Abstract
Background
Globally, case detection of tuberculosis (TB) has stabilized in recent years. Active case finding (ACF) has regained an increased attention as a complementary strategy to fill the case detection gap. In the Philippines, the DetecTB project implemented an innovative ACF strategy that offered a one-stop diagnostic service with a mobile unit equipped with enhanced diagnostic tools including chest X-ray (CXR) and Xpert®MTB/RIF (Xpert). The project targeted the rural poor, the urban poor, prison inmates, indigenous population and high school students.
Methods
This is a retrospective review of TB screening data from 25,103 individuals. A descriptive analysis was carried out to compare screening and treatment outcomes across target populations. Univariate and multivariate analyses were performed to identify predictors of TB for each population. The composition of bacteriologically-confirmed cases by smear and symptom status was further investigated.
Results
The highest yield with lowest number needed to screen (NNS) was found in prison (6.2%, NNS: 16), followed by indigenous population (2.9%, NNS: 34), the rural poor (2.2%, NNS: 45), the urban poor (2.1%, NNS: 48), and high school (0.2%, NNS: 495). The treatment success rate for all populations was high with 89.5% in rifampicin-susceptible patients and 83.3% in rifampicin-resistant patients. A relatively higher loss to follow-up rate was observed in indigenous population (7.5%) and the rural poor (6.4%). Only cough more than two weeks showed a significant association with TB diagnosis in all target populations (Adjusted Odds Ratio ranging from 1.71 to 6.73) while other symptoms and demographic factors varied in their strength of association. The urban poor had the highest proportion of smear-positive patients with cough more than two weeks (72.0%). The proportion of smear-negative (Xpert-positive) patients without cough more than two weeks was the highest in indigenous population (39.3%), followed by prison inmates (27.7%), and the rural poor (22.8%).
Conclusions
The innovative ACF strategy using mobile unit yielded a substantial number of TB patients and achieved successful treatment outcomes. TB screening in prison, indigenous population, and urban and rural poor communities was found to be effective. The combined use of CXR and Xpert largely contributed to increased case detection.
Introduction
Globally, case detection of TB has stabilized in recent years, and 4.3 million cases are estimated to be either not diagnosed or not notified [1]. Limitations of the DOTS strategy which has focused on passive case finding (PCF) by smear microscopy among symptomatic individuals have been increasingly recognized [2–4]. In response to this, targeted active case finding (ACF) has regained an increased attention as a complementary strategy to fill the case detection gap [5,6]. For the last one and half decades, large donor funds have been mobilized worldwide and a number of ACF projects have been implemented in high and intermediate burden countries to stimulate and gather global evidence [7]. The World Health Organization (WHO) facilitated a review of available evidence and provided principles and recommendations on systematic screening for active TB [8]. In principle, systematic screening including ACF should target specific groups that are at high risk of TB or have poor access to TB services, or both [9]. According to the WHO guidelines, it is strongly recommended for people living with human immunodeficiency virus (HIV), close contacts of people with TB, and people exposed to silica, and conditionally recommended for other groups such as prisoners, migrants, people with diabetes mellitus, and urban slums dwellers [8].
Monitoring and evaluation (M&E) is an integral part of any screening programme [9]. A set of basic M&E indicators has been proposed, which includes acceptance rate, retention rate for each screening step, number needed to screen (NNS) and number needed to test (NNT) to detect one case, treatment initiation rate, and treatment success rate (TSR) [9]. In particular, NNS, defined as the number of persons screened divided by the number of persons diagnosed with TB (roughly the inverse of the prevalence), is increasingly used to quantify the effectiveness of screening programmes [9]. In a systematic review on NNS in TB screening, NNS of 100 or lower, in high-incidence settings, was found in several groups such as people living with HIV (PLHIV) including voluntary counselling and testing attendees (NNS 10–37), household contacts (NNS 17–25), drug users (NNS = 20), persons with diabetes (NNS = 35), miners (NNS = 36), and certain community-wide screening in high-incidence countries (NNS = 100) [10]. However the review concluded that NNS is highly variable depending on the different screening approaches and population evaluated [10]. As such, NNS can be more useful when comparing it across different groups in a setting with a fixed screening algorithm, which provides a measure of relative cost-effectiveness [9]. In Nepal, an intensified case finding project screened multiple risk groups for TB using mobile vans that equipped with microscopy and Xpert®MTB/RIF (Xpert) with or without symptom screening. The project had high yield and low NNS in PLHIV (NNS: 17) and household contacts (NNS: 29), followed by urban slum dwellers (NNS: 197) and prisoners (NNS: 261) [11]. Given the increasingly diversified approaches and targets in ACF, individual project assessment is critical to add to evidence-based policy guidance.
When ACF is pursued, it is essential to ensure local health systems have adequate capacity to provide quality TB treatment and care and to respond to the anticipated rise in case detection [8,9]. TSR is an important indicator to monitor and evaluate this capacity. Studies have increasingly reported a high TSR in ACF with more than 85%, being at a similar or higher level as compared to baseline or routine PCF [12–14]. However, careful assessment is needed since several studies showed that ACF led to more unfavourable treatment outcomes due to a high proportion of less symptomatic patients who may be less motivated to complete their treatment [15,16].
The Philippines is one of 30 countries with high burdens of TB, with the estimated incidence rate of 322 per 100,000 population in 2015 [1]. The country also has one of the highest burden of multi-drug resistant (MDR) TB [1]. Case notification has steadily increased since 2008 after eight years of stagnation, however the gap still appears to be substantial [17]. With the increased initiative of the National TB Control Programme (NTP), significant progress has been made in TB control for the past decades. Nationwide DOTS coverage was achieved in 2003 [18]. Programmatic efforts have been undertaken for establishing partnerships with public and private health providers, facilitating nationwide expansion of TB diagnosis in children, and scaling-up Programmatic Management of Drug-resistant TB (PMDT) [18]. More recently, the NTP has been active in bringing national and international partners together to set up interventions including ACF to address the needs of vulnerable populations. However, evidence to suggest the effectiveness of ACF in the Philippines is insufficient.
Since 2012, the DetecTB project has been implemented in the province of Palawan, aiming to increase TB case detection among vulnerable populations. The project implemented an innovative ACF strategy that offered a one-stop diagnostic service with the use of a mobile unit and enhanced diagnostic tools including chest X-ray (CXR) and Xpert. The ACF targeted five vulnerable populations including; (1) residents in rural poor communities, (2) residents in urban poor communities, (3) prison inmates, (4) indigenous population, and (5) high school students. This study primarily aimed to describe yield of TB diagnosis and treatment outcomes by different target populations. Besides, we further examined predictors of TB and the contribution of enhanced diagnostic tools to overall case detection.
Methods
Programmatic information
The DetecTB project has been implemented in Palawan, with the first phase from December 2012 to March 2014 and the second phase from April 2014 to the present. The project was funded by the Korea Foundation for International Healthcare (KOFIH) and implemented in collaboration with the Department of Health of the Philippines, Korean Institute of Tuberculosis (KIT), WHO, and the local government units of Palawan Province and Puerto Princesa City.
Of 23 municipalities and one city in Palawan Province, seven municipalities were selected based on health access and the level of TB case detection, and classified as rural poor communities. Additionally, of 66 barangays (the smallest administrative division) in Puerto Princesa City, three barangays were selected based on the income levels in the official statistics, and classified as urban poor communities. The project also targeted two major indigenous populations (the Palawano peoples in the selected seven municipalities and the Batak tribes in additional three barangays in Puerto Princesa City), inmates from six prison facilities (two jails and the national prison that has four sub-colonies located in Puerto Princesa City), and students from eight high schools.
The project delivered the TB screening and diagnostic services to all target populations through a mobile unit. The Provincial Health Office of Palawan and City Health Office of Puerto Princesa City each formed a mobile team which was composed of two physicians, one nurse, one medical technologist and one radiologic technologist. They were trained on the use of mobile unit for two weeks in the KIT prior to the implementation of the project.
The mobile team utilized a service bus to travel from one project site to another. The mobile unit was equipped with all diagnostic equipment including a digital CXR machine, Light Emitting Diode Fluorescence Microscope (LED-FM), and Xpert machine for molecular diagnosis. The mobile unit also had a slide warmer, biosafety cabinet, and refrigerator to temporarily store sputum specimens. The mobile unit had a capacity to screen over 250 individuals per day at a maximum while it screened around 50–100 individuals per day on average.
ACF activities were coordinated in close collaboration with a point person in each target site (i.e. municipal health officers, barangay health workers, prison/school officials, etc.). They undertook the field preparations including information dissemination and advocacy meetings prior to the pre-scheduled screening sessions. Local health workers were also pre-oriented to the case finding process, and assisted the mobile teams in organizing participants, obtaining consents, conducting interviews, smearing slides, and providing health education.
In the targeted prisons and high schools, all eligible persons were screened by the project. On the other hand, in rural and urban poor communities and indigenous population, only those who voluntarily visited the mobile unit were screened.
During the screening session, all participants aged 15 or above were interviewed for TB symptoms and the known risk factors for TB such as history of TB exposure and previous TB treatment, using a standardized screening form. All interviewed participants then underwent CXR examination. CXR results were read by the physician in the mobile team who were trained on CXR reading by the KIT. The results were classified into five categories; “normal”, suggestive of “active TB” and “inactive TB”, “TB activity undetermined” and “other diseases”. People with suspected TB were determined by clinical judgement of physicians, based primarily on abnormal CXR results (such as “active TB” and “TB activity undetermined”) and additionally on the presence of TB symptoms. They were requested to provide two spot sputum specimens for diagnostic tests. At least one hour interval was given between the first and second specimen collection. Both specimens were examined by sputum smear microscopy using LED-FM, and one of them was tested by Xpert by the trained medical technologist. The test results were released on the same day. Those who had a positive result by smear microscopy and/or an Xpert result with “Mycobacterium Tuberculosis (MTB) detected” were diagnosed as bacteriologically-confirmed TB. Those who had negative sputum microscopy and Xpert results but had strong clinical evidence for active TB, based on presenting signs and symptoms and risk factors, were also treated for TB according to the physician’s best clinical judgment. The project focused on diagnosing only pulmonary TB, but not extra-pulmonary TB.
Both bacteriologically-confirmed and clinically-diagnosed TB patients were referred to local treatment centres for appropriate patient management in line with the national guidelines. CXR results of patients found to have rifampicin resistance (RR) TB were sent a radiologist for reconfirmation, and then they were referred to the PMDT facility for treatment.
Treatment outcomes were reported in line with the national TB guidelines [17] and the latest World Health Organisation's definitions [19]. Treatment success is defined as the sum of “cured” and “completed”.
Study design, analysis and ethical considerations
This is a retrospective review of the screening data from the DetecTB project that covers the period from December 2012 to August 2015.
All raw data were obtained from a paper-based screening form used in the screening session, and then entered into an Epi Info 7 SQL database (CDC Atlanta Georgia, USA) with predefined coding and error-checking formulas. Data processing and statistical analyses were performed using R3.3.0 (CRAN: Comprehensive R Archive Network at https://cran.r-project.org/).
A descriptive analysis was carried out by computing distribution and frequency of screening outcomes and demographic characteristics of participants by different target population. A univariate analysis was performed to identify predictors of TB for detected patients in the rural poor, the urban poor, prison inmates, and indigenous population. Using the variables deemed significant in the univariate analysis and sex and age category as priori variables, a multivariate logistic regression analysis was performed to confirm the observed association. An odds ratio (OR) and adjusted odds ratio (AOR) were calculated to examine the strength of the association, along with 95% Confidence Interval (CI). Statistical significance was set at p <0.05. To see the contribution of CXR and Xpert to overall case detection, we further examined the composition of bacteriologically-confirmed TB cases by smear and symptom status for each target population. Treatment outcomes were then computed, stratified by rifampicin resistant status, excluding patients with ongoing treatment.
As stipulated by the project protocol, a written informed consent was obtained from all screened subjects. If participants were minor, a written informed consent was obtained from their guardians. Patient identifiable information was removed from the dataset before analysis. As this study was part of the routine assessment of the ongoing project by using existing records, ethical clearance was not required according to local regulations.
Results
Characteristics of participants (Table 1)
Table 1. Characteristics of participants by target population.
| Characteristics | Rural poor | Urban poor | Prison inmates | Indigenous population | High school students | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 12907 | (%) | N = 1625 | (%) | N = 6133 | (%) | N = 2145 | (%) | N = 2293 | (%) | N = 25103 | (%) | ||
| Sex | Female | 8055 | (62.4%) | 905 | (55.7%) | 66 | (1.1%) | 1376 | (64.1%) | 1337 | (58.3%) | 11739 | (46.8%) |
| Male | 4852 | (37.6%) | 720 | (44.3%) | 6067 | (98.9%) | 769 | (35.9%) | 956 | (41.7%) | 13364 | (53.2%) | |
| Age | Median | 46.0 | (IQR:33–59) | 40.0 | (IQR:26–52) | 41.0 | (IQR:33–49) | 44.0 | (IQR:32–58) | 16.0 | (IQR:15–16) | 42.0 | (IQR:28–54) |
| Mean | 46.5 | (SD:17.3) | 40.5 | (SD:16.7) | 41.3 | (SD:11.6) | 45.2 | (SD:17.1) | 15.8 | (SD:1) | 41.9 | (SD:17.4) | |
| Age category | 15–24 | 1541 | (11.9%) | 346 | (21.3%) | 439 | (7.2%) | 270 | (12.6%) | 2293 | (100.0%) | 4889 | (19.5%) |
| 25–34 | 1991 | (15.4%) | 298 | (18.3%) | 1443 | (23.5%) | 376 | (17.5%) | 0 | (0.0%) | 4108 | (16.4%) | |
| 35–44 | 2465 | (19.1%) | 341 | (21.0%) | 1878 | (30.6%) | 447 | (20.8%) | 0 | (0.0%) | 5131 | (20.4%) | |
| 45–54 | 2491 | (19.3%) | 282 | (17.4%) | 1535 | (25.0%) | 410 | (19.1%) | 0 | (0.0%) | 4718 | (18.8%) | |
| 55–64 | 2214 | (17.2%) | 205 | (12.6%) | 699 | (11.4%) | 325 | (15.2%) | 0 | (0.0%) | 3443 | (13.7%) | |
| ≥65 | 2205 | (17.1%) | 153 | (9.4%) | 139 | (2.3%) | 317 | (14.8%) | 0 | (0.0%) | 2814 | (11.2%) | |
| History of TB exposure* | No | 10007 | (77.8%) | 1559 | (96.1%) | 4647 | (75.9%) | 1714 | (80.3%) | 2212 | (96.6%) | 20139 | (80.5%) |
| Yes | 2853 | (22.2%) | 63 | (3.9%) | 1472 | (24.1%) | 421 | (19.7%) | 79 | (3.4%) | 4888 | (19.5%) | |
| History of previous TB treatment* | No | 11624 | (90.6%) | 1498 | (92.6%) | 5189 | (84.8%) | 1930 | (91.1%) | 2266 | (98.9%) | 22507 | (90.1%) |
| Yes | 1213 | (9.4%) | 120 | (7.4%) | 931 | (15.2%) | 189 | (8.9%) | 25 | (1.1%) | 2478 | (9.9%) | |
| Smoking status* | No | 7595 | (71.0%) | 770 | (64.1%) | 852 | (17.6%) | 991 | (67.4%) | 1712 | (86.8%) | 11920 | (59.1%) |
| Yes | 3103 | (29.0%) | 431 | (35.9%) | 3980 | (82.4%) | 479 | (32.6%) | 260 | (13.2%) | 8253 | (40.9%) | |
| Location | Palawan | 12907 | (100.0%) | 0 | (0.0%) | 0 | (0.0%) | 1934 | (90.2%) | 326 | (14.2%) | 15167 | (60.4%) |
| Puerto Princesa | 0 | (0.0%) | 1625 | (100.0%) | 6133 | (100.0%) | 211 | (9.8%) | 1967 | (85.8%) | 9936 | (39.6%) | |
* Cases with unknown or missing information were excluded from the calculation.
IQR: Interquartile Range, SD: Standard Deviation
A total of 25103 individuals were screened by the project during the study period. Of them, 12907 were screened from rural poor communities; 1625 from urban poor communities; 6133 from prisons, 2145 from indigenous population; and 2293 from high schools.
The characteristics of the participants were highly heterogeneous across different target populations. For example, males were predominant among prison inmates (98.9% vs 1.1%) whereas more females than males were screened among indigenous population (64.1% vs 35.9%). Participants from high schools were young with the median age of 16 while other groups had their median age of 40 or over. Approximately 20% of participants had a history of TB exposure (close contact within the past two years) in the rural poor, prison inmates and indigenous population whereas only 3–4% were previously exposed to TB in the urban poor and high school students. The proportion of participants with a history of previous TB treatment was the highest in prison inmates (15.2%), which was followed by the rural poor (9.4%), indigenous population (8.9%), and the urban poor (7.4%). The prevalence of smoking was the highest in prison inmates (82.4%), followed by the urban poor (35.9%), indigenous population (32.6%) and the rural poor (29.0%).
Outcomes of screening tests (Table 2)
Table 2. Screening and diagnostic outcomes by target population.
| Screening and diagnostic outcomes | Rural poor | Urban poor | Prison inmates | Indigenous population | High school students | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 12907 | (%) | N = 1625 | (%) | N = 6133 | (%) | N = 2145 | (%) | N = 2293 | (%) | N = 25103 | (%) | ||
| Any TB symptoms | None | 2582 | (20.0%) | 906 | (55.8%) | 2184 | (35.6%) | 266 | (12.4%) | 1688 | (73.6%) | 7626 | (30.4%) |
| Present | 10325 | (80.0%) | 719 | (44.2%) | 3949 | (64.4%) | 1879 | (87.6%) | 605 | (26.4%) | 17477 | (69.6%) | |
| Cough (any duration) | No | 5952 | (46.1%) | 1013 | (62.3%) | 3339 | (54.4%) | 793 | (37.0%) | 1881 | (82.0%) | 12978 | (51.7%) |
| Yes | 6955 | (53.9%) | 612 | (37.7%) | 2794 | (45.6%) | 1352 | (63.0%) | 412 | (18.0%) | 12125 | (48.3%) | |
| Cough (≥2 weeks) | No | 9574 | (74.2%) | 1377 | (84.7%) | 4897 | (79.8%) | 1454 | (67.8%) | 2250 | (98.1%) | 19552 | (77.9%) |
| Yes | 3333 | (25.8%) | 248 | (15.3%) | 1236 | (20.2%) | 691 | (32.2%) | 43 | (1.9%) | 5551 | (22.1%) | |
| Chest X-ray* | Active TB | 134 | (1.0%) | 91 | (5.6%) | 788 | (12.9%) | 33 | (1.5%) | 7 | (0.3%) | 1053 | (4.2%) |
| TB activity undetermined | 1901 | (14.7%) | 273 | (16.8%) | 1582 | (25.8%) | 291 | (13.6%) | 64 | (2.8%) | 4111 | (16.4%) | |
| Inactive TB | 342 | (2.7%) | 8 | (0.5%) | 182 | (3.0%) | 41 | (1.9%) | 3 | (0.1%) | 576 | (2.3%) | |
| No TB findings | 10524 | (81.6%) | 1253 | (77.1%) | 3580 | (58.4%) | 1780 | (83.0%) | 2219 | (96.8%) | 19356 | (77.1%) | |
| Suspected TB† | Not suspected | 10831 | (83.9%) | 1260 | (77.5%) | 3744 | (61.0%) | 1821 | (84.9%) | 2222 | (96.9%) | 19878 | (79.2%) |
| Suspected | 2076 | (16.1%) | 365 | (22.5%) | 2389 | (39.0%) | 324 | (15.1%) | 71 | (3.1%) | 5225 | (20.8%) | |
| Sputum smear test* | Negative | 1598 | (93.9%) | 94 | (79.7%) | 1917 | (89.8%) | 189 | (91.3%) | 39 | (92.9%) | 3837 | (91.3%) |
| Positive | 104 | (6.1%) | 24 | (20.3%) | 218 | (10.2%) | 18 | (8.7%) | 3 | (7.1%) | 367 | (8.7%) | |
| Xpert* | Negative | 1800 | (86.8%) | 311 | (91.5%) | 1999 | (84.7%) | 261 | (80.6%) | 63 | (92.6%) | 4434 | (85.8%) |
| Positive | 274 | (13.2%) | 29 | (8.5%) | 360 | (15.3%) | 63 | (19.4%) | 5 | (7.4%) | 731 | (14.2%) | |
| Rifampicin‡ | Susceptible | 257 | (93.8%) | 26 | (89.7%) | 329 | (91.4%) | 55 | (87.3%) | 5 | (100.0%) | 672 | (91.9%) |
| Resistant | 16 | (5.8%) | 3 | (10.3%) | 29 | (8.1%) | 7 | (11.1%) | 0 | (0.0%) | 55 | (7.5%) | |
| Intermediate | 1 | (0.4%) | 0 | (0.0%) | 2 | (0.6%) | 1 | (1.6%) | 0 | (0.0%) | 4 | (0.5%) | |
| Concordance of lab results§ | Smear + Xpert + | 104 | (6.1%) | 23 | (19.8%) | 212 | (10.0%) | 18 | (8.7%) | 3 | (7.1%) | 360 | (8.6%) |
| Smear − Xpert + | 168 | (9.9%) | 1 | (0.9%) | 146 | (6.9%) | 38 | (18.4%) | 1 | (2.4%) | 354 | (8.4%) | |
| Smear + Xpert − | 0 | (0.0%) | 0 | (0.0%) | 5 | (0.2%) | 0 | (0.0%) | 0 | (0.0%) | 5 | (0.1%) | |
| Smear − Xpert − | 1430 | (84.0%) | 92 | (79.3%) | 1767 | (83.0%) | 151 | (72.9%) | 38 | (90.5%) | 3478 | (82.9%) | |
| Final outcome | All forms of TB | 284 | (2.2%) | 34 | (2.1%) | 378 | (6.2%) | 63 | (2.9%) | 5 | (0.2%) | 764 | (3.0%) |
| - Bacteriologically-confirmed TB | 274 | (2.1%) | 30 | (1.8%) | 366 | (6.0%) | 63 | (2.9%) | 5 | (0.2%) | 738 | (2.9%) | |
| - Clinically-diagnosed TB | 10 | (0.1%) | 4 | (0.2%) | 12 | (0.2%) | 0 | (0.0%) | 0 | (0.0%) | 26 | (0.1%) | |
| NNS | 45 | 48 | 16 | 34 | 459 | 33 | |||||||
* Cases with unknown or missing information were excluded from the calculation.
† Suspected TB was determined by clinical judgement based primarily on X-ray results and additionally on TB symptoms.
‡ Among cases with positive Xpert results.
§ Among cases with results of both sputum smear and Xpert tests.
NNS: Number needed to screen to detect one TB patient (all forms of pulmonary TB)
One or more TB symptoms (any TB symptom) was identified in 69.6% of all participants, with the highest prevalence found in indigenous population (87.6%), followed by the rural poor (80.0%), prisons (64.4%), the urban poor (44.2%), and high schools (26.4%). Cough more than two weeks was identified in 22.1% of all participants, with the highest prevalence reported in indigenous population (32.2%), followed by the rural poor (25.8%), prisons (20.2%), the urban poor (15.3%) and high schools (1.9%).
Prison inmates had a high proportion of abnormal CXR results with suggestive of “active TB” (12.9%) and “TB activity undetermined” (25.8%). A lower proportion of CXR abnormality was found in other populations with the far lowest proportion observed in high school. Accordingly, the highest proportion of people with suspected TB was found in prison (39.0%), followed by the urban poor (22.5%), the rural poor (16.1%), indigenous population (15.1%) and high school (3.1%).
Outcomes of diagnostic tests (Table 2)
A total of 764 patients with all forms of pulmonary TB (3.0% [95%CI 2.8–3.3%]) were detected by the project. Of them, 738 (96.6%) had bacteriologically-confirmed TB, and 26 (3.4%) had clinically-diagnosed TB. The highest yield of TB with the lowest NNS was found in prison (6.2% [95%CI 5.6–6.8%], NNS: 16), followed by indigenous population (2.9% [95%CI 2.3–3.7%], NNS: 34), the rural poor (2.2% [95%CI 2.0–2.5%], NNS: 45), the urban poor (2.1% [95%CI 1.5–2.9%], NNS: 48), and high school (0.2% [95%CI 0.1–0.5%], NNS: 459).
A total of 55 patients (7.5% of Xpert positive patients) had RR-TB, with the highest resistance rate found in indigenous population (11.1%). Of 55 RR-TB patients, 23 patients (41.8%) had no history of previous TB treatment. The highest proportion of smear-negative Xpert-positive patients among patients who had results of both tests was found in indigenous population (18.4%), followed by the rural poor (9.9%) and prisons (6.9%). Five patients (0.2%) in prisons were smear-positive Xpert-negative patients. All of them had abnormal results of CXR (two were suggestive of “active TB” while three were “TB activity undetermined”) and were diagnosed with TB based on the physician’s clinical judgement.
The highest smear positivity rate (the proportion of individuals with a smear-positive result among those who have smear results) was observed in the urban poor (20.3%). In contrast, the highest Xpert positivity rate (the proportion of individuals with an Xpert positive result among those who have Xpert results) was found in indigenous population (19.4%).
Factors associated with diagnosis (yield) of TB (Tables 3–6)
Table 3. Yield of all forms of pulmonary TB by patient’s characteristics (rural poor communities).
| Characteristics | Total | Confirmed TB | Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | % | (95% CI) | Crude OR | (95% CI) | p-value | Adjusted OR | (95% CI) | p-value | |||
| Overall | 12907 | 284 | 2.2 | (1.9–2.4) | |||||||
| Sex | Female | 8055 | 84 | 1 | (0.8–1.3) | 1.00 | |||||
| Male | 4852 | 200 | 4.1 | (3.5–4.5) | 4.08 | (3.17–5.30) | <0.001*** | 2.3 | (1.66–3.19) | <0.001*** | |
| Age category | 15–24 | 1541 | 31 | 2 | (1.4–2.8) | 1.00 | |||||
| 25–34 | 1991 | 31 | 1.6 | (1.1–2.2) | 0.77 | (0.47–1.28) | 0.309 | 0.58 | (0.33–1.04) | 0.066 | |
| 35–44 | 2465 | 50 | 2 | (1.5–2.6) | 1.01 | (0.65–1.60) | 0.971 | 0.77 | (0.47–1.31) | 0.328 | |
| 45–54 | 2491 | 68 | 2.7 | (2.1–3.4) | 1.37 | (0.90–2.13) | 0.154 | 0.86 | (0.53–1.43) | 0.558 | |
| 55–64 | 2214 | 58 | 2.6 | (2.0–3.3) | 1.31 | (0.85–2.06) | 0.230 | 0.62 | (0.38–1.06) | 0.073 | |
| ≥65 | 2205 | 46 | 2.1 | (1.5–2.7) | 1.04 | (0.66–1.66) | 0.874 | 0.48 | (0.28–0.82) | 0.006** | |
| History of TB exposure | No | 10007 | 204 | 2 | (1.7–2.3) | 1.00 | |||||
| Yes | 2853 | 80 | 2.8 | (2.2–3.4) | 1.39 | (1.06–1.79) | 0.015* | 1.17 | (0.86–1.58) | 0.300 | |
| History of previous TB treatment | No | 11624 | 228 | 2 | (1.7–2.2) | 1.00 | |||||
| Yes | 1213 | 56 | 4.6 | (3.4–5.7) | 2.42 | (1.78–3.24) | <0.001*** | 1.29 | (0.90–1.82) | 0.153 | |
| Smoking status | No | 7595 | 108 | 1.4 | (1.2–1.7) | 1.00 | |||||
| Yes | 3103 | 143 | 4.6 | (3.8–5.2) | 3.35 | (2.60–4.32) | <0.001*** | 1.7 | (1.25–2.32) | <0.001*** | |
| Any TB symptoms | None | 2582 | 14 | 0.5 | (0.3–0.9) | 1.00 | |||||
| Present | 10325 | 270 | 2.6 | (2.3–2.9) | 4.93 | (2.99–8.85) | <0.001*** | 1.63 | (0.92–3.11) | 0.115 | |
| Cough (≥2 weeks) | No | 9574 | 89 | 0.9 | (0.8–1.1) | 1.00 | |||||
| Yes | 3333 | 195 | 5.9 | (4.8–6.3) | 6.62 | (5.16–8.57) | <0.001*** | 3.94 | (2.93–5.35) | <0.001*** | |
| Fever | No | 11779 | 209 | 1.8 | (1.5–2.0) | 1.00 | |||||
| Yes | 1128 | 75 | 6.6 | (5.0–7.7) | 3.94 | (2.99–5.15) | <0.001*** | 1.72 | (1.23–2.38) | 0.001** | |
| Night sweats | No | 10725 | 198 | 1.8 | (1.6–2.1) | 1.00 | |||||
| Yes | 2182 | 86 | 3.9 | (3.1–4.7) | 2.18 | (1.68–2.81) | <0.001*** | 1.24 | (0.91–1.67) | 0.170 | |
| Weight loss | No | 11249 | 177 | 1.6 | (1.3–1.8) | 1.00 | |||||
| Yes | 1658 | 107 | 6.5 | (5.0–7.3) | 4.32 | (3.37–5.51) | <0.001*** | 2.37 | (1.77–3.16) | <0.001*** | |
CI: Confidence Interval, OR: Odds Ratio
* Significant difference (0.01 ≤ p < 0.05)
** Significant difference (0.001 ≤ p < 0.01)
*** Significant difference (p < 0.001)
Table 6. Yield of all forms of pulmonary TB by patient’s characteristics (indigenous population).
| Characteristics | Total | Confirmed TB | Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | % | (95% CI) | Crude OR | (95% CI) | p-value | Adjusted OR | (95% CI) | p-value | |||
| Overall | 2145 | 63 | 2.9 | (2.2–3.6) | |||||||
| Sex | Female | 1376 | 23 | 1.7 | (1.1–2.5) | 1.00 | |||||
| Male | 769 | 40 | 5.2 | (3.6–6.7) | 3.23 | (1.93–5.51) | <0.001*** | 2.87 | (1.71–4.93) | <0.001*** | |
| Age category | 15–24 | 270 | 8 | 3 | (1.5–5.6) | 1.00 | |||||
| 25–34 | 376 | 6 | 1.6 | (0.7–3.4) | 0.53 | (0.17–1.54) | 0.246 | 0.52 | (0.17–1.54) | 0.240 | |
| 35–44 | 447 | 11 | 2.5 | (1.4–4.2) | 0.83 | (0.33–2.16) | 0.685 | 0.74 | (0.29–1.96) | 0.533 | |
| 45–54 | 410 | 14 | 3.4 | (2.0–5.5) | 1.16 | (0.49–2.93) | 0.745 | 0.91 | (0.37–2.37) | 0.841 | |
| 55–64 | 325 | 14 | 4.3 | (2.5–6.8) | 1.47 | (0.62–3.74) | 0.389 | 1.15 | (0.47–2.95) | 0.768 | |
| ≥65 | 317 | 10 | 3.2 | (1.7–5.5) | 1.07 | (0.41–2.83) | 0.893 | 0.75 | (0.28–2.03) | 0.561 | |
| History of TB exposure | No | 1714 | 48 | 2.8 | (2.1–3.6) | 1.00 | |||||
| Yes | 421 | 15 | 3.6 | (2.1–5.6) | 1.28 | (0.69–2.26) | 0.409 | ||||
| History of previous TB treatment | No | 1930 | 50 | 2.6 | (1.9–3.3) | 1.00 | |||||
| Yes | 189 | 13 | 6.9 | (3.8–10.7) | 2.78 | (1.42–5.06) | 0.001** | 2.35 | (1.18–4.38) | 0.010* | |
| Smoking status | No | 991 | 19 | 1.9 | (1.2–2.9) | 1.00 | |||||
| Yes | 479 | 16 | 3.3 | (2.0–5.2) | 1.77 | (0.89–3.47) | 0.098 | ||||
| Province | Palawan | 1934 | 54 | 2.8 | (2.1–3.5) | 1.00 | |||||
| Puerto Princesa | 211 | 9 | 4.3 | (2.2–7.6) | 1.55 | (0.71–3.04) | 0.232 | ||||
| Any TB symptoms | None | 266 | 4 | 1.5 | (0.6–3.8) | 1.00 | |||||
| Present | 1879 | 59 | 3.1 | (2.4–3.9) | 2.12 | (0.87–7.04) | 0.148 | ||||
| Cough (≥2 weeks) | No | 1454 | 31 | 2.1 | (1.5–3.0) | 1.00 | |||||
| Yes | 691 | 32 | 4.6 | (3.1–6.2) | 2.23 | (1.35–3.70) | 0.002** | 1.72 | (1.02–2.91) | 0.041* | |
| Fever | No | 1725 | 43 | 2.5 | (1.8–3.3) | 1.00 | |||||
| Yes | 420 | 20 | 4.8 | (3.0–6.9) | 1.96 | (1.12–3.32) | 0.015* | 1.41 | (0.77–2.49) | 0.252 | |
| Night sweats | No | 1483 | 37 | 2.5 | (1.8–3.3) | 1.00 | |||||
| Yes | 662 | 26 | 3.9 | (2.6–5.5) | 1.60 | (0.95–2.65) | 0.072 | ||||
| Weight loss | No | 1747 | 41 | 2.3 | (1.7–3.1) | 1.00 | |||||
| Yes | 398 | 22 | 5.5 | (3.5–7.8) | 2.43 | (1.41–4.09) | <0.001*** | 1.87 | (1.05–3.26) | 0.030* | |
CI: Confidence Interval, OR: Odds Ratio
* Significant difference (0.01 ≤ p < 0.05)
** Significant difference (0.001 ≤ p < 0.01)
*** Significant difference (p < 0.001)
Table 4. Yield of all forms of pulmonary TB by patient’s characteristics (urban poor communities).
| Characteristics | Total | Confirmed TB | Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | % | (95% CI) | Crude OR | (95% CI) | p-value | Adjusted OR | (95% CI) | p-value | |||
| Overall | 1625 | 34 | 2.1 | (1.5–2.9) | |||||||
| Sex | Female | 905 | 11 | 1.2 | (0.7–2.1) | 1.00 | |||||
| Male | 720 | 23 | 3.2 | (2.1–4.6) | 2.68 | (1.33–5.75) | 0.008** | 2.08 | (0.98–4.62) | 0.062 | |
| Age category | 15–24 | 346 | 6 | 1.7 | (0.8–3.7) | 1.00 | |||||
| 25–34 | 298 | 5 | 1.7 | (0.7–3.8) | 0.97 | (0.28–3.24) | 0.956 | 0.96 | (0.26–3.41) | 0.950 | |
| 35–44 | 341 | 10 | 2.9 | (1.6–5.2) | 1.71 | (0.63–5.08) | 0.303 | 1.67 | (0.58–5.26) | 0.351 | |
| 45–54 | 282 | 5 | 1.8 | (0.8–4.0) | 1.02 | (0.29–3.43) | 0.970 | 0.83 | (0.22–2.94) | 0.769 | |
| 55–64 | 205 | 4 | 2 | (0.8–4.8) | 1.13 | (0.29–3.99) | 0.854 | 0.49 | (0.12–1.88) | 0.307 | |
| ≥65 | 153 | 4 | 2.6 | (1.0–6.4) | 1.52 | (0.38–5.40) | 0.520 | 0.91 | (0.21–3.51) | 0.890 | |
| History of TB exposure | No | 1559 | 33 | 2.1 | (1.5–2.9) | 1.00 | |||||
| Yes | 63 | 1 | 1.6 | (0.3–8.3) | 0.75 | (0.04–3.55) | 0.774 | ||||
| History of previous TB treatment | No | 1498 | 28 | 1.9 | (1.3–2.6) | 1.00 | |||||
| Yes | 120 | 6 | 5 | (2.2–10.0) | 2.76 | (1.02–6.38) | 0.027* | 1.69 | (0.57–4.27) | 0.300 | |
| Smoking status | No | 770 | 13 | 1.7 | (1.0–2.8) | 1.00 | |||||
| Yes | 431 | 12 | 2.8 | (1.6–4.7) | 1.67 | (0.74–3.71) | 0.207 | ||||
| Any TB symptoms | None | 906 | 8 | 0.9 | (0.4–1.7) | 1.00 | |||||
| Present | 719 | 26 | 3.6 | (2.4–5.1) | 4.21 | (1.98–10.01) | <0.001*** | 0.93 | (0.27–2.91) | 0.905 | |
| Cough (≥2 weeks) | No | 1377 | 13 | 0.9 | (0.6–1.6) | 1.00 | |||||
| Yes | 248 | 21 | 8.5 | (5.2–11.6) | 9.71 | (4.85–20.16) | <0.001*** | 6.73 | (2.59–20.93) | <0.001*** | |
| Fever | No | 1572 | 29 | 1.8 | (1.3–2.6) | 1.00 | |||||
| Yes | 53 | 5 | 9.4 | (3.7–18.6) | 5.54 | (1.83–13.82) | <0.001*** | 2.34 | (0.66–6.96) | 0.150 | |
| Night sweats | No | 1565 | 27 | 1.7 | (1.2–2.5) | 1.00 | |||||
| Yes | 60 | 7 | 11.7 | (5.2–20.0) | 7.52 | (2.91–17.20) | <0.001*** | 2.41 | (0.79–6.62) | 0.102 | |
| Weight loss | No | 1520 | 24 | 1.6 | (1.1–2.3) | 1.00 | |||||
| Yes | 105 | 10 | 9.5 | (4.8–15.3) | 6.56 | (2.92–13.75) | <0.001*** | 2.28 | (0.88–5.59) | 0.079 | |
CI: Confidence Interval, OR: Odds Ratio
* Significant difference (0.01 ≤ p < 0.05)
** Significant difference (0.001 ≤ p < 0.01)
*** Significant difference (p < 0.001)
Table 5. Yield of all forms of pulmonary TB by patient’s characteristics (prison inmates).
| Characteristics | Total | Confirmed TB | Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | % | (95% CI) | Crude OR | (95% CI) | p-value | Adjusted OR | (95% CI) | p-value | |||
| Overall | 6133 | 378 | 6.2 | (5.3–6.4) | |||||||
| Sex | Female | 66 | 2 | 3 | (0.8–10.1) | 1.00 | |||||
| Male | 6067 | 376 | 6.2 | (5.3–6.4) | 2.11 | (0.66–12.91) | 0.298 | 0.63 | (0.18–3.97) | 0.536 | |
| Age category | 15–24 | 439 | 7 | 1.6 | (0.8–3.2) | 1.00 | |||||
| 25–34 | 1443 | 49 | 3.4 | (2.5–4.3) | 2.17 | (1.04–5.28) | 0.058 | 1.42 | (0.67–3.50) | 0.397 | |
| 35–44 | 1878 | 138 | 7.3 | (5.8–8.0) | 4.89 | (2.45–11.62) | <0.001*** | 2.43 | (1.19–5.86) | 0.027* | |
| 45–54 | 1535 | 108 | 7 | (5.5–7.9) | 4.67 | (2.32–11.13) | <0.001*** | 1.94 | (0.94–4.72) | 0.102 | |
| 55–64 | 699 | 69 | 9.9 | (7.2–11.2) | 6.76 | (3.30–16.30) | <0.001*** | 2.27 | (1.07–5.60) | 0.048* | |
| ≥65 | 139 | 7 | 5 | (2.3–9.6) | 3.27 | (1.10–9.72) | 0.029* | 1.31 | (0.43–4.00) | 0.629 | |
| History of TB exposure | No | 4647 | 261 | 5.6 | (4.7–6.0) | 1.00 | |||||
| Yes | 1472 | 117 | 7.9 | (6.2–8.8) | 1.45 | (1.15–1.82) | 0.001** | 0.86 | (0.67–1.11) | 0.267 | |
| History of previous TB treatment | No | 5189 | 289 | 5.6 | (4.7–5.9) | 1.00 | |||||
| Yes | 931 | 89 | 9.6 | (7.1–10.6) | 1.79 | (1.39–2.29) | <0.001*** | 0.77 | (0.57–1.01) | 0.065 | |
| Smoking status | No | 852 | 54 | 6.3 | (4.6–7.7) | 1.00 | |||||
| Yes | 3980 | 262 | 6.6 | (5.5–6.9) | 1.04 | (0.78–1.42) | 0.793 | ||||
| Prison | Prison A | 688 | 15 | 2.2 | (1.3–3.5) | 1.00 | |||||
| Prison B | 1453 | 28 | 1.9 | (1.3–2.7) | 0.88 | (0.47–1.70) | 0.697 | 0.92 | (0.49–1.80) | 0.797 | |
| Prison C | 2640 | 261 | 9.9 | (8.0–10.1) | 4.92 | (3.01–8.71) | <0.001*** | 4.05 | (2.41–7.32) | <0.001*** | |
| Prison D | 965 | 54 | 5.6 | (4.1–6.8) | 2.66 | (1.53–4.92) | <0.001*** | 2.76 | (1.55–5.22) | 0.001** | |
| Prison E | 343 | 17 | 5 | (3.0–7.4) | 2.34 | (1.15–4.80) | 0.018* | 3.46 | (1.65–7.30) | <0.001*** | |
| Prison F | 44 | 3 | 6.8 | (2.2–17.2) | 3.28 | (0.74–10.45) | 0.069 | 5.12 | (1.13–16.82) | 0.014* | |
| Any TB symptoms | None | 2184 | 61 | 2.8 | (2.1–3.5) | 1.00 | |||||
| Present | 3949 | 317 | 8 | (6.7–8.3) | 3.04 | (2.32–4.05) | <0.001*** | 1.41 | (1.01–1.99) | 0.048* | |
| Cough (≥2 weeks) | No | 4897 | 201 | 4.1 | (3.4–4.5) | 1.00 | |||||
| Yes | 1236 | 177 | 14.3 | (10.9–14.3) | 3.90 | (3.16–4.83) | <0.001*** | 2.41 | (1.87–3.10) | <0.001*** | |
| Fever | No | 5556 | 316 | 5.7 | (4.8–6.0) | 1.00 | |||||
| Yes | 577 | 62 | 10.7 | (7.6–12.2) | 2.00 | (1.49–2.64) | <0.001*** | 1.48 | (1.06–2.04) | 0.018* | |
| Night sweats | No | 5330 | 297 | 5.6 | (4.7–5.9) | 1.00 | |||||
| Yes | 803 | 81 | 10.1 | (7.4–11.2) | 1.90 | (1.46–2.45) | <0.001*** | 1.19 | (0.89–1.59) | 0.242 | |
| Weight loss | No | 4715 | 185 | 3.9 | (3.3–4.3) | 1.00 | |||||
| Yes | 1418 | 193 | 13.6 | (10.5–13.7) | 3.86 | (3.12–4.77) | <0.001*** | 2.11 | (1.65–2.71) | <0.001*** | |
CI: Confidence Interval, OR: Odds Ratio
* Significant difference (0.01 ≤ p < 0.05)
** Significant difference (0.001 ≤ p < 0.01)
*** Significant difference (p < 0.001)
Factors significantly associated with diagnosis of TB varied across different populations. In the rural poor, a significantly higher yield was found in male (AOR 2.3, 95%CI: 1.66–3.19), smokers (AOR 1.7, 95%CI: 1.25–2.32), and those with cough more than two weeks (AOR 3.94, 95%CI: 2.93–5.35), with fever (AOR 1.72, 95%CI: 1.23–2.38), and with weight loss (AOR 2.37, 95%CI: 1.77–3.16). In the urban poor, a significantly higher yield was found only in those with cough more than two weeks (AOR 6.73, 95%CI: 2.59–20.93). In prison, a significantly higher yield was found in middle-to-higher age groups; 35–44 (AOR 2.43, 95%CI: 1.19–5.86) and 55–64 (AOR 2.27, 95%CI: 1.07–5.60), four prison facilities (AOR ranged from 2.76 to 5.12), those with any TB symptoms (AOR 1.41, 95%CI: 1.01–1.99), with cough more than two weeks (AOR 2.41, 95%CI: 1.87–3.10), with fever (AOR 1.48, 95%CI: 1.06–2.04), and with weight loss (AOR 2.11, 95%CI: 1.65–2.71). In indigenous population, a significantly higher yield was found in male (AOR 2.87, 95%CI: 1.71–4.93), those with history of previous TB treatment (AOR 2.35, 95%CI: 1.18–4.38), those with cough more than two weeks (AOR 1.72, 95%CI: 1.02–2.91), and with weight loss (AOR 1.87, 95%CI: 1.05–3.26).
Cough more than two weeks showed a significant association in all target populations after adjustment. History of TB exposure and night sweats were not significantly associated with a higher yield in any of the target populations. Middle-to-higher age groups showed a significant association only in prison. History of previous TB treatment showed a significant association only in indigenous population. Smoking status showed a significant association only in the rural poor. Any symptom showed a significant association only in prison.
Composition of TB patients by smear and symptom status
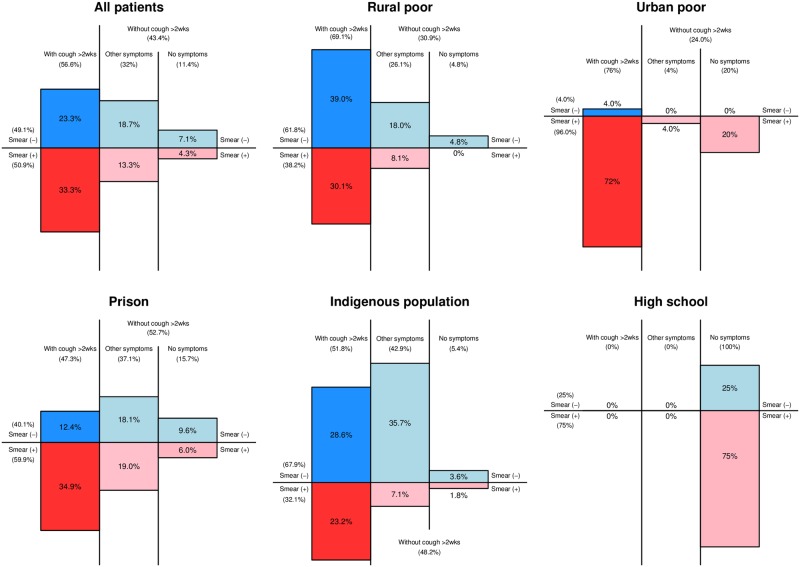
Fig 1 showed the composition of bacteriologically-confirmed TB patients by smear and symptom status for each target population. The proportion of smear-positive patients with cough more than two weeks was the highest in the urban poor (72.0%), followed by prison (34.9%), the rural poor (30.1%), indigenous population (23.2%), and high school (0%). The proportion of patients without cough more than two weeks was the highest in prison (52.7%), followed by indigenous population (48.2%), the rural poor (30.9%), and the urban poor (24.0%) (excluding 100% in high school due to a small number of patients). Among the four populations, the proportion of smear-negative (Xpert-positive) patients was low in the urban poor (4.0%) while it accounted for the substantial proportion in the rural poor (61.8%), prison (40.1%), and indigenous population (67.9%). The proportion of smear-negative patients without cough more than two weeks was the highest in indigenous population (39.3%), followed by prison (27.7%) and the rural poor (22.8%).
Fig 1. Composition of bacteriologically-confirmed TB patients by smear and symptom status, by target populations.
Patients with unknown or missing sputum smear results were excluded from the calculation.
Treatment outcomes (Table 7)
Table 7. Treatment outcomes of TB patients by target population.
| Type of TB | Treatment outcomes | Rural poor | Urban poor | Prison inmates | Indigenous population | High school students | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 252 | (%) | N = 23 | (%) | N = 300 | (%) | N = 57 | (%) | N = 4 | (%) | N = 636 | (%) | ||
| Rifampicin-susceptible TB | Number of cohort | 249 | 21 | 285 | 53 | 4 | 612 | ||||||
| Treatment success | 215 | (86.3%) | 20 | (95.2%) | 263 | (92.3%) | 46 | (86.8%) | 4 | (100.0%) | 548 | (89.5%) | |
| - Cured | 136 | (54.6%) | 6 | (28.6%) | 243 | (85.3%) | 27 | (50.9%) | 4 | (100.0%) | 416 | (68.0%) | |
| - Completed | 79 | (31.7%) | 14 | (66.7%) | 20 | (7.0%) | 19 | (35.8%) | 0 | (0.0%) | 132 | (21.6%) | |
| Died | 5 | (2.0%) | 1 | (4.8%) | 4 | (1.4%) | 1 | (1.9%) | 0 | (0.0%) | 11 | (1.8%) | |
| Failure | 3 | (1.2%) | 0 | (0.0%) | 3 | (1.1%) | 1 | (1.9%) | 0 | (0.0%) | 7 | (1.1%) | |
| Loss to follow-up | 16 | (6.4%) | 0 | (0.0%) | 0 | (0.0%) | 4 | (7.5%) | 0 | (0.0%) | 20 | (3.3%) | |
| Transfer-out | 10 | (4.0%) | 0 | (0.0%) | 15 | (5.3%) | 1 | (1.9%) | 0 | (0.0%) | 26 | (4.2%) | |
| Rifampicin-resistant TB | Number of cohort | 3 | 2 | 15 | 4 | 0 | 24 | ||||||
| Treatment success | 1 | (33.3%) | 1 | (50.0%) | 14 | (93.3%) | 4 | (100.0%) | 0 | (0.0%) | 20 | (83.3%) | |
| - Cured | 0 | (0.0%) | 1 | (50.0%) | 2 | (13.3%) | 3 | (75.0%) | 0 | (0.0%) | 6 | (25.0%) | |
| - Completed | 1 | (33.3%) | 0 | (0.0%) | 12 | (80.0%) | 1 | (25.0%) | 0 | (0.0%) | 14 | (58.3%) | |
| Died | 0 | (0.0%) | 0 | (0.0%) | 1 | (6.7%) | 0 | (0.0%) | 0 | (0.0%) | 1 | (4.2%) | |
| Failure | 0 | (0.0%) | 0 | (0.0%) | 0 | (0.0%) | 0 | (0.0%) | 0 | (0.0%) | 0 | (0.0%) | |
| Loss to follow-up | 2 | (66.7%) | 1 | (50.0%) | 0 | (0.0%) | 0 | (0.0%) | 0 | (0.0%) | 3 | (12.5%) | |
| Transfer-out | 0 | (0.0%) | 0 | (0.0%) | 0 | (0.0%) | 0 | (0.0%) | 0 | (0.0%) | 0 | (0.0%) | |
TSR was found to be high with 89.5% for patients with Rifampicin-susceptible (RS) TB and 83.3% for patients with RR-TB. For RS-TB patients, a higher TSR was observed in high school (100%), the urban poor (95.2%) and prison (92.3%). In contrast, a relatively higher loss to follow-up rate was reported in indigenous population (7.5%) and the rural poor (6.4%).
Discussion
Our analysis showed a wide range of demographic profiles and prevalence of symptoms and predictors of TB across five different target populations. As a result, we found a wide variation of yield, ranging from 0.2% (NNS: 495) in high school, 2.1% (NNS: 48) in the urban poor, 2.2% (NNS: 45) in the rural poor, 2.9% (NNS: 34) in indigenous population and to 6.2% (NNS: 16) in prison. We also identified some commonalities and differences between target populations in the predictor analysis, composition of the patients by smear and symptom status, as well as in treatment outcomes.
In this project, screening in prison was found to be most effective among all target populations given the lowest NNS observed. A systematic review on NNS in TB screening showed that the median NNS for prisoners was 43 (IQR 21–123) in medium and high incidence settings which was among the lowest across different risk groups [10]. In the Philippines, excessive overcrowding in prisons has been well recognized [20], and mass-screening for TB has been conducted in some prisons in other regions of the country too, with a yield ranging from 2.5% (NNS: 40) to 3.2% (NNS: 31) [21,22]. Given that our project also employed mass screening in prisons, the results confirmed the high prevalence of TB among prison inmates in the Palawan settings. It is noteworthy that the prison with the highest yield had an over five-fold higher rate than the prison with the lowest yield (9.9% [NNS: 10] vs 1.9% [NNS: 53]), suggesting a wide range of TB prevalence across different prisons.
The highest NNS in high school suggested ineffectiveness as compared to other target populations. In the Philippines, the prevalence of culture positive TB for those aged 10–29 was estimated to be 0.28% [23], which was almost equivalent to the yield reported in high school students aged 15–23 (0.2%). School-based mass screening usually involves subjects from a specific age group, therefore an age-group specific prevalence rate from the national survey could represent expected yield in such screening activities. Although the prevalence in those with younger age varies depending on epidemiological profiles of countries, it is generally lower than that of general population in many countries [4,24–26] including the Philippines [23]. They are often categorized as a low risk group in which systematic screening for active TB is not recommended [9]. Some studies indeed showed a low yield in their TB screening among adolescents in high burden settings [27–29], being consistent with our results. However diagnosing and treating a younger patient would achieve more life-years saved than treating an older patient, and in some settings, may contribute to addressing health inequity and filling programmatic gaps. A study in Kenya demonstrated a high TB prevalence and low patient diagnostic rate in adolescents [30]. In such context with a strong evidence-base, screening adolescents including high school students may be justifiable. In the Palawan setting where TB is obviously less concentrated in high school students, a priority for ACF should be given to other populations with a higher risk of TB.
According to WHO, systematic screening for active TB may be considered for geographically defined subpopulations with extremely high levels of undetected TB (1% prevalence or higher), other subpopulations that have very poor access to health care, and other vulnerable or marginalized groups [8]. Our ACF among the urban poor, the rural poor and indigenous population is likely to fall in these categories, given the high yield of TB with more than 2%. In other countries with a high burden of TB, systematic screening also achieved a relatively high yield in the urban poor and slums [11,31–35], in the rural poor [13,36] and in indigenous population [37], being consistent with our results. These studies may not be directly comparable due to considerable differences in the definition of screening (denominator of the yield calculation), the disease prevalence in target population, and screening approaches including diagnostic tools and algorithms. In the Palawan setting, the high yield of TB among these populations could be explained by several factors. First, underlying TB prevalence was likely to be high due to poor access to health services and low socio-economic status. In particular, indigenous populations are likely to have the most limited health access as they usually reside in mountain areas [38], which could have led the highest yield among the three. Second, the robust diagnostic algorithm using a rapid sensitive test with a combination of digital CXR could have greatly increased the diagnostic accuracy. In the systematic review on NNS, inclusion of CXR in the screening tools was associated with low NNS [10], which supported our results. Furthermore, people who have TB symptoms could be more likely to visit the mobile unit for screening since the participation was based on a voluntary visit (except prison and high school screening where mass screening was conducted). This might have increased the yield by facilitating the spontaneous selection of people with a higher risk of TB. Indeed, the prevalence of TB symptomatics among them was higher than the national average of 13.3% reported in the 2007 national prevalence survey [23].
Our ACF approach was found to be effective in the four populations in terms of yield. However there are many other considerations in order to rationalize the project and further inform the continuation or scale-up of the ACF activities. First, cost-effectiveness and cost-utility of the project should be investigated and compared between target populations. Indicators such as cost per case detected and incremental cost-effectiveness ratio will provide important financial implications [39,40]. These cost-effectiveness indicators can also represent the efficiency of the screening including the average number of people screened per day and travel time and distance needed for screening. Second, the additionality of the ACF to case detection could be explored to evaluate the contribution of the project. Not all patients detected by the project can be regarded as true additional cases as some could have been detected by the routine PCF even if there was no project intervention [41]. An analysis of case notification trend in intervention areas before and during intervention periods as well as its comparison with control areas enables to estimate the number of true additional cases and provides a clearer picture of the contribution of ACF to increased case detection [13,42].
Our analysis of the composition of bacteriologically-confirmed TB by smear and symptom status enabled to quantify the contribution of CXR, Xpert and its combination to overall case detection. First, the patients without cough more than two weeks accounted for a substantial proportion, ranging from 24.0% to 52.7% (except high school students). If they underwent a routine health-seeking pathway, they would not have been presumed to have TB since the national guidelines use cough more than two weeks as criteria [17]. The use of CXR in initial screening helped identify many TB patients with mild or no symptoms. Given the nature of ACF where many asymptomatic individuals may be subject to screening [16], CXR plays a critically important role in ACF with its high sensitivity [43]. Positioning CXR in initial screening can be costly [44], however the use of digital X-ray machine, in place of conventional film X-ray, can significantly reduce running costs and simplify logistics [45], making it a feasible and cost-effective option even in a large scale ACF in resource-constrained programmes.
Second, the proportion of smear-negative (Xpert-positive) patients was high in three populations (61.8% in the rural poor, 40.1% in prison and 67.9% in indigenous population). They also would not have been detected in the routine diagnostic algorithm that employs sputum smear microscopy as a main tool for bacteriological confirmation [17]. Given that smear-negatives often indicate less severity of the disease [12,46,47], these patients might be detected at the early stage of their disease by using Xpert. In contrast, the high proportion of smear-positives was found in the urban poor, consistent with the results from the national prevalence survey [23]. This implies a considerable diagnostic delay in this population, which needs further investigation and addressing.
Third, smear-negative patients without cough more than two weeks also accounted for a substantial proportion in three populations (39.3% in indigenous population, 27.7% in prison and 22.8% in the rural poor). These patients would not have been diagnosed if either CXR or Xpert was not available in the project, thereby representing a shared contribution of CXR and Xpert. The contribution of CXR can be maximized when it is used in combination with Xpert, and vice versa. In our project, indigenous population appears to be most benefited from increased diagnostic sensitivity due to the combined use of CXR and Xpert. Less-severe and less-symptomatic TB patients may be most prevalent in this population.
In this project, the use of Xpert facilitates bacteriological confirmation among smear-negatives, which also could have led to the low proportion of clinically-diagnosed TB patients (3.4%). The national average in the proportion of clinically-diagnosed TB among all pulmonary TB patients was high with 64% in 2015 [1]. This may indicate the high detection of less or non-infectious cases but also reflect the issue of low sensitivity of sputum smear microscopy that is mainly used for bacteriological confirmation in the routine setting. Our results suggested that continued efforts to increase the availability of Xpert would make clinicians more convinced to rule out TB and reduce possible unnecessary TB treatment.
This study has several limitations. First, 5–10% of the participant records in the study period were missing, which could have influenced the study results. Second, some participants from indigenous population were selected from the defined areas of the rural poor communities. Hence their demographic and clinical characteristics could have showed some similarities with those of the rural poor. Third, five smear-positive Xpert-negative cases that we considered bacteriologically-confirmed cases may be possible non-tuberculosis mycobacteria, which could have slightly overestimated the yield in prison. Fourth, we highlighted the contribution of Xpert by showing the composition of patient by smear status. However our project used LED-FM that has a higher sensitivity than conventional light microscopy [48], which could have underestimated the contribution of Xpert. Finally, our study did not aim to achieve national representation for each target population therefore the results may not be generalized to other areas.
The results from our predictor analysis help provide additional screening criteria for each target population in the study setting. Our results showed a tendency that symptoms criteria had generally stronger association than demographic and other known risk factors. In particular, a significantly higher yield was found in those with cough more than two weeks in all populations. This reaffirmed the importance of continued emphasis on cough more than weeks that should remain the priority symptom criteria regardless of the presence of other risk factors. However focusing on only this symptom could miss a large number of cases as explained above. To find more cases missed in routine PCF, non- or less-symptomatic individuals should be included in screening based on other factors that had stronger associations with higher yield in addition to their relative risk of TB inherent in their respective populations. In our study setting, fever and weight loss were found to be more useful symptom criteria than night sweat. Demographic factors also have the potential to serve as effective screening criteria. Examining diagnostic power of each symptom by stratifying patient socio-demographic characteristics will enable to identify possible population-specific predictors of TB, which helps develop locally-defined screening criteria that can be used not only in ACF but also in routine PCF.
In this study, RS-TB patients had a TSR of 89.5%. This was almost equivalent to the TSR reported at the national level (92% for new and relapse cases in 2015) [1]. The TSR among RR-TB patients detected by the project was much higher than the national average of RR-/MDR-TB patients (83.3% vs 49%) [1], showing that ACF did not compromise treatment outcome. This finding is consistent with other studies [12,49] as well as a systematic review that concluded treatment outcome is similar between ACF and PCF [50]. However, the relatively lower TSR and higher loss to follow-up rate were found in indigenous population and the rural poor. In a study from South India, a higher loss to follow-up rate was particularly reported in smear-negative patients in ACF [16]. Given the higher proportion of smear-negative patients found in these two populations, they could have more patients with less severe disease, leading to higher loss to follow-up. The difference in accessibility to care across target populations could have also influenced treatment outcomes.
The United Nation’s Sustainable Development Goals (SDGs) aims to achieve Universal Health Coverage (UHC) which includes financial risk protection, access to quality essential health-care services and access to safe, effective, quality and affordable essential medicines and vaccines for all [51]. Well-designed ACF brings quality TB diagnostic service to high-risk and most vulnerable populations with no patient cost sharing, which could facilitate early case detection and help mitigate financial burden of the disease [52]. Such pro-poor case finding interventions intend to address health inequity, which is in line with the objectives of UHC and further supports achieving the SDGs. However, ACF, if poorly designed, may carry a risk of over-diagnosis [12] and false positive results due to less targeted screening involving people with low risk of TB [9], increasing unsuccessful treatment outcomes including initial loss to follow-up [16,50] and depriving resources for a poor yield. In this regard, careful assessment is essential to inform evidence-based decisions about target populations and diagnostic approaches that ensure the effectiveness of screening.
Conclusions
In the Philippines, the ACF project that targeted vulnerable populations using mobile unit yielded a substantial number of TB patients and achieved successful treatment outcomes. In particular, screening in prison, indigenous population, and urban and rural poor communities was found to be effective and they can be considered high-risk populations in the study setting. The combined use of CXR and Xpert largely contributed to increased case detection. Further research is needed to explore cost-effectiveness of the project and diagnostic power of different symptoms to accelerate evidence-based interventions as well as to inform future TB screening policies in the Philippines.
Supporting information
(XLSX)
Acknowledgments
The authors would like to thank Ms Chan Ju Kim (Korea Foundation for International Healthcare, Seoul) for her invaluable support throughout the project implementation and this evaluation study. The authors are grateful to Ms May Antonnette Lebanan who supported the data collation. The authors extend their appreciation to all project staff, health officials, prison and school officials, and local healthcare workers who supported the project implementation.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The DetecTB project was funded by the Government of the Republic of Korea through the Korean Foundation for International Healthcare (KOFIH). This study was financially supported by the Government of the Republic of Korea through Korean Centers for Disease Control & Prevention, and the Government of Japan through Ministry of Health, Labour and Welfare. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the views of the authors' organization or affiliations.
References
- 1.World Health Organization. Global Tuberculosis Report. Geneva; 2016. [Google Scholar]
- 2.Böttger EC, Springer B. Tuberculosis: drug resistance, fitness, and strategies for global control. Eur J Pediatr. Springer-Verlag; 2007;167: 141–148. [DOI] [PubMed] [Google Scholar]
- 3.Brewer TF. To control and beyond: moving towards eliminating the global tuberculosis threat. Journal of Epidemiology & Community Health. 2004;58: 822–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao TE, Okada K, Yamada N, Peou S, Ota M, Saint S, et al. Cross-sectional studies of tuberculosis prevalence in Cambodia between 2002 and 2011. Bull World Health Org. 2014;92: 573–581. 10.2471/BLT.13.131581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golub JE, Mohan CI, Comstock GW, Chaisson RE. Active case finding of tuberculosis: historical perspective and future prospects. int j tuberc lung dis. 2005;9: 1183–1203. [PMC free article] [PubMed] [Google Scholar]
- 6.Lonnroth K, Corbett E, Golub J, Godfrey-Faussett P, Uplekar M, Weil D, et al. Systematic screening for active tuberculosis: rationale, definitions and key considerations [State of the art series. Active case finding/screening. Number 1 in the series]. int j tuberc lung dis. 2013;17: 289–298. 10.5588/ijtld.12.0797 [DOI] [PubMed] [Google Scholar]
- 7.Creswell J, Sahu S, Blok L, Bakker MI, Stevens R, Ditiu L. A Multi-Site Evaluation of Innovative Approaches to Increase Tuberculosis Case Notification: Summary Results. Hoshino Y, editor. PLoS ONE. 2014;9: e94465–11. 10.1371/journal.pone.0094465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Systematic screening for active tuberculosis. Geneva; 2013. Report No.: WHO/HTM/TB/2013.04. [PubMed]
- 9.World Health Organization. Systematic screening for active tuberculosis: an operational guide. Geneva; 2015. Report No.: WHO/HTM/TB/2015.16.
- 10.Shapiro AE, Chakravorty R, Akande T, Lönnroth K, Golub JE. A systematic review of the number needed to screen to detect a case of active tuberculosis in different risk groups [Internet]. Geneva: World Health Organization; Available: http://www.who.int/tb/Review3NNS_case_active_TB_riskgroups.pdf?ua=1 [Google Scholar]
- 11.Khanal S, Baral S, Shrestha P, Puri M, Kandel S, Lamichanne B, et al. Yield of intensified tuberculosis case-finding activities using Xpert® MTB/RIF among risk groups in Nepal. public health action. 2016;6: 136–141. 10.5588/pha.16.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eang MT, Satha P, Yadav RP, Morishita F, Nishikiori N, van-Maaren P, et al. Early detection of tuberculosis through community-based active case finding in Cambodia. BMC Public Health. 2012;12: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yassin MA, Datiko DG, Tulloch O, Markos P, Aschalew M, Shargie EB, et al. Innovative Community-Based Approaches Doubled Tuberculosis Case Notification and Improve Treatment Outcome in Southern Ethiopia. Pai M, editor. PLoS ONE. 2013;8: e63174–8. 10.1371/journal.pone.0063174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John S, Gidado M, Dahiru T, Fanning A, Codlin AJ, Creswell J. Tuberculosis among nomads in Adamawa, Nigeria: outcomes from two years of active case finding. int j tuberc lung dis. 2015;19: 463–468. 10.5588/ijtld.14.0679 [DOI] [PubMed] [Google Scholar]
- 15.Cassels A, Heineman E, LeClerq S, Gurung PK, Rahut CB. Tuberculosis case-finding in Eastern Nepal. Tubercle. 1982;63: 175–185. [DOI] [PubMed] [Google Scholar]
- 16.Santha T, Renu G, Frieden TR, Subramani R, Gopi PG, Chandrasekaran V, et al. Are community surveys to detect tuberculosis in high prevalence areas useful? Results of a comparative study from Tiruvallur District, South India. int j tuberc lung dis. 2003;7: 258–265. [PubMed] [Google Scholar]
- 17.Department of Health the Philippines. Manual of Procedures of the National Tuberculosis Control Program, 5th Edition Manila; 2014. [Google Scholar]
- 18.Vianzon R, Garfin AMC, Lagos A, Belen R. The tuberculosis profile of the Philippines, 2003–2011: advancing DOTS and beyond. WPSAR. 2013;4: 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Definitions and reporting framework for tuberculosis-2013 revision. Geneva; 2013. Report No.: WHO/HTM/TB/2013 2.
- 20.International Committee of the Red Cross. Philippines: protecting life and dignity in places of detention [Internet]. 3 Feb 2010 [cited 31 Aug 2016]. Available: https://www.icrc.org/eng/resources/documents/update/philippines-update-030210.htm
- 21.International Union Against Tuberculosis and Lung Disease. The Philippines TB Society Publishes a Study on TB Prevalence in Prisons [Internet]. 2009 [cited 31 Aug 2016]. Available: http://old.theunion.org/Regions/AsiaPacific/index.php/Member-News/The-Philippines-TB-Society-Publishes-a-Study-on-TB-Prevalence-in-Prisons.html
- 22.International Committee of the Red Cross. Philippines: Largest mass screening for TB held in New Bilibid Prison [Internet]. 29 Mar 2016 [cited 31 Aug 2016]. Available: https://www.icrc.org/en/document/philippines-largest-mass-screening-tb-held-new-bilibid-prison
- 23.Tropical Disease Foundation Inc., Department of Health the Philippines. Nationwide Tuberculosis Prevalence Survey 2007 for the Philippines. Navotas City, Philippines; 2008.
- 24.Kapata N, Chanda-Kapata P, Ngosa W, Metitiri M, Klinkenberg E, Kalisvaart N, et al. The Prevalence of Tuberculosis in Zambia: Results from the First National TB Prevalence Survey, 2013–2014. Deribe K, editor. PLoS ONE. 2016;11: e0146392–14. 10.1371/journal.pone.0146392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law I, Sylavanh P, Bounmala S, Nzabintwali F, Paboriboune P, Iem V, et al. The first national tuberculosis prevalence survey of Lao PDR (2010–2011). Trop Med Int Health. 2015;20: 1146–1154. 10.1111/tmi.12536 [DOI] [PubMed] [Google Scholar]
- 26.Adetifa IM, Kendall L, Bashorun A, Linda C, Omoleke S, Jeffries D, et al. A tuberculosis nationwide prevalence survey in Gambia, 2012. Bull World Health Org. 2016;94: 433–441. 10.2471/BLT.14.151670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahomed H, Ehrlich R, Hawkridge T, Hatherill M, Geiter L, Kafaar F, et al. Screening for TB in high school adolescents in a high burden setting in South Africa. Tuberculosis. Elsevier Ltd; 2013;93: 357–362. [DOI] [PubMed] [Google Scholar]
- 28.Mahomed H, Ehrlich R, Hawkridge T, Hatherill M, Geiter L, Kafaar F, et al. TB Incidence in an Adolescent Cohort in South Africa. Ruhwald M, editor. PLoS ONE. 2013;8: e59652–7. 10.1371/journal.pone.0059652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waako J, Verver S, Wajja A, Ssengooba W, Joloba ML, Colebunders R, et al. Burden of tuberculosis disease among adolescents in a rural cohort in Eastern Uganda. BMC Infect Dis. 2013;13: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nduba V, Hoog AHV, Mitchell E, Onyango P, Laserson K, Borgdorff M. Prevalence of tuberculosis in adolescents, western Kenya: implications for control programs. International Journal of Infectious Diseases. International Society for Infectious Diseases; 2015;35: 11–17. [DOI] [PubMed] [Google Scholar]
- 31.Ogbudebe CL, Chukwu JN, Nwafor CC, Meka AO, Ekeke N, Madichie NO, et al. Reaching the underserved: Active tuberculosis case finding in urban slums in southeastern Nigeria. International Journal of Mycobacteriology. Asian-African Society for Mycobacteriology; 2015;4: 18–24. [DOI] [PubMed] [Google Scholar]
- 32.Sekandi JN, Neuhauser D, Whalen CC. Active case finding of undetected tuberculosis among chronic coughers in a slum setting in Kampala, Uganda. int j tuberc lung dis. 2009;13: 508–513. [PMC free article] [PubMed] [Google Scholar]
- 33.Fatima R, Qadeer E, Enarson DA, Creswell J, Stevens R, Hinderaker SG, et al. Success of active tuberculosis case detection among high-risk groups in urban slums in Pakistan. int j tuberc lung dis. 2014;18: 1099–1104. 10.5588/ijtld.14.0001 [DOI] [PubMed] [Google Scholar]
- 34.Banu S, Rahman MT, Uddin MKM, Khatun R, Ahmed T, Rahman MM, et al. Epidemiology of Tuberculosis in an Urban Slum of Dhaka City, Bangladesh. Wilkinson KA, editor. PLoS ONE. Public Library of Science; 2013;8: e77721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorent N, Choun K, Thai S, Kim T, Huy S, Pe R, et al. Community-Based Active Tuberculosis Case Finding in Poor Urban Settlements of Phnom Penh, Cambodia: A Feasible and Effective Strategy. Hoshino Y, editor. PLoS ONE. 2014;9: e92754–12. 10.1371/journal.pone.0092754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad BM, Satyanarayana S, Chadha SS, Das A, Thapa B, Mohanty S, et al. Experience of active tuberculosis case finding in nearly 5 million households in India. public health action. 2016;6: 15–18. 10.5588/pha.15.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tollefson D, Bloss E, Fanning A, Redd JT, Barker K, McCray E. Burden of tuberculosis in indigenous peoples globally: a systematic review [Review article]. int j tuberc lung dis. 2013;17: 1139–1150. 10.5588/ijtld.12.0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romualdez AG Jr, Rosa dela JFE, Flavier JDA, Quimbo SLA, Hartigan-Go KY, Lagrada LP, et al. The Philippines Health System Review [Internet]. Health Systems in Transition. 2011. Available: http://www.wpro.who.int/philippines/areas/health_systems/financing/philippines_health_system_review.pdf [Google Scholar]
- 39.Yadav RP, Nishikiori N, Satha P, Eang MT, Lubell Y. Cost-effectiveness of a tuberculosis active case finding program targeting household and neighborhood contacts in Cambodia. Am J Trop Med Hyg. American Society of Tropical Medicine and Hygiene; 2014;90: 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mupere E, Schiltz NK, Mulogo E, Katamba A, Nabbuye-Sekandi J, Singer ME. Effectiveness of active case-finding strategies in tuberculosis control in Kampala, Uganda. int j tuberc lung dis. 2013;17: 207–213. 10.5588/ijtld.12.0160 [DOI] [PubMed] [Google Scholar]
- 41.Blok L, Creswell J, Stevens R, Brouwer M, Ramis O, Weil O, et al. A pragmatic approach to measuring, monitoring and evaluating interventions for improved tuberculosis case detection. International Health. Oxford University Press; 2014;6: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morishita F, Eang MT, Nishikiori N, Yadav RP. Increased Case Notification through Active Case Finding of Tuberculosis among Household and Neighbourhood Contacts in Cambodia. Hozbor DF, editor. PLoS ONE. 2016;11: e0150405–14. 10.1371/journal.pone.0150405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van 't Hoog A, Langendam MW, Mitchell E, Cobelens FG, Sinclair D, Leeflang M, et al. A systemat- ic review of the sensitivity and specificity of symptom- and chest-radiography screening for active pul- monary tuberculosis in HIV-negative persons and persons with unknown HIV status [Internet]. 2013. Available: http://www.who.int/tb/Review2Accuracyofscreeningtests.pdf
- 44.Nishikiori N, Van Weezenbeek C. Target prioritization and strategy selection for active case-finding of pulmonary tuberculosis: a tool to support country-level project planning. BMC Public Health. 2013;13: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.KNCV Tuberculosis Foundation. Working document on chest X-ray equipment for use in TB prevalence surveys [Internet]. 2008 Sep. Available: http://www.who.int/tb/advisory_bodies/impact_measurement_taskforce/meetings/prevalence_survey/chest_x_ray_eqpt.pdf
- 46.Colebunders R, Bastian I. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. int j tuberc lung dis. 2000;4: 97–107. [PubMed] [Google Scholar]
- 47.Cavanaugh JS, Shah NS, Cain KP, Winston CA. Survival among Patients with HIV Infection and Smear-Negative Pulmonary Tuberculosis—United States, 1993–2006. Cattamanchi A, editor. PLoS ONE. 2012;7: e47855–7. 10.1371/journal.pone.0047855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cuevas LE, Al-Sonboli N, Lawson L, Yassin MA, Arbide I, Al-Aghbari N, et al. LED fluorescence microscopy for the diagnosis of pulmonary tuberculosis: a multi-country cross-sectional evaluation. Wilson D, editor. PLoS Med. Public Library of Science; 2011;8: e1001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boon den S, Verver S, Lombard CJ, Bateman ED, Irusen EM, Enarson DA, et al. Comparison of symptoms and treatment outcomes between actively and passively detected tuberculosis cases: the additional value of active case finding. Epidemiol Infect. 2008;136: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kranzer K, Afnan-Holmes H, Tomlin K, Golub JE, Shapiro AE, Schaap A, et al. The benefits to communities and individuals of screening for active tuberculosis disease: a systematic review [State of the art series. Case finding/screening. Number 2 in the series]. int j tuberc lung dis. 2013;17: 432–446. 10.5588/ijtld.12.0743 [DOI] [PubMed] [Google Scholar]
- 51.United Nations. Transforming our world: the 2030 agenda for sustainable development. 2015.
- 52.Morishita F, Yadav RP, Eang MT, Saint S, Nishikiori N. Mitigating Financial Burden of Tuberculosis through Active Case Finding Targeting Household and Neighbourhood Contacts in Cambodia. Lubell Y, editor. PLoS ONE. Public Library of Science; 2016;11: e0162796–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.