ABSTRACT
Apple is a fleshy fruit distinguished by a climacteric type of ripening, since most of the relevant physiological changes are triggered and governed by the action of ethylene. After its production, this hormone is perceived by a series of receptors to regulate, through a signaling cascade, downstream ethylene related genes. The possibility to control the effect of ethylene opened new horizons to the improvement of the postharvest fruit quality. To this end, 1-methylcyclopropene (1-MCP), an ethylene antagonist, is routinely used to modulate the ripening progression increasing storage life. In a recent work published in The Plant Journal, the whole transcriptome variation throughout fruit development and ripening, with the adjunct comparison between normal and impaired postharvest ripening, has been illustrated. In particular, besides the expected downregulation of ethylene-regulated genes, we shed light on a regulatory circuit leading to de-repressing the expression of a specific set of genes following 1-MCP treatment, such as AUX/IAA, NAC and MADS. These findings suggested the existence of a possible ethylene/auxin cross-talk in apple, regulated by a transcriptional circuit stimulated by the interference at the ethylene receptor level.
KEYWORDS: Apple, fruit ripening, ethylene, auxin, transcription factor, NAC, 1-methylcyclopropene
Fleshy fruit ripening is a physiological process ongoing at the end of the fruits’ life cycle, and comprehends a series of modifications leading to the establishment of important quality properties.1 Depending to their type of maturation and ripening, fruits can be classified either as climacteric and non-climacteric.2,3,4 In climacteric fruits (such as tomato, banana, apple and peach) the ripening progression is controlled by ethylene, a plant hormone highly synthetized during the late ripening stage. This burst is moreover accompanied by a rise in respiration rate.5 On the contrary, non-climacteric fruits are distinguished by a continuous and basal production of this hormone and a linear decrease in the respiration rate.
The ethylene pathway
Ethylene is synthesized by the Yang's cycle with the involvement of two fundamental enzymes, 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) and ACC oxidase (ACO6,7). The binding of ethylene to the receptors (ERSs and ETRs) turns off the active suppression exerted on CTR1, allowing the ethylene responses to occurs.8,9 The ethylene signaling, due to a MAPKKKs system, modulates a downstream cascade of ethylene-related genes, including elements involved in the control of fundamental processes, such as fruit texture.10,11,12 In tomato, the starting signal of this pathway is however caused by the upstream action of RIN,13 a MADSbox gene triggering the transition from the autoinhibitory production of ethylene (system 1) to the autocatalytic phase (system 214). The role of this transcription factor, together with that of NOR, has been comprehensively elucidated in tomato, a model species for fruit ripening investigation.15 Taking into account the impact of these genes, the possibility to control the ripening process has represented a major goal, especially to improve the quality of fruits through postharvest storage. Although specific and characterized apple ripening mutants are unknown yet, the ripening process governed by ethylene can be regulated by the exogenous application of 1-methylcyclopropene (1-MCP), an ethylene inhibitor competing at the level of the receptor binding site.16
Auxin pathway
Another important hormone in the fruit physiology is represented by auxin. Initially proposed as antagonistically inhibiting ethylene, auxin is primarily accumulated during the initial growing and developmental phases.17,18,19 The regulatory pathway of auxin is substantially short, since the hormone action within the cell depends on the regulation of ARF transcription factors through the binding by Aux/IAA transcription modulators. In addition to this mechanism and the well-known polar transport, the auxin homeostasis is also regulated by a conjugation/degradation process. In particular, GH3 proteins can conjugate auxin with amino acids and sugars mainly.20
Hormonal interplay and transcription factor regulatory circuits
In a recent work21 a transcriptional deviation occurring after 1-MCP application, with regards to the normal condition, was shown. Interestingly, the artificial interference at the ethylene receptor level by 1-MCP, besides down-regulating a set of known genes, also induced the de-repression of genes usually expressed in pre-mature stages rather than in the full-ripening phase. As illustrated in Fig. 1, the genes repressed by the ethylene inhibitor are mainly related to the ethylene pathway. Among them, genes involved in the ethylene biosynthesis, as well as in the responsive pathway (such as ACS, ACO and AP2-ERF) are highly expressed at harvest and after 1 week of shelf-life ripening (PHCTRL), coincident with the climacteric ethylene burst. In addition to these, other genes, related to ethylene receptors (ERS1 and ERS2) and one ERF, show, instead, an expression pattern characterized by two phases, with a first peak at the beginning of fruit development and a second at the beginning of the climacteric accumulation of ethylene. Within the genes positively correlated with the ethylene increase, it is also worth noting the expression of the apple homologous of the tomato RIN (MDP0000326390). Although it expression follows the transcription dynamics observed for the full-climacteric genes, the application of 1-MCP only slightly decreases its expression level. The second group of genes, induced by 1-MCP, is interestingly represented by transcription factors and auxin related genes (Aux/IAA and AUX1). Within the class of transcription factors, NAC are the most abundant. Among them a specific element (MDP0000173636) is supposed to be homologous of the tomato NOR, whose mutation induces a non-ripening phenotype similar to what was observed for RIN.4 In tomato, both rin and nor mutants fail to produce climacteric ethylene. For all these genes the expression pattern suggests a functional de-repression mechanism. While their activity is stimulated during the initial developmental pre-climacteric phase, their expressions tend to show a decrease at the onset of the climacteric ripening, till a complete repression during the ethylene burst. Application of 1-MCP re-establishes the gene transcription at late ripening stage, in some cases at a higher level compared with the control stage, such as for the MADSbox gene (MDP0000152821).
Figure 1.
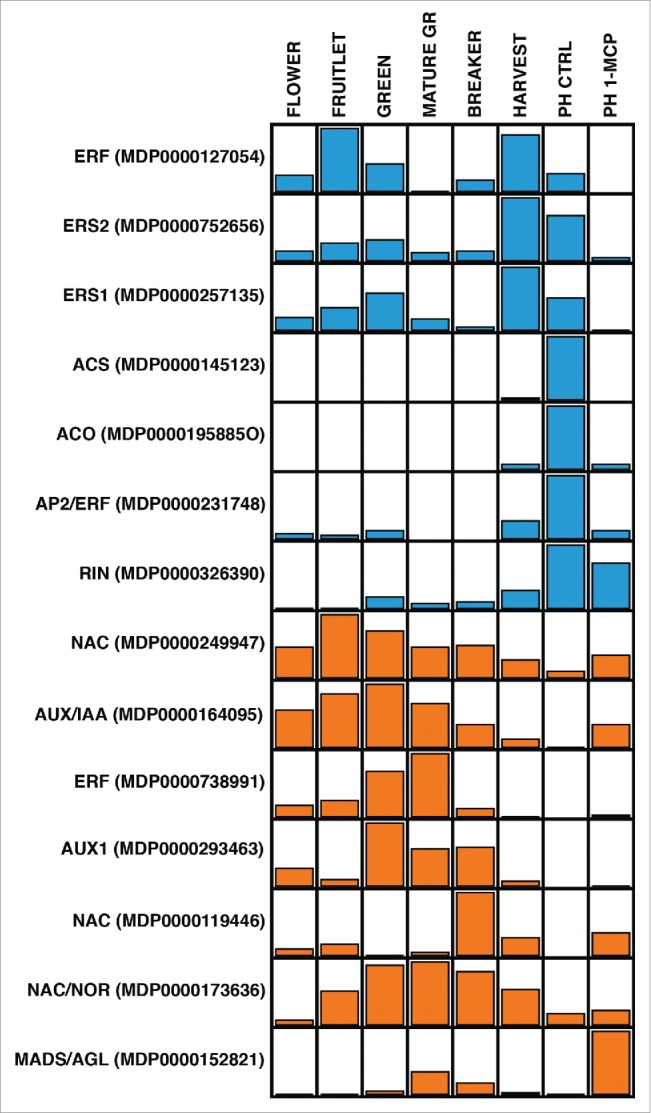
Expression profile of 14 candidate genes throughout the apple fruit development and ripening. Stages are reported on the top of the figure. The last stage PH (postharvest) is further distinguished in control (CTRL) and treated with 1-methylcyclopropene (1-MCP). For each gene the name and the ID (according to the GDR database) are reported. The pattern of gene expression is moreover characterized by different colors. While in blue are highlighted genes induced by ethylene and repressed by 1-MCP, in orange are instead depicted the elements with an opposite regulation, thus stimulated by the ethylene competitor 1-MCP.
Model of action
Our recent results, together with those of other Authors,22,23,24,25 shed light on the possible ethylene/auxin cross-talk in rosaceae species triggered by the interference at the receptor level (depicted in Fig. 2). As already documented,10,26 1-MCP efficiently delays the general fruit ripening in apple, especially due to the hampered accumulation of ethylene and the reduced dismantling of the cell-wall polysaccharide architecture, a process leading to fruit softening. However, it needs to be taken into account that fruits are organs originally programmed to disperse seeds. To this end, fruits with an artificially induced impaired ethylene perception machinery attempt to restore the normal progression of physiological ripening. Since the entire ethylene perception system is compromised by the binding of 1-MCP to the receptors, a parallel pathway seems to be triggered. This alternative pathway toward the production of ethylene involves the initial de-repression of NAC, encoding transcription factors. In other species (Arabidopsis), the connection between NAC and auxin-related genes has been already suggested.27 In this scenario, a set of NAC transcription factors can be re-activated to stimulate a late synthesis of auxin, with the final purpose to induce the expression of ACS genes21,28 and the final accumulation of the hormone ethylene.
Figure 2.
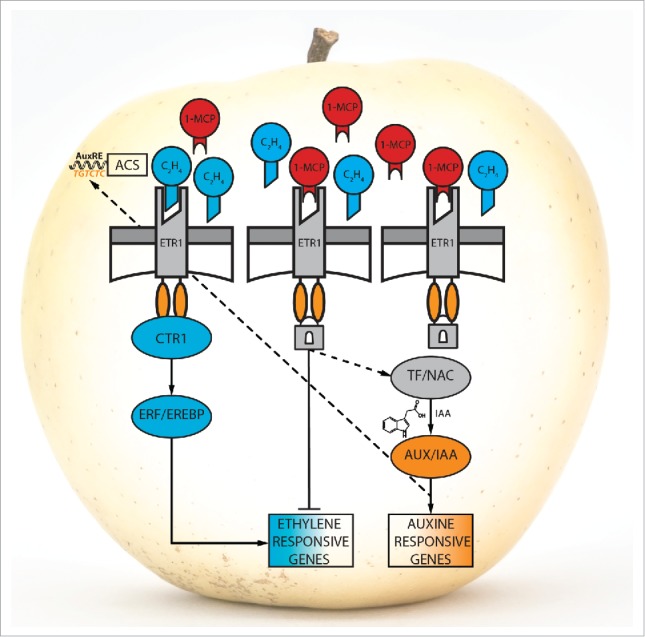
Tentative model of action of the ethylene/auxin cross-talk. In blue and orange are indicated the ethylene and auxin pathway, respectively.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Giovannoni J. Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol 2001; 52:725-49; PMID:11337414; http://dx.doi.org/ 10.1146/annurev.arplant.52.1.725 [DOI] [PubMed] [Google Scholar]
- 2.Lelievre JM, Latche A, Jones B, Bouzayen M, Pech JC. Ethylene and fruit ripening. Physiol Plant 1997; 101:727-39; http://dx.doi.org/ 10.1111/j.1399-3054.1997.tb01057.x [DOI] [Google Scholar]
- 3.Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot 2002; 53:2039-55; PMID:12324528; http://dx.doi.org/ 10.1093/jxb/erf072 [DOI] [PubMed] [Google Scholar]
- 4.Barry CS, Giovannoni JJ. Ethylene and fruit ripening. J Plant Growth Regul 2007; 6:143-59; http://dx.doi.org/ 10.1007/s00344-007-9002-y [DOI] [Google Scholar]
- 5.Bleecker AB, Kende H. Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 2000; 16:1-18; PMID:11031228; http://dx.doi.org/ 10.1146/annurev.cellbio.16.1.1 [DOI] [PubMed] [Google Scholar]
- 6.Adams DO, Yang SF. Ethylene biosynthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci USA 1979; 76:170-4; PMID:16592605; http://dx.doi.org/ 10.1073/pnas.76.1.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higherplants. Annu Rev Plant Physiol 1984; 35:155-89; http://dx.doi.org/ 10.1146/annurev.pp.35.060184.001103 [DOI] [Google Scholar]
- 8.Ciardi JA, Tieman DM, Jones JB, Klee HJ. Reduced expression of the tomato ethylene receptor gene LeETR4 enhances the hypersensitive response to Xanthomonas campestris pv. vesicatoria. Mol Plant Microbe Interact 2001; 14:487-95; PMID:11310736; http://dx.doi.org/ 10.1094/MPMI.2001.14.4.487 [DOI] [PubMed] [Google Scholar]
- 9.Klee HJ. Control of ethylene-mediated processes in tomato at the level of receptors. J Exp Bot 2002; 53:2057-63; PMID:12324529; http://dx.doi.org/ 10.1093/jxb/erf062 [DOI] [PubMed] [Google Scholar]
- 10.Costa F, Alba R, Schouten H, Soglio V, Gianfranceschi L, Serra S, Musacchi S, Sansavini S, Costa G, Fei ZJ, et al.. Use of homologous and heterologous gene expression profiling tools to characterize transcription dynamics during apple fruit maturation and ripening. BMC Plant Biol 2010; 10:229; PMID:20973957; http://dx.doi.org/ 10.1186/1471-2229-10-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alba R, Payton P, Fei Z, McQuinn R, Debbie P, Martin GB, Tanksley SD, Giovannoni JJ. Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell 2005; 17:2954-65; PMID:16243903; http://dx.doi.org/ 10.1105/tpc.105.036053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiyama K, Guis M, Rose JKC, Kubo Y, Bennett KA, Wangjin L, Kato K, Ushijima K, Nakano R, Inaba A, et al.. Ethylene regulation of fruit softening and cell wall disassembly in Charentais melon. J Exp Bot 2007; 58:1281-90; PMID:17308329; http://dx.doi.org/ 10.1093/jxb/erl283 [DOI] [PubMed] [Google Scholar]
- 13.Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (Rin) locus. Science 2002; 296:3436; http://dx.doi.org/ 10.1126/science.1068181 [DOI] [PubMed] [Google Scholar]
- 14.Barry CS, Llop-Tous MI, Grierson D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol 2000; 123:979-86; PMID:10889246; http://dx.doi.org/ 10.1104/pp.123.3.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giovannoni JJ. Fruit ripening mutants yield insights into ripening control. Curr Opin Plant Biol 2007; 10:283-9; PMID:17442612; http://dx.doi.org/ 10.1016/j.pbi.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 16.Sisler EC, Serek M. Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiol Plant 1997; 100:577-82; http://dx.doi.org/ 10.1111/j.1399-3054.1997.tb03063.x [DOI] [Google Scholar]
- 17.Leyser O. The power of auxin in plants. Plant Physiol 2010;155:501-5; http://dx.doi.org/ 10.1104/pp.110.161323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YH, Irving HR. Developing a model of plant hormone interactions. Plant Signal Behav 2011; 6:494-500; PMID:21406974; http://dx.doi.org/ 10.4161/psb.6.4.14558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kende H, Zeevaart JAD. The five “classical” plant hormones. Plant Cell 1997; 9:1197-210; PMID:12237383; http://dx.doi.org/ 10.1105/tpc.9.7.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ljung K. Auxin metabolism and homeostasis during plant development. Development 2013; 140:943-50; PMID:23404103; http://dx.doi.org/ 10.1242/dev.086363 [DOI] [PubMed] [Google Scholar]
- 21.Tadiello A, Longhi S, Moretto M, Ferrarini A, Tononi P, Farneti B, Busatto N, Vrhovsek U, dal Molin A, Avanzato C, et al.. Interference with ethylene perception at receptor level sheds light on auxin and transcriptional circuits associated with climacteric ripening of apple fruit (Malus x domestica Borkh.). Plant J 2016; PMID:27531564; http://dx.doi.org/ 10.1111/tpj.13306 [DOI] [PubMed] [Google Scholar]
- 22.Trainotti L, Tadiello A, Casadoro G. The involvement of auxin in the ripening of climacteric fruits comes of age: the hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J Exp Bot 2007; 58:3299-308; PMID:17925301; http://dx.doi.org/ 10.1093/jxb/erm178 [DOI] [PubMed] [Google Scholar]
- 23.Tadiello A, Ziosi V, Negri AS, Noferini M, Fiori G, Busatto N, Espen L, Costa G. Trainotti L. On the role of ethylene, auxin and a GOLVEN-like peptide hormone in the regulation of peach ripening. BMC Plant Biol 2016; 14:44; http://dx.doi.org/ 10.1186/s12870-016-0730-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin S, Lee J, Rudell D, Evans K, Zhu Y. Transcriptional regulation of auxin metabolism and ethylene biosynthesis activation during apple (Malus × domestica) fruit maturation. J Plant Growth Regulat 2016; 35(3): 655-666; http://dx.doi.org/ 10.1007/s00344-015-9568-8 [DOI] [Google Scholar]
- 25.Shin S, Lee J, Rudell D, Evans K, Zhu Y. Transcript profiles of auxin efflux carrier and IAA-Amido synthetase genes suggest the role of auxin on apple (Malus domestica) fruit maturation patterns. Am J Plant Scie 2015; 06:620-32; http://dx.doi.org/ 10.4236/ajps.2015.65067 [DOI] [Google Scholar]
- 26.Watkins CB. Overview of 1-methylcyclopropene trials and uses for edible horticultural crops. HortScience 2008; 43:86-94. [Google Scholar]
- 27.Olsen AN, Ernst HA, Leggio LL, Skriver K. NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci 2005; 10:79-87; PMID:15708345; http://dx.doi.org/ 10.1016/j.tplants.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 28.Abel S, Theologis A. Early genes and auxin action. Plant Physiol 1996; 111:9-17; PMID:8685277; http://dx.doi.org/ 10.1104/pp.111.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]


