ABSTRACT
The excessive xylem phenotype of acaulis5 (acl5), an Arabidopsis mutant defective in the synthesis of thermospermine, indicates that thermospermine is required for negative regulation of xylem differentiation. SAC51 was identified from a dominant suppressor of acl5, sac51-d, and encodes a basic helix-loop-helix (bHLH) protein. sac51-d has a premature termination codon in one of upstream open-reading frames (uORFs) of the SAC51 mRNA that is conserved among the SAC51 family members. Thermospermine may act to bypass the inhibitory effect of the uORF on main ORF translation. Another suppressor, sac57-d, also has a mutation in the conserved uORF of SACL3, a member of the SAC51 family. On the other hand, the double knockout of SAC51 and SACL3 is insensitive to thermospermine, suggesting their key role in the response to thermospermine. However, we found that thermospermine enhances mRNA translation of SAC51 and SACL1 but not of SACL2 and SACL3. Taken together with recent findings from other groups, we propose a mechanism by which thermospermine diffused from xylem precursor cells acts non-cell-autonomously to restrict their proliferation.
KEYWORDS: Arabidopsis, thermospermine, translation, uORF, xylem differentiation
Thermospermine, a structural isomer of spermine, is widely present in the plant kingdom and in extremophiles.1,2 The acaulis5 (acl5) mutant of Arabidopsis thaliana, which is defective in the biosynthesis of thermospermine, shows excessive xylem differentiation with a stunted growth phenotype, indicating that thermospermine is required for proper xylem differentiation.3 Our previous studies of suppressor mutants of acl5 that restore the phenotype without thermospermine have revealed that thermospermine may be involved in enhancing the translation of the SAC51 mRNA, which encodes a basic helix-loop-helix (bHLH) protein.4,5 Instead of thermospermine, a dominant mutant, sac51-d, which has a premature termination codon in one of upstream open-reading frames (uORFs) of the SAC51 mRNA, cancels the inhibitory effect of the uORF on main ORF translation and may result in overproduction of the SAC51 bHLH protein. This uORF is highly conserved in all four members of the SAC51 family in Arabidopsis and classified as a homology group 15.6 Dominant mutations that cause an amino acid substitution in the corresponding uORF of SACL1 and SACL3 have also been shown to suppress the dwarf phenotype of acl5.7 We also identified another suppressor mutation, sac57-d, in the conserved uORF of SACL3.8 These results suggest that thermospermine act to bypass the inhibitory effect of these conserved uORFs on main ORF translation. To check the regulatory role of these uORFs in the response to thermospermine, we generated transgenic plants carrying the GUS reporter gene under the control of each gene promoter followed by the 5′ leader region and revealed that SAC51 and SACL1 were responsive to 24-h treatment of seedlings with 100 µM thermospermine but SACL2 and SACL3 were not.8 Unlike our results, two recent studies using GUS staining or GFP fluorescence have shown that SACL3 was responsive to thermospermine7,9 but their experimental conditions have not been clearly defined. Thus, although it might be possible that SACL3 translation is enhanced by thermospermine under some conditions, we discuss here how the difference in the responsiveness to thermospermine between SAC51 family members can occur. A detailed comparison of 5′ leader sequences of SAC51 family mRNAs reveals that 6 AUG codons are present in the 5′ leader region of SAC51, SACL1, and SACL2 mRNAs while 17 and 8 are in two alternative forms of SACL3 mRNAs, respectively (Fig. 1). The 6th AUG codons in SAC51 and SACL1 mRNAs are in the same reading frame as the conserved uORF. These AUG codons are conserved within some uORFs of the homology group 15 in other plants but have not been assigned as a start codon of an additional uORF.6 As one possibility, these shorter uORFs conserved in SAC51 and SACL1 might be indeed involved in the response to thermospermine. Because polyamines mainly exist as a polyamine-RNA complex,10 thermospermine might have an effect on the secondary structure of these uORF coding regions of the mRNA or ribosomal RNAs. However, our in vitro translation assays using wheat germ extracts have so far not succeeded in reproducing the translation enhancement of the SAC51 5′-reporter mRNA by thermospermine. Thermospermine might be required to be incorporated into ribosomes. It is interesting to speculate that thermospermine-bound translating ribosomes could not be stalled by the 6th uORF-encoded nascent polypeptides of SAC51 and SACL1 and/or reinitiate the translation of the main ORF with higher efficiency. A major role of uncommon polyamines in bacteria living under extreme environmental conditions is proposed to be in the formation of the initiation complex during translation.11 Further studies focusing on the responsiveness of these uORFs to thermospermine will be necessary to clarify the mode of action of thermospermine during translation.
Figure 1.
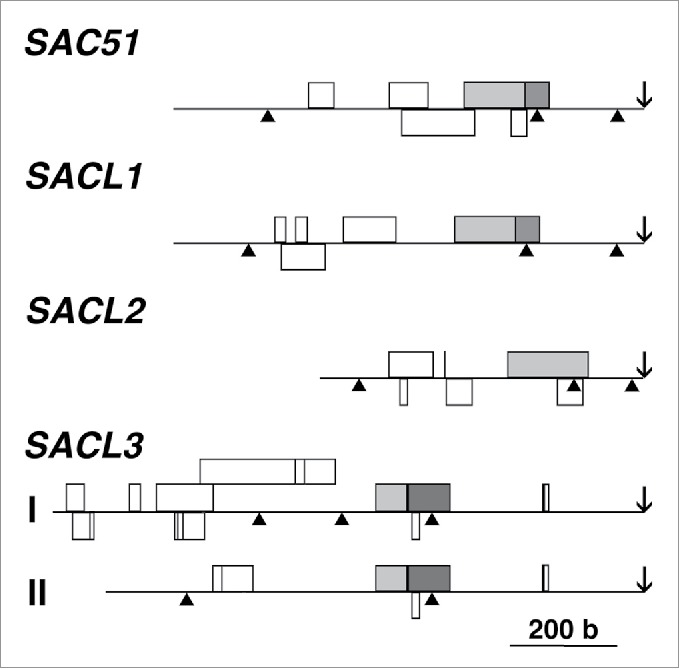
uORF arrangement in 5′ leader regions of SAC51 family mRNAs in Arabidopsis. Boxes represent uORFs. Overlapped but different reading frames are shown in different rows. The uORFs classified as a homology group 156 and those in the same reading frame as them are shaded in light and dark gray, respectively. Arrowheads and arrows indicate positions of introns and the start codon of the main ORF, respectively. SACL3 I and II indicate alternatively spliced forms of the mRNA.
Expression of ACL5 and SACL3 is directly activated by LHW-TMO5 or LHW-T5L1 bHLH heterodimers in xylem precursor cells of the root.9 On the other hand, SAC51, SACL1, and SACL2 are expressed in whole vascular tissues, phloem cells, and procambial cells, respectively.7 Furthermore, SAC51 and SACL3 proteins have been shown to antagonize TMO5 and T5L1 to form heterodimers with LHW.7 LHW-TMO5 and LHW-T5L1 heterodimers also direct expression of LOG4 for cytokinin synthesis and AHP6 for blocking of cytokinin signaling by which cytokinin functions exclusively in procambial cells for proliferation while xylem precursor cells differentiate into xylem vessels.12 In analogy with the cytokinin signaling, we would like to propose a model that thermospermine non-cell-autonomously enhances translation of SAC51 and SACL1 mRNAs and these gene products antagonize other bHLH combinations while SACL3 blocks the heterodimer formation of TMO5 or T5L1 with LHW in xylem precursor cells, independently of thermospermine (Fig. 2A). Although at close range, the non-cell-autonomous action of thermospermine would be in line with the definition of plant hormones. There are some reports on polyamine transporters in plants,14 whereas cell-to-cell transport of thermospermine remains to be addressed and requires further investigation.
Figure 2.
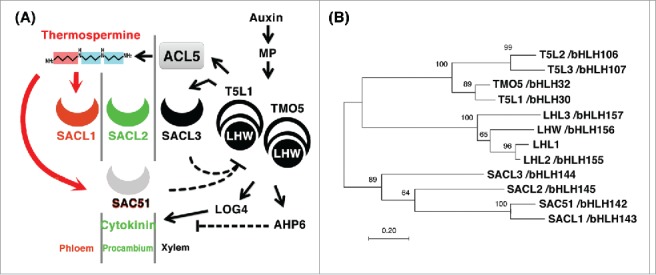
Relationship between bHLH proteins belonging to SAC51, TMO5, and LHW families in Arabidopsis. (A) A model of thermospermine-mediated repression of xylem differentiation in the root. The auxin signaling in the formation of vascular tissues shown here is not comprehensive. MP is an auxin-responsive transcription factor MONOPTEROS, which directly regulates expression of TMO5 and T5L1.13 Dashed lines indicate inhibitory actions. (B) Evolutionary relationship of bHLH domains of SAC51, TMO5, and LHW family proteins in Arabidopsis. The phylogenetic tree based on amino acid sequences of the bHLH domain of each protein was constructed using the neighbor-joining method of the MEGA7 software.15 Bootstrap values (1000 replicates) are shown at the branching points. The scale bar indicates the number of amino acid substitutions per site. The bHLH protein numbering is according to a previous publication.16
There are three homologs to LHW, LHL1-LHL3, and those to TMO5, T5L1-T5L3, in the Arabidopsis genome (Fig. 2B).13 Combinatorial interactions of these transcription factors with SAC51 family proteins might have different roles to fine-tune the vascular formation. It is also noted that LHW, LHL1, and LHL2 mRNAs have another highly conserved uORF classified as a homology group 2,6 although their regulatory functions remain to be studied.
The fact that double but not single knockouts of SAC51 and SACL3 are insensitive to a high concentration of exogenous thermospermine that completely represses xylem differentiation in the wild-type root suggests a redundant or additive role for SAC51 and SACL3 in the negative feedback control of auxin-dependent LHW-TMO5/T5L1-mediated xylem differentiation pathways.8 Furthermore, given that the SACL3 mRNA is not responsive to thermospermine, the response to thermospermine in the knockout of SAC51, sac51–1, could be attributed to the role of SACL1. It is alternatively possible that another unidentified target of thermospermine is involved in the SACL3 regulatory loop.
In conclusion, our study identified SAC51 family members as a central mediator of thermospermine signaling and also the difference in the responsiveness to thermospermine between them. It is still an open question how and why thermospermine was employed for translational regulation of a few specific mRNAs during the evolution of the plant vascular system. The original function of thermospermine in bacteria and algae undoubtedly has no relation to vascular formation and its target also in these organisms remains to be identified.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research [No. 26113516, No. 16H0124518] from the Japan Society for the Promotion of Science (JSPS) to TT.
References
- 1.Oshima T. Enigmas of biosyntheses of unusual polyamines in an extreme thermophile, Thermus thermophiles. Plant Physiol Biochem 2010; 48:521-526; PMID: 20417109; http://dx.doi.org/ 10.1016/j.plaphy.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 2.Takano A, Kakehi J, Takahashi T. Thermospermine is not a minor polyamine in the plant kingdom. Plant Cell Physiol 2012; 53:606-616; PMID: 22366038; http://dx.doi.org/ 10.1093/pcp/pcs019 [DOI] [PubMed] [Google Scholar]
- 3.Kakehi J, Kuwashiro Y, Niitsu M, Takahashi T. Thermospermine is required for stem elongation in Arabidopsis thaliana. Plant Cell Physiol 2008; 49:1342-49; PMID: 18669523; http://dx.doi.org/ 10.1093/pcp/pcn109 [DOI] [PubMed] [Google Scholar]
- 4.Kakehi J, Kawano E, Yoshimoto K, Cai Q, Imai A, Takahashi T. Mutations in ribosomal proteins, RPL4 and RACK1, suppress the phenotype of a thermospermine-deficient mutant of Arabidopsis thaliana. PLoS One 2015; 27:e0117309; PMID: 25625317; http://dx.doi.org/ 10.1371/journal.pone.0117309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imai A, Hanzawa Y, Komura M, Yamamoto KT, Komeda Y, Takahashi T. The dwarf phenotype of the Arabidopsis acl5 mutant is suppressed by a mutation in an upstream ORF of a bHLH gene. Development 2006; 133:3575-85; PMID: 16936072; http://dx.doi.org/ 10.1242/dev.02535 [DOI] [PubMed] [Google Scholar]
- 6.Hayden CA, Jorgensen RA. Identification of novel conserved peptide uORF homology groups in Arabidopsis and rice reveals ancient eukaryotic origin of select groups and preferential association with transcription factor-encoding genes. BMC Biol 2007; 30:32; PMID: 17663791; http://dx.doi.org/ 10.1186/1741-7007-5-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vera-Sirera F, De Rybel B, Úrbez C, Kouklas E, Pesquera M, Álvarez-Mahecha JC, Minguet EG, Tuominen H, Carbonell J, Borst JW, Weijers D, Blázquez MA. A bHLH-based feedback loop restricts vascular cell proliferation in plants. Dev Cell 2015; 35:432-443; PMID: 26609958; http://dx.doi.org/ 10.1016/j.devcel.2015.10.022 [DOI] [PubMed] [Google Scholar]
- 8.Cai Q, Fukushima H, Yamamoto M, Ishii N, Sakamoto T, Kurata T, Motose H, Takahashi T. The SAC51 family plays a central role in thermospermine responses in Arabidopsis. Plant Cell Physiol 2016; 57:1583-92; PMID: 27388339; http://dx.doi.org/ 10.1093/pcp/pcw113 [DOI] [PubMed] [Google Scholar]
- 9.Katayama H, Iwamoto K, Kariya Y, Asakawa T, Kan T, Fukuda H, Ohashi-Ito K. A negative feedback loop controlling bHLH complexes is involved in vascular cell division and differentiation in the root apical meristem. Curr Biol 2015; 25:3144-50; PMID: 26616019; http://dx.doi.org/ 10.1016/j.cub.2015.10.051 [DOI] [PubMed] [Google Scholar]
- 10.Igarashi K, Kashiwagi K. Modulation of protein synthesis by polyamines. IUBMB Life 2015; 67:160-9; PMID: 25906835; http://dx.doi.org/ 10.1002/iub.1363 [DOI] [PubMed] [Google Scholar]
- 11.Oshima T. Unique polyamines produced by an extreme thermophile, Thermus thermophilus. Amino Acids 2007; 33:367-372; PMID: 17429571; http://dx.doi.org/ 10.1007/s00726-007-0526-z [DOI] [PubMed] [Google Scholar]
- 12.Ohashi-Ito K, Saegusa M, Iwamoto K, Oda Y, Katayama H, Kojima M, Sakakibara H, Fukuda H. A bHLH complex activates vascular cell division via cytokinin action in root apical meristem. Curr Biol 2014; 24:2053-58; PMID: 25131670; http://dx.doi.org/ 10.1016/j.cub.2014.07.050 [DOI] [PubMed] [Google Scholar]
- 13.De Rybel B, Möller B, Yoshida S, Grabowicz I, Barbier de Reuille P, Boeren S, Smith RS, Borst JW, Weijers D. A bHLH complex controls embryonic vascular tissue establishment and indeterminate growth in Arabidopsis. Dev Cell 2013; 24:426-437; PMID: 23415953; http://dx.doi.org/ 10.1016/j.devcel.2012.12.013 [DOI] [PubMed] [Google Scholar]
- 14.Fujita M, Shinozaki K. Identification of polyamine transporters in plants: paraquat transport provides crucial clues. Plant Cell Physiol 2014; 55:855-861; PMID: 24590488; http://dx.doi.org/ 10.1093/pcp/pcu032 [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016; 33:1870-74; PMID: 27004904; http://dx.doi.org/ 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey PC, Martin C, Toledo-Ortiz G, Quail PH, Huq E, Heim MA, Jakoby M, Werber M, Weisshaar B. Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana. Plant Cell 2003; 15:2497-2502; PMID: 14600211; http://dx.doi.org/ 10.1105/tpc.151140 [DOI] [PMC free article] [PubMed] [Google Scholar]


