbs is a factor determining whether root cells undergo acclimation (in meristematic and differentiating cells) or avoidance (in fully differentiated cells) during hypoxia. Meristematic and differentiating cells experienced more general and larger increases in Pgb expression during hypoxia, whereas in mature cells Pgb expression was more localized to specific cell types.
One debatable question resulting from Mira et al.6 is whether the effects of NO occur upstream of the ethylene synthesis and response pathways or downstream of them. The suggestion that Pgbs and NO act downstream of ERFs in the hypoxic stress response 2 is difficult to reconcile with the observation that, in the meristematic tissue of hypoxic roots, an elevation of NO by suppressing Pgb increases ethylene synthesis and response genes. This discrepancy might be the result of the different physiologic state of the cells where these processes occur.
If NO is acting in signal transduction pathways27 and Pgbs are involved in regulating signal transduction they must be present in the cell nucleus. This has been shown to be the case in rice 28 and alfalfa.29 Furthermore, the concentration of Pgb in the alfalfa cell nucleus was found to be higher than that in the cytoplasm. The concept of Pgb regulating signal transduction is supported by recent work from our laboratory showing that Pgb is effective in enhancing Arabidopsis somatic embryogenesis only when it is targeted to the nucleus.30
Phytoglobins at the crossroads of hormonal responses
In attempting to determine how the expression of Pgbs, by regulating cellular NO levels, could affect ethylene-induced events during hypoxic stress, it may be instructive to look at research examining the effects of Pgb expression on hormonal pathways regulating somatic embryogenesis. Suppression of Arabidopsis Pgb2 enhanced auxin-induced somatic embryo formation by increasing the expression of genes related to auxin synthesis.31 This enhancement was attributed to increase cellular NO levels, as a result of reduced Pgbs, that inhibited the transcription factor MYC2, a repressor of the auxin biosynthetic pathways.32 Further studies indicated that the more immediate effect of NO was on genes related to jasmonic acid synthesis, yielding elevated jasmonic acid levels that in turn suppressed MYC2 and increased JAZ1, resulting in stimulated auxin synthesis.33,34 ERFs integrate signals from ethylene and jasmonate in plant defense 35 and there is some evidence that MYC2 may interfere upstream of ERF1 to regulate these responses.36 In addition, MYC2 antagonizes ethylene-promoted apical hook formation in Arabidopsis by repressing EIN3.37
A model accounting for the cell-specific regulation of acclimation and avoidance in hypoxic roots
Cell-specific management of ethylene synthesis and response appears to be a determinant factor in acclimation and avoidance strategies of hypoxic roots (Fig. 1). Our results suggest that this management occurs through Pgbs controlling NO levels in specific cell types. In fully differentiated cortical cells of hypoxic root tissue, the low expression of Pgbs allows accumulation of NO, a suppressor of MYC2.31,33,34 As MYC2 represses ethylene responses,38 its suppression promotes ethylene responses and the up regulation of NADPH oxidase resulting in the death of cortical cells by PCD. These processes, possibly mediated by metacaspases which are executors of the death program and inhibited by Pgbs (Fig. 2A), lead to the formation of aerenchyma, an avoidance strategy. In meristematic or early differentiating cells the ethylene-induction of PCD is repressed due to the scavenging of NO by Pgbs that are preferentially expressed at the root tip. Presence of Pgbs in the meristematic cells of hypoxic roots might also be required to prevent the accumulation of indole-acetic acid (IAA), possibly by suppressing the expression of the last IAA biosynthetic enzyme indole-3-acetamide hydroxylase (AMI) (Fig. 2B). Over-production of IAA in the RAM induces the differentiation and consumption of the stem cells leading to an arrest in root growth.39 These acclimation strategies ensure the survival of the stem cells, which upon the re-establishment of non-stress conditions can resume their activity and contribute to the growth of a functional root. If it holds true, this model argues that execution of acclimation and avoidance strategies is cell specific and dependent on the “physiologic” state and age of the cells. While the presence of Pgbs in young cells triggers acclimation responses, their absence in more mature cells triggers avoidance responses.
Figure 1.
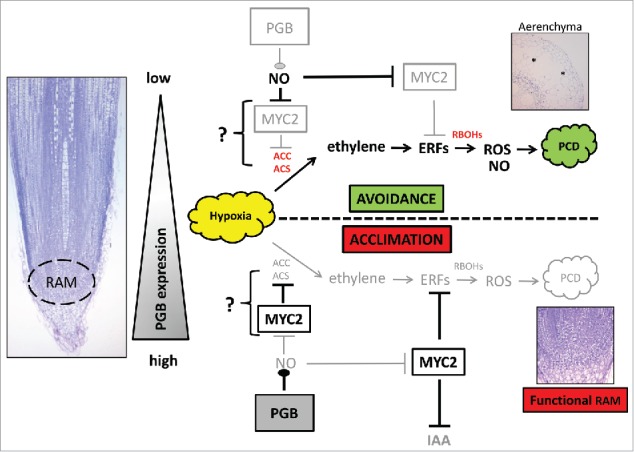
Schematic representation of Pgb-regulation of avoidance (aerenchyma formation) and acclimation (maintenance of a functional root meristem) responses in hypoxic maize roots. The gradient of Pgb along the root profile determines the type of response. High levels of Pgbs at the root tip lower the hypoxia-induced accumulation of ethylene by scavenging NO, and attenuate ethylene responses possibly through regulation of MYC2. These effects reduce ROS-induced PCD and auxin over-production in the meristematic cells that remain functional. In mature tissue, characterized by low expression of Pgb, the accumulation of ethylene triggers PCD contributing to the formation of aerenchyma (*). In this response NO production is also induced by ethylene. Repressed responses are faded. ACS, 1-aminocyclopropane-1-carboxylic acid synthase; ACO, 1-aminocyclopropane-1-carboxylic acid oxidase; ERFs, ethylene response factors; RBHOs, respiratory burst oxidase homologs.
Figure 2.
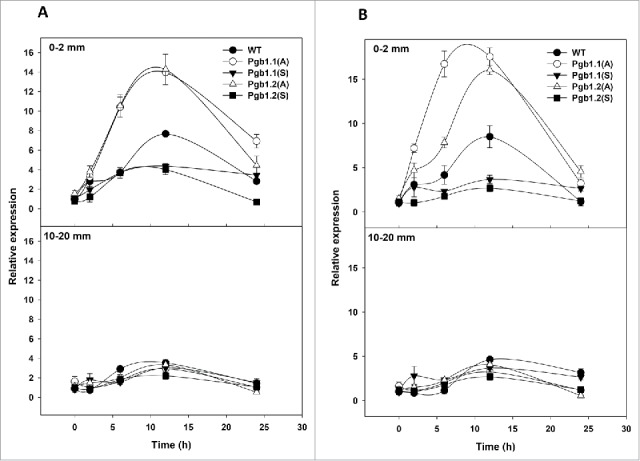
Relative expression of metacaspase 9 (A) and indole-3-acetamide hydroxylase (AMI) (B) in root tips (0–2mm) and mature root segments (10–20 mm) of maize seedlings subjected to 4% oxygen treatments. Values ± SE are means of 3 biologic replicates and are normalized to the WT value of 0 hours (set at 1). Root segments were harvested from WT seedlings and seedlings over-expressing (S) or down-regulating (A) Pgb1.1 and Pgb1.2.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Manitoba Corn Growers Association.
References
- 1.Bailey-Serres J, Voesenek LA. Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 2008; 59:313-39; http://dx.doi.org/10.1146/annurev.arplant.59.032607.092752; PMID:18444902, [Web of Science ®]. [DOI] [PubMed] [Google Scholar]
- 2.Voesenek LA, Bailey-Serres J. Flood adaptive traits and processes: an overview. New Phytol 2015; 206: 57-73; PMID:25580769; http://dx.doi.org/ 10.1111/nph.13209 [DOI] [PubMed] [Google Scholar]
- 3.Olsen JE, Junttila O, Nilsen J, Eriksson ME, Martinussen I, Olsson O, Sandberg G, Moritz T. Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimatization. Plant J 1997; 12: 1339-50; http://dx.doi.org/ 10.1046/j.1365-313x.1997.12061339.x. [DOI] [Google Scholar]
- 4.Guy CL. Cold acclimation and freezing stress tolerance: role of protein metabolism. Annu Rev Plant Physiol Plant Mol Biol 1990; 41: 187-223; http://dx.doi.org/ 10.1146/annurev.pp.41.060190.00115 [Web of Science ®] [DOI] [Google Scholar]
- 5.Evans DE. Aerenchyma formation. New Phytol 2003; 161: 35-49; http://dx.doi.org/ 10.1046/j.1469-8137.2003.00907.x. [DOI] [Google Scholar]
- 6.Mira MM, Hill RD, Stasolla C. Phytoglobins alleviate growth inhibition of hypoxic maize roots by suppressing death at the apical meristem. Plant Physiol 2016a; 172: 2044-2056 ; PMID:27702845; http://dx.doi.org/ 10.1104/pp.16.01150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drew MC, Jackson MB, Giffard S. Ethylene-promoted adventitious rooting and development of cortical air spaces (aerenchyma) in roots may be adaptive responses to flooding in Zea mays L. Planta 1979; 147: 83-88; PMID:24310899; http://dx.doi.org/ 10.1007/BF00384595 [DOI] [PubMed] [Google Scholar]
- 8.Cox MC, Benschop JJ, Vreeburg RA, Wagemaker CA, Moritz T, Peeters AJ, Voesenek LA. The roles of ethylene, auxin, abscisic acid, and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiol 2004; 136: 2948-60; PMID:15466223; http://dx.doi.org/ 10.1104/pp.104.049197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benschop JJ, Jackson MB, Guhl K, Vreeburg RA, Croker SJ, Peeters AJ, Voesenek LA. Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. Plant 2005; J 44: 756-768; PMID:16297068; http://dx.doi.org/ 10.1111/j.1365-313X.2005.02563.x [DOI] [PubMed] [Google Scholar]
- 10.Kende H, van der Knaap E, Cho HT. Deepwater rice: a model plant to study stem elongation. Plant Physiol 1998; 118: 1105-10; PMID:9847084; http://dx.doi.org/ 10.1104/pp.118.4.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukao T, Xu K, Ronald PC, Bailey-Serres J. A. variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 2006; 18: 2021-34; PMID:16816135; http://dx.doi.org/ 10.1105/tpc.106.043000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, Matsuoka M, Mori H, Ashikari M. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009; 460: 1026-30; PMID:19693083; http://dx.doi.org/ 10.1038/nature08258 [DOI] [PubMed] [Google Scholar]
- 13.Gibbs DJ, Lee SC, Md Isa N, Gramuglia S, Fukao T, Bassel GW, Correia CS, Corbineau F, Theodoulou FL, Bailey-Serres J, et al.. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 2011; 479: 415-8; PMID:22020279; http://dx.doi.org/ 10.1038/nature10534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LACJ, Perata P, van Dongen JT. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 2011; 479: 419-22; PMID:22020282; http://dx.doi.org/ 10.1038/nature10536.15 [DOI] [PubMed] [Google Scholar]
- 15.Gibbs DJ, Md IN, Movahedi M, Lozano-Juste J, Mendiondo GM, Berckhan S, Marin-de la Rosa N, Vicente CJ, Sousa CC, Pearce SP, et al.. Nitric oxide sensing in plants is mediated by proteolytic control of group VII ERF transcription factors. Mol Cell 2014; 53: 369-379; PMID:24462115; http://dx.doi.org/ 10.1016/j.molcel.2013.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dongen JT, Licausi F. Oxygen sensing and signaling. Annu Rev Plant Biol 2015; 66: 345-67; PMID:25580837; http://dx.doi.org/ 10.1146/annurev-arplant-043014-114813 [DOI] [PubMed] [Google Scholar]
- 17.Voesenek LA, Sasidharan R, Visser EJ, Bailey-Serres J. Flooding stress signaling through perturbations in oxygen, ethylene, nitric oxide and light. New Phytol 2016; 209: 39-43; PMID:26625347; http://dx.doi.org/ 10.1111/nph.13775 [DOI] [PubMed] [Google Scholar]
- 18.Parent C, Berger A, Capelli N, Crevecoeur M, Dat JF. A novel non-symbiotic hemoglobin from oak: roles in root signalling and development?. Plant Signal Behav 2008; 3: 819-20; PMID:26625347; http://dx.doi.org/ 10.1111/nph.13775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L, Gu RL, Gao P, Wang GY. A nonsymbiotic hemoglobin gene from maize, ZmHb, is involved in response to submergence, high-salt and osmotic stresses. Plant Cell Tiss Org 2008; 95: 227-37; http://dx.doi.org/ 10.1007/s11240-008-9436-3. [DOI] [Google Scholar]
- 20.Smagghe BJ, Hoy JA, Percifield R, Kundu S, Hargrove MS, Sarath G, Hilbert JL, Watts RA, Dennis ES, Peacock WJ, et al.. v 2009; 91: 1083-96; PMID:19441024; http://dx.doi.org/18799663 10.1002/bip.21256 [DOI] [PubMed] [Google Scholar]
- 21.Mur LA, Laarhoven LJ, Harren FJ, Hall MA, Smith AR. Nitric oxide interacts with salicylate to regulate biphasic ethylene production during the hypersensitive response. Plant Physiol 2008; 148: 1537-46; PMID:18799663; http://dx.doi.org/ 10.1104/pp.108.124404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mur LA, Sivakumaran A, Mandon J, Cristescu SM, Harren FJ, Hebelstrup KH. Haemoglobin modulates salicylate and jasmonate/ethylene-mediated resistance mechanisms against pathogens. J Exp Bot 2012; 63: 4375-87; PMID:22641422; http://dx.doi.org/ 10.1093/jxb/ers116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dordas C, Hasinoff BB, Igamberdiev AU, Manac'h N, Rivoal J, Hill RD. Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J 2003; 35: 763-70; PMID:12969429; http://dx.doi.org/ 10.1046/j.1365-313X.2003.01846.x [DOI] [PubMed] [Google Scholar]
- 24.Manac'h-Little N, Igamberdiev AU, Hill RD. Hemoglobin expression affects ethylene production in maize cell cultures. Plant Physiol Biochem 2005; 43: 485-9; PMID:15914016; http://dx.doi.org/ 10.1016/j.plaphy.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 25.Hebelstrup KH, Van ZM, Mandon J, Voesenek LA, Harren FJ, Cristescu SM, Moller IM, Mur LA. Haemoglobin modulates NO emission and hyponasty under hypoxia-related stress in Arabidopsis thaliana. J Exp Bot 2012; 63: 5581-91; PMID:22915746; http://dx.doi.org/ 10.1093/jxb/ers210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajhi I, Yamauchi T, Takahashi H, Nishiuchi S, Shiono K, Watanabe R, Mliki A, Nagamura Y, Tsutsumi N, Nishizawa NK, et al.. Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol 2011; 190: 351-68; PMID:21091694; http://dx.doi.org/ 10.1111/j.1469-8137.2010.03535.x [DOI] [PubMed] [Google Scholar]
- 27.Neill SJ, Desikan R, Hancock JT. Nitric oxide signalling in plants. New Phytol 2003; 159: 11-35; PMID:24474956; http://dx.doi.org/ 10.3389/fpls.2013.00553 [DOI] [PubMed] [Google Scholar]
- 28.Ross EJ, Shearman L, Mathiesen M, Zhou YJ, Arredondo-Peter R, Sarath G, Klucas RV. Nonsymbiotic hemoglobins in rice are synthesized during germination and in differentiating cell types. Protoplasma 2001; 218: 125-33; PMID:11770429; http://dx.doi.org/ 10.1007/BF01306602 [DOI] [PubMed] [Google Scholar]
- 29.Seregélyes C, Mustárdy L, Ayaydin F, Sass L, Kovács L, Endre G, Lukács N, Kovács I, Vass I, Kiss GB, et al.. Nuclear localization of a hypoxia-inducible novel non-symbiotic hemoglobin in cultured alfalfa cells. FEBS Lett 2000; 482: 125-130; PMID:411018535; http://dx.doi.org/ 10.1016/S0014-5793(00)02049 [DOI] [PubMed] [Google Scholar]
- 30.Godee C, Mira MM, Wally O, Hill RD, Stasolla C. Cellular localization of the Arabidopsis class 2 phytoglobin influences somatic embryogenesis. J Exp Bot 2017 (Accepted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elhiti M, Hebelstrup KH, Wang A, Li C, Cui Y, Hill RD, Stasolla C. Function of the type-2 Arabidopsis hemoglobin in the auxin-mediated formation of embryogenic cells during morphogenesis. Plant 2013; J 74: 946-58; PMID:23510449; http://dx.doi.org/ 10.1111/tpj.12181 [DOI] [PubMed] [Google Scholar]
- 32.Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, Reid JB, Fitt GP, Sewelam N, Schenk PM, Manners JM, et al.. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 2007; 19:2225-45; PMID:17616737; http://dx.doi.org/ 10.1105/tpc.106.048017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mira MM, Wally OS, Elhiti M, El-Shanshory A, Reddy DS, Hill RD, Stasolla C. Jasmonic acid is a downstream component in the modulation of somatic embryogenesis by Arabidopsis Class 2 phytoglobin. J Exp Bot. 2016; 67:2231-46; PMID:26962208; http://dx.doi.org/ 10.1093/jxb/erw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mira MM, Hill RD, Stasolla C. Regulation of programmed cell death by phytoglobins. J Exp Bot. 2016; 67(20):5901-08; PMID:27371712; http://dx.doi.org/12509529 10.1093/jxb/erw259. [DOI] [PubMed] [Google Scholar]
- 35.Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 2003; 15: 165-78; PMID:12509529; http://dx.doi.org/ 10.1105/tpc.007468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 2004; 16: 3460-79; PMID:15548743; http://dx.doi.org/ 10.1105/tpc.104.025833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Zhu Z, An F, Hao D, Li P, Song J, Yi C, Guo H. Jasmonate-activated MYC2 represses ETHYLENE INSENSITIVE3 activity to antagonize ethylene-promoted apical hook formation in Arabidopsis. Plant Cell 2014; 26: 1105-17; PMID:24668749; http://dx.doi.org/ 10.1105/tpc.113.122002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill RD. Non-symbiotic haemoglobins – What's happening beyond nitric oxide scavenging? AoB Plants; 2012:plos004; PMID:22479675; http://dx.doi.org/ 10.1093/aobpla/pls004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee Y, Lee WS, Kim S-H. Hormonal regulation of stem maintenance in the roots. J Exp Bot 2013; 64: 1153-65; PMID:23183258; http://dx.doi.org/ 10.1093/jxb/ers331 [DOI] [PubMed] [Google Scholar]


