Abstract
Rotavirus NSP5 is a nonstructural protein that localizes in viroplasms of virus-infected cells. NSP5 interacts with NSP2 and undergoes a complex posttranslational hyperphosphorylation, generating species with reduced PAGE mobility. Here we show that NSP5 operates as an autoregulator of its own phosphorylation as a consequence of two distinct activities of the protein: substrate and activator. We developed an in vivo hyperphosphorylation assay in which two NSP5 mutant constructs are cotransfected. One of them, fused to an 11-aa tag, served as substrate whereas the other was used to map NSP5 domains required for activation. The activation and substrate activity could be uncoupled, demonstrating a hyperphosphorylation process in trans between the activator and substratum. This process involved dimerization of the two components through the 18-aa C-terminal tail. Phosphorylation of Ser-67 within the SDSAS motif (amino acids 63-67) was required to trigger hyperphosphorylation by promoting the activation function. We present evidence of casein kinase 1α being the protein kinase responsible for this key step as well as for the consecutive ones leading to NSP5 hyperphosphorylation.
Rotavirus is a nonenveloped virus with a genome composed of 11 segments of double-stranded RNA. Within a few hours after infection (2-3 h), discrete cytoplasmic structures called viroplasms are formed (1). These structures contain the nonstructural proteins NSP2 and NSP5 together with other viral proteins, including the polymerase VP1, the guanyltransferase methylase VP3, and the main inner-core protein VP2 (2, 3). Viroplasms are considered the site of virus replication and where the initial steps of virus morphogenesis occur, in a process involving the concerted packaging and replication of the 11 positive polarity single-stranded RNAs, to generate the viral double-stranded RNA genomic segments (4-6).
NSP5, encoded by genomic segment 11, is a 198-aa protein with a high content of Ser and Thr, that is O-glycosylated and highly phosphorylated in infected cells (7-10). The hyperphosphorylation of NSP5 produces slower migration isoforms with apparent molecular masses ranging from 28 to 32-34 kDa, which are easily visualized in SDS/PAGE (9). NSP5 localizes to viroplasms of virus-infected cells where it interacts with other viral proteins like NSP2 (11, 12), VP1 (11), and VP2 (13). It has also been described that NSP5 can bind double-stranded RNA (14). The in vivo interaction with NSP2, which we recently demonstrated to involve the N- and C-terminal regions of NSP5 (15, 16), leads to the formation of viroplasms-like structures (17) and NSP5 hyperphosphorylation (11). This result was proposed to be the consequence of activation by NSP5 itself with involvement of a cellular kinase, in a process resembling autophosphorylation (18).
To determine whether NSP5 could mediate both processes, we performed a mutational analysis of NSP5. In this article, we describe the uncoupling of the two roles of NSP5, namely: to function as a substrate and an activator for its own phosphorylation. In addition, we describe a mechanism involving Ser-67 of NSP5, the phosphorylation of which is required for the hyperphosphorylation of this protein as a dimer. In vitro experiments with recombinant protein kinase casein kinase 1α (CK1α) demonstrate that this enzyme can carry out both the priming of Ser-67 and the subsequent hyperphosphorylation of NSP5.
Materials and Methods
Cells and Viruses and Abs. MA104 cells were cultured in DMEM supplemented with 10% FCS (Life Technologies, Grand Island, NY). Rotavirus porcine strain SA11 (serotype 3) was propagated and grown in MA104 cells as described (19). Guinea pig anti-NSP5 was obtained as described by Eichwald et al. (18), and monoclonal anti-SV5 was obtained as described by Hanke et al. (20).
In Vivo Hyperphosphorylation Assay. To prepare cellular extracts, cells were transfected essentially as described by Afrikanova et al. (11). In brief, 5 × 105 cells growing on a 35-mm diameter Petri dish were infected with T7 recombinant vaccinia virus (strain VTF7.3) (21) and 1 h later transfected with 5 μl of Transfectam reagent (Promega) containing 2 μg of total plasmid DNA (1 μg of substrate and 1 μg of activator). At 16 h after transfection, cells were washed twice with PBS, lysed in 60 μl of TNN lysis buffer (100 mM Tris·HCl, pH 8.0/250 mM NaCl/0.5% Nonidet P-40/1 mM PMSF) for 10 min at 4°C, and centrifugated at 10,000 × g for 5 min. The supernatants were loaded in SDS/PAGE (22) and transferred onto a poly(vinylidene difluoride) membrane (Immobilon-P, Millipore). A Western immunoblot was performed as described by Afrikanova et al. (9).
Plasmid Constructs. pT7v-NSP5, pT7v-Δ1, pT7v-Δ2, pT7v-Δ3, pT7v-Δ3/ΔT, pT7v-Δ4, pT7v-ΔT, pT7v-Δ1/Δ3, and pT7v-His-6-Δ1/Δ3 have been described (11, 15, 17, 18). Internal deletion mutants were obtained by PCR by using specific primers for the construction of pT7v-Δ3/ΔT, pT7v-Δ1/Δ3/ΔT, and pT7v-Δ1/ΔT and cloned as KpnI/BamHI fragments in pcDNA3 (Invitrogen). pT7v-Δ3a, pT7v-Δ3b, pT7v-Δ3/S63A, pT7v-Δ3/S65A, pT7v-Δ3/S67A, and pT7v-Δ3(S63,65A/S67D) were obtained by double-step PCR by using internal oligonucleotides to amplified regions 1 and 2 and cloned KpnI/ClaI in pT7v-(4TclaI5′). pT7v-(4TclaI5′) was obtained by PCR of the regions 4 and T, inserting the restriction sites KpnI and ClaI at the 5′ and BamHI at the 3′. The fragment was cloned as KpnI/BamHI in pcDNA3. pT7v-SV5Δ2 and pT7v-SV5Δ4 were obtained by inserting at the N terminus the SV5 tag with oligonucleotides, 5′-AGCTTGTACCATGGGCAAACCAATCCCAAACCCACTGCTGGGTCTGGATGGTAC-3′ and 5′-CATCCAGACCCAGCAGTGGGTTTGGGATTGGTTTGCCCATGGTACA-3′, and into HindIII/KpnI. pT7v-NSP5a, pT7v-NSP5/S57A, and pT7v-NSP5(S63,65A/S67D) were obtained by PCR of the respective pT7v-Δ3 point mutation by using specific primers to incorporate KpnI and BstBI restriction sites at the 5′ and 3′ ends of the 1 + 2 region, respectively. The fragments were cloned KpnI/BstBI into pT7v-Δ1Δ2(KpnI/BstBI). This construct was obtained by PCR with specific primer to insert KpnI/BstBI and BamHI restriction sites at the 5′ and 3′ ends of the Δ1Δ2 region, respectively.
pT7v-SV5Δ2/ΔT and pT7v-NSP5(S63,65A/S67D)/ΔT were obtained by PCR from pT7v-Δ2 and pT7v-NSP5(S63,65A/S67D) with specific primers and cloned KpnI/BamHI in pT7v-SV5[K/B] and pcDNA3, respectively.
pQE-Δ3, pQE-Δ3a, pQE-Δ3/S67A, pQE-NSP5, pQENSP5(S63,65A/S67D), and pQE-NSP5a were obtained by digestion KpnI/EcoRV of above described pcDNA3 construct and cloned KpnI/SmaI in pQE-30 (Qiagen, Valencia, CA).
Oligonucleotides. The internal primers used for the construction of pT7v mutants were 5′-CGGGGTACCATGTCTCTCAGC-3′ and 5′-CGCGGATCCTTAGTACTTTTTCTTA-3′ for pT7v-Δ3/ΔT, pT7v-SV5Δ2/ΔT, and pT7v-NSP5/(S63,65A/S67D)/ΔT, and 5′-AATGGTACCATGATTGGTAGGAG-3′ and 5′-CGCGGATCCTTAGTACTTTTTCTTA-3′ for pT7v-Δ1/Δ3/ΔT and pT7v-Δ1/ΔT. For double-step PCR of pT7v-Δ3a, pT7v-Δ3/S36A, pT7v-Δ3/S65A, and pT7v-Δ3/S67A were used as common primers 5′-CGGGGTACCATGTCTCTCAGC-3′ and 5′-CCATCGATTGCATTCGATCTAATCGAA-3′, and the specific oligonucleotides to introduce point mutations were 5′-GAGGATATTGGACCAGCAGATGCTGCTGCAAACGATCCACTAACCAGCTTTTCGATTAGATCGAATGCA-3′, 5′-GAGGATATTGGACCAGCAGATTCTGCTTCAAACGATCACTAACCAGCTTTTCGATTAGATCGAATGCA-3′, 5′-GAGGATATTGGACCATCTGATGCTGCTTCAAACGATCCACTAACCAGCTTTTCGATTAGATCGAATGCA-3′, 5′-GAGGATATTGGACCATCTGATTCTCTGCAAACGATCCACTAACCAGCTTTTCGATTAGATCGAATGCA-3′, and 5′-GAGGATATTGGACCAGCAGATGCTGCTGACAACGATCCACTAACCAGCTTTTCGATTAGATCGAATGCA-3′, respectively.
λ-Ppase Assay. Cellular extracts were treated essentially as described by Afrikanova et al. (9). In brief, cellular extracts were incubated with 1,600 units of λ-protein phosphatase (λ-Ppase) (New England Biolabs) in 50 mM Tris·HCl (pH 7.5), 0.1 mM EDTA, 5 mM DTT, 0.01% Brij 35, and 2 mM MnCl2. The mixture was incubated at 30°C for 2 h. Samples were loaded in SDS/PAGE (22) and analyzed by Western blot.
Expression and Purification of His-6-Tagged Proteins. pQE-Δ3, pQE-Δ3a, and pQE-Δ3/S67A were expressed in Escherichia coli M15[pREP4]. Culture was induced with isopropyl β-d-thiogalactoside (1 mM), grown for 4 h, and centrifuged at 3,500 rpm for 10 min. Pellet was resuspended in 6 ml of PBS and incubated for 15 min on ice with 0.1 mg/ml lysozyme, 5 mM DTT, 1.5% laurylsarcosine, and mixture inhibitor protease (Sigma). Lysate was sonicated (six times for 10 s each) and centrifuged at 10,000 rpm for 10 min. Supernatant was supplemented with 1% Triton X-100 and loaded onto a nickel column (Novagen) previously equilibrated in 5 vol of loading buffer (20 mM imidazol in PBS). The beads were washed in 10 vol of washing buffer (35 mM imidazol in PBS) and eluted in 2 vol of elution buffer (250 mM imidazol in PBS). The eluted protein was dialyzed against PBS and analyzed by SDS/PAGE and Coomassie blue staining.
pQE-wtNSP5, pQE-NSP5/(S63,65A/S67D), and pQE-NSP5a were expressed as described above. Pellet was resuspended in 4 ml of 20 mM Tris·HCl (pH 8.0) and inhibitor protease mixture, sonicated (four times for 10 s each), and centrifuged at 10,000 rpm for 10 min. Inclusion bodies were resuspended in 3 ml of cold 2 M urea, 20 mM Tris·HCl, 0.5 M NaCl, and 2% Triton X-100 (pH 8.0), sonicated, and centrifuged as above. A second wash in urea was performed, and a last wash was performed in a buffer lacking urea. The pellet was resuspended in 5 ml of 20 mM Tris·HCl, 0.5 M NaCl, 5 mM imidazol, and 6 M guanidine hydrochloride (pH 8.0), stirred for 45 min at room temperature, and centrifuged 15 min at 10,000 rpm. Supernatant was passed on a 0.45-μm filter and loaded onto a preequilibrated nickel column (Novagen). Column was washed five times in 20 mM Tris·HCl, 0.5 M NaCl, 20 mM imidazol, and 6 M urea (pH 8.0) and eluted in 10 ml of 20 mM Tris·HCl, 0.5 M NaCl, 6 M urea, and 150 mM imidazol, pH 8.0. The eluted protein was refolded by consecutive dialysis in 3, 1.5, and 0 M urea in 20 mM Tris·HCl (pH 8.0), 250 mM NaCl, and 1 mM DTT.
Peptides Synthesis. Peptides were synthesized by a solid-phase method by using fluorenylmethoxycarbonl (Fmoc) chemistry. Peptide DMA-GG was synthesized by using Fmoc-N,N-dimethylarginine(Mts)-OH as described (23). Peptides were purified by RPHPLC on a C-18 column (Waters RCM, 25 × 100 mm) by using a linear gradient of acetonitrile in water (0-35% over 70 min) containing 0.1% trifluoroacetic acid, and their identity was confirmed by electrospray ionization MS (PerkinElmer SCIEX API-150EX) and stored as lyophilized powder.
In Vitro CK1 Phosphorylation Assay. The assay has been described by Marin et al. (24). In brief, synthetic peptides substrates (0.2 mM) were phosphorylated by incubation in a medium (30 μl of final volume) containing 50 mM Hepes buffer (pH 7.5), 10 mM MgCl2, 150 mM NaCl, and 50 μM (9)γ-ATP (specific radioactivity 4,500-8,000 cpm/pmol). The reaction was started with addition of 16 units (107 units/pmol) of recombinant CK1α from zebrafish (Danio rerio) (25). After incubation at 30°C for the times indicated, the mixture was spotted onto phosphocellulose paper, which was washed three times in cold 75 mM H3PO4. Filter-associated radioactivity was measured by scintillation counting. Kinetic constants were determined by regression analysis of double-reciprocal plots constructed from initial-rate measurements. Protein phosphorylation was carried out in a reaction buffer containing 50 mM Hepes buffer (pH 7.5), 10 mM MgCl2, 150 mM NaCl, 50 μM[32P]-γ-ATP (12,000 cpm/pmol), and 1-2 μM of protein substrate, in a total volume of 14 μl. Then 23 units of recombinant CK1α (65 units/pmol) was added to initiate reactions; incubation was for 30 min at 30°C. Samples were analyzed by 12.5% SDS/PAGE and radioactive bands were detected in a Molecular Imager FX (Bio-Rad) apparatus.
Dimerization Assay. Cells were cotransfected and lysed as described above. The cellular extracts were loaded onto a nickel-agarose column for 30 min at 4°C equilibrated in loading buffer (20 mM imidazol in PBS). The samples were washed with 10 vol of washing buffer (35 mM imidazol in PBS) and eluted with 2 vol of elution buffer (250 mM imidazol in PBS). The recovered protein was analyzed by Western immunoblotting using anti-SV5 mAb (1:5,000).
Results
Activation of NSP5 Hyperphosphorylation in Vivo. Previous experiments have suggested that NSP5 hyperphosphorylation is the consequence of a complex autoregulatory mechanism in which the protein is, at the same time, substrate and activator of the process that leads to its hyperphosphorylation (18).
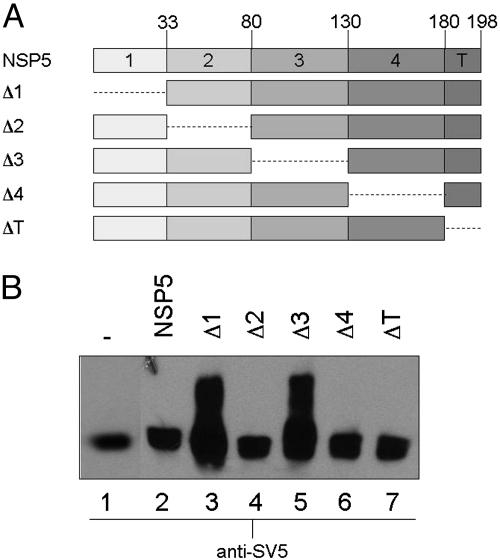
With the aim of understanding the molecular mechanism involved, we developed an in vivo coexpression assay with two plasmid constructs containing NSP5 or different NSP5 mutants. One of such constructs, used as substrate, contained an N-terminal fused SV5 tag (11 aa), to monitor hyperphosphorylation by PAGE mobility shift (a characteristic of NSP5 hyperphosphorylation) by Western blot, without interference of the protein encoded on the second construct, which was used as an activator of the hyperphosphorylation. NSP5 is not hyperphosphorylated when expressed alone, but it undergoes hyperphosphorylation when coexpressed with NSP2. Similarly, deletion of regions 1, 3, or both (mutants Δ1, Δ3, or Δ1/Δ3) renders transfected NSP5 able to be hyperphosphorylated, suggesting that the two domains act as internal inhibitors (11, 17, 18). We first tested two SV5-tagged NSP5 deletion mutants, SV5-Δ2 and SV5-Δ4 (Fig. 1), for their ability to be substrates when coexpressed with the putative activators mutants Δ3 or Δ1/Δ3. The two substrates were chosen because they were unable to be hyperphosphorylated when expressed alone.
Fig. 1.
Coexpression of SV5-Δ2 or SV5-Δ4 mutants with activators Δ1/Δ3 and Δ3. (A) Schematic representation of NSP5 and the deletion mutants constructs. (B) Coexpression assay in MA104 cells of SV5-Δ2 (lanes 1-3) or SV5-Δ4 (lanes 4-6) substrates with or without the indicated activators. (C) λ-Ppase treatment of SV5-Δ2 cotransfected with activator Δ3. Samples were loaded in a 15% SDS/PAGE, and substrates were visualized by Western blot.
As shown in Fig. 1, both substrates migrated as a single (not phosphorylated) band when transfected alone, whereas when coexpressed with either of the two activators, they completely differed in their behavior. Mutant SV5-Δ2 was sensitive to both activators, whereas SV5-Δ4 was not. λ-Ppase treatment confirmed that the PAGE mobility-shift was caused by phosphorylation (Fig. 1C). Among the single-domain deletion mutants, Δ1 and Δ3 were the only ones able to behave as activators of hyperphosphorylation (Fig. 2). These results indicated that the activator molecule was acting in trans in relation to the substrate.
Fig. 2.
Activation by NSP5 single domain deletion mutants. (A) Schematic representation of the single domain deletion mutants. (B) Anti-SV5 immunoblot of total cellular extracts from MA104 cells cotransfected with SV5-Δ2 substrate and the indicated mutants. Samples were run in a 15% SDS/PAGE.
Ser-67 and the Activation Function. From the results presented above, activation and substrate activities could be clearly distinguished. They also suggested that the activation function was associated to region 2. We therefore focused our attention into two different Ser-rich motifs within this domain, namely: Ser-Asp-Ser-Ala-Ser from residue 63 to residue 67 and Ser-Phe-Ser-Ile-Arg-Ser from residue 73 to residue 78, that we termed motifs a and b, respectively (Fig. 3A). To investigate whether they were involved in the activation step, both motifs were independently mutated, in the activator construct Δ3, transforming Ser residues into Ala. As shown in Fig. 3, mutation of all three Sers of motif a (Ser-63, Ser-65, and Ser-67, construct Δ3a) completely abolished activation function, whereas mutation of Ser in motif b (construct Δ3b) had no effect. A more detailed analysis of Ser in motif a revealed a key role for Ser-67. In fact, the single Ser-67Ala mutation eliminated activation, suggesting that phosphorylation of this residue was required for activation function (Fig. 3C). This was further confirmed with a construct in which Ser-67 was mutated to Asp to mimic a phosphorylated residue, with the two other Sers in motif a, Ser-63 and Ser-65, mutated to Ala. As shown in Fig. 3C, lane 5, the Ser-67Asp mutation (construct Δ3/S63,65A/S67D) restored activation. Furthermore, the single Ser-67Asp mutation was sufficient to render, the otherwise inactive full-length NSP5, able to be hyperphosphorylated despite the presence of the inhibitory domains 1 and 3 (Fig. 3D).
Fig. 3.
Ser-67 from motif a is essential for activation. (A) Scheme of point mutations in the activator Δ3. Motifs a and b are indicated. The S→ A and S→ D modifications are indicated in red and green, respectively. (B) Coexpression of SV5-Δ2 substrate with activators Δ3a, Δ3b, or Δ3. (C) Coexpression of SV5-Δ2 substrate with activators Δ3, Δ3a, Δ3/S67A, or Δ3(S63,65A/S67D). (D) Scheme of full-length NSP5 with point mutations on Sers in motif a (Left), and coexpression of SV5-Δ2 with the NSP5 point mutants (Right). Cellular extracts were loaded on a 15% SDS/PAGE, and substrate was visualized by Western blot.
Taken together these results demonstrated that in a first step, phosphorylation of Ser-67 is necessary for NSP5 to become an activator: once phosphorylation of Ser-67 has taken place, both domains 1 and 3 do not hinder hyperphosphorylation any longer. It appears that these domains prevent phosphorylation of Ser-67, probably by impairing its accessibility. This interpretation was supported by the fact that wtNSP5, and more importantly the activation negative NSP5a, (with Ser in motif a mutated to Ala), were efficiently hyperphosphorylated when coexpressed with an activator (such as Δ1/Δ3), but not when expressed alone (Fig. 4A). Furthermore, transfected NSP5/S67D, but not NSP5/S67A or NSP5a, became hyperphosphorylated, in a way similar to the protein obtained from virus-infected cells (Fig. 4B).
Fig. 4.
Activator-dependent wt NSP5 phosphorylation. (A) Coexpression in MA104 cells of wtNSP5 or NSP5a with the activator Δ1/Δ3. Immunoblot developed with anti-NSP5 serum. The arrow indicates the position of Δ1/Δ3. Bracket indicates NSP5 phosphorylated forms. (B) Expression of full-length wtNSP5 and NSP5 point mutants. Lane 5 corresponds to NSP5 from rotavirus-infected MA104 cells. Samples were migrated in a 15% SDS/PAGE and visualized by Western blot.
Ser-67 Phosphorylation and CK1. The sequence similarity between the consensus phosphorylation sites of protein kinase 1, also known as CK1 and motif a, suggested that CK1 could be involved in the phosphorylation of Ser-67. This possibility was investigated in an in vitro kinase assay by using recombinant CK1α (from zebrafish) and two synthetic substrate peptides of 14 residues encompassing motif a, from residues E58 to L71: peptide NSP5wt had the NSP5 wt sequence whereas peptide NSP5(S67A) contained a Ser→Ala mutation in the position corresponding to Ser-67. As shown in Fig. 5A, NSP5wt was substrate of CK1α, whereas peptide NSP5(S67A) was not phosphorylated at all. When compared to a CK1 consensus-specific substrate peptide (RRKHMIGDDDDAYSITA) (25), phosphorylation of peptide NSP5wt by CK1α showed a similar Km (specific peptide Km = 283 μM; NSP5wt Km = 218 μM) but a lower catalytic constant of 1.7 min-1 for the NSP5wt peptide vs. 50 min-1 for the specific substrate. This result indicated CK1 as a candidate enzyme involved in the phosphorylation of Ser-67 to render NSP5 susceptible to hyperphosphorylation. Similar experiments with recombinant CK2 showed that this kinase does not phosphorylate Ser-67 (data not shown).
Fig. 5.
NSP5 phosphorylation by CK1. (A) Time course of CK1α phosphorylation of motif a peptides derived from NSP5. Phosphorylations were carried out as described in Materials and Methods by using peptides at 200 μM concentration and recombinant zebrafish CK1α. The amino acidic sequence of both peptides is indicated. NSP5wt (•) and NSP5(S67A) (○). (B) SDS/PAGE autoradiography of in vitro phosphorylation assay of mutants Δ3, Δ3/S67A, and Δ3a (Left) or wtNSP5,NSP5/(S63,65A/S67D) and NSP5a (Right), with recombinant CK1α and [32P]-γATP. The 38-kDa band corresponds to autophosphorylated CK1α. Reactions were carried out at the indicated concentrations of substrate proteins. Negative control reactions containing either CK1α alone (lanes 1 and 9) or substrate Δ3 alone (lane 8) are shown.
However, when the involvement of CK1 in NSP5 hyperphosphorylation was further investigated in the in vitro assay by using as substrate the bacteria-expressed proteins, a surprising result was obtained. CK1α was able to phosphorylate only the activation positive substrates Δ3 or NSP5/(S63,65A/S67D) generating mobility shifted species with higher apparent molecular weights. The whole process depended on the phosphorylation of Ser-67 because the Ser-67Ala point mutation (construct Δ3/S67A) as well as mutation into Ala of all three Ser residues of motif a (constructs Δ3a and NSP5a) completely abolished phosphorylation activity (Fig. 5B). This result strongly suggested that CK1 or a CK1-like activity, directly participates in NSP5 phosphorylation in vivo.
The C-Terminal Tail in Substrate and Activator. Because of the transactivation shown above, we investigated the role of the 18-aa C-terminal tail, which has been described to be responsible of NSP5 dimerization (18, 26). We found the presence of the tail was essential, both in the activator and the substrate. As seen in Fig. 6A, deletion of the tail in the activators Δ1, Δ1/Δ3, and Δ3 completely abolished their activity. This effect was not caused by deficient phosphorylation of Ser-67, because the tailless mutant carrying the Ser-67Asp activation mark (Δ3/S67D/ΔT) was also activation deficient (Fig. 6A, lanes 9 and 10).
Fig. 6.
The C-terminal tail is required for substrate and activator functions. (A) Coexpression of SV5-Δ2 in the presence of activator deletion mutants of NSP5 with and without the 18-aa tail region (lanes 3, 5, 8, and 10). The immunoblots were detected with an anti-SV5 Ab. (B) Western immunoblots. Coexpression of substrates SV5-Δ2 (lanes 1 and 2) and SV5-Δ2/ΔT (lanes 3 and 4) with and without activator Δ3(Left) and expression of NSP5 or NSP5 point mutants with (lanes 5, 6, 9, and 10) or without (lanes 7, 8, 11, and 12) the C-terminal tailpiece, in the presence or absence of the activator Δ1/Δ3, as indicated (Right). The arrow shows the position of the activator protein, and bracket shows the phosphorylated forms of NSP5. (C) Pull-down of SV5-Δ2 or SV5-Δ2/ΔT substrates coexpressed with the activator His-6-Δ1/Δ3. Cellular extracts were passed through a nickel column, and the indicated fractions were run in a 15% SDS/PAGE.
Substrate activity was also severely impaired by deletion of the tail as shown for substrates SV5-Δ2/ΔT and NSP5/ΔT (Fig. 6B). As in the case of the activators, deletion of the tail in the NSP5/S67D mutant did not yield hyperphosphorylation (Fig. 6B, lanes 9-12). Taken together these results suggested that an interaction between activator and substrate was required for the final hyperphosphorylation outcome. This interaction was confirmed in pull-down experiments. Coexpression of the His-6-tagged activator Δ1/Δ3 (His-6-Δ1/Δ3) with substrates SV5-Δ2 or SV5-Δ2/ΔT, followed by purification on a nickel column and Western blot with anti-SV5, revealed efficient copurification of SV5-Δ2 (>80% retention) but not of the substrate-negative mutant SV5-Δ2/ΔT (Fig. 6C).
Discussion
Using different NSP5 mutants, in this article we show uncoupling of two functions associated to NSP5 phosphorylation, namely, substrate and activation. For instance, mutant SV5-Δ2 was an excellent substrate but not an activator, whereas mutant Δ3 was both and activator and a substrate. Furthermore, we have shown that NSP5, which is not hyperphosphorylated when expressed alone, can acquire this property if only Ser-67 is mutated into Asp. It is worth noting that Ser-67, as well as motif a, are highly conserved in all NSP5 from group A rotaviruses.
The dissociation of the two NSP5 functions allowed us to have an insight into the mechanism, indicating that the activator molecule can act in trans in relation to the substrate. This fact was further supported by the demonstration that interaction between both components, activator and substrate, through the previously characterized dimerizing tailpiece was crucial.
The use of deletion mutants proved to be a useful strategy to understanding the complex process of NSP5 hyperphosphorylation. In several reports, NSP5 was attributed to be itself a kinase leading to its autophosphorylation (8-12, 27). Instead, NSP5 appears to autoregulate its own phosphorylation, in a process that requires at least two different steps. In the first step, NSP5 acquires its activation function through the phosphorylation of Ser-67, most likely through the action of protein kinase CK1. As seen when NSP5 is expressed alone, this event cannot take place unless NSP5 is interacting with NSP2 (11), a circumstance occurring during the virus infectious cycle. Presumably, as a consequence of this interaction, region 2, and in particular Ser-67, becomes exposed and available for phosphorylation. In this case NSP2 would work as a molecular matchmaker, similar to the phosphorylation of NS5A of hepatitis C virus, which gets phosphorylated by a cellular kinase, in a process determined by the interaction of NS5A with NS3 (28).
Interestingly, in vitro phosphorylation of NSP5 by CK1 depended on the negative charge in residue 67 (Ser-67Asp mutation), resembling the performance in vivo. Moreover, mutants involving deletion of domains 1, 3, or both have a similar effect to the interaction with NSP2, in terms of rendering these molecules efficient activators. It is possible that these two domains interact between them, hiding region 2 for Ser-67 phosphorylation. From the experiments in which Ser-67 was mutated to Asp we can conclude that the introduction of the negative charge in that position is an event required only for activation, because also Ser-67Ala mutants were good substrates for hyperphosphorylation. Furthermore, mutant SV5-Δ2 was also hyperphosphorylated when expressed in cells sustaining rotavirus replication, in which wtNSP5, after interaction with NSP2, would perform the activator function (data not shown). Interestingly, CK1α was also able to phosphorylate other regions of NSP5 in a Ser-67-dependent mode, suggesting a sort of hierarchical phosphorylation. This event was reproduced in vitro, with the wtNSP5 carrying the activation mutation Ser-67Asp, further confirming that phosphorylation of Ser-67 was the first step of the process. These results strongly suggested that CK1, or a CK1-like activity, may be the enzyme required for both phosphorylation steps in vivo.
It is worth mentioning that mutations involving motif a appear not to alter NSP5 structure, as revealed by the capacity of NSP5a, NSP5/S67A, or NSP5/(S63,65A/S67D) to form viroplasms-like structures when coexpressed with NSP2 (C.E. and O.R.B., unpublished results). Moreover, it has recently been reported that the NTPase activity of NSP2 is not linked to NSP5 hyperphosphorylation or the formation of viroplasms-like structures (29).
We can also rule out that the results here reported depend on the alternative ORF present in NSP5-coding region, that in some strains constitute the nonstructural protein NSP6 of 91 aa. Our constructs were all derived from the coding region of the rotavirus strain OSU, which contains a truncated ORF2 version coding for only 51 aa. In addition, such ORF2 is present neither in the substrate mutant SV5-Δ2, nor in the activator mutant Δ3. Furthermore, an OSU NSP5/S67D variant in which the AUG initiation codon of the ORF2 was deliberately mutated to ACG without changing the amino acid sequence of NSP5, was hyperphosphorylated in vivo as efficiently as the original NSP5 (data not shown).
We would like to propose a model of NSP5 hyperphosphorylation (Fig. 7), involving phosphorylation of Ser-67 by CK1, followed by, or concomitantly with dimerization of activator and substrate through the C-terminal tailpiece. The Ser-67 phosphorylated component activates the other monomer of the dimeric complex rendering it (or both) susceptible to hyperphosphorylation, by the same CK1 or a CK1-like enzyme.
Fig. 7.
Model for rotavirus NSP5 hyperphosphorylation. Interaction of NSP2 with monomeric (a) or dimeric (b) NSP5 promotes phosphorylation of Ser-67 by CK1 (in either one or both subunits), which in the case of the monomer may induce dimerization (c). A phosphorylated Ser-67 in the dimer induces conformational changes (d) to render the other partner molecule (and probably both) susceptible to hyperphosphorylation by the CK1-like cellular kinase (e).
Acknowledgments
We are grateful to M. Campagna for pT7v-NSP5/NSP6- construct. C.E. was supported by a Scuola Internazionale Superiore di Studi Avanzati predoctoral fellowship, European Community Grant QLK2-CT-2000-00739, and an International Center for Genetic Engineering and Biotechnology postdoctoral fellowship. This work was supported in part by European Community Grant QLK2-CT-2000-00739, Fondo Nacional de Investigacion Científica y Tecnologica-Chile Grant 1030462, and the Wellcome Trust (to J.E.A.).
Author contributions: C.E., G.J., J.E.A., and O.R.B. designed research; C.E., G.J., and B.M. performed research; C.E., G.J., J.E.A., and O.R.B. analyzed data; and C.E., J.E.A., and O.R.B. wrote the paper.
Abbreviation: CK1α, casein kinase 1α.
References
- 1.Petrie, B. L., Greenberg, H. B., Graham, D. Y. & Estes, M. K. (1984) Virus Res. 1, 133-152. [DOI] [PubMed] [Google Scholar]
- 2.Gallegos, C. O. & Patton, J. T. (1989) Virology 172, 616-627. [DOI] [PubMed] [Google Scholar]
- 3.Zeng, C. Q., Estes, M. K., Charpilienne, A. & Cohen, J. (1998) J. Virol. 72, 201-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patton, J. T., Jones, M. T., Kalbach, A. N., He, Y. W. & Xiaobo, J. (1997) J. Virol. 71, 9618-9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wentz, M. J., Zeng, C. Q., Patton, J. T., Estes, M. K. & Ramig, R. F. (1996) Arch. Virol. Suppl. 12, 59-67. [DOI] [PubMed] [Google Scholar]
- 6.Patton, J. T. & Gallegos, C. O. (1990) J. Gen. Virol. 71, 1087-1094. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez, S. A. & Burrone, O. R. (1991) Virology 182, 8-16. [DOI] [PubMed] [Google Scholar]
- 8.Welch, S. K., Crawford, S. E. & Estes, M. K. (1989) J. Virol. 63, 3974-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afrikanova, I., Miozzo, M. C., Giambiagi, S. & Burrone, O. (1996) J. Gen. Virol. 77, 2059-2065. [DOI] [PubMed] [Google Scholar]
- 10.Blackhall, J., Munoz, M., Fuentes, A. & Magnusson, G. (1998) J. Virol. 72, 6398-6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afrikanova, I., Fabbretti, E., Miozzo, M. C. & Burrone, O. R. (1998) J. Gen. Virol. 79, 2679-2686. [DOI] [PubMed] [Google Scholar]
- 12.Poncet, D., Lindenbaum, P., L'Haridon, R. & Cohen, J. (1997) J. Virol. 71, 34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berois, M., Sapin, C., Erk, I., Poncet, D. & Cohen, J. (2003) J. Virol. 77, 1757-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vende, P., Taraporewala, Z. F. & Patton, J. T. (2002) J. Virol. 76, 5291-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichwald, C., Rodriguez, J. F. & Burrone, O. R. (2004) J. Gen. Virol. 85, 625-634. [DOI] [PubMed] [Google Scholar]
- 16.Mohan, K. V., Muller, J., Som, I. & Atreya, C. D. (2003) J. Virol. 77, 12184-12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabbretti, E., Afrikanova, I., Vascotto, F. & Burrone, O. R. (1999) J. Gen. Virol. 80, 333-339. [DOI] [PubMed] [Google Scholar]
- 18.Eichwald, C., Vascotto, F., Fabbretti, E. & Burrone, O. R. (2002) J. Virol. 76, 3461-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estes, M. K., Graham, D. Y., Gerba, C. P. & Smith, E. M. (1979) J. Virol. 31, 810-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanke, T., Szawlowski, P. & Randall, R. E. (1992) J. Gen. Virol. 73, 653-660. [DOI] [PubMed] [Google Scholar]
- 21.Fuerst, T. R., Niles, E. G., Studier, F. W. & Moss, B. (1986) Proc. Natl. Acad. Sci. USA 83, 8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli, U. K. (1970) Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- 23.Szekelt, Z., Zakhariev, S., Guarnaccia, C., Antcheva, N. & Pongor, S. (1999) Tetrahedron Lett. 40, 4439-4442. [Google Scholar]
- 24.Marin, O., Burzio, V., Boschetti, M., Meggio, F., Allende, C. C., Allende, J. E. & Pinna, L. A. (2002) Biochemistry 41, 618-627. [DOI] [PubMed] [Google Scholar]
- 25.Marin, O., Bustos, V. H., Cesaro, L., Meggio, F., Pagano, M. A., Antonelli, M., Allende, C. C., Pinna, L. A. & Allende, J. E. (2003) Proc. Natl. Acad. Sci. USA 100, 10193-10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres-Vega, M. A., Gonzalez, R. A., Duarte, M., Poncet, D., Lopez, S. & Arias, C. F. (2000) J. Gen. Virol. 81, 821-830. [DOI] [PubMed] [Google Scholar]
- 27.Blackhall, J., Fuentes, A., Hansen, K. & Magnusson, G. (1997) J. Virol. 71, 138-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulze zur Wiesch, J., Schmitz, H., Borowski, E. & Borowski, P. (2003) Arch. Virol. 148, 1247-1267. [DOI] [PubMed] [Google Scholar]
- 29.Carpio, R. V., Gonzalez-Nilo, F. D., Jayaram, H., Spencer, E., Prasad, B. V., Patton, J. T. & Taraporewala, Z. F. (2004) J. Biol. Chem. 279, 10624-10633. [DOI] [PubMed] [Google Scholar]