Abstract
Pattern recognition by Toll-like receptors (TLRs) is known to be important for the induction of dendritic cell (DC) maturation. DCs, in turn, are critically important in the initiation of T cell responses. However, most viruses do not infect DCs. This recognition system poses a biological problem in ensuring that most viral infections be detected by pattern recognition receptors. Furthermore, it is unknown what, if any, is the contribution of TLRs expressed by cells that are infected by a virus, versus TLRs expressed by DCs, in the initiation of antiviral adaptive immunity. Here we address these issues using a physiologically relevant model of mucosal infection with herpes simplex virus type 2. We demonstrate that innate immune recognition of viral infection occurs in two distinct stages, one at the level of the infected epithelial cells and the other at the level of the noninfected DCs. Importantly, both TLR-mediated recognition events are required for the induction of effector T cells. Our results demonstrate that virally infected tissues instruct DCs to initiate the appropriate class of effector T cell responses and reveal the critical importance of the stromal cells in detecting infectious agents through their own pattern recognition receptors.
Keywords: mucosal immunity, pattern recognition, viral infection
Dendritic cells (DCs) represent the most effective antigen (Ag)-presenting cells (APCs) capable of inducing robust CD4+ and CD8+ T cell immunity in vivo (1). One of the major pathways of DC activation and maturation involves the recognition of conserved molecular patterns, collectively known as pathogen-associated molecular pattern (PAMP), by means of the Toll-like receptors (TLRs) (2). A critical requirement for TLR-mediated recognition of pathogens by DCs in T helper (Th) 1 induction was demonstrated in mice lacking the MyD88 molecule (3-6), an adapter protein involved in most TLR signaling. Although the recognition of patterns associated with bacterial, fungal, and parasitic pathogens has been described to occur through binding to distinct TLRs (7), the mechanism of viral recognition is also beginning to be revealed. Both respiratory syncytial virus and mouse mammary tumor virus induce activation of TLR4 (8-11), whereas measles viruses and human cytomegalovirus were reported to trigger TLR2 activation and stimulate induction of proinflammatory cytokines such as IL-6 (12, 13). A recent report showed that a strain of herpes simplex virus (HSV)-1, KOS, activates TLR2 and causes herpes encephalitis in mice (14). The double-stranded RNA isolated from reovirus is recognized by TLR3 (15). The plasmacytoid DCs (16) and other types of DCs (17) recognize HSV-1 and HSV-2 by means of the TLR9, likely through the recognition of the CpG motifs highly present in the double-stranded genomes of these viruses (18), whereas single-stranded RNA viruses are detected by TLR7 (19-21). Based on these findings, we hypothesized that TLR-mediated signals are important in the induction of Th1 responses by DCs after HSV infection.
Although the importance of DCs in immune generation is well accepted, the types of activation signals DCs need to receive to induce antimicrobial immune effector cells are assumed to come from direct recognition of PAMPs during infection. This recognition system raises a problem in detecting strictly intracellular pathogens such as viruses. Moreover, most viruses infect and replicate in cells other than DCs. To address how the mammalian immune system deals with viruses that predominantly infect stromal cells, we used an in vivo model of mucosal viral infection. Using a mouse model of genital herpes infection (22), we have previously demonstrated that vaginal infection with thymidine kinase-deficient HSV-2 resulted in a rapid recruitment of the CD11b+ DCs to the submucosa just beneath the infected epithelium, followed by the subsequent appearance of the CD11b+ DCs in the draining lymph nodes presenting viral peptides to CD4+ T cells (23). Virus replication occurred within the vaginal keratinocytes, and no virions were detected in the draining lymph nodes or within the Ag-presenting DC populations. The DC-T cell interaction led to the secretion of IFN-γ by CD4+ T cells (23). IFN-γ secreted from CD4+ T cells mediates protective immunity against subsequent challenges with virulent WT HSV-2 (24, 25). Using this viral infection model, we demonstrate the critical importance of viral recognition by both the infected stromal cells and uninfected Ag-presenting DCs in the generation of antiviral immunity.
Methods
Virus. The thymidine kinase mutant HSV-2 strain 186TKΔKpn was constructed as described in ref. 26 and propagated and assayed on Vero cells (27). All stocks were titered on the Vero cell line before use. Virus-infected Vero cell lysate was heat-inactivated at 56°C for 30 min and used for stimulation of HSV-2-specific CD4+ T cells by splenic APCs.
Animals and HSV-2 Infection. Female MyD88-/- (28) and TLR9-/- (29) mice were previously described and were gifts from S. Akira (Osaka University, Osaka). ICE-/- mice were a gift from R. A. Flavell (Yale University). IL-12 p40-/- mice were obtained from the Taconic Farms/National Institute of Allergy and Infectious Diseases mouse repository. IFN-γR-/- mice were originally described by Aguet and colleagues (30) and were generously provided by H. Virgin (Washington University, St. Louis). WT control littermates (F2 generations from 129/svJ × C57/BL6 or BALB/c mice) were purchased from The Jackson Laboratory. For virus infection studies, mice were injected s.c. in the neck ruff with Depo Provera (Pharmacia & Upjohn) at 2 mg per mouse in a 100-μl volume 5-7 days before infection, swabbed with calcium alginate, and inoculated intravaginally (ivag) with either 1 × 107 plaque-forming units of HSV-2 strain 186TKΔKpn or inoculated with noninfected Vero cell lysate (mock infection) in a 10-μl volume by using a blunt-ended micropipette tip. All procedures used in this study complied with federal guidelines and institutional policies by the Yale University animal care and use committee.
Abs. The following Abs were used for the identification of cell populations: anti-CD11c (HL3), anti-CD11b (M1/70), anti-CD8α (53-6.7), and anti-CD86 (GL1). These Abs were purchased from BD Bioscience. To identify viral presence on tissue sections, FITC-conjugated Ab against HSV (ViroStat, Portland, ME) was used.
Immunofluorescence Staining. To examine the DC recruitment to the vagina epithelium, frozen sections of vagina were stained with the specific Abs in a procedure similar to that described in ref. 23.
Preparation of DCs. DCs were prepared from the draining lymph nodes of ivag HSV-2-infected mice as described (23). Briefly, draining lymph nodes (inguinal and iliac) were excised from infected mice at 4 d postinfection (p.i.). Lymph nodes were digested with collagenase D and DNase I and incubated in the presence of 5 mM EDTA at 37°C for 5 min. A single-cell suspension was prepared, and cells were incubated with anti-mouse CD11c-coated magnetic beads (Miltenyi Biotec, Auburn, CA) and selected on magnetic-activated cell sorting separation columns.
Isolation of CD4+ T Cells. Draining lymph nodes were excised from infected mice at 4 d p.i.Single-cell suspensions of lymph node cells were made by dissociating cells through a cell strainer. Cells were washed twice with PBS and selected with magnetic beads conjugated to anti-CD4 Ab (Miltenyi Biotec) according to the manufacturer's instructions.
Stimulation of HSV-2-Specific CD4+ T Cells by DC or APC. To determine the effector functions of the Th cells, CD4+ T cells were stimulated for 72 h in vitro with irradiated syngeneic splenocytes as APCs in the presence of heat-inactivated virus Ags as described (23). To determine the ability of the DCs to stimulate HSV-2-specific T cells, 105 CD4+ T cells from draining lymph nodes of WT mice infected ivag with HSV-2 for 4 d were cocultured with 5 × 104 DCs for 72 h in vitro in the absence of exogenously added Ags as described (23).
RT-PCR Analysis of TLR Expression by Vaginal Epithelial Layer. Vagina was excised from naïve WT mice. A sheet of vagina was prepared by cutting longitudinally near the urethra. Excess fat, blood vessels, and urethra were removed with forceps. The vaginal sheet was placed with epithelium side down in 10 ml of buffer containing 0.5 M NH4SCN, 0.5 M Na2HPO4, and 0.5 M K2HPO4 and incubated at 37°C for 20 min according to an established method (31). The epithelium sheet was gently separated from lamina propria with forceps and washed with PBS. Total RNA was isolated from the vaginal epithelial layer by RNeasy (Qiagen, Valencia, CA). Single-stranded cDNA was synthesized by using the SuperScript preamplification system (Invitrogen). PCR was conducted for 35 cycles by using primer pairs specific for each TLR (Table 1). All primer sets produced PCR products of expected sizes and have been independently confirmed to be specific for the intended TLRs.
Table 1. Sequence for TLR-specific primers.
| Primer sequences
|
||
|---|---|---|
| TLR | Forward | Reverse |
| TLR1 | 5′-CCAAACGCAAACCTTACCAGA-3′ | 5′-CCAGGAACAGTAGTTGGGAC-3′ |
| TLR2 | 5′-CTCTGTCATGTGATGCTTCTG-3′ | 5′-ATGTTACCCCCAGTGTCTGG-3′ |
| TLR3 | 5′-ACAACGTAGCTGACTGCAGC-3′ | 5′-GAGTTCTGTCAGGTTCGTGC-3′ |
| TLR4 | 5′-CCCTGATGACATTCCTTCTT-3′ | 5′-GATCCACATGTACTAGGTTCG-3′ |
| TLR5 | 5′-GCTCCTGCTCAGCTTCAACT-3′ | 5′-ATGGAGGCGGAGGCTGTGAA-3′ |
| TLR6 | 5′-CGCCCTGGCCTTAATAGTCG-3′ | 5′-CCAAATTCCTTACACACAGGC-3′ |
| TLR7 | 5′-CGATGTGGACACGGAAGAGA-3′ | 5′-CTGCAGCCTCTTGGTACACA-3′ |
| TLR8 | 5′-TGTCTGCTGTCCTCTGGAAC-3′ | 5′-GGCAACCCAGCAGGTATAGT-3′ |
| TLR9 | 5′-CCCTCCTGGTACAGGCTGC-3′ | 5′-TCTTGTAGTAGCAGTTCCCG-3′ |
Construction of Bone Marrow (BM) Chimeric Mice. BM chimeric mice were constructed by a standard method described in ref. 32. Briefly, MyD88-/- mice and WT mice were lethally irradiated with 9 Gy of total-body irradiation. BM was obtained from the femur and tibia of donor mice and collected in RPMI medium 1640 containing 1% penicillin/streptomycin, 0.1% 2-mercaptoethanol, and 10 mM Hepes. The cells were subsequently washed twice, counted, and resuspended in sterile PBS at a concentration of 5.0 × 107 cells per ml. Irradiated recipient mice were reconstituted with 1.0 × 107 cells of the appropriate cell suspension by means of tail vein injection. The reconstituted mice were maintained in a clean facility for at least 8 weeks to allow for complete engraftment with donor BM. To avoid death of the irradiated mice by bacterial infection, recipient mice were treated with antibiotics before and after the irradiation until complete reconstitution by the donor BM-derived cells had taken place. To assay BM reconstitution, lymphocytes were harvested from the spleen of these BM chimeric mice, and genomic DNA isolates were prepared. The presence or absence of the neomycin resistance gene was determined by PCR.
Results
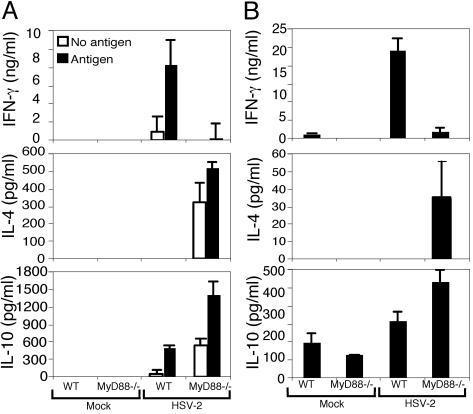
MyD88 Is Required for Th1 Induction After HSV Infection. To determine the importance of TLR-mediated viral recognition in Th1 generation, we first examined whether immune responses are generated in mice deficient for MyD88 after HSV-2 infection. CD4+ T cells were isolated from the draining lymph nodes of WT or MyD88-/- mice infected with HSV-2 for 4 d and restimulated in vitro with irradiated WT syngeneic splenocytes. In the presence of HSV-2 Ag, CD4+ T cells from the WT mice secreted high levels of IFN-γ but undetectable levels of IL-4 at this time point (Fig. 1A). In contrast, CD4+ T cells from the draining lymph nodes of HSV-2-infected MyD88-/- mice secreted minimal IFN-γ. Instead, these cells secreted higher levels of IL-4 and IL-10 (Fig. 1A). Therefore, CD4+ T cells generated in the absence of MyD88 were skewed toward a Th2 phenotype and failed to secrete IFN-γ in response to viral Ag stimulation. Some secretion of IL-4 and IL-10 was observed from CD4+ T cells of the infected MyD88-/- mice even in the absence of HSV-2 Ags (Fig. 1A), consistent with the spontaneous secretion of Th2 cytokines observed in these mice (3).
Fig. 1.
MyD88 is required for Th1 induction after ivag HSV-2 infection. (A) CD4+ T cells isolated from the draining lymph nodes of WT or MyD88-/- mice infected ivag with HSV-2 for 4 d or inoculated with mock control cell lysate were cocultured with irradiated syngeneic splenocytes in the presence (filled bars) or absence (open bars) of HSV-2 Ags, and supernatants were analyzed for cytokines. (B) CD11c+ DCs isolated from draining lymph nodes of WT or MyD88-/- mice infected ivag with HSV-2 for 4 d or mock-infected controls were cocultured with HSV-2-primed WT CD4+ T cells in the absence of exogenous Ags, and supernatants were analyzed for cytokines. Data are representative of nine separate experiments producing similar results.
We hypothesized that the failure of the MyD88-/- mice to generate Th1 responses was caused by the defect in the function of DCs (3). Thus, we examined the requirement for intact TLR signaling pathways in DCs in the induction of Th1 responses. To this end, DCs were isolated from the draining lymph nodes of WT or MyD88-/- mice infected ivag with HSV-2 at 4 d p.i. Purified DCs were cocultured with HSV-2-primed WT CD4+ T cells in the absence of exogenously added viral Ags to examine the presentation of viral peptides acquired in vivo by these cells (23). DCs isolated from infected WT mice induced CD4+ T cells to secrete high levels of IFN-γ but not IL-4 (Fig. 1B). In contrast, despite their ability to secrete high levels of IFN-γ after in vitro stimulation with irradiated splenocytes (Fig. 1A), HSV-primed WT CD4+ T cells were induced to secrete IL-4, but not IFN-γ, upon incubation with DCs isolated from the infected MyD88-/- mice (Fig. 1B). Thus, in the absence of MyD88 signal pathways, DCs had acquired the ability to induce pro-Th1 cells to instead secrete only Th2 cytokines, and the failure of MyD88-/- mice to mount effective Th1 responses after HSV-2 infection reflects the defect in the DC function.
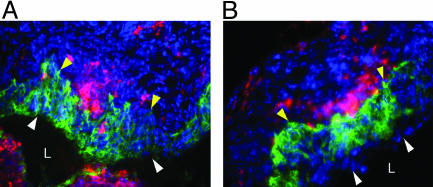
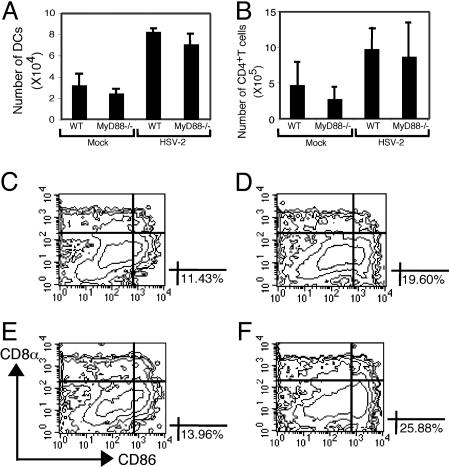
Migration of DCs Occurs in the Absence of MyD88. To further dissect the mechanism of the DC defect in MyD88-/- mice in response to HSV-2 infection, we examined the migration of the DCs. After encountering pathogens in the peripheral tissues, DCs undergo a maturation program that leads to their migration into secondary lymphoid tissues. This migration is accompanied by the increase in the expression of MHC and costimulatory molecules and the production of cytokines leading to their enhanced ability to prime naïve T cells. Neither the recruitment of DCs to the genital mucosa (Fig. 2) nor their subsequent migration to the draining lymph nodes (Fig. 3A) was impaired in the MyD88-/- mice. Consequently, in vivo expansion of CD4+ T cell numbers were comparable between MyD88-/- and WT mice (Fig. 3B). These data suggested that chemotactic signals that direct the migration of DCs to the sites of infection and the subsequent migration of Ag-loaded DCs to the draining lymph nodes were intact in MyD88-/- mice after HSV-2 infection.
Fig. 2.
DC recruitment in MyD88-/- mice after ivag HSV-2 infection. Vaginal tissues were collected from WT (A) and MyD88-/- (B) mice at 24 h p.i. and stained for DCs (CD11c; red) and the virus (HSV-2; green). The nuclei were visualized by DAPI staining (blue). The epithelial layers are indicated by the white (luminal edge) and yellow (basement membrane) arrowheads. L, lumen.
Fig. 3.
DC migration and activation in MyD88-/- mice after ivag HSV-2 infection. (A and B) CD11c+ DCs and CD4+ T cells were isolated from the draining lymph nodes of 4-d-infected or mock-infected WT or MyD88-/- mice, and the average numbers of DCs (A) and CD4+ T cells (B) per draining lymph node were measured. (C-F) CD11c+ DCs were isolated from the draining lymph nodes of WT (C and D) or MyD88-/- (E and F) mice infected ivag with HSV-2 at 3 d p.i. (D and F) or mock-infected (C and E). The expression profiles of CD8α and CD86 of the B220-/CD11c+ DCs are depicted as contour plots. Percentages refer to the CD8α-/B220-/CD11c+/CD86hi populations in the lower right quadrant. Data are representative of five separate experiments producing similar results.
DC Maturation in the MyD88-/- Mice. Immature DCs express low levels of CD80, CD86, and MHC class II and are inefficient in activating naïve T cells. Activation of DCs is thought to occur by recognition of PAMPs by means of the pattern recognition receptors (PRRs). Thus, we next examined the activation status of DCs after ivag HSV-2 infection. The CD11b+/CD11c+/B220-/CD8α- DC population, which has been shown to mediate Th1 induction after HSV-2 infection (23), was found to express high levels of CD86 in the infected MyD88-/- mice (25.88%) (Fig. 3F), similar in frequency and number to the infected WT mice (19.60%) (Fig. 3D). Furthermore, up-regulation of other costimulatory molecules occurred normally in the relevant DC population in the MyD88-/- mice (data not shown). Thus, neither the migratory ability nor the maturation as evidenced by the surface expression of activation markers on the Ag-presenting DCs was affected by the lack of MyD88.
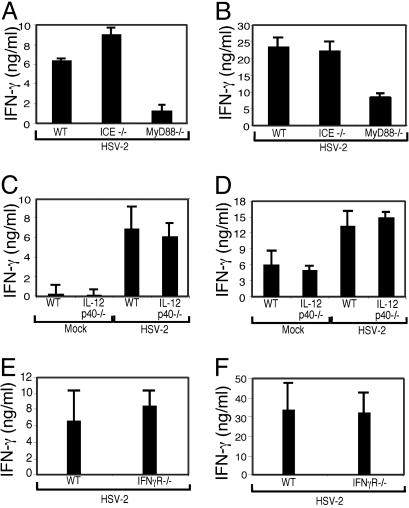
Intact Th1 Response in the Absence of Caspase-1, IL-12 or IFN-γR. Next, we examined potential MyD88-dependent mediators of Th1 differentiation after HSV infection. In addition to TLR signaling, MyD88 is involved in IL-1 and IL-18 signaling (28). Biologically active forms of IL-1β and IL-18 require the protease caspase-1 for their processing (33). Therefore, we used IL-1 β-converting enzyme knockout (ICE-/-) mice to examine whether the observations made in the MyD88-/- mice can be explained by the action of these two cytokines. CD4+ T cells isolated from HSV-2-infected ICE-/- mice produced IFN-γ normally (Fig. 4A), and DCs isolated from these mice also induced HSV-primed WT CD4+ T cells to secrete normal levels of IFN-γ (Fig. 4B). Therefore, the lack of Th1 responses in the MyD88-/- mice could not be attributed to the deficiencies in the cytokines IL-1β and IL-18 and suggested the involvement of TLRs in this process.
Fig. 4.
Th1 responses are intact in mice deficient for caspase-1, IL-12 p40, or IFN-γR. CD4+ T cells isolated from the draining lymph nodes of WT, ICE-/- (A), IL-12 p40-/- (C), or IFN-γR-/- (E) mice infected ivag with HSV-2 at 4 d p.i. were cocultured with irradiated syngeneic splenocytes in the presence of HSV-2 Ags, and supernatants were analyzed for IFN-γ secretion. DCs isolated from the draining lymph nodes of WT, ICE-/- (B), IL-12 p40-/- (D), or IFN-γR-/- (F) mice were cocultured with CD4+ T cells from d-4 HSV-2-primed WT mice in the absence of exogenous Ags, and supernatants were analyzed for IFN-γ. Data are representative of two separate experiments with similar results.
One of the key mediators of Th1 differentiation is IL-12, a cytokine secreted by DC in response to microbial stimuli (1). DCs have been shown to secrete IL-12 in response to HSV-2 in a TLR9-dependent manner (17). Thus, the failure of MyD88-deficient DCs to generate a Th1 response could be caused by their inability to secrete IL-12. To address whether IL-12 production by DCs is required for Th1 induction after HSV-2 infection, we examined T cell responses in the IL-12 p40-/- mice. Unlike the MyD88-/- mice, CD4+ T cells isolated from HSV-2-infected IL-12 p40-/- mice were found to secrete WT levels of IFN-γ upon stimulation with HSV-2 Ags (Fig. 4C). DCs isolated from IL-12 p40-/- mice were also found to induce HSV-primed WT CD4+ T cells to secrete IFN-γ at levels similar to WT DCs in vitro (Fig. 4D).
Previous studies have shown that IFN-γ can stabilize Th1 responses (34). To address whether IFN-γ is important in the Th1 differentiation after HSV-2 infection, we examined the abilities of DCs that are themselves unresponsive to IFN-γ in stimulating IFN-γ secretion from WT CD4+ T cells. The DCs isolated from the draining lymph nodes of IFN-γR-/- mice were found to induce IFN-γ secretion from WT HSV-2 specific CD4+ T cells at a level comparable to those from WT mice (Fig. 4F). Consequently, WT levels of Th1 responses were generated in HSV-2-infected IFN-γR-/- mice (Fig. 4E). Thus, neither IL-12 nor IFN-γR was required for the generation of IFN-γ-secreting CD4+ T cells after ivag HSV-2 infection.
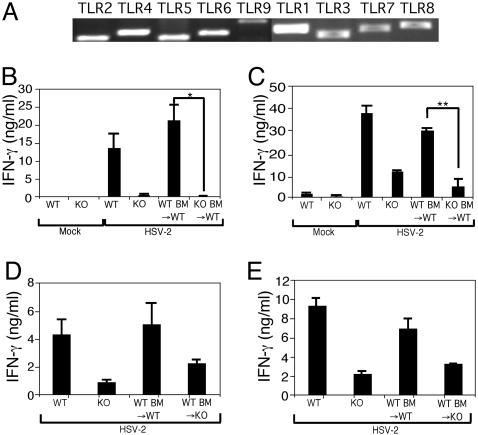
TLR Recognition of Viral Infection by Hematopoietic and Stromal Cells. Thus far, our results indicated that DCs are programmed during infection to induce Th1 responses in a MyD88-dependent manner. However, DCs that present viral Ags to CD4+ T cells in the draining lymph nodes are not themselves infected (23). On the other hand, the first cells with which the HSV-2 virions come in contact and establish infection are the vaginal epithelial cells. Previous studies have demonstrated that certain TLRs are expressed by epithelial cells lining various body cavities (35-38), and pathogen recognition by the stromal cells can result in inflammatory responses after bacterial stimulation (39, 40). We hypothesized that viral recognition by stromal cells might contribute to the development of the adaptive immune responses. To examine whether TLRs are expressed by the vaginal epithelial layer, RT-PCR analysis of the mRNA expression for TLR1 through TLR9 was conducted. Cells within the vaginal epithelial layer were found to express all TLRs examined (Fig. 5A). Considering the facts that (i) the vaginal epithelial layer provides the first line of defense against HSV-2, (ii) keratinocytes are the primary target of this virus infection, and (iii) cells within this layer express TLR1 through TLR9, we hypothesized that TLR-mediated viral recognition by the vaginal epithelial cells could play a role in the development of the Th1 responses against HSV-2 infection.
Fig. 5.
Th1 generation requires MyD88 in both hematopoietic and stromal compartments. (A) Total RNA was prepared from vaginal epithelium of naïve WT mice and analyzed for the mRNA expression of TLR1 through TLR9 by RT-PCR. (B and D) CD4+ T cells from the draining lymph nodes of mice infected ivag with HSV-2 for 4 d were cocultured with irradiated syngeneic WT splenocytes in the presence of HSV-2 Ags, and supernatants were analyzed for IFN-γ. (C and E) DCs isolated from draining lymph nodes of mice at 4 d p.i. were cocultured with HSV-2-specific WT CD4+ T cells in the absence of exogenously added Ags, and supernatants were analyzed for IFN-γ secretion.*, P < 0.05;**, P < 0.01. Data are representative of three separate experiments producing similar results.
To address the relative contributions of TLR-mediated viral recognition in Th1 immunity by DCs and vaginal epithelial cells in vivo, we constructed a set of BM chimeric mice. In irradiated WT mice reconstituted with MyD88-/- BM cells [knockout (KO) BM→WT], the professional APCs such as DCs lack the MyD88 molecule, whereas stromal cells such as the vaginal keratinocytes express intact TLR pathways. HSV-2 infection of MyD88-/- BM→WT mice resulted in CD4+ T cells that failed to secrete IFN-γ in response to antigenic stimulation (Fig. 5B). In contrast, CD4+ T cells from WT BM→WT mice secreted high levels of IFN-γ, comparable to WT mice, eliminating the possibility that the process of constructing BM chimera, such as irradiation, negatively affected IFN-γ secretion from CD4+ T cells. DCs isolated from the infected MyD88-/- BM→WT mice failed to induce CD4+ T cells to secrete IFN-γ, similar to those isolated from the MyD88-/- mice (Fig. 5C). Because lethal irradiation does not induce reconstitution of skin-resident Langerhans cell precursor population (41), these results suggested that MyD88 responsiveness is required for BM-derived DCs to prime Th1 cells and that TLR-mediated viral recognition by nonhematopoietic cells or Langerhans cells of the host origin is not sufficient for this process.
Next, the role of the stromal cells in the induction of antiviral CD4+ T cell responses was examined. To this end, we constructed irradiation-induced chimeric mice in which the BM from WT mice were used to reconstitute lethally irradiated MyD88-/- mice (WT BM→KO). To our surprise, CD4+ T cells from the HSV-infected WT BM→MyD88-/- mice secreted reduced levels of IFN-γ compared with those of WT mice (Fig. 5D). Moreover, DCs isolated from the infected WT BM→MyD88-/- mice had a significantly reduced ability to induce WT CD4+ T cells to secrete IFN-γ compared with the WT counterpart (Fig. 5E). These results revealed that TLR-mediated recognition of HSV-2 infection by the tissue stromal cells is a critical step in the generation of adaptive immunity and that, in the absence of this recognition, DCs fail to generate robust Th1 responses in vivo.
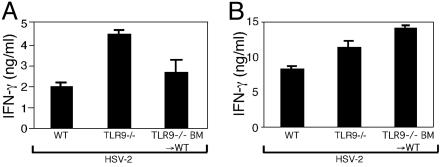
TLR9 Is Dispensable for Th1 Generation. To further characterize the mechanism responsible for these observations, we used BM chimeric mice lacking TLR9 in specific compartments. Various types of DCs (16, 17) have been shown to recognize HSV by means of TLR9, likely through the recognition of the CpG motifs highly present in the double-stranded genomes of these viruses (18). Because TLR9 is required for HSV recognition by DCs, we specifically tested the importance of TLR9-mediated HSV-2 recognition by DCs in Th1 induction. Unlike similar experiments conducted with the MyD88-/- mice, the TLR9-/- BM→WT mice possessed DCs that stimulated CD4+ T cells to secrete high levels of IFN-γ in the draining lymph nodes (Fig. 6B). Consequently, these infected mice generated normal Th1 responses in vivo (Fig. 6A). Similarly, mice lacking TLR9 in all compartments (TLR9-/-) generated WT levels of Th1 responses in vivo (Fig. 6). A recent report indicated that a strain of HSV-1, KOS, activates TLR2 and causes neuropathology in mice (14). To examine whether other TLRs mediate HSV-2 recognition and initiate Th1 generation, we examined mice deficient in TLR2, TLR3, or TLR4. These analyses revealed that mice deficient in TLR2, TLR3, or TLR4 were able to generate WT levels of Th1 responses after HSV-2 infection (data not shown). Thus, these results indicated that, although DCs require TLR9 for HSV recognition in vitro, TLR9 is dispensable for DCs to generate Th1 responses during in vivo viral infection. Similarly, single deficiency in TLR2, TLR3, or TLR4 does not recapitulate the phenotype of the MyD88-/- mice. Moreover, non-DCs can support DCs to become Th1 inducers in the absence of these receptors.
Fig. 6.
Th1 generation does not require TLR9. (A) CD4+ T cells from the draining lymph nodes of mice infected ivag with HSV-2 for 4 d were cocultured with irradiated syngeneic WT splenocytes in the presence of HSV-2 Ags, and supernatants were analyzed for IFN-γ. (B) DCs isolated from draining lymph nodes of mice at 4 d p.i. were cocultured with HSV-2-specific WT CD4+ T cells in the absence of exogenously added Ags, and supernatants were analyzed for IFN-γ secretion. Data are representative of two separate experiments with similar results.
Discussion
The ability of DCs to initiate adaptive immunity has become well established over the past decades (1). Through recognition of PAMPs, DCs link TLR-mediated innate immune detection of pathogens to the initiation of T cell activation (2). Consequently, investigation of the importance of pathogen recognition through TLRs by DCs in immune induction has become a major focus of study. However, most pathogens avoid directly infecting DCs, whereas the first cells to harbor viral replication are usually the mucosal epithelial cells lining various body cavities. Placing PRRs solely on DCs raises a problem for pathogen detection and induction of antimicrobial immunity.
To examine how the immune system combats such infectious agents, we used an in vivo model of mucosal viral infection. In this system, HSV-2 is introduced into the vaginal mucosa, where replication of the virus is confined within the keratinocytes (stromal cells), whereas BM-derived myeloid DCs exclusively mediate Th1 generation without themselves becoming infected (23). We first demonstrated that TLR signaling through MyD88 is required for the generation of Th1 responses after mucosal infection with HSV. These results are consistent with several other reports demonstrating the requirement of MyD88 in Th1 induction (3-5, 42). Furthermore, using BM chimeric mice, we provided evidence that MyD88-mediated stimulation of hematopoietic cells is required for the generation of Th1 cells. These data demonstrated an absolute requirement for TLR recognition of viral infection by DCs in Th1 generation and showed that the presence of MyD88+ epithelial cells and Langerhans cells (41) is not sufficient to induce CD4+ T cells to secrete high levels of IFN-γ. These findings were not surprising, based on a previous observation that Langerhans cells do not participate in CD4+ T cell priming after genital HSV-2 infection (23).
More importantly, we showed that MyD88-mediated viral recognition by the stromal cells was also required for the generating robust Th1 responses. In the absence of pattern recognition by the infected epithelial cells, DCs' ability to stimulate secretion of IFN-γ from HSV-specific T cells was significantly reduced. Collectively, these results revealed that, although required, direct recognition of PAMPs by DCs alone is not sufficient for the generation of full Th1 response after viral infection. Instead, DCs require signals from the stromal cells, and the provision of this signal depends on the detection of virus infection through PRRs on the stromal cells.
Our results revealed that innate immune recognition of viral infection occurs in two distinct stages, one at the level of the infected epithelial cells and the other at the level of the noninfected DCs. Although the precise mechanism of the two-stage recognition is yet to be elucidated, our current study provides several important insights. First, we demonstrated that multiple PAMPs are involved in this process. DCs are recruited just below the epithelium, where they could potentially make direct contact with the virus or virus-infected cells, which could stimulate TLRs expressed by DCs. One such PAMP is the CpG motifs present in the genome of HSV, which are recognized through TLR9 expressed by DCs (16, 17). However, our demonstration that TLR9-/- BM→WT mice generated full Th1 responses after HSV-2 infection suggests that DCs must possess PRRs other than TLR9 that can sense virus infection and that the HSV genome may be only one of the viral signatures generated during mucosal infection. A strain of HSV-1 has been shown to activate TLR2 (14). However, normal Th1 induction was observed in TLR2-deficient mice (data not shown), eliminating TLR2 as a sole PRR for HSV infection.
Second, we showed that Th1 differentiation by TLR-activated DCs is mediated by multiple factors. Thus, IL-1β, IL-18, IL-12, or IFN-γ does not singly account for the Th1-inducing ability of DCs. These data are consistent with previous studies demonstrating that Th1 responses to Toxoplasma gondii (6) and lipopolysaccharide (5) required MyD88 but not IL-12. It is possible that other Th1-promoting factors (43) such as IL-23, IL-27, and IL-18 compensate for the lack of IL-12 in the IL-12 p40-/- mice and that all of these factors are diminished in the absence of MyD88. Consistent with this is the observation that IFN-γ secretion from T cells required both IL-12 and IL-18 to be expressed by DCs upon lipopolysaccharide stimulation (5). Because IL-23 shares the p40 subunit, which is defective in the IL-12 p40-/- mice, our results indicated that MyD88 signals in DCs induce expression of molecules in addition to IL-12 and IL-23 that are required for Th1 generation.
Third, our data demonstrated that the MyD88-dependent contribution of the radioresistant stromal cells in Th1 generation was mediated through the activation of DCs. However, the stromal cell control of DC activation was not mediated at the level of DC recruitment. Thus, normal migration of DCs to the sites of infection occurred in the infected MyD88-/- mice. Furthermore, the absence of MyD88 did not alter either the appearance of submucosal DC subset in the draining lymph nodes or the maturation status of the Ag-presenting DC population. Thus, the stromal cell support of DCs does not rely on the secretion of chemokines that promote recruitment of the appropriate DC populations to the infected tissue or to the draining lymph nodes or on factors that induce up-regulation of costimulatory molecules on DCs. Because the cells of the vaginal epithelial layer expressed TLR1 through TLR9, the PAMPs responsible for activating these cells to support DC1 generation may involve multiple ligands generated during viral infection. Uninfected DCs ingest infected epithelial cells and acquire viral Ags. It is possible that DCs also acquire a virally induced PAMPs from the infected keratinocytes in this process. Future studies must focus on identifying the nature of the receptor ligand responsible for DC1 activation by the stromal cells.
Our data provide the first evidence for the critical role played by the stromal cells in TLR-mediated viral recognition in providing help to DCs in generating adaptive immune responses after a viral infection in vivo. We showed that TLR signaling of DCs is required, but not sufficient, for inducing Th1 responses and that TLR signaling of the radio-resistant cells such as the vaginal epithelial cells is critical for the activation of DCs to promote Th1 generation. These results reveal the importance of the interaction and cooperation between DCs and peripheral tissue stromal cells in the generation of optimal adaptive immunity and show that pattern recognition is mediated in two distinct stages, one involving pattern recognition within the infected tissue stroma and the other at the level of the noninfected DCs. This concept has critical implications on vaccine design and immunotherapeutic approaches involving DCs, because it illuminates the need to trigger activation of both the DCs and the tissue TLRs for optimal effector immunity.
Acknowledgments
We are grateful to R. Medzhitov for critical review of the manuscript and X. Y. Zhao for technical assistance. A.S. is supported by a James Hudson Brown-Alexander Brown Coxe postdoctoral fellowship. A.I. is a recipient of the Wyeth-Lederle Vaccine Young Investigator Award. This work was supported by National Institutes of Health Grant AI054395.
Author contributions: A.S. performed research; A.S. and A.I. analyzed data; A.S. and A.I. wrote the paper; and A.I. designed research.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Ag, antigen; APC, Ag-presenting cell; BM, bone marrow; DC, dendritic cell; HSV, herpes simplex virus; ivag, intravaginal(ly); KO, knockout; PAMP, pathogen-associated molecular pattern; p.i., postinfection; PRR, pattern recognition receptor; Th, T helper; TLR, Toll-like receptor.
See Commentary on page 16083.
References
- 1.Banchereau, J. & Steinman, R. M. (1998) Nature 392, 245-252. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov, R. (2001) Nat. Rev. Immunol. 1, 135-145. [DOI] [PubMed] [Google Scholar]
- 3.Schnare, M., Barton, G. M., Holt, A. C., Takeda, K., Akira, S. & Medzhitov, R. (2001) Nat. Immunol. 2, 947-950. [DOI] [PubMed] [Google Scholar]
- 4.Muraille, E., De Trez, C., Brait, M., De Baetselier, P., Leo, O. & Carlier, Y. (2003) J. Immunol. 170, 4237-4241. [DOI] [PubMed] [Google Scholar]
- 5.Kaisho, T., Hoshino, K., Iwabe, T., Takeuchi, O., Yasui, T. & Akira, S. (2002) Int. Immunol. 14, 695-700. [DOI] [PubMed] [Google Scholar]
- 6.Jankovic, D., Kullberg, M. C., Hieny, S., Caspar, P., Collazo, C. M. & Sher, A. (2002) Immunity 16, 429-439. [DOI] [PubMed] [Google Scholar]
- 7.Takeda, K., Kaisho, T. & Akira, S. (2003) Annu. Rev. Immunol. 21, 335-376. [DOI] [PubMed] [Google Scholar]
- 8.Kurt-Jones, E. A., Popova, L., Kwinn, L., Haynes, L. M., Jones, L. P., Tripp, R. A., Walsh, E. E., Freeman, M. W., Golenbock, D. T., Anderson, L. J. & Finberg, R. W. (2000) Nat. Immunol. 1, 398-401. [DOI] [PubMed] [Google Scholar]
- 9.Haynes, L. M., Moore, D. D., Kurt-Jones, E. A., Finberg, R. W., Anderson, L. J. & Tripp, R. A. (2001) J. Virol. 75, 10730-10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rassa, J. C., Meyers, J. L., Zhang, Y., Kudaravalli, R. & Ross, S. R. (2002) Proc. Natl. Acad. Sci. USA 99, 2281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jude, B. A., Pobezinskaya, Y., Bishop, J., Parke, S., Medzhitov, R. M., Chervonsky, A. V. & Golovkina, T. V. (2003) Nat. Immunol. 4, 573-578. [DOI] [PubMed] [Google Scholar]
- 12.Bieback, K., Lien, E., Klagge, I. M., Avota, E., Schneider-Schaulies, J., Duprex, W. P., Wagner, H., Kirschning, C. J., Ter Meulen, V. & Schneider-Schaulies, S. (2002) J. Virol. 76, 8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compton, T., Kurt-Jones, E. A., Boehme, K. W., Belko, J., Latz, E., Golenbock, D. T. & Finberg, R. W. (2003) J. Virol. 77, 4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurt-Jones, E. A., Chan, M., Zhou, S., Wang, J., Reed, G., Bronson, R., Arnold, M. M., Knipe, D. M. & Finberg, R. W. (2004) Proc. Natl. Acad. Sci. USA 101, 1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. (2001) Nature 413, 732-738. [DOI] [PubMed] [Google Scholar]
- 16.Lund, J., Sato, A., Akira, S., Medzhitov, R. & Iwasaki, A. (2003) J. Exp. Med. 198, 513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krug, A., Luker, G. D., Barchet, W., Leib, D. A., Akira, S. & Colonna, M. (2004) Blood 103, 1433-1437. [DOI] [PubMed] [Google Scholar]
- 18.Karlin, S., Doerfler, W. & Cardon, L. R. (1994) J. Virol. 68, 2889-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund, J. M., Alexopoulou, L., Sato, A., Karow, M., Adams, N. C., Gale, N. W., Iwasaki, A. & Flavell, R. A. (2004) Proc. Natl. Acad. Sci. USA 101, 5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heil, F., Hemmi, H., Hochrein, H., Ampenberger, F., Kirschning, C., Akira, S., Lipford, G., Wagner, H. & Bauer, S. (2004) Science 303, 1526-1529. [DOI] [PubMed] [Google Scholar]
- 21.Diebold, S. S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. (2004) Science 303, 1529-1531. [DOI] [PubMed] [Google Scholar]
- 22.Parr, M. B., Kepple, L., McDermott, M. R., Drew, M. D., Bozzola, J. J. & Parr, E. L. (1994) Lab. Invest. 70, 369-380. [PubMed] [Google Scholar]
- 23.Zhao, X., Deak, E., Soderberg, K., Linehan, M., Spezzano, D., Zhu, J., Knipe, D. M. & Iwasaki, A. (2003) J. Exp. Med. 197, 153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milligan, G. N. & Bernstein, D. I. (1997) Virology 229, 259-268. [DOI] [PubMed] [Google Scholar]
- 25.Milligan, G. N., Bernstein, D. I. & Bourne, N. (1998) J. Immunol. 160, 6093-6100. [PubMed] [Google Scholar]
- 26.Jones, C. A., Taylor, T. J. & Knipe, D. M. (2000) Virology 278, 137-150. [DOI] [PubMed] [Google Scholar]
- 27.Gao, M., Bouchey, J., Curtin, K. & Knipe, D. M. (1988) Virology 163, 319-329. [DOI] [PubMed] [Google Scholar]
- 28.Adachi, O., Kawai, T., Takeda, K., Matsumoto, M., Tsutsui, H., Sakagami, M., Nakanishi, K. & Akira, S. (1998) Immunity 9, 143-150. [DOI] [PubMed] [Google Scholar]
- 29.Hemmi, H., Takeuchi, O., Kawai, T., Kaisho, T., Sato, S., Sanjo, H., Matsumoto, M., Hoshino, K., Wagner, H., Takeda, K. & Akira, S. (2000) Nature 408, 740-745. [DOI] [PubMed] [Google Scholar]
- 30.Huang, S., Hendriks, W., Althage, A., Hemmi, S., Bluethmann, H., Kamijo, R., Vilcek, J., Zinkernagel, R. M. & Aguet, M. (1993) Science 259, 1742-1745. [DOI] [PubMed] [Google Scholar]
- 31.Tamaki, K., Fujiwara, H. & Katz, S. I. (1981) J. Invest. Dermatol. 76, 275-278. [DOI] [PubMed] [Google Scholar]
- 32.Iwasaki, A. & Barber, B. H. (1999) Methods in Molecular Medicine: DNA Vaccines: Methods and Protocols, eds. Lowrie, D. B. & Whalen R. (Humana, Totowa, NJ), pp. 133-144.
- 33.Fantuzzi, G. & Dinarello, C. A. (1999) J. Clin. Immunol. 19, 1-11. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, Y., Apilado, R., Coleman, J., Ben-Sasson, S., Tsang, S., Hu-Li, J., Paul, W. E. & Huang, H. (2001) J. Exp. Med. 194, 165-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cario, E., Rosenberg, I. M., Brandwein, S. L., Beck, P. L., Reinecker, H. C. & Podolsky, D. K. (2000) J. Immunol. 164, 966-972. [DOI] [PubMed] [Google Scholar]
- 36.Schilling, J. D., Mulvey, M. A., Vincent, C. D., Lorenz, R. G. & Hultgren, S. J. (2001) J. Immunol. 166, 1148-1155. [DOI] [PubMed] [Google Scholar]
- 37.Hedlund, M., Frendeus, B., Wachtler, C., Hang, L., Fischer, H. & Svanborg, C. (2001) Mol. Microbiol. 39, 542-552. [DOI] [PubMed] [Google Scholar]
- 38.Backhed, F., Soderhall, M., Ekman, P., Normark, S. & Richter-Dahlfors, A. (2001) Cell Microbiol. 3, 153-158. [DOI] [PubMed] [Google Scholar]
- 39.Kagnoff, M. F. & Eckmann, L. (1997) J. Clin. Invest. 100, 6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andonegui, G., Bonder, C. S., Green, F., Mullaly, S. C., Zbytnuik, L., Raharjo, E. & Kubes, P. (2003) J. Clin. Invest. 111, 1011-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merad, M., Manz, M. G., Karsunky, H., Wagers, A., Peters, W., Charo, I., Weissman, I. L., Cyster, J. G. & Engleman, E. G. (2002) Nat. Immunol. 3, 1135-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldstein, D. R., Tesar, B. M., Akira, S. & Lakkis, F. G. (2003) J. Clin. Invest. 111, 1571-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trinchieri, G. (2003) Nat. Rev. Immunol. 3, 133-146. [DOI] [PubMed] [Google Scholar]